Abstract
Streptococcus pyogenes, also known as group A beta-hemolytic strep, is a Gram positive coccus responsible for several million infections every year. The types of infections vary widely from pharyngitis to myositis, but all can advance to severe life threatening invasive disease. Of those infected, approximately 1100 to 1600 people die each year due to invasive disease. Why certain individuals contract severe infections is not known, but many strains of Streptococcus pyogenes are known to produce toxins and superantigens. Invasive Streptococcus pyogenes infections have been shown to cause significant morbidity and rapid mortality. In many cases, patients expire before full antemortem testing can be performed, causing physicians and families to look to forensic pathologists for answers. Understanding the pathogenesis of invasive group A strep infections, relevant gross and microscopic findings, and proper culturing techniques is critical for forensic pathologists to diagnosis this condition and assist in the education and protection of the communities they serve.
Keywords: Forensic pathology, Streptococcus, Group A strep, Infection, Postmortem cultures
Introduction
Streptococcus pyogenes, also known as strep pyogenes, group A beta-hemolytic strep, or GAS, is an exceedingly common human pathogen that causes disease in everyone and anyone from the very young to very old and the overall healthy to the chronically ill. It is ubiquitous in human civilization and manifests in a wide range of presentations including pharyngitis and impetigo to cellulitis and necrotizing fasciitis. The Centers for Disease Control and Prevention (CDC) estimates that several million cases of GAS infection occur annually (1). Its common nature leads some to classify it as a minor annoyance, such as the common cold or viral gastroenteritis, and many may delay or forgo seeking medical treatment. Reluctance to seek treatment may be one factor in the steady rise in invasive and life-threatening cases of GAS infection within the last few decades. Though it may seem common and underwhelming, GAS infections can lead to significant morbidity and mortality, with between 1100 to 1600 deaths occurring in the United States each year (1). Worldwide, the death toll is estimated at 500 000 annually (2). Those numbers include thousands of people who initially complained of a strep throat or injury-induced muscle pain and then rapidly declined and expired. Due to the acuteness of death, forensic pathologists may encounter these cases with concerned families and physicians awaiting answers. Pathologists must know how to recognize potential cases and take appropriate steps to secure the diagnosis to counsel and console grieving families. This manuscript seeks to outline and explain the who, what, and how of GAS infections and deaths, and provide useful references and tools for the practicing forensic pathologist. Two case examples will also be provided to outline common findings both within the autopsy suite and in the pages of an individual's medical record.
Discussion
What is Streptococcus pyogenes?
Streptococcus pyogenes is a Gram positive, beta hemolytic bacteria (Image 1). It is a round-oval encapsulated coccus occurring in chains or pairs in Gram stain preparations (3) (Image 2). Humans are the natural reservoir, with the organism commonly residing in the oropharynx and female genital tract (3–6). It is believed that between 5 and 15% of the general population are asymptomatic carriers (4). The organism is classified as a facultative anaerobe, meaning it can produce energy by aerobic respiration in the presence of oxygen (such as in the throat or lungs), but is also capable of switching to fermentation in the absence of oxygen (as in deep tissue infections) (3). The bacterium has a host of virulence factors that mediate attachment, invasion, destruction, and survival within the infected host (5, 7). Certain strains of S. pyogenes produce toxins and superantigens, which intensify its deleterious nature.
Image 1.
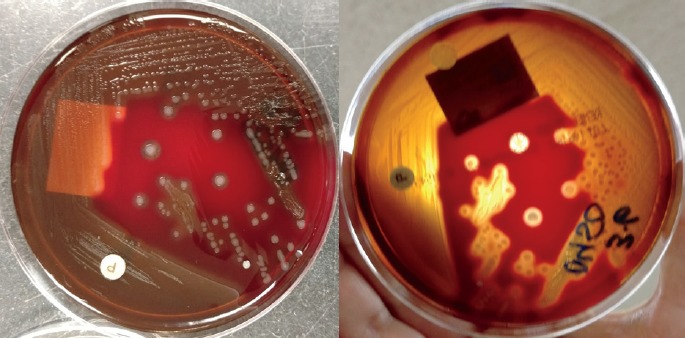
Culture of Streptococcus pyogenes showing white-clear colonies on blood agar (left), with a zone of clearing in the agar called beta hemolysis (right).
Image 2.
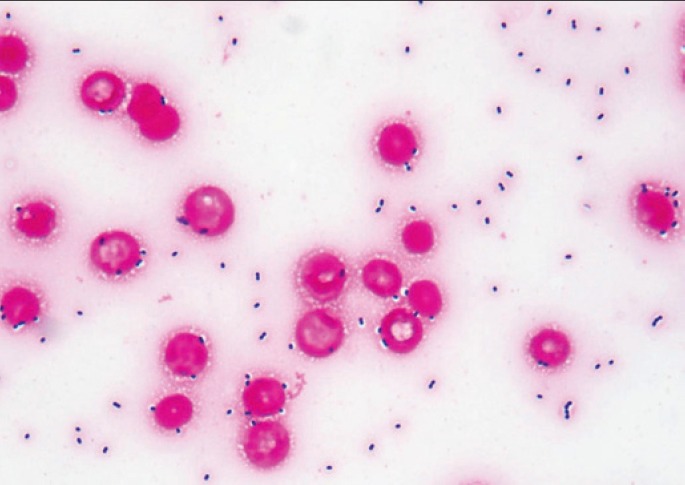
Gram stain of a blood smear from a septic patient with group A streptococcus infection showing abundant Gram positive cocci in pairs and chains (Gram Stain, x1000).
