Abstract
From 2000 to 2014, drug overdose deaths increased 137% in the United States, and 61% of these deaths included some form of opiate. The vast majority of opiate-related drug fatalities include multiple drugs, although there is scant data quantitatively describing the exact drugs that contribute to deaths due to multiple drugs. In the present study, we sought to quantitatively identify the drugs that occur with opiates in accidental multidrug-related fatalities. We retrospectively explored fatal drug trends in four Michigan counties, with a focus on profiling drugs present concurrently with opiates. Blood and urine toxicology reports for mixed drug fatalities (N=180) were analyzed using frequent item analysis approaches to identify common analyte trends in opiate-related fatalities. Within our cohort, the most prevalent serum analytes included caffeine (n=147), morphine (n=90), alprazolam (n=69), gabapentin (n=46), and tetrahydrocannabinol (n=44). In 100% of cases where gabapentin was present (n=46), an opiate was also present in the serum or urine. The average gabapentin serum concentration was 13.56 μg/mL (SEM =0.33 μg/mL), with a range of 0.5-88.7 μg/mL. Gabapentin was found at very high frequency in accidental mixed drug fatalities. Gabapentin concentrations were generally within the normal therapeutic range (2-20 μg/mL). It is unknown whether a synergistic effect with opioids may contribute to central respiratory depression. Further research is warranted to determine any contributory role of gabapentin in these deaths. Confirmed interactions could have broad implications for future reporting by forensic pathologists as well as prescribing practices by clinicians.
Keywords: Forensic pathology, Gabapentin, Opioid overdose, Hydrocodone, Substance abuse, Mixed-drug fatality
Introduction
The opiate abuse epidemic in the United States is a growing health crisis that shows little evidence of subsiding in the near future. Opioid substances now account for more than half of all accidental overdose deaths (1). From 1999 to 2014, opioid overdose deaths rose by almost four-fold, closely mirroring an approximate four-fold increase in the sales of prescription opioids. Importantly, this rise in opioid use did not affect reports of subjective levels of pain (2, 3). Nearly half a million people have died during this time frame as a result of opioid abuse (1).
There have been a plethora of studies confirming this opioid use phenomenon, specifically in the postmortem toxicology analysis of accidental drug-related fatalities. Often, these drug-related fatalities include multiple pharmacologic agents in addition to opiates. However, while data on opiate-related fatalities are abundant, there is a paucity of data describing the nonopiate substances found concurrently with opioids in multidrug fatalities. By determining what substances are present with opioids, we can better appreciate the toxicological context in which these deaths are occurring.
The primary mechanism of death in an opioid-induced overdose is central respiratory depression. It is well known that other central nervous system depressants, including ethanol and benzodiazepines, can act synergistically with opioids to exacerbate respiratory depression, resulting in hypoxia and eventual death (4). There is less clarity regarding how other nonopiates may interact with opiates. In addition to exacerbating respiratory depression through synergistic central nervous system depression, nonopiates may have pharmacokinetic interactions with opiates. Such interactions could increase the concentration of opiate in the blood, increasing the likelihood of undesired or fatal effects. The extent to which combined perimortem blood concentrations of opioids and other substances contribute to death has not been well-described. In the present study, we sought to identify potential pairings between opiates and nonopiates that may contribute to death.
Methods
Study Subjects
Deceased adults (greater than or equal to 18 years of age) that met inclusion criteria for cause of death, manner of death, and county of death were identified from the death investigation database currently used by the Office of the Medical Examiner in southwest Michigan. Study subjects were deceased in Allegan, Calhoun, Kalamazoo, or Muskegon counties in Michigan from January 1, 2013 to December 31, 2015. The cause of death for all study subjects was classified as “drug-related” and the manner of death was determined to be “accident.” All study subjects had a toxicology report on file within the database. Additional demographic information was obtained for each study subject, including age, sex, and race (Table 1).
Table 1.
