Abstract
Oriented behaviour is present in almost all animals, indicating that it is an ancient feature that has emerged from animal brains hundreds of millions of years ago. Although many complex navigation strategies have been described, each strategy can be broken down into a series of elementary navigational decisions. In each moment in time an animal has to compare its current heading with its desired direction and compensate for any mismatch by producing a steering response either to the right or to the left. Different from reflex driven movements, target directed navigation is not only initiated in response to sensory input, but also takes into account previous experience and motivational state. Once a series of elementary decisions are chained together to form one of many coherent navigation strategies, the animal can pursue a navigational target, e.g. a food source, a nest entrance, or a constant flight direction during migrations. Insects show a great variety of complex navigation behaviours and, owing to their small brains, the pursuit of the neural circuits controlling navigation has made substantial progress over the last years. A brain region as ancient as insects themselves, called the central complex, has emerged as the likely navigation centre of the brain. Research across many species has shown that the central complex contains the circuitry that might comprise the neural substrate of elementary navigational decisions. While this region is also involved in a wide range of other functions, we hypothesise in this review that its role in mediating the animal’s next move during target directed behaviour is its ancestral function, around which other functions have been layered over the course of evolution.
Introduction
A defining feature of animals is their ability to move. Movements range from reflex-driven escape responses to highly optimized foraging trips in complex environments. All oriented movements that are not solely controlled by reflexes are considered navigational behaviours for the purpose of this review. Irrespective of whether these behaviours last for seconds, hours, or months, they have in common that they are directed towards either a transient or a stable navigational target. This target can be a randomly chosen heading relative to a landmark or compass direction, an inherited migratory bearing, a food stimulus, or the site of an animal’s nest (Heinze, 2017). While diverse strategies can be used to generate a coherent, target directed navigation behaviour, for each of them an animal has to internally define a desired direction. To be able to align its body with this direction, it additionally has to establish its own heading angle. If the two directions do not match, a body turn has to be initiated to compensate for the difference.
While this basic idea applies to most animals, insects have proven to be powerful model species to illuminate the underlying behavioural and neural principles. This can be attributed to the rich repertoire of sophisticated navigation behaviours that insects achieve despite their comparably simple brains. The insect brain is uniquely accessible for detailed functional and anatomical examination at the level of single cells, neural circuits and entire brain regions. Large strides have thus been made recently to uncover the neural basis of navigation in a range of species, findings that can now be related to the results from decades of behavioural work.
A brain region called the central complex (CX) has shifted into the focus of this work and has gained the status of the navigation centre of insect brains (Pfeiffer and Homberg, 2014; Turner-Evans and Jayaraman, 2016; Heinze, 2017). Whereas navigational control is clearly not the only function of the CX, we propose the idea that the neural circuits responsible for fundamental navigation decisions might represent the ancestral role of this brain region, to which secondary functions have been subsequently added in response to increasingly complex demands of different species’ ecologies.
The central complex
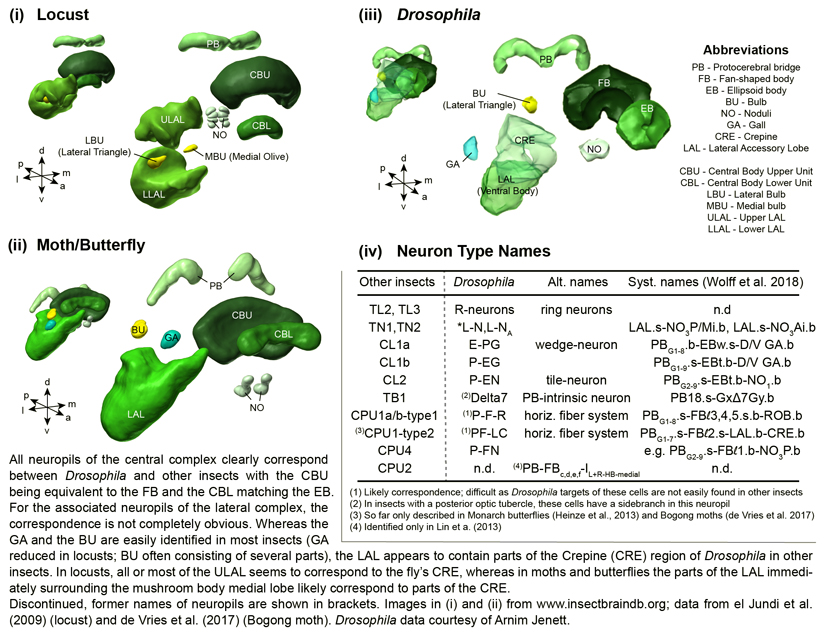
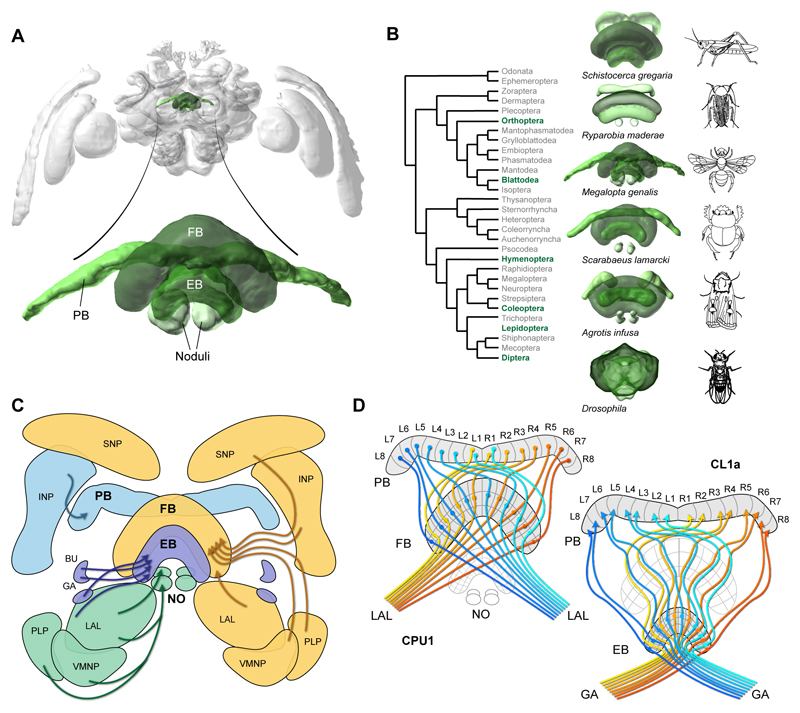
The CX is a unique midline neuropil found in all insects examined to date. It originated more than 400 million years ago and has changed intriguingly little over all this time (Homberg, 2008; Strausfeld, 2009). In all species it consists of the fan-shaped body and ellipsoid body (FB, EB; for alterative names see Box 1), the protocerebral bridge (PB) and a pair of noduli (Pfeiffer and Homberg, 2014; Strausfeld, 2012). A characteristic feature of the CX is its highly regular neuroarchitecture consisting of 16-18 vertical columns intersected by horizontal layers (Heinze and Homberg, 2008; Homberg, 2008; Williams, 1975; Wolff et al., 2015, Lin et al., 2013). Tangential cells provide input to entire horizontal layers of each CX neuropil from a great variety of brain regions (Fig.1C). Different types of columnar cells innervate single columns and, with each cell type existing in sets of eight to nine individual neurons per hemisphere, in principle one for each column (e.g. Heinze and Homberg, 2008; Wolff and Rubin, 2018) (Fig.1D). They link single PB-columns with corresponding columns in either the FB or the EB, thereby producing a stereotypical, interhemispheric projection matrix (Fig.1D). The main output pathway connecting the CX to downstream brain centres is also composed of large columnar cells projecting to the lateral accessory lobes (LAL; Franconville et al., 2018; Heinze and Homberg, 2008; Heinze et al., 2013).
Box 1. Nomenclature of CX neuropils and neurons across insect species.
Figure.
Fig. 1. Anatomy of the central complex (CX).
(A) Location of the CX (colour) in the insect brain (sweatbee Megalopta genalis, from Stone et al., 2017). Image source: www.insectbraindb.org. (B) The CX is conserved across a wide range of insects. Data from el Jundi et al. (2009) (locust), Wei et al. (2010) (cockroach), Stone et al. (2017) (sweat bee), Immonen et al. (2017) (dung beetle), de Vries et al. (2017) (Bogong moth), Jenett et al. (2012) (Drosophila). Images from www.insectbraindb.org for all species except cockroach and Drosophila. (C) Input pathways to the different CX-components. Input to the PB, EB, and noduli (NO) is shown on the left side, while FB-input is shown on the right. (D) Columnar neurons form highly stereotypical intrinsic connections between the PB and the FB/EB. Note that different cell types form projection patterns that are shifted with respect to one another, so that identical PB-columns are mapped to different FB/EB-columns. Based on locusts and Monarch butterfly (Heinze and Homberg, 2008; Heinze et al., 2013).
As is required for navigational control, the CX has a variety of sensory inputs. The coding of celestial compass cues has been most thoroughly investigated, but neurons of the CX also respond to mechanosensory information from the antenna and wings, visual features of the environment, as well as large-field motion cues. Yet, the CX is not primarily a sensory brain region, but has long been known to be involved in locomotor control (Strausfeld, 1999; Strauss and Heisenberg, 1993). Evidence was obtained from Drosophila mutants with structural CX defects and associated locomotor deficiencies (Strauss, 2002; Triphan et al., 2010), as well as from surgical lesions, electrophysiological recordings and injection of electrical current in behaving cockroaches (Harley and Ritzmann, 2010; Ridgel et al., 2007; Bender et al., 2010; Guo and Ritzmann, 2013, Martin et al. 2015). The latter experiments established a causal relation between CX activity and control of specific movement of the animals.