Who is at Risk for Infection?
Group A beta-hemolytic strep is a non-discriminatory organism. Infections occur at any age, with more severe illnesses affecting the extremes of age (less than 2 and greater than 65) (5, 6, 8, 9). Most patients become infected by human to human transmission via respiratory droplets (4, 5). Others may become infected by introducing the organisms into the tissues through injury or penetration by a contaminated object. Finally, some asymptomatic carriers may develop infection from their own flora due to a break in the immune system or the development of a virulence factor. Repeated exposure to GAS increases the risk of infection, demonstrated by the increased rates of infection in schools and nursing homes (9). Certain particularly virulent strains have been known to cause outbreaks in these settings (9, 10). Finally, GAS infections occur year-round, but rates are highest in the winter and early spring. There are several factors that may contribute to this seasonal pattern, including human crowding indoors, close contact with infected individuals, and concurrent acute viral infections that weaken an individual's defenses and lead to bacterial superinfections (2, 11). Overall, GAS is an infection of the masses.
At this point, we will introduce our case subjects, Mr. X and Ms. A. Mr. X was a 31-year-old man who was in generally good health. He had a history of acute lymphocytic leukemia at the age of two that was treated and had been in remission for decades. Ms. A was a 37-year-old woman with a mental disability resulting in a lack of speech development, and Klippel-Trenaunay-Weber (KTW) syndrome, a genetic condition that results in port-wine stains, varicose veins, and soft tissue hypertrophy of the extremities (12). She had been mobile and in general good overall health.
How Does Infection Present?
Streptococcus pyogenes causes a wide range of infections in several areas of the body. The most common areas infected include the oropharynx, lungs, skin, soft tissue, and endometrium (13). Classically, infections are categorized as noninvasive or invasive, depending on location and severity. Noninvasive infections include pharyngitis (also known as strep throat), sinusitis, impetigo, ecthyma (cutaneous ulceration), otitis media, scarlet fever (toxin-produced rash), cellulitis, endometritis (especially in pregnant women), and pneumonia (4, 14, 15). Invasive infections include peritonsillar abscess, myositis, septic arthritis, necrotizing fasciitis, meningitis, bacteriemia, and streptococcal toxic shock syndrome (STSS) (4, 15). Though these categories exist to help us better stratify patients, it must be remembered that any type of infection with S. pyogenes may ultimately prove fatal.
Regardless of location of infection, most patients present with influenza-like symptoms, with or without musculoskeletal pain (6, 13). These symptoms are most often due to host reactions to the underlying infection. Every patient who proceeds to a severe or invasive infection will develop a characteristic high fever at some point during the illness (8). Patients with soft tissue infection will begin with pain in the affected area that grows out of proportion to the visual appearance of the infection (6, 16). Leukocyte count can be quite variable (8). One study evaluated leukocyte counts in children with STSS and concluded that approximately 51% of patients had a leukopenia and 31% had leukocytosis during the course of disease (8).
Mr. X presented in the early morning to the local emergency department with complaints of fatigue, fever, upper respiratory symptoms, diarrhea, and generalized muscle pain for two days. His physical examination showed flushing with slight erythema of the oral mucosa and coarse lung sounds. Influenza PCR testing was negative and he received intravenous fluids for dehydration, which improved his symptoms. Ms. A presented to an urgent care clinic after stubbing her great toe, resulting in disruption of the toe nail. The clinic staff proceeded to clean and dress the wound as well as provide Ms. A with a topical antibiotic cream. Both were discharged home with instructions to return if symptoms worsened.
Who is at Higher Risk for Invasive/Severe Infection?
Though it has been emphasized that severe or invasive disease can develop in anyone, GAS infections, like other bacterial diseases in general, progress in patients with underlying chinks in their defenses. Intact humoral immunity plays a very important role in preventing and combating GAS infections (17). Extremes of age (less than 2 or greater than 65), concurrent acute viral infection, preexisting skin/soft tissue lesions, diabetes mellitus, heart disease, chronic obstructive pulmonary disease, underlying malignancy, and immune suppression from HIV infection or corticosteroids are all associated with increased risk of infection and progression to invasive disease (5, 6, 8, 9, 11, 16, 18). Repeated exposure to GAS also increases the risk of invasive disease. Intravenous drug use, trauma, young school age children in the household, and residence in nursing home facilities all result in repeated exposure to GAS, increasing the likelihood of coming into contact with more virulent strains (5, 6, 16, 18). A few other risk factors for invasive disease have been proposed including male sex, ethanol abuse, and nonsteroidal anti-inflammatory drug (NSAID) use; but these factors remain controversial (6, 8, 16, 18).