Demographic Data on Study Subjects
| All Decedents | N = 180 (100%) |
|---|---|
| Age (Years) | |
| Mean | 42 |
| Range | 18-81 |
| Age Category (Years) | |
| 18-35 | 57(31.6) |
| 36-65 | 116(64.4) |
| 66+ | 7 (3.8) |
| Sex | |
| Male | 113(62.8) |
| Female | 67 (37.2) |
| Race | |
| White | 148 (82.2) |
| Hispanic | 5 (2.8) |
| Black or African American | 26 (14.4) |
| American Indian or Alaskan | 1 (0.6) |
Analyte Prevalence
Each toxicology report was de-identified and reviewed by at least two individuals. Analytes data were recorded and automatically converted to a binary database to generate “present” or “not present” calls for each analyte across all the cases. Prevalence values are the frequency of occurrence for each analyte within the toxicology report subtype (n=180 for blood and urine combined, n=178 for blood reports, and n=144 for urine reports). A prevalence of 1.0 would indicate that the analyte is present in every case.
Descriptive Analysis: Frequent Item Analysis
Frequent item analysis (FIA) is used to identify common combinations (subset or whole) within a “transaction” (5). For example, consider the following four transactions at a grocery store:
Transaction #1: milk, bread, butter, spaghetti noodles, pasta sauce
Transaction #2: eggs, spaghetti noodles, pasta sauce, bananas, apples
Transaction #3: garlic bread, spaghetti noodles, pasta sauce
Transaction #4: spaghetti noodles, eggs
All four transactions have a unique combination of items purchased, but three out of the four have a common subset combination of spaghetti noodles and pasta sauce. Frequent item analysis is used to identify these common combinations within multiple transactions. In this case, our “transaction” is an overdose case, and our “items” are drug analytes. In frequent item analysis, there are two parameters of interest for which the investigator sets: support and confidence. Support is the proportion of cases in which the combination of items (X|Y) is found. Confidence is the conditional probability in which item Y was present given that item X was present. For instance, going back to our grocery transaction example, we had a support of 0.75 and a confidence of 1.00 for the combination of (pasta sauce | spaghetti noodles), indicating 75% of transactions included pasta sauce and spaghetti noodles, and that 100% of transactions with pasta sauce also included spaghetti noodles. These parameters are set by the investigator, and for the analyses presented herein, support=0.2 and confidence=0.7. The FIAs presented here use a candidate-based approach in which only the cases with the candidate of interest are included in each respective analysis, allowing for combinations with that candidate to be explored. In this analysis we were primarily interested with support given that the candidate was always part of the combination, resulting in a confidence of 1.0. It is important to note that FIA is a purely descriptive analysis, and as a descriptive analysis, there is no p-value or confidence level associated with the FIA analysis.
Inferential Statistical Analysis
Blood gabapentin concentrations were compared in those cases involving hydrocodone versus those cases that did not involve hydrocodone using a two-tailed heteroscedastic t-test. For concentration correlations between gabapentin and hydrocodone, concentrations were plotted on two-dimensional axes and a linear regression with Pearson's correlation coefficient was determined.
Results
Prevalence of Analytes
The first analysis performed was a simple frequency analysis to identify the most common analytes present in blood toxicology reports, urine toxicology reports, or both. Of these N=180 cases, we had both a urine and blood sample for n=143 cases. We had a blood sample for n=178 cases and a urine sample for n=144 cases. The number of analytes detected in the blood and urinalysis of each individual ranged from 0-18, with an average of 7.44±3.05 analytes per individual. One individual had no analytes present in either the blood or the urine toxicology reports but was included in the analysis because the death was ruled an accidental multidrug-related fatality. The single most common analyte detected was caffeine, occurring with a relative frequency of 82% (n=148) in the combined blood and urinalysis reports. Because this common analyte was so prevalent and generally does not contribute to death, we excluded this analyte from further analysis in this study.
Opiate analytes were present in 90.6% of all cases. The frequency of opiate analytes across the blood and urine analyses combined occurred as follows: morphine (55%), monoacetylmorphine (34%), codeine (28%), hydromorphone (26%), methadone (23%), and fentanyl (21%) (Figure 1). Less frequent opiates include hydrocodone (18%), oxycodone (11%), and oxymorphone (9%). All other opiates were found at frequencies less than 5%. Heroin is infrequently detectable in the blood because it rapidly undergoes deacetylation into 6-monoacetylmorphine, which can be detected in the blood and urine and is used as a specific and proven biomarker for heroin (6, 7). Opioids can present differently in the blood and urine because they are generally converted into polar metabolites eliminated in the urine, and urine volume can vary widely. This change in volume influences urine analyte concentration and renders it generally less reliable than blood analysis. To that end, we also report analyte frequency in the blood and urine separately. The frequency of opiates in the urine occurred as follows: morphine (51%), monoacetylmorphine (32%), hydromorphone (31%), codeine (27%), methadone (24%), fentanyl (21%), and hydrocodone (20%) (Figure 2). The frequency of opiates in the blood occurred as follows: morphine (51%), methadone (22%), and monoacetylmorphine (20%) (Figure 3).