In addition to sensory-motor integration, the CX is instrumental for a range of other phenomena. The Drosophila CX is required for spatial working memory during navigation tasks (Ofstad et al., 2011; Neuser et al., 2008). Changes in properties of CX responses according to the animal’s behavioural and motivational state were found in Drosophila (Weir and Dickinson, 2015; Weir et al., 2014) and cockroaches (Martin et al., 2015). Additionally, the insect’s arousal level affects whether CX-output leads to motor action or not, as shown by data on sleep control and gating of locomotor behaviour in the Drosophila CX (Donlea et al., 2018). Finally, the CX plays a direct role in sensing the nutritional state of the animal (Drosophila) (Park et al., 2016) and encodes memory of behaviourally relevant (aversive) visual shapes (Liu et al., 2006).
Navigational decisions as common framework for CX function
How can all these data be aligned within a common framework of CX-function? As outlined above, a comparison between the desired heading and the current body orientation is essential for all planned, targeted behaviours. If these two angles do not align, compensatory steering decisions have to be generated. These elementary navigational decisions have to be carried out on a moment to moment basis and are independent of the overall navigation strategy. If this hypothesis is true, navigation strategies should only differ in the way the intended heading is computed and in the sensory information used to compute the current heading. The identical basic circuit could thus mediate all navigation behaviours, with slight modifications adjusting for subtly different demands of specific strategies. Additionally, whether a sensory input is selected as a potential target for navigation during this elementary decision process can be expected to depend on the animal’s motivational state and its previous experience; features that attach relevance to sensory information. In the light of this hypothesis, we will review diverse behavioural strategies and the role of the CX-circuits in mediating them in the following sections.
Behavioural strategies
Straight line orientation
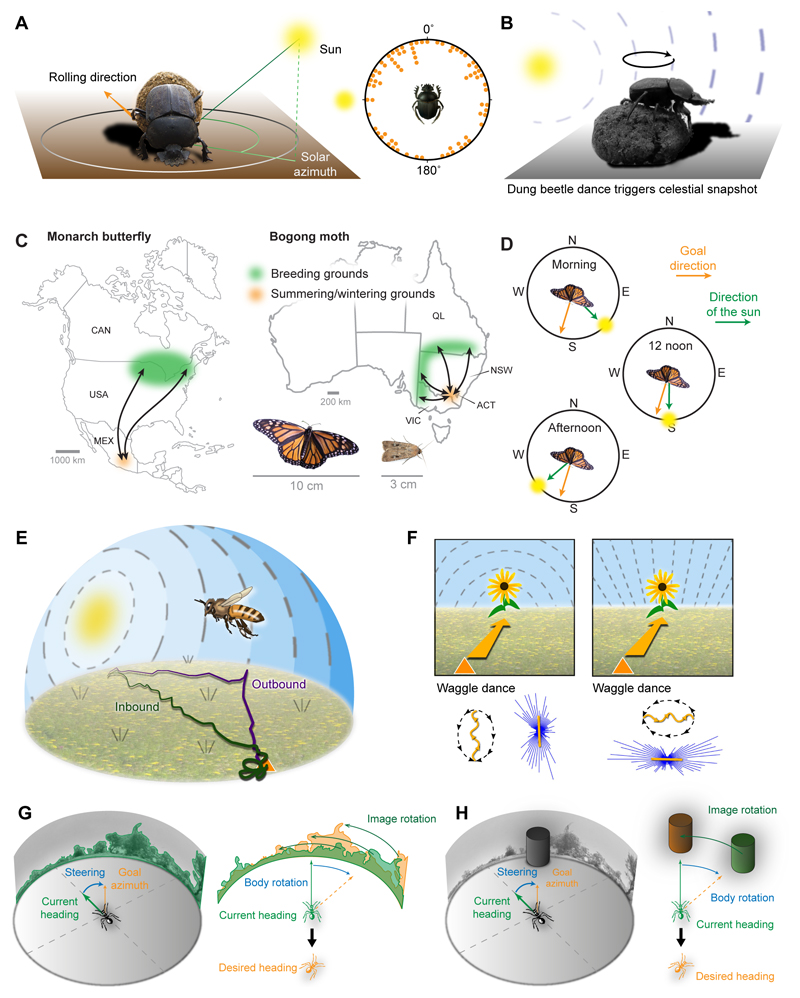
Ball rolling dung beetles depend on moving along a straight course (Byrne et al., 2003). They shape their dung balls when they arrive at a dung pile and have to escape this area of fierce competition as quickly as possible to permanently secure their ball as food reservoir. The most efficient way to get away from the dung pile is to roll along a straight course into a randomly chosen direction (Fig.2A) (Byrne et al., 2003; el Jundi et al., 2016). Yet, this seemingly simple task is far from trivial. Without external reference, any animal, including humans, will very quickly walk in circles (Cheung et al., 2007). Indeed, a variety of celestial cues keep these beetles on a straight path. They not only use the Sun (Dacke et al., 2014) and the skylight polarization pattern (el Jundi et al., 2014b), but also the skylight intensity gradient (el Jundi et al., 2014b) and the spectral gradient (el Jundi et al., 2015a). Nocturnal species use the Moon (Dacke et al., 2004), the nocturnal polarization pattern (Dacke et al., 2003), and the Milky Way (Dacke et al., 2013) for keeping their ball-rolling trajectories straight. Similarly to dung beetles, Drosophila was recently shown to follow straight courses in randomly chosen directions relative to a simulated Sun (Giraldo et al., 2018) or a stable landmark (Green et al., 2018). In both insects this behaviour depends on a functioning CX.
Fig. 2. Insect navigation behaviours.
(A) Ball-rolling dung beetles use celestial cues for choosing a random travel direction. Left: illustration of behaviour; right: rolling directions are random with respect to the Sun (data from Byrne et al., 2003). (B) During a circular dance on the dung ball these beetles take a snapshot of the celestial cue constellation to decide on a rolling direction. Beetle images in A/B courtesy of B. el Jundi and M. Dacke. (C) The Monarch butterfly and the Bogong moth migratory routes. Both insects fly over 1000 km from their breeding grounds to the wintering/summering sites. (D) When navigating by celestial cues, migrating insects need to compensate for the time of day. (E) A honeybee foraging flight with navigation-relevant visual cues used to compute a vector representation of the hive’s position (path integration). Triangle: hive. Looping pattern at the end of inbound flight: systematic search to locate nest entrance. Flight track adapted from Degen et al. (2016). (F) Skylight polarization is an ambiguous directional cue. Bees communicate their directional knowledge from the outbound flight (top panels) via the waggle dance (bottom panels). Dance distributions are bidirectional when only polarized light cues are available; adapted from Evangelista et al. (2014). (G) Image matching, as performed by ants in rich visual environments (Collett et al., 2013), is used for following habitual routes. A series of snapshots of the visual panorama are compared to current views and both are brought to a best match via body rotations (Zeil, 2012). (H) Landmark based navigation uses salient visual features independent of the surrounding panorama in a similar way to overall image matching. G/H adapted from Heinze, 2017.
Long distance migration
Long distance migrations are seasonal movements of animals from one region to another to escape unfavourable conditions in their habitats. While this strategy is vastly different from the dung beetle’s rolling behaviour in geographical and temporal scales, both involve following a straight course until an animal reaches the anticipated favourable conditions, without the need to measure distance. The basic navigation decisions at each moment in time are thus similar between the strategies. A key difference is, however, that the direction of migration is not randomly chosen, but genetically fixed across the entire population of migrating animals within a species. The best described long-distance migrators among insects are the Monarch butterfly (Danaus plexippus) (Reppert et al., 2010), the Bogong moth (Agrotis infusa) (Warrant et al., 2016) and the desert locust (Schistocerca gregaria) (Homberg, 2015) (Fig.2C). Indeed, in all three species, the CX has been implicated in processing navigation relevant sensory information. While much of the evidence for the involvement of the CX has resulted from locust studies (Homberg et al., 2011), the migratory patterns of this species are less well understood than those of the Monarch butterfly or the Bogong moth, with locust swarms often migrating downwind rather than towards a specific target (Homberg, 2015).
Migrating over thousands of kilometres to a specific region is challenging. First, the migratory heading needs to be followed precisely, as any deviation would mean missing the target. Second, after weeks of following their migratory heading, the insects have to stop close to the target area to find their precise resting site – certain mountain tops in the case of the Monarch butterfly (Merlin et al., 2012) and specific alpine caves for the Bogong moth (Warrant et al., 2016). It is unknown what triggers the switch between the long-range migratory behaviour and the short-distance searching behaviour. While both species might use olfactory cues to find their target sites, concrete evidence supporting this hypothesis is still lacking and neural work has focused on the long-range component of migration.
Path integration
Different from the previous two behaviours, finding back to a place of origin (homing) requires distance tracking in addition to directional information. Path integration is a computational strategy that enables homing. During path integration, direction and distance information are continuously integrated to update the animal’s internal estimate of its current position in relation to a fixed origin. Arthropods, especially central-place foraging hymenopterans such as ants and bees, have long been known to use this strategy for returning home in a straight line after a tortuous outbound trip to find food (Fig.2E) (for review, see e.g. Heinze, et al. 2018, Wehner and Srinivasan, 2003). Recently, path integration was found to also occur without the context of homing, when Drosophila was shown to use it for returning back to a food source after performing short exploratory excursions (Kim and Dickinson, 2017).