Despite taking care of her wound, Ms. A began to develop pain and erythema of her left foot and leg emanating from her great toe, consistent with cellulitis. Ms. A's underlying genetic vascular lesions and history of trauma (stubbing her toe) both increased her likelihood of developing more severe disease.
How Does Group a Streptococcus Cause Death?
The question of why certain people die from GAS and others live is not a question that can be easily answered. What is known, and is currently being researched, is how GAS affects the body resulting in morbidity and mortality. The virulence factors belonging to GAS are the weapons it uses to evade and destroy the human immune defenses. The most significant and devastating of these virulence factors are toxins and superantigens. Toxins, including pyrogenic toxins, streptolysins S and O, and NAD-glycohydrolase cause frank tissue destruction and disrupt cellular function (15). Superantigens, including the aforementioned toxins, mitogenic factors, and products of bacteriophage-encoded genes, are nonspecific proteins that cause an exponential overreaction of the host immune defenses (4, 6, 7, 19). Binding of superantigens to common structural aspects of T cell receptors basically bypasses the specificity mechanisms of the humoral immune system. These common structural aspects are present on up to 20% of all T cells (20). This uninhibited binding leads to T cell activation with release of cytokines, such as interleukins and tumor necrosis factors, in vast quantities (19). The markedly elevated cytokines induce an intense, misdirected, autodestructive systemic inflammatory reaction (21). This results in multiorgan failure including respiratory failure, kidney failure, neurologic dysfunction, shock, coagulopathy, and ultimately death (6, 8, 16, 17).
Most deaths occur within seven days of infection, with the highest risk for mortality occurring within the first 48 hours (6, 17, 22). Due to this, lethal cases may lack antemortem diagnosis and treatment.
Mr. X returned to the emergency department less than 24 hours after first presenting. He had altered mental status, shallow breathing, and a dusky complexion. Physical examination showed tachycardia, hypotension, severe leukopenia, and fever, indicating shock. Blood cultures were drawn and set a record within the microbiology department by turning positive for S. pyogenes in less than 90 minutes. The high level of bacteremia was corroborated by a grossly positive blood smear showing a bacterial count greater than the total red blood cell count (Image 2). Unfortunately, the patient experienced cardiac arrest without return of spontaneous circulation and expired less than one hour after arriving. Ms. A delayed seeking treatment for a few days for her ascending cellulitis due to a winter storm and began to develop abdominal discomfort, sweats, decreased appetite, and high fever. The storm cleared and she sought medical attention after the cellulitis spread to both lower limbs, making ambulation impossible. Blood cultures were positive for S. pyogenes. She was started on antibiotics but quickly progressed into acute respiratory distress and multiorgan failure. She expired three days after presenting to the emergency department.
Who is at Higher Risk for Death Due to Streptococcus pyogenes?
Overall death rates for all invasive S. pyogenes infections range from 10–30% (18). For severe invasive infections, such as necrotizing fasciitis and STSS, death rates can rise as high as 80% (6, 16, 17). Patients at higher risk for death include those with invasive disease, greater than 65 years of age, underlying liver or kidney dysfunction, nonspecific presentation/delayed treatment, gastrointestinal symptoms (indicating toxin production), and residence in nursing home facilities (2, 5, 6, 17, 22). The CDC tracks invasive S. pyogenes infections using the Active Bacterial Core Surveillance (ABCs) system (1, 23). The system tracks invasive infections in certain areas throughout the United States. The data is then tabulated and extrapolated to give an overall national estimate. Data from the CDC for 2015 show an increase in infection at the extremes of age as well as an increased mortality rate in patients over 65 years of age (Figure 1). The national number of invasive infections and deaths was estimated at 15 540 and 1570, respectively, showing a 10% mortality rate for invasive disease (23).
Figure 1.
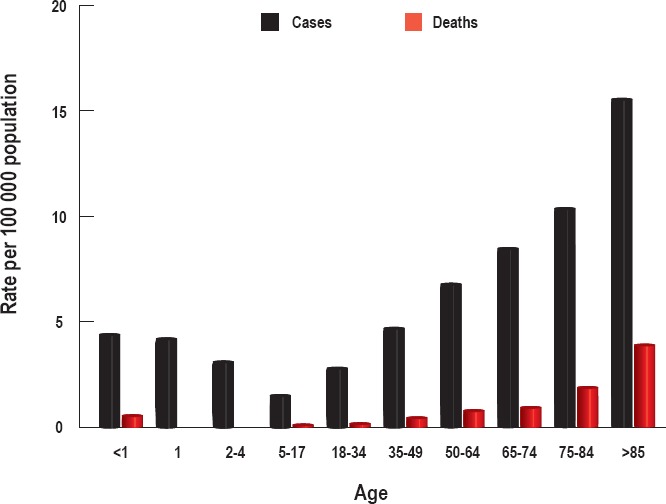
Authors' graphical representation of data obtained from a Centers for Disease Control and Prevention Active Bacterial Core Surveillance Report for group A streptococcal infections in 2015 (23). The national numbers of invasive infections and deaths in 2015 were estimated at 15 540 and 1570, respectively.