Figure 1.
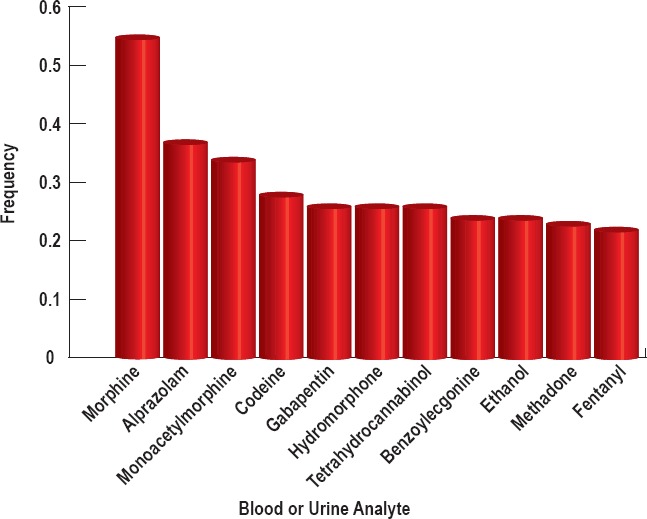
The most common analytes when considering both blood and urine toxicology of N=180 subjects that occur with a frequency >0.2.
Figure 2.
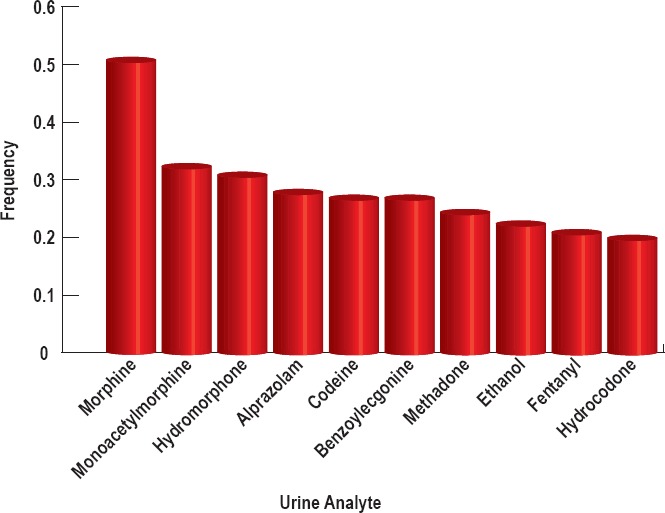
The most common analytes from the urine samples of n=144 subjects for whom urinalysis was available that occur with a frequency >0.2.
Figure 3.
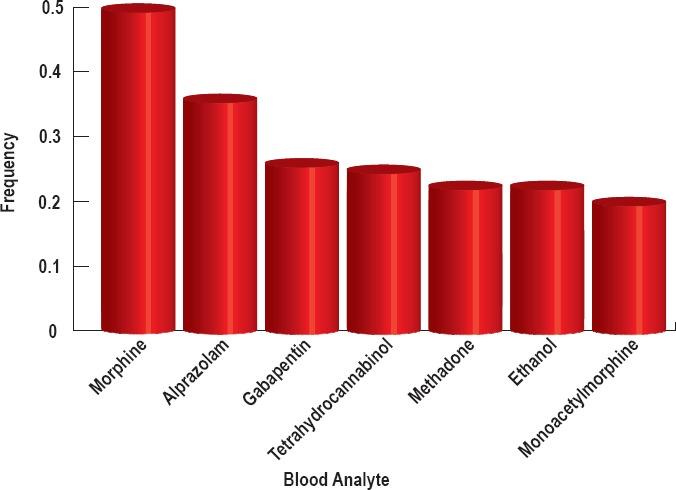
The most common analytes from the blood samples of n=178 subjects for whom blood toxicology was available that occur with a frequency >0.2.