The internal estimate of the animal’s position with respect to an origin contains information about the shortest path to that point, i.e. the home vector. It is computed by continually integrating memories of the distances travelled in each direction during the outbound trip (Fig.2E) (Wehner and Srinivasan, 2003; Heinze et al. 2018). The directional component of the home vector becomes the desired heading as soon as an insect on a foraging trip has found enough food to warrant carrying it back to the nest. The path integrator thus switches from ‘accumulating’ to ‘following’. Once the end of the vector has been reached a systematic search for the nest entrance is initiated (Fig.2E). Search behaviour is key for successful homing, as it compensates for the errors accumulated during path integration (Cheng et al., 1999; Wehner and Srinivasan, 1981; Heinze et al. 2018).
Behavioural readouts, i.e. homing flights, search behaviour, and the honeybee waggle dance (Fig.2E,F), are visible manifestations of the internal position estimates acquired via path integration. Through studying these behaviours in bees and ants we have gained substantial insight into the nature of the insect’s path integrator. Directional (compass) information is obtained by observing celestial cues (Fig.2E,F) (Evangelista et al., 2014; Wehner and Müller, 2006), generating an internal reference frame in global coordinates. By accumulating optic flow, i.e. the apparent movement of the environment across the retina resulting from self-motion, during flight, bees also derive distance information from visual information (Esch and Burns, 1996; Srinivasan et al., 2000). In contrast, the pedestrian ants primarily use a step integrator for estimating distance (Wittlinger et al., 2006).
Visual route following
One feature shared between all previously described navigation strategies is that they do not involve long term memory. Either the target direction is inherited (long distance migrants) or it is maintained for a short time only, indicating that the information is stored in working memory. The latter applies to the home vector during path integration as well as to the direction of ball rolling that a dung beetle chooses for a particular trip. Yet, there are other navigation behaviours that require long term memory (Collett and Collett, 2002). The most prominent such strategy is route following, using landmarks or visual snapshots (Collett, 2010; Zeil et al., 2014). Like path integration, route following is used for homing in hymenopteran insects (ants, bees and wasps) and many species rely on a combination of both strategies to return to the nest (Collett, 2012). Particularly in landmark rich environments, these insects learn the arrangement of visual features along the foraging path (Narendra, 2007; Narendra et al., 2013). The established routes are stable over time and override information from path integration, especially close to the nest (Wystrach et al., 2015, Wehner et al., 1996; Kohler and Wehner, 2005). The memory of such routes likely comprises snapshots of the visual panorama around the nest and along the taken path, which are matched to the currently perceived visual input (Fig.2G,H; Zeil et al., 2014). By attempting to maximize the match between the current view and the memorized view, the insect can follow a valley of minimal image difference and thus locate home using a familiar route (Zeil et al., 2003).
Directional information in the central complex – the internal compass
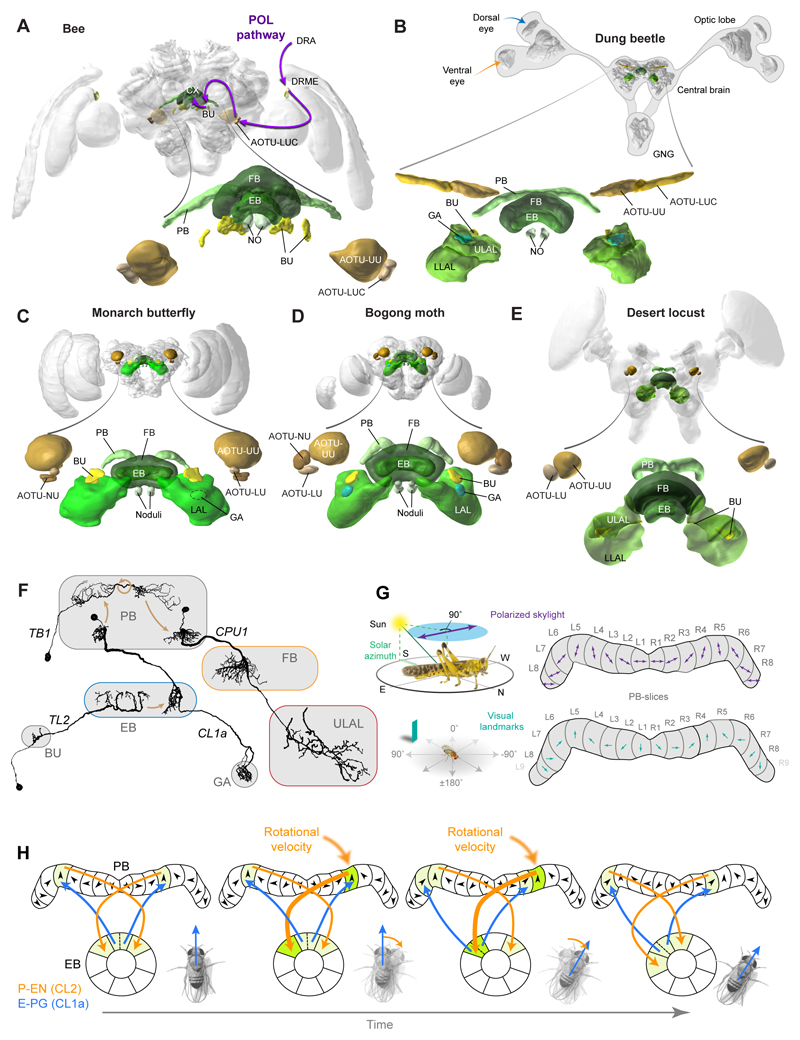
One piece of information that is required for all of the described strategies is directional input that is used to compute the body orientation with respect to the environment. Across locusts, butterflies, beetles, flies and bees this direction coding involves highly conserved brain regions: the AOTU, the lateral complex, and the CX (Fig.3A-E). Many neurons in these regions are tuned to celestial compass cues and indicate the animal’s azimuth by modulating their firing rates (Homberg et al., 2011; Heinze and Reppert, 2011; Stone et al., 2017; el Jundi et al., 2015b). In flies, visual landmarks (Seelig and Jayaraman, 2013), rotational optic flow, and non-visual angular velocity cues (Green et al., 2017; Turner-Evans et al., 2017) were shown to mediate direction coding in neurons homologous to the sky compass neurons. While cockroach neurons were recorded that responded similarly to those of flies, their morphological identity was not yet identified (Varga and Ritzmann, 2016). Importantly, allothetic (external) cues are represented in the CX at the same time as idiothetic (internally generated) cues, suggesting that diverse sources of information are continuously integrated to generate a robust representation of body orientation.
Fig. 3. Compass encoding in the insect CX.
(A) Brain of tropical bee Megalopta genalis. Highlighted are neuropils involved in compass encoding (CX, lateral complex, AOTU) as well as the pathway for processing polarized skylight (data from Zeller et al. 2015, Pfeiffer and Kinoshita, 2012). (B-E) Brains with highlighted navigation relevant regions for dung beetle (B), Monarch butterfly (C), Bogong moth (D), and desert locust (E). Data from Immonen et al. (2017), Heinze et al. (2013), de Vries et al. (2017), el Jundi et al. (2009). Images from www.insectbraindb.org. (F) Neurons involved in processing of compass cues in the dung beetle (images courtesy of B. el Jundi). (G) Top: Representation of the angles of polarized skylight in the PB-columns of the locust (Heinze and Homberg, 2007). Bottom: Corresponding mapping of body orientation of Drosophila with respect to surrounding landmarks (Seelig and Jayaraman, 2015). (H) Tracking of heading-directions in Drosophila: Asymmetrical activity in the P-EN neurons (CL2 in other insects) of the right and left PB hemispheres during turning shifts the E-PG-neuron encoded activity bump in the EB, allowing angular integration of body rotations. Arrowheads: directional tunings of E-PG neurons; adapted from Green et al. (2017).
Neurons of the compass pathway upstream of the CX (Fig.3A) already encode heading based on multiple celestial cues in an integrated way, such as the position of the Sun combined with the skylight polarization pattern (Heinze and Reppert, 2011; el Jundi et al., 2014a; el Jundi et al., 2015b; Pegel et al., 2018) or polarized light combined with spectral information (Pfeiffer and Homberg, 2007). This use of several, partly redundant visual compass cues likely increases the accuracy of the heading estimate. Additionally, shown via behavioural studies, the geomagnetic field is used by migratory butterflies (Guerra et al., 2014), ants (Fleischmann et al., 2018) and moths (Dreyer et al., 2018), information that might feed into the same circuit. In Bogong moths magnetic fields are used in conjunction with visual landmark cues, suggesting that the integration of multiple sensory modalities indeed provides an advantage when elaborate navigation abilities are required (Dreyer et al., 2018).
After directional information reaches the CX it is translated into a coordinated activity pattern across a population of columnar neurons connecting the EB with the PB (E-PG cells), yielding a single activity bump across the width of both structures that tracks the animal’s current heading (Seelig and Jayaraman, 2015). Each of the eight PB-columns in each hemisphere thus represents one azimuth direction, together tiling the entire horizon and conveying meaning to the regular, columnar neuroarchitecture (Fig.3G). In flies this heading signal was found to be tethered to visual features of the environment, i.e. provide directional reference based on local cues (Seelig and Jayaraman, 2015). In migratory locusts, a similar mapping of directions was found in PB neurons tuned to polarized skylight, thus indicating a heading code rooted in a global, Sun-based reference frame (Heinze and Homberg, 2007). Different from flies, in which the zero-point of the direction map (phase) shifts arbitrarily between individuals and experiments, the polarized-light based direction map in locusts is identical across individuals. Flies therefor appear to remap their compass based on local cues in each new environment, while locusts appear to possess a fixed reference frame (Fig.3G).