Mr. X complained of gastrointestinal symptoms when he first presented to the emergency department. These symptoms are consistent with infection by a toxin-producing strain of S. pyogenes. He later developed signs of toxic shock including high fever, hypotension, tachycardia, and leukopenia. Ms. A delayed seeking treatment for her cellulitis due to inclement weather. Both ended up developing bacteremia.
How to Recognize a Potential Group a Streptococcus Case at Autopsy
Since any type of GAS infection can lead to mortality, the history and presentations at autopsy can vary widely, from frankly obvious to imperceptibly subtle. Some infections, such as meningitis, pneumonia, or endometritis, may not be easily seen until histologic sections are obtained and reviewed. Cutaneous and soft tissue infections will be the most common grossly identified manifestation, and need to be recognized and documented. Diligence is important, but it should be noted that 50% of invasive GAS infections have no identifiable primary infection or source (21).
The appearance of many GAS infections may be altered by the time of postmortem examination. Cellulitis, for example, loses its characteristic tense, bright red erythematous nature and distinct borders (Image 3A). This presents a challenge to forensic pathologists as the infection may mimic other postmortem changes, including livor mortis. Some signs that help indicate postmortem cellulitis include circumferential discoloration of limbs or continuation of discoloration over areas exposed to pressure. Dot-like folliculitis patterning around the lesional borders and associated ulceration or bullae formation are also helpful features (Image 3B). Another potentially confusing presentation includes impetigo, a classic cutaneous infection often involving the face (14). At the time of postmortem examination, the infection can lose it characteristic golden yellow crusting (Image 4A). The resulting erythematous erosions may be mistaken for abrasions or insect activity. Some indications of infectious etiology include erosions in nonprotruding areas such as the supra alar creases or nostrils, arguing against abrasions, and evidence of vital reaction, disputing postmortem insect activity (Image 4B) (24).
Image 3.
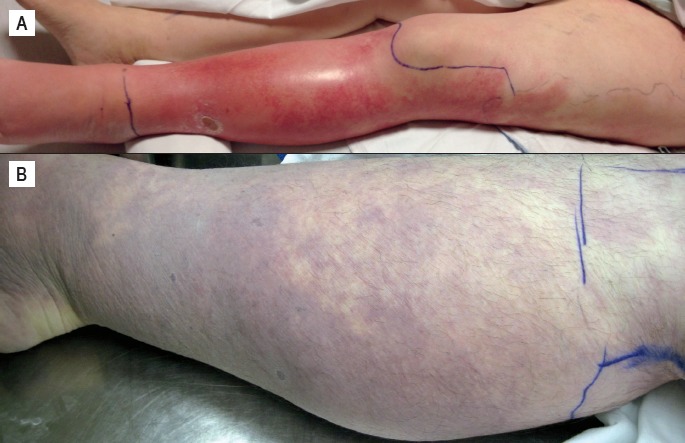
A) Antemortem cellulitis of the leg. Note characteristic tense, bright red erythematous nature and distinct boarders; B) Postmortem cellulitis of the leg. Differentiation between this and livor mortis may be problematic.
Image 4.
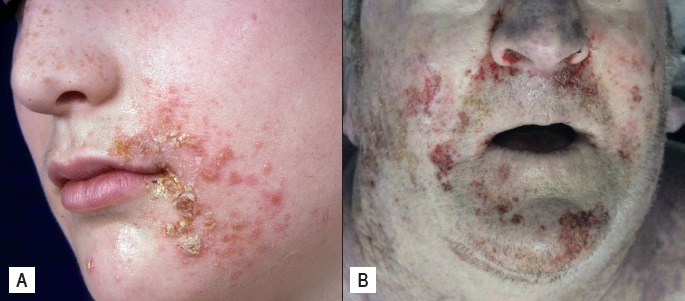
A) Antemortem impetigo with characteristic golden yellow crusting; B) Postmortem impetigo may mimic abrasions or postmortem insect activity.
Some infections may be obscured by postmortem artifacts, making the identification of a source difficult if not impossible. Stasis of blood within the head and neck may obscure the visualization of erythema in the pharynx, indicating pharyngitis. Skin slippage can destroy antemortem bullous formation pointing toward underlying myositis. Conversely, true antemortem bullae associated with infection maybe interpreted as postmortem fluid blister formation. Putrefaction may overrun and blur cutaneous manifestations of soft tissue necrosis seen in necrotizing fasciitis (Image 5).
Image 5.
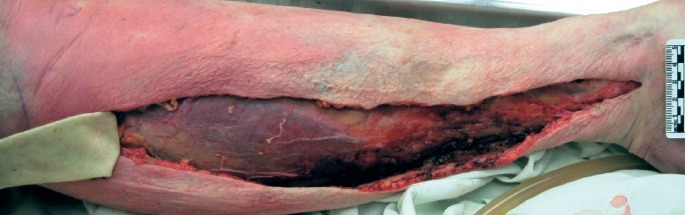
Necrotizing fasciitis showing black, liquid necrosis within deep tissue of the leg, which maybe obscured by decompositional changes.