The analyte concentrations of all nonopiates in the blood and urine were also examined. A combined analysis of blood and urine nonopiates revealed analyte prevalence of 20% or greater as follows: alprazolam (36%), gabapentin (26%), tetrahydrocannabinol (THC, 26%), benzoylecgonine (24%), and ethanol (24%) (Figure 1). Alprazolam is a commonly prescribed benzodiazepine manufactured most commonly under the trade name Xanax. Cocaine is metabolized into benzoylecgonine, which can be detected in the urine. The prevalence levels of all nonopiates in the urine for which the prevalence was 20% or greater occurred as follows: alprazolam (28%), benzoylecgonine (27%), and ethanol (22%) (Figure 2). All of the cases in which urinalysis was positive for alprazolam also tested positive for alpha-hydroxy-alprazolam. The prevalence levels of all nonopiates in the blood (for which the prevalence was 20% or greater) occurred as follows: alprazolam (36%), gabapentin (26%), THC (25%), and ethanol (22%) (Figure 3).
Frequent Item Associations
We initially performed an unbiased frequent item association (support ≥0.20, confidence ≥0.7) to identify multidrug combinations of frequently occurring analytes. This analysis yielded only combinations of common opiates (data not shown), and did not provide any additional information regarding associations with nonopiate analytes. To assess the potential contribution of nonopiates to the seemingly opiate-mediated mortality, we identified the most common combinations that included frequent nonopiate analytes. Frequent nonopiate analytes were defined as nonopiate analytes that appeared in the combined blood and urine analyses at a relative frequency of 20% or greater. This included alprazolam, benzoylecgonine, ethanol, gabapentin, and THC. For each of these analytes, a data subset was created to include information for only cases that involved the candidate analyte. Frequent item analysis was then employed using a support of 0.2 and a confidence of 0.7 for each candidate.
Frequent item analysis revealed that many of the most common opiate analytes are present in a similar proportion across the cases containing each of the candidate nonopiates we examined (Table 2). For example, morphine occurred with a relative frequency of 0.52 in the alprazolam-containing cases. (This means that 52% of all cases containing alprazolam also contained morphine). Likewise, morphine was present with a relative frequency of 0.66, 0.57, 0.57, and 0.62 in cases containing benzoylecgonine, ethanol, gabapentin, and THC, respectively. None of these relative frequencies are especially remarkable considering that morphine was present with a relative frequency of 0.55 across all cases.
Table 2.
Frequent Item Analysis of Opiate Associations with Each Nonopiate Examined Using a Candidate-Based Approach
| Alprazolam | Benzoylecgonine | Ethanol | Gabapentin | Tetrahydrocannabinol | |
|---|---|---|---|---|---|
| Alprazolam | 1.00 | 0.32 | 0.20 | 0.35 | 0.43 |
| Aminoclonazepam | 0.20 | <0.20 | <0.20 | 0.22 | 0.30 |
| Benzoylecgonine | 0.22 | 1.00 | 0.23 | <0.20 | <0.20 |
| Codeine | 0.25 | 0.41 | 0.23 | 0.30 | 0.32 |
| Ethanol | <0.20 | 0.23 | 1.00 | 0.22 | 0.26 |
| Fentanyl | 0.20 | 0.20 | 0.20 | <0.20 | 0.22 |
| Gabapentin | 0.25 | <0.20 | 0.23 | 1.00 | <0.20 |
| Hydrocodone | <0.20 | <0.20 | 0.20 | 0.26 | <0.20 |
| Hydromorphone | 0.26 | 0.32 | 0.20 | 0.30 | 0.23 |
| Methadone | 0.29 | 0.25 | <0.20 | 0.22 | <0.20 |
| Monoacetylmorphine | 0.28 | 0.50 | 0.45 | 0.33 | 0.36 |
| Morphine | 0.52 | 0.66 | 0.57 | 0.57 | 0.62 |
| Tetrahydrocannabinol | 0.31 | 0.20 | 0.27 | <0.20 | 1.00 |
| Alprazolam, morphine | <0.20 | <0.20 | <0.20 | <0.20 | 0.21 |
| Codeine, monoacetylmorphine | 0.23 | 0.41 | 0.20 | 0.26 | 0.26 |
| Codeine, morphine | 0.25 | 0.41 | 0.20 | 0.30 | 0.30 |
| Hydrocodone, hydromorphone | <0.20 | <0.20 | <0.20 | 0.22 | <0.20 |
| Hydromorphone, morphine | <0.20 | 0.20 | <0.20 | <0.20 | <0.20 |
| Monoacetylmorphine, morphine | 0.28 | 0.50 | 0.45 | 0.33 | 0.36 |
| Codeine, monoacetylmorphine, morphine | 0.23 | 0.41 | 0.20 | 0.26 | 0.26 |
Support values are given. Row indicates A and column indicates B in the conditional (A|B). All combinations where support ≥ 0.2 are shown. Hydrocodone and hydrocodone-containing multidrug combinations occur more frequently with gabapentin than the other nonopiates we examined.