While the heading direction activity bump is linked to external cues, in Drosophila and cockroaches, corresponding activity was also detected in darkness, revealing that idiothetic rotational cues can also be used to generate and update the position of the observed activity bump (Green et al., 2017; Turner-Evans et al., 2017). Angular movement of the animal in one direction yields increased activity in columnar cells called P-EN neurons in one hemisphere of the PB. Between the PB and EB, these cells are recurrently connected with the E-PG-cells and an offset in the columnar projections between both cell types (Wolff et al., 2015) leads to a shift of the activity-bump position along the heading map, whenever the activity of P-EN cells in one hemisphere exceeds that of the other hemisphere (Fig.3H). Due to the excitatory recurrence between E-PG and P-EN cells that underlies this bump shifting, inhibition is required to prevent the excitation from spreading through all CX-columns. Indeed, in Drosophila, global inhibition, likely mediated via Delta7-cells (Franconville et al., 2018), is combined with local recurrent excitation to form a ring attractor circuit (Kim et al., 2017). While this circuit allows for tracking the fly’s angular movements even in darkness, it does not yet explain how external directional cues are integrated with idiothetic rotation cues.
Integration of external cues is essential to avoid the accumulation of errors resulting from continuous estimate of body rotations (Cheung et al., 2007). Without allothetic calibration, the described Drosophila circuit by itself cannot be used for tracking body angles for path integration over distances relevant to bee homing (Heinze et al, 2018) or for long-range migration.
Even though the systematic mapping of headings in the PB found in locusts and Drosophila has not yet been extended to other insects, all the major neuron types identified as components of the compass circuit across the different CX-neuropils (TL, CL, TB1 and CPU1-neurons) also exist in dung beetles (el Jundi et al., 2015b), Monarch butterflies (Heinze and Reppert, 2011) and bees (Stone et al., 2017). As these cells resemble their locust counterparts in great detail (Fig.3F), it seems likely that the ordered heading representation in the PB-columns is conserved across insects. If true, this general encoding of body orientation solves the fundamental problem of representing the current heading of the animal. How is this direction code combined with other information and ultimately used to drive concrete navigation behaviours?
Integrating directions and distance
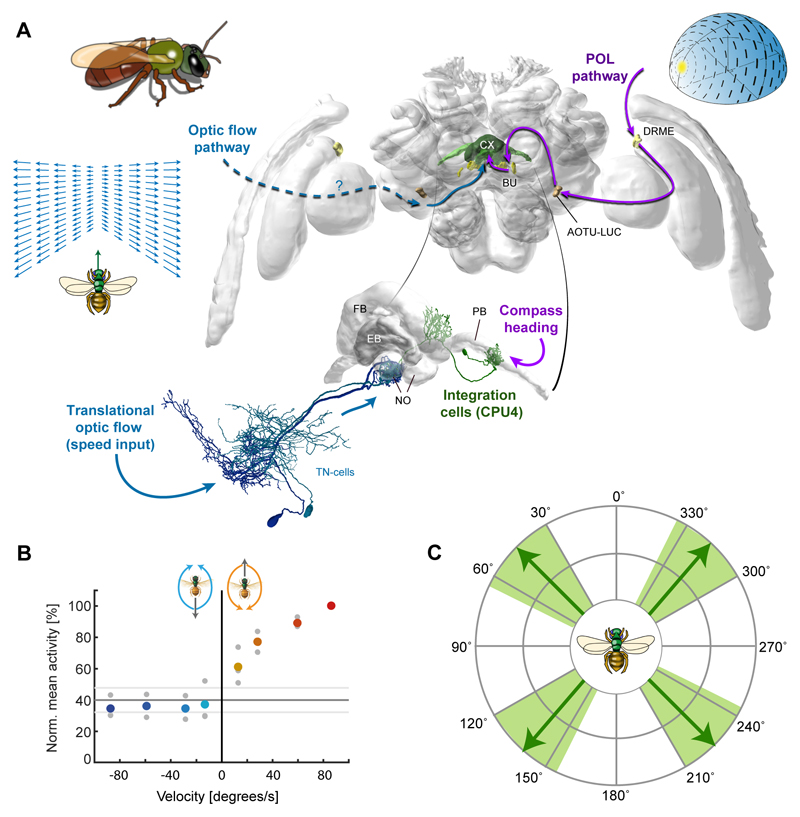
How the current-heading representation is integrated with other information is not known. However, examining path integration in bees with a combination of electrophysiology, anatomy and modelling has led to a testable hypothesis about neural circuit mechanisms downstream of the described heading encoding. For path integration, information about the animal’s forward velocity has to be combined with heading information (Srinivasan, 2015). In the tropical bee Megalopta optic-flow based speed signals were shown to arrive at the CX via two types of noduli neurons (TN-neurons; Fig.4A), revealing that optic flow converges with compass information in the CX (Stone et al., 2017). While one type is inhibited by simulated forward flight and excited by simulated backward flight, the other behaves the opposite way (Fig.4B). Together, the detailed response characteristics of these cells (Fig.4C) allow the faithful encoding of holonomic movements, i.e. bee-typical movements during which the movement direction is misaligned with body orientation.
Fig. 4. Convergence of sensory information in the CX as a basis for path integration.
(A) Convergence of speed and direction input in the CX of the bee Megalopta (left). CX-location (green) in the brain is shown alongside polarized-light input pathway (purple arrows) and the likely route for optic flow information (blue arrows). Insert: proposed cellular substrate for convergence for speed and direction inputs are the CPU4 columnar neurons, one of which is depicted (green). At least 18 CPU4-cells exist for each CX-column. (B) Normalized mean activity of three TN2 ‘speed’-neurons in response to different velocities of translational optic flow. Coloured circles, mean activity; grey circles, individual data points; solid lines, background activity ± SD. Figure from Stone et al, (2017), with permission from Elsevier. (C) The four TN-neurons in the Megalopta CX possess four preferred expansion points of translational optic flow (as predicted by local motion tunings), providing the basis for holonomic motion encoding; based on Stone et al. (2017).
The converging distance and direction information must be continuously integrated and stored to generate a path integration memory. Anatomical data suggests a columnar cell type of the FB, the CPU4-neurons, as neural substrate for this integration (P-FN in Drosophila) (Stone et al., 2017). These neurons have arborisations in the PB, the noduli and the FB (Fig.4A), an anatomy that, in theory, could allow compass information from the PB and optic flow information from the noduli to reach these cells. Direct synaptic contacts between TN ‘speed’-neurons and fibres likely belonging to CPU4-cells in the noduli (Stone et al., 2017), combined with reported polarized-light responses in locust CPU4-cells (Heinze and Homberg, 2009), support this hypothesis. Dendritic arbours overlapping with CPU4 output branches suggest the main CX output cells (CPU1-cells; PF-LC cells in Drosophila) as principal targets of these cells.
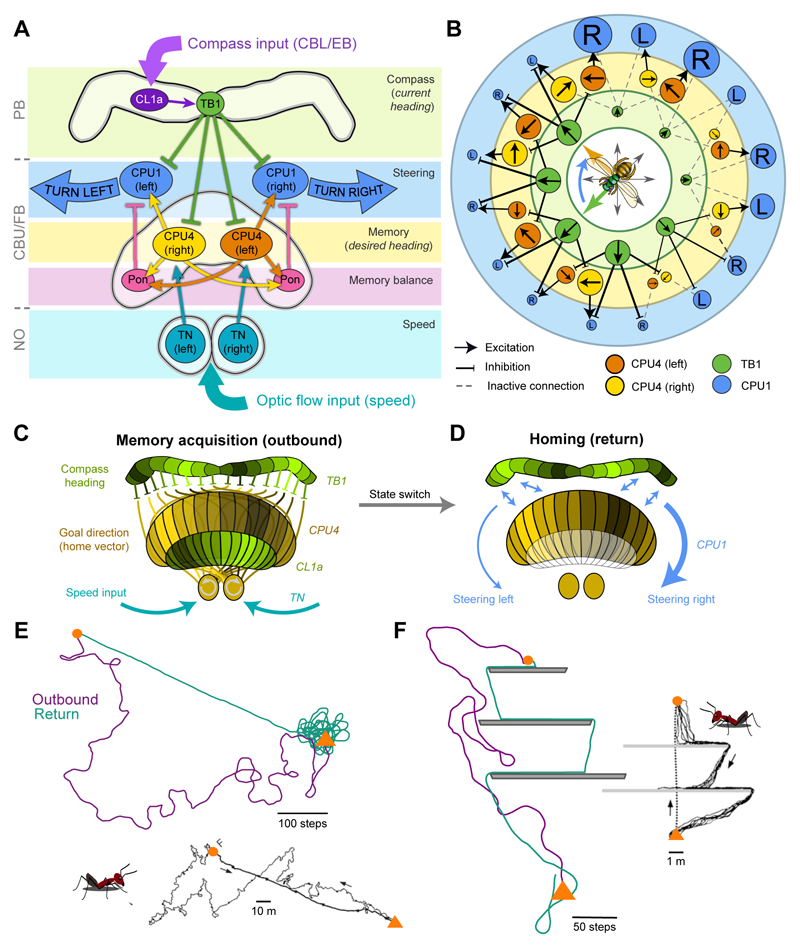
To generate an activity-based working memory by accumulating optic flow over time, Stone et al. (2017) postulate recurrent connectivity between the PB and noduli between all CPU4-cells of one column. To gain sufficient capacity for encoding and maintaining accumulated neural activity, a large number of neurons would be needed. Supporting this idea, each CX-column in the bumblebee (Bombus terrestris) contains at least 18 individual CPU4-neurons (Stone et al., 2017). In the model, TB1 direction-cells directly inhibit the proposed memory cells (CPU4), which additionally receive speed input in the noduli. The least inhibited memory cells can thus accumulate optic-flow-based speed information. Across the CPU4-population such a hypothetical memory mechanism would yield an array of direction-locked odometers (Figs.5B/C,6A). Throughout any foraging trip, the population of these cells could thus encode distance and direction of the animal with respect to the nest (the home vector) as a pattern of neural activity distributed across the FB-columns.