Finally, medical and surgical interventions may obscure or remove infectious tissue. Intubation may remove gray-white exudate from pharyngitis or artificially cause oropharyngeal erythema. Surgical debridement will remove necrotic tissue in necrotizing fasciitis (Image 6). Even with aggressive treatment, 25–35% of patients with necrotizing fasciitis die due to the severity of disease (20). Treatment with broad spectrum antibiotics can result in negative cultures, commonly rendering a diagnosis of culture negative sepsis. In these cases, it is important to contact health providers to obtain any pretreatment culture results.
Image 6.
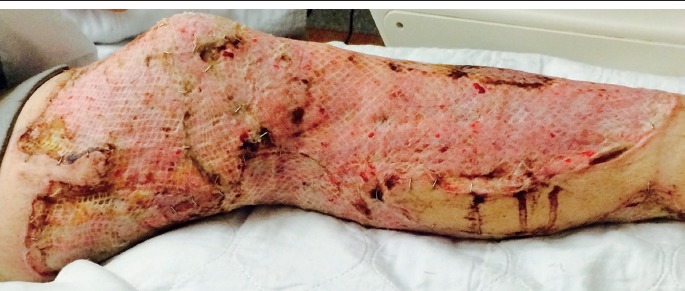
Patient with necrotizing fasciitis postsurgical debridement. Risk of death due to invasive infection is still relatively high after treatment due to severity of disease.
Histologic evidence of infection may not be easily recognizable as no pathognomonic features of GAS exist (5). End organ damage due to infection generally appears histologically identical to end organ damage due to other conditions such as diffuse alveolar damage or ischemia/infarction. The presence of bacterial colonies on H&E stained sections should be accompanied by vital reaction to preclude the possibility of postmortem contamination and bacterial overgrowth (14).
At the time of autopsy, Mr. X showed significant erythema of the upper chest and face as well as antemortem marbling of the torso and arms. Marbling is generally considered an early decompositional change and maybe seen in the antemortem period in cases with significant bacteremia. Internally, the organs were grossly normal except for significant edema of the lungs. Histology showed significant bacterial colonies in all sections (except for the brain) along with diffuse intravascular fibrin deposition. Even though the patient clinically had severe leukopenia, vital inflammatory reaction with bacterial phagocytosis can be seen in many sections, precluding postmortem bacterial overgrowth (Images 7 and 8). Acute kidney damage in the form of thrombotic microangiopathy was identified histologically and characterized by electron microscopy (Image 9). Ms. A had patchy red discoloration of the chest and neck with mild cyanosis. Severe edema of the feet was noted bilaterally with red discoloration and swelling surrounding the left great toe. Internally, there was mild edema of both lungs, patchy hemorrhages throughout the gastrointestinal tract, and necrosis of the liver. Histology showed diffuse alveolar damage and centrilobular necrosis of the liver.
Image 7.
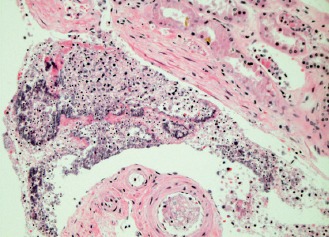
Perirenal soft tissues with large bacterial colonies in association with a neutrophilic reaction (H&E, x200).
Image 8.
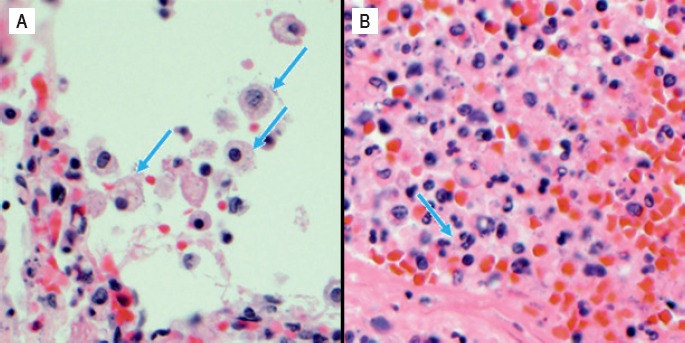
Bacterial phagocytosis by pulmonary macrophages (A) and neutrophils within the liver (B) (H&E, x400).
Image 9.