More remarkable, however, is the case of hydrocodone. Hydrocodone was present at a relative frequency of 0.18 across all cases. However, in gabapentin-containing cases, hydrocodone was present with a relative frequency of 0.26. In addition, the hydrocodone-containing drug combination of hydrocodone and hydromorphone was present at a frequency of 0.22 in the cases with gabapentin (Table 2). In contrast, hydrocodone and hydrocodone-containing drug combinations were present at a frequency of ≤0.2 in cases containing alprazolam, benzoylecgonine, ethanol, and THC. Thus, hydrocodone is observed to be present more frequently in cases with gabapentin toxicity than across the regular population of mixed drug fatalities, although these are not mutually exclusive groups so a chi-square test cannot be used to determine the significance of this observation.
Gabapentin and Hydrocodone Concentrations
Because it is reported that hydrocodone and gabapentin have a pharmacokinetic interaction whereby hydrocodone increases gabapentin drug exposure (8), we next sought to determine whether gabapentin concentrations in the blood are increased to supratherapeutic concentrations in decedents who also tested positive for hydrocodone. To assess this, we examined the blood concentrations of gabapentin in decedents who concurrently tested positive for hydrocodone against the blood concentrations of gabapentin in decedents who did not test positive for hydrocodone (Figure 4). The average blood gabapentin concentration in decedents with positive hydrocodone toxicology (n=12) was 11.98 ± 2.52 μg/mL. The range of blood gabapentin concentrations in this population was from 11 μg/mL to 172 μg/mL. Meanwhile, the average blood concentration of gabapentin in decedents with negative hydrocodone toxicology (n=34) was 14.11 ± 2.92 μg/mL. The range of blood gabapentin concentrations in these decedents ranged from 0.9 μg/mL to 27.5 μg/mL. Gabapentin is generally considered therapeutic at concentrations <20 μg/mL, although concentrations are often <10 μg/mL (9, 10). Overall, the gabapentin concentrations between these two groups were not statistically different (p=0.592), and the means were below 20 μg/mL, suggesting that the presence of hydrocodone does not cause gabapentin to be increased to supratherapeutic concentrations in these patients.
Figure 4.
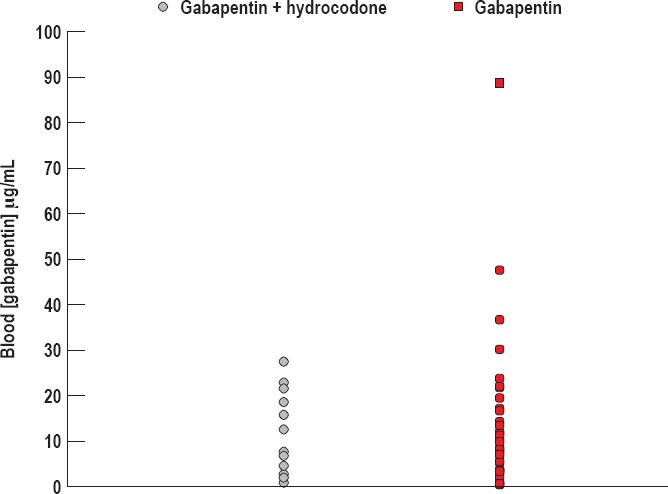
Gabapentin concentration in the presence or absence of hydrocodone. The average blood gabapentin concentration in decedents with positive hydrocodone toxicology (n=12) was 11.98 μg/mL ± 2.52. The average blood concentration of gabapentin in decedents with negative hydrocodone toxicology (n=34) was 14.11 μg/mL ± 2.92.