Fig. 5. The CX as neural substrate for path integration.
(A) Schematic connections between cell types in the proposed Megalopta path integrator network (Stone et al., 2017). Arrows: excitation; blunt ends: inhibition. Pon, Pontine neurons. (B) Topology of the path integration model circuit. Circles represent neurons, size of neurons indicate activity level, colour-code for model layers and cell groups as in (A). Arrows in the circles: directional tuning (TB1) and integrated direction preference (CPU4); R: right turn; L: left turn; compass rose: current (green) and desired heading (orange). (C) Illustration of the two activity bumps resulting from encoding current heading (green, in PB and EB) and target direction (yellow, in FB). The target direction results from integrating speed and compass information. (D) Steering is induced by comparing PB-activity with FB-activity column by column. The resulting imbalance in CPU1-neuron activity between the right and left side causes steering. (E) Homing behaviour produced by the path integrator model compared to desert ant data (below). Near the nest site (triangle) the model initiates searching behaviour. (F) Comparison of a desert ant (right) and the model negotiating obstacles during the homeward journey. E/F from Stone et al. (2017); inserts in E/F modified from Wehner (2003) (reproduced with permission from Elsevier).
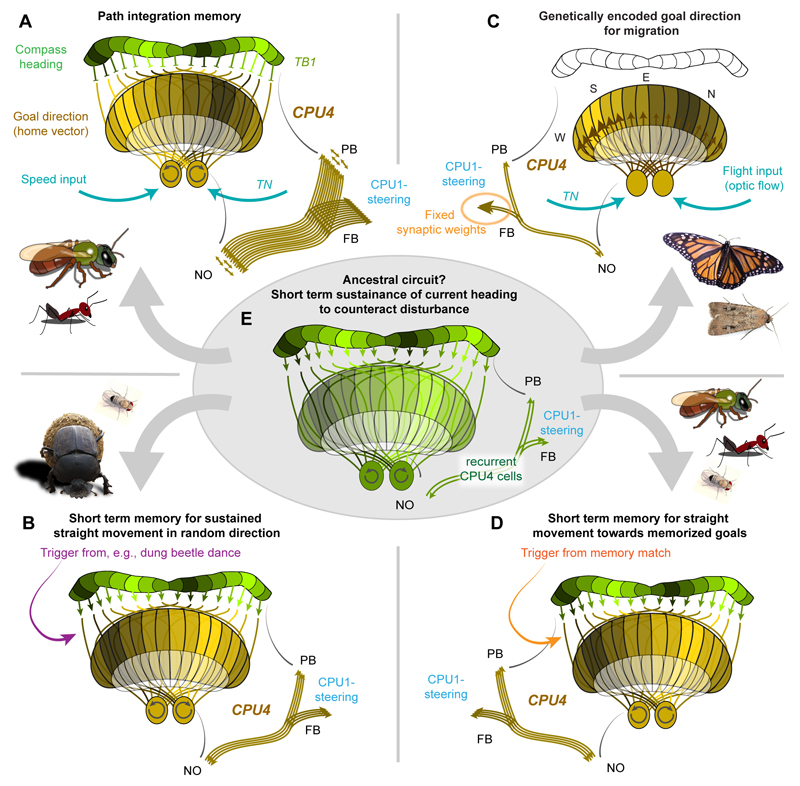
Fig. 6. A common framework for encoding navigational decisions in the insect CX.
(A) Illustration of how compass signals from the PB could be integrated with a speed signal arriving at the noduli to produce a distributed representation of the home vector across FB-columns during path integration, based on the model by Stone et al. (2017). In the model, the home-vector memory resides in CPU4-neurons, which form recurrent connections between PB-columns and the NO. At least 18 individual CPU4-cells per column exist in bumblebees (Stone et al., 2017), suited to form local microcircuits to sustain a lasting, activity-based working memory. CPU4-output in the FB could directly signal the desired heading to steering cells. (B) Similarly, a direction code can be achieved for straight-line orientation in dung beetles (or menotactic orientation in random directions in general) by transferring the current heading signal from the PB to CPU4-neurons. A modulatory signal, e.g. during the dung beetle dance, could trigger the imprinting of the direction code on the CPU4-population. Stability of the memory over the time of the behaviour would require several CPU4-neurons per column. (C) Adjusting synaptic weights between CPU4 and CPU1-cells in a sinusoid manner would allow genetic encoding of migratory directions. Evenly distributed activity across all CPU4-cells (driven by optic flow input) would signal an activity bump to the CPU1-cells according to synaptic weight distribution, guiding the animal towards the migratory heading upon deviation. (D) A similar mechanism could be used for route following based on memorized visual snapshots. If the current view of the animal matches a memorized snapshot, the resulting positive valence output from the memory centres (e.g. mushroom body) could serve as trigger to imprint the current view as temporary desired heading (Collett and Collett, 2018). (E) Based on the path integration model by Stone et al. (2017) we propose a simplified circuit, lacking memory, speed input and the large number of CPU4-cells as possible ancestral circuit, suited to store a copy of the current heading representation in CPU4-neurons for a short amount of time. In case of disturbance, the original heading can be regained via the CPU1 based steering mechanism, using the information stored in CPU4-cells.
Towards a general concept of encoding desired headings
How generally applicable is the outlined representation of the home vector? In dung beetles, the suggested similarity of current-heading encoding would allow the navigational control circuitry that uses this information to be laid out in a similar way as well. Different from homing, the beetle’s intended heading is a random direction (Byrne et al., 2003; el Jundi et al., 2016). The ball-rolling direction is chosen when the beetle performs a short circular dance on top of its freshly formed dung ball, just before embarking on its trip (Fig.2B) (el Jundi et al., 2016). During this time the beetles take a celestial snapshot, i.e. memorize the current configuration of all available celestial cues. Whenever the beetle gets disturbed during the subsequent trip, it repeats this dance to realign itself with the originally chosen direction (el Jundi et al., 2016). These animals thus have to store a single angle within the reference frame provided by the available celestial information. Whereas this desired direction does not contain any distance information, it nevertheless resembles the angular component of the home vector resulting from path integration. It therefore seems conceivable that the same neurons proposed to encode the home vector as a population-coded activity bump across the FB-columns (CPU4) also have this function in dung beetles. How could the activity bump in these neurons be created during the beetle’s orientation dance? While the answer is unknown, one can speculate that the activity pattern generated by the heading code in the PB is transferred to CPU4-cells in a randomly chosen moment during the dance. Similar to the home vector, this activity bump would be maintained by the recurrent CPU4 connections between noduli and PB (Fig.6B). To allow the imprinting of the current heading onto the CPU4-population, the dance behaviour would have to generate a short sensitive period, e.g. by causing the release of neuromodulators that facilitate synaptic transmission between the head-direction cells and the CPU4-cells.
In the context of migration, the described path integration model also suggests a possibility of how a genetically fixed migratory heading might be encoded in the CX. It could involve the same CPU4-neurons suggested to encode the home vector. In migrants, a CPU4 mediated desired heading would have to be genetically fixed to always point towards the migratory target. In theory, this could be realized by sinusoidally adjusting the weights of the CPU4 output synapses. Any cell receiving their population activity would sense an activity bump, even though the ongoing activity of each CPU4-cell would be identical due to shared, flight driven optic-flow input (Fig.6C) (Heinze, 2017). To reverse the target direction for the return migration the synaptic weight distribution across the FB-columns could be inverted by external stimuli during the resting period. Indeed, in Monarch butterflies, the reversal of their migratory direction is triggered by exposure to cold temperatures mimicking conditions in their overwintering grounds (Guerra and Reppert, 2013).
During long range migration, using celestial cues as a reference for determining headings presents an additional problem that can be solved in one of several ways using the presented model. The position of celestial cues depends on the Earth’s rotation and thus on daytime. If an animal were to simply follow the Sun, its apparent movement throughout the day would lead the animal in circles. Therefore, the Sun’s azimuth needs to be reinterpreted according to time of day to maintain a constant bearing (Fig.2D). This means that either the compass itself has to shift its zero-point relative to the Sun over the course of the day (allowing the representation of the target direction to remain constant), or the representation of the target direction would have to shift with respect to the compass. Alternatively, the output of the entire circuit could be modulated according to time of day. While a recent study models how the Sun-compass may be time compensated (Shlizerman et al., 2016), it does not suggest a specific neural substrate and it remains to be shown whether the neural circuitry for representing current and desired headings is involved in azimuth compensation.