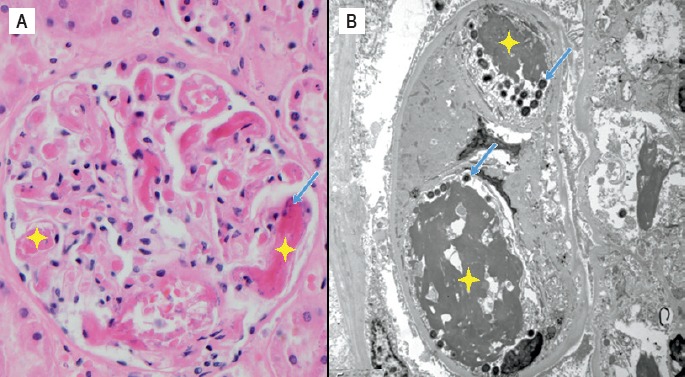
Thrombotic microangiopathy of the glomeruli on H&E stained section (x400) (A) as well as electron microscopy (B). Bacteria (blue arrows) are shown in association with deposited fibrin (yellow stars).
Obtaining Postmortem Cultures
Culturing of organisms is the gold standard for identifying bacterial infection. As the procurement of cultures is not standard procedure for most autopsy cases, knowing when cultures are necessary and the appropriate techniques for obtaining them is of the utmost importance. As it is the last chance to obtain any medically relevant organisms, the forensic pathologist should have a low threshold for obtaining and submitting cultures. Any patient with a history of vague flu-like symptoms, cutaneous or soft tissue lesions, or a previously healthy individual who declines rapidly despite a relatively benign presentation should raise concern and consideration for postmortem cultures (13, 16).
When taking cultures, certain rules should always be followed to prevent contamination or false negative results. Cultures should be taken before any dissection begins. Fluids, such as blood, cerebrospinal fluid (CSF), or effusions can be taken percutaneously via syringe before opening the body. Most importantly, before inserting a syringe into the body, the overlying skin should be cleaned of debris and sterilized using alcohol or iodine. Fluids can then be obtained using the same techniques as antemortem collection such as phlebotomy (usually the jugular, femoral, or subclavian vein), lumbar puncture, or thoracentesis/paracentesis. If cultures from internal organs are warranted, they should be taken immediately after opening. Maintenance of sterility is difficult during postmortem examination due to general decomposition and other postmortem changes, but factors that can be controlled by the pathologist include prepping the surface overlying the desired site with iodine or alcohol, use of sterile tools, including syringes, scalpels, and forceps, and placing specimens in the appropriate sterile containers as quickly as possible. Taking cultures from multiple sites can help indicate true infection versus contamination (5). If multiple sites are positive for a single organism, this leans more toward true infection. When culturing deep soft tissue wounds, taking tissue from both the center of the wound as well as the edge increases the chance of culturing an organism. Submitting a 1 cm sample of tissue is preferred over swabs as the chance of recovering organisms from simple swabs is relatively low. It is important to keep tissue moist with sterile saline to prevent drying, as desiccation will result in a negative culture (14). Finally, timely delivery to the microbiology lab is critical. If a delay is unavoidable, the specimen should be refrigerated for no more than a few hours. Having a checklist, like the one provided in Table 1, may help in improving overall postmortem culturing technique.
Table 1.
Example of Postmortem Culture Collection Checklist for Tissue, Fluid, and Swab Samples
| Tissue | Label sterile container with patient information |
| Gather sterile scalpel and forceps | |
| Clean overlying area with water to remove debris | |
| Dry and clean overlying area with alcohol or iodine | |
|
Cut and remove a 1 cm size piece of tissue For wounds/abscess: take pieces from both center and edge | |
| Place tissue in sterile container | |
| Add small amount of sterile saline or saline soaked gauze | |
| Send tissue sample to microbiology lab or store in refrigerator for no more than a few hours | |
| Fluid | Label sterile vials with patient information |
| Gather sterile needle, syringe, and scalpel | |
| Clean overlying area with water to remove debris | |
| Dry and clean overlying area with alcohol or iodine | |
| For abscess fluid collection, make small incision in skin with scalpel | |
| Aspirate 5 mL of fluid (minimum of 1 mL) | |
| Clean top of vial cap with alcohol | |
| Transfer fluid into vial | |
| Send fluid sample to microbiology lab or store in refrigerator for no more than a few hours | |
| Swab (use only if absolutely necessary) | Label sterile container with patient information |
| Gather sterile scalpel | |
|
If going through skin or organ surface Clean overlying area with water to remove debris Dry and clean overlying area with alcohol or iodine Make small incision in skin with scalpel | |
| Insert swab up to hilt deep into nose, wound, or organ | |
| Rotate swab | |
| Remove and place in sterile container | |
| Send swab to microbiology lab or store in refrigerator for no more than a few hours |
It should be noted that taking cultures from normally sterile sites, such as blood, CSF, deep soft tissue, and internal cavities is highly recommended. If cultures need to be obtained from normally nonsterile sites such as the throat, skin, or vaginal tract, the culture will most likely return with multiple organisms. Discussion with a microbiologist will help distinguish what organisms are normal flora and what are pathogens and can be considered true infections. In the case of S. pyogenes, positive cultures from the oropharynx or vaginal tract do not indicate infection, as a significant portion of the population may be asymptomatic carriers. Culturing of S. pyogenes from sterile sites is more indicative of true infection.