It has been reported that gabapentin increases hydrocodone elimination (8), so we next investigated whether gabapentin decreased the blood concentrations of hydrocodone. Blood hydrocodone concentrations in decedents who tested positive for both gabapentin and hydrocodone ranged from 0 μg/mL to 514 μg/mL, with the average blood hydrocodone concentration being 99.03 ± 40.93 μg/mL (Figure 5). Hydrocodone detection was negative in two blood samples for which the corresponding decedent urine specimens tested positive. In these two cases, the urine hydrocodone concentrations were 192 μg/mL and 875 μg/mL, with corresponding blood gabapentin concentrations of 4.6 μg/mL and 15.8 μg/mL, respectively.
Figure 5.
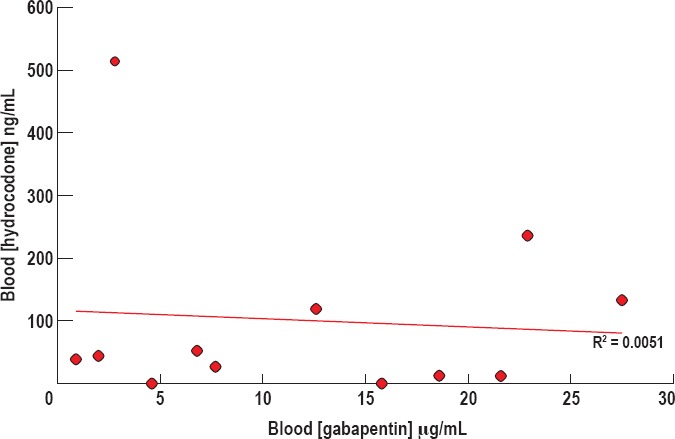
Hydrocodone concentration in the blood versus gabapentin concentration. There appears to be no significant direct or inverse association between concentrations of blood hydrocodone and blood gabapentin (R2 = 0.0051).
There appears to be no significant direct or inverse association between concentrations of blood hydrocodone and blood gabapentin (Pearson correlation coefficient = 0.0051).
Although there seemed to be no correlation in blood gabapentin with decreasing blood hydrocodone concentrations, we investigated the relationship between blood gabapentin and urine hydrocodone concentration to detect whether urine hydrocodone concentrations may be higher in cases with positive gabapentin toxicology. The urine hydrocodone concentrations in decedents who tested positive for both gabapentin and hydrocodone ranged from 192 ng/mL to 10 000 ng/mL, with an average urine hydrocodone concentration of 3365.50 ng/mL ± 873 (Figure 6). When values of urine hydrocodone are plotted with respect to blood gabapentin concentrations, there does not seem to be a direct or inverse correlation between the two concentrations (Pearson correlation coefficient = 0.2812).
Figure 6.
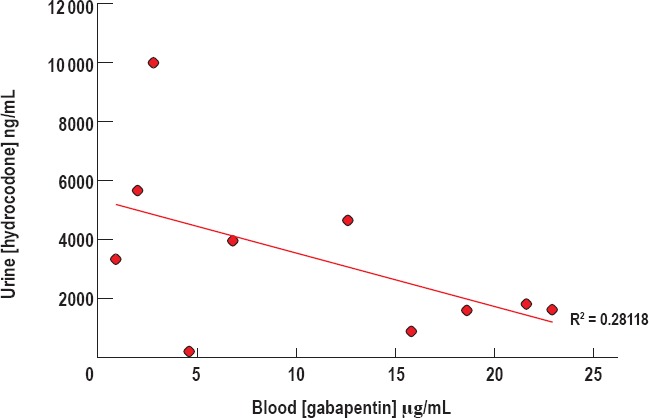
Hydrocodone concentration in the urine versus gabapentin concentration. There appears to be no significant direct or inverse association between concentrations of urine hydrocodone and blood gabapentin (R2 = 0.28118).
Discussion
Gabapentin, or 1-(aminomethyl)cyclohexaneacetic acid, was first approved for use in the United States in 1993. It is widely prescribed as a treatment for seizure disorders and neuropathic pain syndromes, including postherpetic neuralgia and diabetic neuropathy. Additionally it is prescribed for off-label use in managing conditions such as restless legs syndrome and anxiety disorders (11, 12). Prescribed dosages of gabapentin vary with the condition it is intended to treat, but generally fall between 300-3600 mg per day (13). The effective therapeutic concentration in blood is 2-20 μg/mL, with concentrations greater than 25 μg/mL noted as being toxic (9, 10). The recorded side effects of people experiencing a gabapentin overdose include dizziness, drowsiness, ataxia, nausea, tremor, and increased risk of suicide (8, 14).