Route following differs from the three behaviours already described in that the navigational target is recorded in long term memory. These memories can last for a lifetime and have been proposed to reside in the mushroom body (Ardin et al., 2016). As no direct connection exists between the mushroom bodies and the CX, it is currently unknown whether or how this strategy can be aligned with the above described circuitry. In principle however, during route following the insect also has to match current and desired headings. Given that the current heading is likely encoded in the PB and steering likely involves the CX-output, it would be most parsimonious to assume that the desired heading for route following is also encoded in the FB-columns (Fig.6E). Indeed, tangential FB input-neurons possess dendrites in regions of the brain that receive mushroom body output projections (Heinze et al., 2013; Young and Armstrong, 2010). These output cells of the mushroom body generally encode the valence of a sensory cue, i.e. indicate whether a perceived cue had been associated with a positive or negative experience (Aso et al., 2014). During route following this would mean that whenever the image similarity between the current view and a remembered view is high, the output would signal a positive valence, which could be interpreted as a trigger to fix the current heading, e.g. based on skylight polarization or visual landmarks, as goal direction (Collett and Collett, 2018). A positive match could thus trigger an imprinting of the current head-direction activity pattern in the PB onto the population of CPU4-neurons in the FB in a way similar to that proposed for the dung beetle (Fig.6E).
Navigational steering
Across all strategies, once the navigating animal has determined its desired and current headings, it has to steer to compensate mismatches. During homing via path integration, steering commands have been proposed to originate in the CX output-cells (CPU1). These neurons are anatomically suited to combine the signals of CPU4 memory-neurons with the input from TB1 compass-neurons (Figs.5A-C,7B; Stone et al., 2017), thereby comparing activity patterns representing the current and desired headings. As the projection pattern of CPU1-cells is shifted by at least one column with respect to CPU4-neurons (in opposite directions in each hemisphere) (Heinze and Homberg, 2008; Wolff et al., 2015), the comparison of TB1 and CPU4 activity patterns will lead to an imbalance in the total activation of CPU1-cells between the right and the left hemispheres, whenever the two activity patterns are not aligned (Stone et al., 2017). This uneven activation will cause steering into the direction that will push the animal back towards its goal (Fig.5).
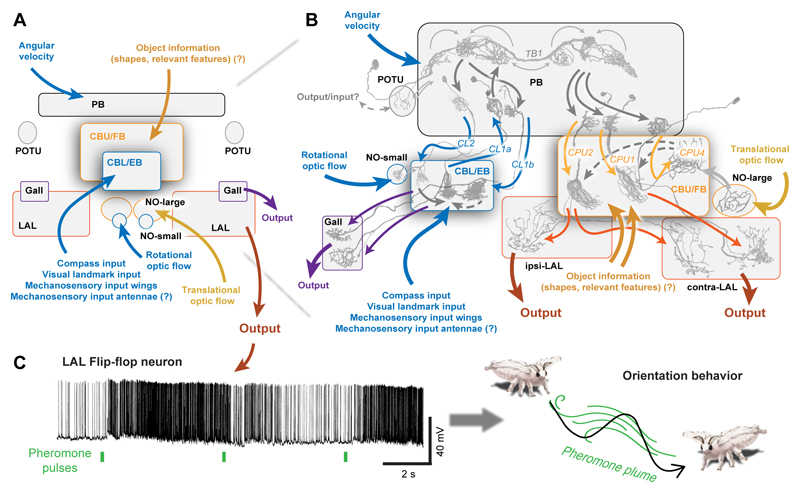
Fig. 7. Sensory-motor transformation in the CX as basis for navigational decisions.
(A) Summary of sensory input and possible output pathways of the CX. (B) Detailed information flow within the CX. Several sensory pathways convey different information to different levels of the two main CX-circuits, the FB-circuit (orange) and the EB-circuit (blue). Question marks: proposed input pathways inferred from physiological data lacking anatomical confirmation. Grey arrows: connections verified in Drosophila (Franconville et al., 2018). Dashed grey arrows: proposed connections. Coloured arrows: information flow. (C) Left: A flip-flop LAL-neuron in the moth Bombyx mori responds to pheromone pulses with inversion of activity. Adapted from Mishima and Kanzaki (1998). Right: Illustration of the zig-zagging plume tracking behaviour initiated by the flip-flop neurons.
While this direct impact of CPU1-cells on steering is hypothetical, two findings support the proposed model. First, data from cockroaches has revealed direct effects of CX-neurons on turning behaviour (Martin et al., 2015). And second, studies in the moth Bombyx mori revealed that the LAL contains descending “flip-flop” cells that are directly involved in steering (Namiki and Kanzaki, 2016; Olberg, 1983). These bi-stable neurons “flip” between two activity states, a highly active and a less active one, in response to a pheromone pulse or a light flash, and the contralateral flip-flop neuron is usually in the opposite state to the ipsilateral one. As the flip-flop neurons project directly onto neck motor neurons, each flip of activity leads to changed activity of the neck motor neurons, which ultimately leads to a head turn (Mishima and Kanzaki, 1998, 1999) (Fig.7C). Flip-flop neurons have so far only been shown to be involved in short-distance pheromone following, where they underlie the zig-zagging trajectory the male moth uses to follow a female’s pheromone trail. However, they are also ideally suited to transform the activity imbalance between the right and left CPU1-populations (CX-output) into a steering decision during homing, straight-line orientation and for long distance migration (Namiki and Kanzaki, 2016). Moreover, the possible convergence of path integration memory and the desired heading for route following on the same CPU1-neurons would also allow dynamic weighting of the target directions signalled by both strategies during homing.
Elementary navigation decisions as ancestral function of the CX?
As outlined in the beginning, navigation can be broken down into elementary decisions. At each moment in time, the animal has to decide whether a right turn or a left turn will bring its current heading in better alignment with its target direction. This applies equally to straight-line orientation, long-range navigation, homing by path integration or homing by route following. The main difference between navigation strategies is how the desired heading is computed (Fig.6A-D). Once a neural representation of that direction exists, the process of comparing it to the current heading and the generation of steering commands faces the same constraints and, therefore, could be solved in a similar way. This suggests that, in principle, the circuit motifs found in the CX could be the basis for elementary navigational decisions.
Given that all main types of CX-cells known from across species are required for the outlined circuit to function, the computation it performs likely emerged when the CX evolved as a neuropil and might constitute the principle computation of this brain area since the origin of insects. However, it is difficult to conceive that the complex navigational strategies of today’s insects are equally ancient. What relevance could this circuit have had when it originally evolved?
We propose that elementary navigation decisions were the prime challenge faced by early arthropods. Having evolved keen senses and matching motor abilities, purely reflex-driven chains of automatic movements would not have put these new abilities to adequate use. Planned, target directed navigation became necessary, a task achievable by a simplified CX path integration circuit (Fig.6E). Whenever the animal moves into a specific direction (towards or relative to a visual feature), the heading signal of the PB would be transferred to a recurrent circuit formed by the CPU4-cells. The resulting activity bump resonating between the PB and the noduli would be more stable than the actual heading direction bump in the PB and mismatches between the two could be compared by the CPU1 steering-cells. Upon deviation from the stored direction, the circuit would push the movement direction back on track, an idea that is consistent with the findings surrounding the principle of insect “turn alternation” (e.g. Dingle, 1964). Without sophisticated working memory implemented in the CPU4-circuit, the stored activity bump would only allow to return to the original course for a short time, and a new target would be followed once the previous memory has faded. This would enable a strategy during which an early insect could have explored its environment in a series of short, straight movements, selecting targets based on its sensory abilities and species-specific preferences (Fig.6E).
Given the fundamental nature of elementary navigational decisions and the existence of unpaired midline neuropils beyond insects (Homberg, 2008; Thoen et al., 2017), this circuit might have even preceded the CX. A simpler circuit with lower spatial resolution than the one resulting from the population code based on eight static reference vectors found in today’s insect CX would still have enabled navigation decisions, albeit with less precision. Systematically exploring the structure and function of CX-circuits in basal insects and more distantly related arthropods could thus lead the way towards identifying the origins of elementary behavioural decisions.
Acknowledgements
The authors are grateful for financial support from the following organisations: the Swedish Research Council (VR, 621-2012-2213, to S.H.), the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 714599, to S.H.), the Air Force Office for Scientific Research (AFOSR) (2012-02205 to Eric Warrant and S.H.), and the Wenner-Gren Foundation to A.H.