At autopsy, Mr. X had several cultures obtained, including CSF, lung, and blood. Both lung and blood cultures grew S. pyogenes organisms, similar to the antemortem cultures. The CSF culture was negative for S. pyogenes, but positive for Staphylococcus epidermidis. As this was the only culture to show S. epidermidis, the patient showed no gross or histologic signs of meningitis, and the organism is a common colonizer of the skin, the culture was considered contaminated. Improper sterilization of the site prior to lumber puncture was most likely to blame.
Why Should Forensic Pathologists be Concerned with Streptococcus pyogenes Infections?
Apart from the obvious aspiration for correct and complete postmortem diagnosis, identifying S. pyogenes infections and related deaths should initiate a follow-up with close contacts of the decedent. Informing contacts of the signs and symptoms of infection can prompt otherwise reluctant individuals to seek medical attention earlier and prevent further loss of life. Prophylactic treatment with antibiotics is not currently a standard of care for S. pyogenes exposure, but treatment of early noninvasive infections is common (20). As strains that cause severe infection and mortality are usually more virulent, stressing to family the importance of communication with the decedents' work or school is recommended. Along with tracking invasive infection through the ABCs, the CDC has also recorded cases of STSS every year since 1995 (1, 25). The data are gathered through reporting by medical providers. If a case of STSS is not recorded by the treating physician due to the acuteness of death, the forensic pathologist has the responsibility to inform the CDC. Streptococcal toxic shock syndrome is defined as the isolation of S. pyogenes from a normally sterile site, record of hypotension, and two or more of the following: tissue necrosis, desquamation of skin, acute respiratory distress syndrome, liver impairment, coagulopathy, or renal impairment (6). Apart from the CDC, many state and local health departments collect data surrounding invasive S. pyogenes infections. Requirements for reporting differ from state to state and forensic pathologists should be aware of requirements within their jurisdictions. Accurate and timely reporting at both the local and national levels allows for better surveillance and tracking of severe invasive infections. Data can also be tabulated, formatted, and distributed to the community to aid in the improvement of overall public health.
Conclusion
Streptococcus pyogenes is a ubiquitous human pathogen with a propensity for severe life-threatening infections. Becoming complacent in our investigation and management of this organism only leads to higher morbidity and mortality. Understanding how to recognize potential S. pyogenes infections and properly obtain postmortem cultures better prepares forensic pathologists to support and enhance the communities they serve.
Acknowledgments
The authors would like to thank Dr. Gary P. Williams MD from the Department of Pediatrics, University of Wisconsin School of Medicine and Public Health for providing the antemortem photograph of impetigo.
Footnotes
ETHICAL APPROVAL
As per Journal Policies, ethical approval was not required for this manuscript
STATEMENT OF HUMAN AND ANIMAL RIGHTS
This article does not contain any studies conducted with animals or on living human subjects
STATEMENT OF INFORMED CONSENT
No identifiable personal data were presented in this manuscsript
DISCLOSURES & DECLARATION OF CONFLICTS OF INTEREST
This work was presented at the 2017 NAME Annual Meeting. The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
FINANCIAL DISCLOSURE The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1).Centers for Disease Control and Prevention [Internet]. Atlanta: Centers for Disease Control and Prevention; c2017. Group A streptococcal (GAS) disease; [updated 2016. Sep 16; cited 2017 Aug 29]. Available from: https://www.cdc.gov/groupastrep/surveillance.html. [Google Scholar]
- 2).Nelson G.E., Pondo T., Toews K.A. et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis. 2016. Aug 15; 63(4): 478–86. PMID: 27105747. PMCID: PMC5776658. 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Todar's online textbook of bacteriology [Internet]. Madison (WI): Kenneth Todar, PhD; c2008–2012. Streptococcus pyogenes and streptococcal disease; [cited 2017. Aug 29]. Available from: http://textbookofbacteriology.net/streptococcus.html. [Google Scholar]
- 4).Koneman E.W. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia: LWW; 1997. 1488 p. [Google Scholar]
- 5).Batalis N.I., Caplan M.J., Schandl C.A. Acute deaths in nonpregnant adults due to invasive streptococcal infections. Am J Forensic Med Pathol. 2007. Mar; 28(1): 63–8. PMID: 17325468. 10.1097/01.paf.0000248775.34108.da. [DOI] [PubMed] [Google Scholar]