Gabapentin binds to the α2δ subunit of N-type voltage-gated calcium ion channels (15) and modulates synthesis of both GABA and glutamate through interactions with glutamate decarboxylase and branched chain aminotransferase (16). While many sites of gabapentin binding such as these have been identified, the precise mechanism by which gabapentin-mediated modulation of calcium channels may influence neuropathic pain remains unknown. The co-occurrence of gabapentin with opioid metabolites in our study begs the question of whether gabapentin had any contributory effect to the mechanism of death in the subjects. Opioids have been shown to close N-type voltage-gated calcium channels through indirect OP2-receptor interactions, thereby causing decreased neuronal excitability through hyperpolarization (17). Since this α2δ subunit of N-type channels is also the target of gabapentin inhibition, it stands to reason that the co-occurrence of gabapentin and opioids in the body could affect the function of these N-type channels in unintended ways. Opioids with a higher affinity for the OP2 receptor could possibly have a more pronounced interaction through this mechanism.
In our study, gabapentin was most frequently found to be associated with hydrocodone as a co-occurring metabolite. It has been documented that the presence of gabapentin decreases hydrocodone exposure in a dose-dependent manner. Conversely, hydrocodone has been reported to increase the drug exposure of gabapentin by 14% (8). In our study, however, the presence or absence of hydrocodone did not correlate with differences in the average concentration of gabapentin. It has also been reported that co-administration of gabapentin and other opiates such as morphine may alter the pharmacokinetic profile of gabapentin. We did not focus on that work here, as the drug exposure of the opiate is unchanged in these cases, (8) and we did not identify an increased association between gabapentin and any other opiate besides hydrocodone.
Perhaps one of the more intriguing aspects of gabapentin in our study is the significant co-occurrence of gabapentin with other metabolites of drugs prone to abuse. This discovery prompted the exploration of the manner in which gabapentin is used, whether as prescribed or as a potential drug of abuse. A previous study of prison inmates showed that of the population sampled who had an opiate use disorder, 26% reported intentional abuse of gabapentin (18). The reasons for gabapentin abuse remain unclear, although reports suggest that gabapentin may help relieve symptoms of opioid withdrawal (19), potentially contributing to the high co-occurrence with opioids in our study.
Interestingly, a 2010 report from the Scottish Drug and Crime Enforcement Agency illustrated an appreciable amount of gabapentin retrieved from prisons and on the street, largely in the form as a cutting agent with heroin (20). On a molecular level, gabapentin would appear to be an appropriate cutting agent. Both are odorless white crystalline solids, and the melting temperature of gabapentin (162-166°C) is similar to that of diacetylmorphine (172°C). This is noteworthy as the process of injecting heroin involves melting the substance in preparation for injection. Subjectively, the effects of gabapentin would complement or augment those of diacetylmorphine, making it potentially less likely for the user to notice the adulterant.
Conclusion
While the significant occurrence of gabapentin deserves acknowledgement, our data does not definitively suggest that gabapentin was a causative or contributory factor in the deaths of our sample population. With the exception of minimal outliers, the gabapentin concentration of the vast majority of our sample population was well within the therapeutic range, preventing suspicion that gabapentin toxicity alone played a role in the deaths. Numerous drug interactions have been identified and/or suspected with gabapentin, but because the exact mechanism has not been elucidated, definitive conclusions regarding its contribution to overdose deaths cannot be made. Further research exploring these interactions would allow for more complete understanding of the mechanisms of gabapentin. Verified interactions that produce undesirable effects could significantly change prescribing practices of gabapentin, especially if they are more likely to contribute to accidental death.