Abbreviations
- FB
fan-shaped body
- EB
ellipsoid body
- CX
central complex
- PB
protocerebral bridge
- NO
noduli
- AOTU
anterior optic tubercle
- BU
bulb
- GA
gall
- LAL
lateral accessory lobe
- LU
lower unit
- NU
nodular unit
- UU
upper unit
- ULAL
upper lateral accessory lobe
- LLAL
lower lateral accessory lobe
- GNG
gnathal ganglion
- PLP
posterior lateral protocerebrum
- VMNP
ventromedial neuropils
- INP
inferior neuropils
- SNP
superior neuropils
- DRA
dorsal rim area
- DRME
dorsal rim medulla
- AOTU-LUC
lower unit complex of the anterior optic tubercle
References
- Ardin P, Peng F, Mangan M, Lagogiannis K, Webb B. Using an Insect Mushroom Body Circuit to Encode Route Memory in Complex Natural Environments. PLoS Comput Biol. 2016;12:e1004683. doi: 10.1371/journal.pcbi.1004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20:921–926. doi: 10.1016/j.cub.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Byrne MJ, Dacke M, Nordström P, Scholtz CH, Warrant EJ. Visual cues used by ball-rolling dung beetles for orientation. J Comp Physiol A. 2003;189:411–418. doi: 10.1007/s00359-003-0415-1. [DOI] [PubMed] [Google Scholar]
- Cheng K, Srinivasan MV, Zhang S. Error is proportional to distance measured by honeybees: Weber’s law in the odometer. Anim Cogn. 1999;2:11–16. [Google Scholar]
- Cheung A, Zhang S, Stricker C, Srinivasan MV. Animal navigation: the difficulty of moving in a straight line. Biological Cybernetics. 2007;97:47–61. doi: 10.1007/s00422-007-0158-0. [DOI] [PubMed] [Google Scholar]
- Collett M. How desert ants use a visual landmark for guidance along a habitual route. Proc Natl Acad Sci USA. 2010;107:11638–11643. doi: 10.1073/pnas.1001401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. How navigational guidance systems are combined in a desert ant. Curr Biol. 2012;22:927–932. doi: 10.1016/j.cub.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Collett M, Collett TS. How does the insect central complex use mushroom body output for steering? Curr Biol. 2018;28:R733–R734. doi: 10.1016/j.cub.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Collett TS, Collett M. Memory use in insect visual navigation. Nat Rev Neurosci. 2002;3:542–552. doi: 10.1038/nrn872. [DOI] [PubMed] [Google Scholar]
- Collett M, Chittka L, Collett TS. Spatial memory in insect navigation. Curr Biol. 2013;23:R789–800. doi: 10.1016/j.cub.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Dacke M, Baird E, Byrne MJ, Scholtz CH, Warrant EJ. Dung beetles use the Milky Way for orientation. Curr Biol. 2013;23:298–300. doi: 10.1016/j.cub.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Dacke M, Byrne MJ, Scholtz CH, Warrant EJ. Lunar orientation in a beetle. Proc Biol Sci. 2004;271:361–365. doi: 10.1098/rspb.2003.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacke M, el Jundi B, Smolka J, Byrne MJ, Baird E. The role of the sun in the celestial compass of dung beetles. Phil Trans R Soc B. 2014;369 doi: 10.1098/rstb.2013.0036. 20130036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacke M, Nilsson D-E, Scholtz CH, Byrne MJ, Warrant EJ. Animal behaviour: insect orientation to polarized moonlight. Nature. 2003;424:33–33. doi: 10.1038/424033a. [DOI] [PubMed] [Google Scholar]
- de Vries L, Pfeiffer K, Trebels B, Adden AK, Green K, Warrant EJ, Heinze S. Comparison of navigation-related brain regions in migratory versus non-migratory noctuid moths. Front Behav Neurosci. 2017;11:158. doi: 10.3389/fnbeh.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J, Kirbach A, Reiter L, Lehmann K, Norton P, Storms M, Koblofsky M, Winter S, Georgieva PB, Nguyen H, et al. Honeybees learn landscape features during exploratory orientation flights. Curr Biol. 2016;26:2800–2804. doi: 10.1016/j.cub.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Dingle H. Turn alternation by bugs on causeways as a delayed compensatory response and the effects of varying visual inputs and length of straight path. Animal Behaviour. 1964;13:171–177. [Google Scholar]
- Donlea JM, Piment D, Talbot CB, Kempf A, Omoto JJ, Hartenstein V, Miesenböck G. Recurrent circuitry for balancing sleep need and sleep. Neuron. 2018;97:378–389.e4. doi: 10.1016/j.neuron.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer D, Frost B, Mouritsen H, Günther A, Green K, Whitehouse M, Johnsen S, Heinze S, Warrant EJ. The Earth’s magnetic field and visual landmarks steer migratory flight behavior in the nocturnal Australian Bogong moth. Curr Biol. 2018;28:2160–2166.e5. doi: 10.1016/j.cub.2018.05.030. [DOI] [PubMed] [Google Scholar]
- Esch H, Burns J. Distance estimation by foraging honeybees. J J Exp Biol. 1996;199:155–162. doi: 10.1242/jeb.199.1.155. [DOI] [PubMed] [Google Scholar]
- Evangelista C, Kraft P, Dacke M, Labhart T, Srinivasan MV. Honeybee navigation: critically examining the role of the polarization compass. Philos Trans R Soc Lond, B. 2014;369:20130037–20130037. doi: 10.1098/rstb.2013.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconville R, Beron C, Jayaraman V. Building a functional connectome of the Drosophila central complex. eLife. 2018;7:e04577. doi: 10.7554/eLife.37017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, Maimon G. A neural circuit architecture for angular integration in Drosophila. Nature. 2017;546:101–106. doi: 10.1038/nature22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Vijayan V, Mussells Pires P, Adachi A, Maimon G. Walking Drosophila aim to maintain a neural heading estimate at an internal goal angle. bioRxiv. 2018:1–43. [Google Scholar]
- Fleischmann PN, Grob R, Müller VL, Wehner R, Rössler W. The geomagnetic field is a compass cue in Cataglyphis ant navigation. Curr Biol. 2018;28:1440–1444.e2. doi: 10.1016/j.cub.2018.03.043. [DOI] [PubMed] [Google Scholar]
- Guerra PA, Reppert SM. Coldness triggers northward flight in remigrant monarch butterflies. Curr Biol. 2013;23:419–423. doi: 10.1016/j.cub.2013.01.052. [DOI] [PubMed] [Google Scholar]
- Guerra PA, Gegear RJ, Reppert SM. A magnetic compass aids monarch butterfly migration. Nat Comm. 2014;5:4164. doi: 10.1038/ncomms5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Ritzmann RE. Neural activity in the central complex of the cockroach brain is linked to turning behaviours. J Exp Biol. 2013;216:992–1002. doi: 10.1242/jeb.080473. [DOI] [PubMed] [Google Scholar]
- Harley CM, Ritzmann RE. Electrolytic lesions within central complex neuropils of the cockroach brain affect negotiation of barriers. J Exp Biol. 2010;213:2851–2864. doi: 10.1242/jeb.042499. [DOI] [PubMed] [Google Scholar]
- Heinze S. Unraveling the neural basis of insect navigation. Curr Opin Insect Sci. 2017;24:58–67. doi: 10.1016/j.cois.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Narendra A, Cheung A. Principles of insect path integration. Curr Biol. 2018 doi: 10.1016/j.cub.2018.04.058. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: Intrinsic and columnar neurons. J Comp Neurol. 2008;511:454–478. doi: 10.1002/cne.21842. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Linking the input to the output: new sets of neurons complement the polarisation vision network in the locust central complex. J Neurosci. 2009;29:4911–4921. doi: 10.1523/JNEUROSCI.0332-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron. 2011;69:345–358. doi: 10.1016/j.neuron.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Heinze S, Florman J, Asokaraj S, el Jundi B, Reppert SM. Anatomical basis of sun compass navigation II: the neuronal composition of the central complex of the monarch butterfly. J Comp Neurol. 2013;521:267–298. doi: 10.1002/cne.23214. [DOI] [PubMed] [Google Scholar]
- Homberg U. Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct Dev. 2008;37:347–362. doi: 10.1016/j.asd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Homberg U. Sky compass orientation in desert locusts—Evidence from field and laboratory studies. Front Behav Neurosci. 2015;9:346. doi: 10.3389/fnbeh.2015.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U, Heinze S, Pfeiffer K, Kinoshita M, el Jundi B. Central neural coding of sky polarisation in insects. Phil Trans R Soc B. 2011;366:680–687. doi: 10.1098/rstb.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen E-V, Dacke M, Heinze S, el Jundi B. Anatomical organization of the brain of a diurnal and a nocturnal dung beetle. J Comp Neurol. 2017;525:1879–1908. doi: 10.1002/cne.24169. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B, Foster JJ, Byrne MJ, Baird E, Dacke M. Spectral information as an orientation cue in dung beetles. Biology Letters. 2015a;11 doi: 10.1098/rsbl.2015.0656. 20150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B, Foster JJ, Khaldy L, Byrne MJ, Dacke M, Baird E. A snapshot-based mechanism for celestial orientation. Curr Biol. 2016;26:1456–1462. doi: 10.1016/j.cub.2016.03.030. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Heinze S, Lenschow C, Kurylas A, Rohlfing T, Homberg U. The locust standard brain: A 3D standard of the central complex as a platform for neural network analysis. Front Syst Neurosci. 2009;3:21. doi: 10.3389/neuro.06.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B, Pfeiffer K, Heinze S, Homberg U. Integration of polarisation and chromatic cues in the insect sky compass. J Comp Physiol A. 2014a;200:575–589. doi: 10.1007/s00359-014-0890-6. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Smolka J, Baird E, Byrne MJ, Dacke M. Diurnal dung beetles use the intensity gradient and the polarisation pattern of the sky for orientation. J Exp Biol. 2014b;217:2422–2429. doi: 10.1242/jeb.101154. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Warrant EJ, Byrne MJ, Khaldy L, Baird E, Smolka J, Dacke M. Neural coding underlying the cue preference for celestial orientation. Proc Natl Acad Sci USA. 2015b;112:11395–11400. doi: 10.1073/pnas.1501272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo YM, Leitch KJ, Ros IK, Warren TL, Weir PT, Dickinson MH. Sun navigation requires compass neurons in Drosophila. Current Biology. 2018 doi: 10.1016/j.cub.2018.07.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Wehner R. Idiosyncratic route-based memories in desert ants, Melophorus bagoti: how do they interact with path-integration vectors? Neurobiol Learn Mem. 2005;83:1–12. doi: 10.1016/j.nlm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Kim IS, Dickinson MH. Idiothetic path integration in the fruit fly Drosophila melanogaster. Curr Biol. 2017;27:2227–2238.e3. doi: 10.1016/j.cub.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Kim SS, Rouault H, Druckmann S, Jayaraman V. Ring attractor dynamics in the Drosophila central brain. Science. 2017;356:849–853. doi: 10.1126/science.aal4835. [DOI] [PubMed] [Google Scholar]