- 6).Chua W.C., Mazlan M.Z., Ali S. et al. Post-partum streptococcal toxic shock syndrome associated with necrotizing fasciitis. IDCases. 2017. Jun 27; 9: 91–94. PMID: 28725564. PMCID: PMC5506869. 10.1016/j.idcr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).de Almeida Torres R.S., Fedalto L.E., de Almeida Torres R.F. et al. Group A streptococcus meningitis in children. Pediatr Infect Dis J. 2013. Feb; 32(2): 110–4. PMID: 22926217. 10.1097/INF.0b013e31826fd4af. [DOI] [PubMed] [Google Scholar]
- 8).Rodríguez-Nuñez A., Dosil-Gallardo S., Jordan I., ad hoc Streptococcal Toxic Shock Syndrome collaborative group of Spanish Society of Pediatric Intensive Care. Clinical characteristics of children with group A streptococcal toxic shock syndrome admitted to pediatric intensive care units. Eur J Pediatr. 2011. May; 170(5): 639–44. PMID: 20981441. 10.1007/s00431-010-1337-x. [DOI] [PubMed] [Google Scholar]
- 9).Rainbow J., Jewell B., Danila R.N. et al. Invasive group a streptococcal disease in nursing homes, Minnesota, 1995–2006. Emerg Infect Dis. 2008. May; 14(5): 772–7. PMID: 18439360. PMCID: PMC2600262. 10.3201/eid1405.070407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Centers for Disease Control and Prevention [Internet]. Atlanta: Centers for Disease Control and Prevention; c2018. Nosocomial group A streptococcal infections associated with asymptomatic health-care workers – Maryland and California, 1997; [cited 2017 Aug 29]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/00056612.htm. [Google Scholar]
- 11).Hidalgo-Carballal A., Suárez-Mier M.P. Sudden unexpected death in a child with varicella caused by necrotizing fasciitis and streptococcal toxic shock syndrome. Am J Forensic Med Pathol. 2006. Mar; 27(1): 93–6. PMID: 16501360. 10.1097/01.paf.0000203152.62134.1f. [DOI] [PubMed] [Google Scholar]
- 12).Medscape [Internet]. New York: WebMD LLC; c1994–2018. Klippel-Trenaunay-Weber syndrome; [cited 2017. Aug 29]. Available from: http://reference.medscape.com/article/1084257-overview. [Google Scholar]
- 13).Hasegawa J., Sekizawa A., Yoshimatsu J. et al. Cases of death due to serious group A streptococcal toxic shock syndrome in pregnant females in Japan. Arch Gynecol Obstet. 2015. Jan; 291(1): 5–7. PMID: 25194311. 10.1007/s00404-014-3440-0. [DOI] [PubMed] [Google Scholar]
- 14).Kradin R.L. Diagnostic pathology of infectious disease. Philadelphia: Saunders; 2010. 660 p. [Google Scholar]
- 15).Bastiat-Sempe B., Love J.F., Lomayesva N., Wessels M.R. Streptolysin O and NAD-glycohydrolase prevent phagolysosome acidification and promote group A Streptococcus survival in macrophages. MBio. 2014. Sep 16; 5(5): e01690–14. PMID: 25227466. PMCID: PMC4172074. 10.1128/mBio.01690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Heinze S., Püschel K., Tsokos M. Necrotizing fasciitis with fatal outcome: a report of two cases. Forensic Sci Med Pathol. 2011. Sep; 7(3): 278–82. PMID: 21170756. 10.1007/s12024-010-9211-8. [DOI] [PubMed] [Google Scholar]
- 17).Mehta S., McGeer A., Low D.E. et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest. 2006. Dec; 130(6): 1679–86. PMID: 17166982. 10.1378/chest.130.6.1679. [DOI] [PubMed] [Google Scholar]
- 18).Factor S.H., Levine O.S., Schwartz B. et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003. Aug; 9(8): 970–7. PMID: 12967496. PMCID: PMC3020599. 10.3201/eid0908.020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Proft T., Sriskandan S., Yang L., Fraser J.D. Superantigens and streptococcal toxic shock syndrome. Emerg Infect Dis. 2003. Oct; 9(10): 1211–8. PMID: 14609454. PMCID: PMC3033064. 10.3201/eid0910.030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hakkarainen T.W., Kopari N.M., Pham T.N., Evans H.L. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014. Aug; 51(8): 344–62. PMID: 25069713. PMCID: PMC4199388. 10.1067/j.cpsurg.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Saito M., Kajiwara H., Iida K. et al. Systemic cytokine response in moribund mice of streptococcal toxic shock syndrome model. Microb Pathog. 2011. Feb; 50(2): 109–13. PMID: 21146602. 10.1016/j.micpath.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 22).Lamagni T.L., Neal S., Keshishian C. et al. Predictors of death after severe Streptococcus pyogenes infection. Emerg Infect Dis. 2009. Aug; 15(8): 1304–7. PMID: 19751599. 10.3201/eid1508.090264. [DOI] [PubMed] [Google Scholar]
- 23).Centers for Disease Control and Prevention [Internet]. Atlanta: Centers for Disease Control and Prevention; c2017. ABCs report: group A streptococcus, 2015; [cited 2017 Aug 29]. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/gas15.html. [Google Scholar]
- 24).Spitz W.U. Spitz and Fisher's medicolegal investigation of death: guidelines for the application of pathology to crime investigation. 4th ed. Springfield (IL): Charles C Thomas; 2005. 1358 p. [Google Scholar]
- 25).Centers for Disease Control and Prevention [Internet]. Atlanta: Centers for Disease Control and Prevention; c2017. Notifiable diseases and mortality tables; [cited 2017. Aug 29]. Available from: https://www.cdc.gov/mmwr/volumes/66/wr/mm6626md.htm#table-1. [Google Scholar]