Footnotes
ETHICAL APPROVAL
As per Journal Policies, ethical approval was not required for this manuscript
STATEMENT OF HUMAN AND ANIMAL RIGHTS
This article does not contain any studies conducted with animals or on living human subjects
STATEMENT OF INFORMED CONSENT
No identifiable personal data were presented in this manuscsript
DISCLOSURES & DECLARATION OF CONFLICTS OF INTEREST
The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
FINANCIAL DISCLOSURE The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1.Rudd R.A., Aleshire N., Zibbell J.E., Gladden R.M. Increases in drug and opioid overdose deaths–United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016. Jan 1; 64(50-51): 1378–82. PMID: 26720857. 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 2.Chang H.Y., Daubresse M., Kruszewski S.P., Alexander G.C. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014. May; 32(5): 421–31. PMID: 24560834. 10.1016/j.ajem.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Daubresse M., Chang H.Y., Yu Y. et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013. Oct; 51(10): 870–8. PMID: 24025657. PMCID: PMC3845222. 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J.M., Irvine R.J. Mechanisms of fatal opioid overdose. Addiction. 1999. Jul; 94(7): 961–72. PMID: 10707430. 10.1046/j.1360-0443.1999.9479612.x. [DOI] [PubMed] [Google Scholar]
- 5.Hahsler M., Grün B., Hornik K., Buchta C. Introduction to arules – a computational environment for mining association rules and frequent item sets. J Stat Softw [Internet]. 2005. [cited 2016 Oct 10]; 14(15): 1–25. Available from: https://cran.r-project.org/web/packages/arules/vignettes/arules.pdf. [Google Scholar]
- 6.Paul B.D., Mitchell J.M., Mell L.D. Jr., Irving J. Gas chromatography/electron impact mass fragmentometric determination of urinary 6-acetylmorphine, a metabolite of heroin. J Anal Toxicol. 1989. Jan-Feb; 13(1): 2–7. PMID: 2709823. 10.1093/jat/13.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Kolbrich E.A., Barnes A.J., Gorelick D.A. et al. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J Anal Toxicol. 2006. Oct; 30(8): 501–10. PMID: 17132243. 10.1093/jat/30.8.501. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration [Internet]. Washington: U.S. Food and Drug Administration; c2011. Neurontin (gabapentin) capsules; [cited 2016. Oct 10]. 34 p. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020235s036,020882s022,021129s0221bl.pdf. [Google Scholar]
- 9.Cantrell F.L., Mena O., Gary R.D., McIntyre I.M. An acute gabapentin fatality: a case report with postmortem concentrations. Int J Legal Med. 2015. Jul; 129(4): 771–5. PMID: 25904080. 10.1007/s00414-015-1193-3. [DOI] [PubMed] [Google Scholar]
- 10.Tjandrawinata R.R., Setiawati E., Putri R.S. et al. Single dose pharmacokinetic equivalence study of two gabapentin preparations in healthy subjects. Drug Des Devel Ther. 2014. Sep 4; 8: 1249–55. PMID: 25214768. PMCID: PMC4159312. 10.2147/DDDT.S69326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm. 2003. Nov-Dec; 9(6): 559–68. PMID: 14664664. 10.18553/jmcp.2003.9.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mula M., Pini S., Cassano G.B. The role of anticonvulsant drugs in anxiety disorders: a critical review of the evidence. J Clin Psychopharmacol. 2007. Jun; 27(3): 263–72. PMID: 17502773. 10.1097/jcp.0b013e318059361a. [DOI] [PubMed] [Google Scholar]
- 13.Backonja M., Glanzman R.L. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003. Jan; 25(1): 81–104. PMID: 12637113. 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 14.Middleton O. Suicide by gabapentin overdose. J Forensic Sci. 2011. Sep; 56(5): 1373–5. PMID: 21554310. 10.1111/j.1556-4029.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendrich J., Van Minh A.T., Heblich F. et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008. Mar 4; 105(9): 3628–33. PMID: 18299583. PMCID: PMC2265195. 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor C.P., Gee N.S., Su T.Z. et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998. Feb; 29(3): 233–49. PMID: 9551785. 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 17.Tallent M., Dichter M.A., Bell G.I., Reisine T. The cloned kappa opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells. Neuroscience. 1994. Dec; 63(4): 1033–40. PMID: 7700508. 10.1016/0306-4522(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 18.Bastiaens L., Galus J., Mazur C. Abuse of gabapentin is associated with opioid addiction. Psychiatr Q. 2016. Dec; 87(4): 763–7. PMID: 26887855. 10.1007/s11126-016-9421-7. [DOI] [PubMed] [Google Scholar]
- 19.Stoicea N., Russell D., Weidner G. et al. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front Pharmacol. 2015. May 27; 6: 104 PMID: 26074817. PMCID: PMC4444749. 10.3389/fphar.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith B.H., Higgins C., Baldacchino A. et al. Substance misuse of gabapentin. Br J Gen Pract. 2012. Aug; 62(601): 406–7. PMID: 22867659. PMCID: PMC3404313. 10.3399/bjgp12X653516. [DOI] [PMC free article] [PubMed] [Google Scholar]