- Lin C-Y, Chuang C-C, Hua T-E, Chen C-C, Dickson BJ, Greenspan RJ, Chiang A-S. A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Rep. 2013;3:1739–1753. doi: 10.1016/j.celrep.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Martin J, Guo P, Mu L, Harley CM, Ritzmann RE. Central-complex control of movement in the freely walking cockroach. Curr Biol. 2015;25:2795–2803. doi: 10.1016/j.cub.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Merlin C, Heinze S, Reppert SM. Unraveling navigational strategies in migratory insects. Curr Opin Neurobiol. 2012;22:353–361. doi: 10.1016/j.conb.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T, Kanzaki R. Coordination of flipflopping neural signals and head turning during pheromone-mediated walking in a male silkworm moth Bombyx mori. J Comp Physiol A. 1998;183:273–282. [Google Scholar]
- Mishima T, Kanzaki R. Physiological and morphological characterization of olfactory descending interneurons of the male silkworm moth, Bombyx mori. J Comp Physiol A. 1999;184:143–160. [Google Scholar]
- Namiki S, Kanzaki R. The neurobiological basis of orientation in insects: insights from the silkmoth mating dance. Curr Opin Insect Sci. 2016;15:16–26. doi: 10.1016/j.cois.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Narendra A. Homing strategies of the Australian desert ant Melophorus bagoti II. Interaction of the path integrator with visual cue information. J Exp Biol. 2007;210:1804–1812. doi: 10.1242/jeb.02769. [DOI] [PubMed] [Google Scholar]
- Narendra A, Gourmaud S, Zeil J. Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc R Soc London B. 2013;280 doi: 10.1098/rspb.2013.0683. 20130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberg RM. Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol A. 1983;152:297–307. [Google Scholar]
- Park J-Y, Dus M, Kim S, Abu F, Kanai MI, Rudy B, Suh GSB. Drosophila SLC5A11 mediates hunger by regulating K(+) channel activity. Curr Biol. 2016;26:1965–1974. doi: 10.1016/j.cub.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegel U, Pfeiffer K, Homberg U. Integration of celestial compass cues in the central complex of the locust brain. J J Exp Biol. 2018;221 doi: 10.1242/jeb.171207. jeb171207. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr Biol. 2007;17:960–965. doi: 10.1016/j.cub.2007.04.059. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol. 2014;59:165–184. doi: 10.1146/annurev-ento-011613-162031. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Kinoshita M. Segregation of visual inputs from different regions of the compound eye in two parallel pathways through the anterior optic tubercle of the bumblebee (Bombus ignitus) J Comp Neurol. 2012;520:212–229. doi: 10.1002/cne.22776. [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Alexander BE, Ritzmann RE. Descending control of turning behaviour in the cockroach, Blaberus discoidalis. J Comp Physiol A. 2007;193:385–402. doi: 10.1007/s00359-006-0193-7. [DOI] [PubMed] [Google Scholar]
- Seelig JD, Jayaraman V. Feature detection and orientation tuning in the Drosophila central complex. Nature. 2013;503:262–266. doi: 10.1038/nature12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521:186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlizerman E, Phillips-Portillo J, Forger DB, Reppert SM. Neural Integration Underlying a Time-Compensated Sun Compass in the Migratory Monarch Butterfly. Cell Rep. 2016;15:683–691. doi: 10.1016/j.celrep.2016.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan MV. Where paths meet and cross: navigation by path integration in the desert ant and the honeybee. J Comp Physiol A. 2015;201:533–546. doi: 10.1007/s00359-015-1000-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Zhang S, Altwein M, Tautz J. Honeybee navigation: Nature and calibration of the “odometer”. Science. 2000;287:851–853. doi: 10.1126/science.287.5454.851. [DOI] [PubMed] [Google Scholar]
- Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant EJ, Heinze S. An anatomically constrained model for path integration in the bee brain. Curr Biol. 2017;27:3069–3085.e11. doi: 10.1016/j.cub.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. A brain region in insects that supervises walking. Progress in Brain Research. 1999;123:273–284. doi: 10.1016/s0079-6123(08)62863-0. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Brain organization and the origin of insects: an assessment. Proc Biol Sci. 2009;276:1929–1937. doi: 10.1098/rspb.2008.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. Arthropod Brains. Harvard University Press. Cambridge, Massachusetts: 2012. [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control centre of locomotor behaviour in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen HH, Marshall J, Wolff GH, Strausfeld NJ. Insect-like organization of the stomatopod central complex: Functional and phylogenetic implications. Front Behav Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr Biol. 2010;20:663–668. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Turner-Evans DB, Jayaraman V. The insect central complex. Curr Biol. 2016;26:R453–R457. doi: 10.1016/j.cub.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, Druckmann S, Jayaraman V. Angular velocity integration in a fly heading circuit. Elife. 2017;6:e04577. doi: 10.7554/eLife.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AG, Ritzmann RE. Cellular Basis of Head Direction and Contextual Cues in the Insect Brain. Curr Biol. 2016;26:1816–1828. doi: 10.1016/j.cub.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Warrant EJ, Frost B, Green K, Mouritsen H, Dreyer D, Adden A, Brauburger K, Heinze S. The Australian Bogong moth Agrotis infusa: A long-distance nocturnal navigator. Front Behav Neurosci. 2016;10:77. doi: 10.3389/fnbeh.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R. Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A. 2003;189:579–588. doi: 10.1007/s00359-003-0431-1. [DOI] [PubMed] [Google Scholar]
- Wehner R, Michel B, Antonsen P. Visual navigation in insects: coupling of egocentric and geocentric information. Journal of Experimental Biology. 1996;199:129–140. doi: 10.1242/jeb.199.1.129. [DOI] [PubMed] [Google Scholar]
- Wehner R, Müller M. The significance of direct sunlight and polarized skylight in the ant's celestial system of navigation. Proc Natl Acad Sci USA. 2006;103:12575–12579. doi: 10.1073/pnas.0604430103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R, Srinivasan MV. Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1981;142:315–338. [Google Scholar]
- Wehner R, Srinivasan MV. Path Integration in Insects. In: Jeffrey KK, editor. The neurobiology of spatial behaviour. Oxford University Press; 2003. pp. 9–30. [Google Scholar]
- Wei H, el Jundi B, Homberg U, Stengl M. Implementation of pigment-dispersing factor-immunoreactive neurons in a standardized atlas of the brain of the cockroach Leucophaea maderae. J Comp Neurol. 2010;518:4113–4133. doi: 10.1002/cne.22471. [DOI] [PubMed] [Google Scholar]
- Weir PT, Dickinson MH. Functional divisions for visual processing in the central brain of flying Drosophila. Proc Natl Acad Sci USA. 2015;112:E5523–32. doi: 10.1073/pnas.1514415112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir PT, Schnell B, Dickinson MH. Central complex neurons exhibit behaviourally gated responses to visual motion in Drosophila. J Neurophysiol. 2014;111:62–71. doi: 10.1152/jn.00593.2013. [DOI] [PubMed] [Google Scholar]
- Williams L. Anatomical studies of the insect central nervous system: A ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera) Journal of Zoology. 1975;176:67–86. [Google Scholar]
- Wittlinger M, Wehner R, Wolf H. The ant odometer: stepping on stilts and stumps. Science. 2006;312:1965–1967. doi: 10.1126/science.1126912. [DOI] [PubMed] [Google Scholar]
- Wolff T, Iyer NA, Rubin GM. Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits. J Comp Neurol. 2015;523:997–1037. doi: 10.1002/cne.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J Comp Neurol. 2018 doi: 10.1002/cne.24512. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystrach A, Mangan M, Webb B. Optimal cue integration in ants. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2015.1484. 20151484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Armstrong JD. Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J Comp Neurol. 2010;518:1500–1524. doi: 10.1002/cne.22284. [DOI] [PubMed] [Google Scholar]
- Zeil J. Visual homing: an insect perspective. Curr Opinion Neurobiol. 2012;22:285–293. doi: 10.1016/j.conb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Zeil J, Hofmann MI, Chahl JS. Catchment areas of panoramic snapshots in outdoor scenes. J Opt Soc Am A. 2003;20:450–469. doi: 10.1364/josaa.20.000450. [DOI] [PubMed] [Google Scholar]
- Zeil J, Narendra A, Stürzl W. Looking and homing: how displaced ants decide where to go. Philos Trans R Soc Lond, B. 2014;369 doi: 10.1098/rstb.2013.0034. 20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller M, Held M, Bender J, Berz A, Heinloth T, Hellfritz T, Pfeiffer K. Transmedulla neurons in the sky compass network of the honeybee (Apis mellifera) are a possible site of circadian input. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0143244. e0143244–25. [DOI] [PMC free article] [PubMed] [Google Scholar]