Abstract
Neurotrophins have been shown to be involved in functional strengthening of central nervous system synapses. Although their general importance in this process is undisputed, it remains unresolved whether neurotrophins are truly mediators of synaptic strengthening or merely important cofactors. To address this question, we have devised a method to inactivate endogenous brain-derived neurotrophic factor (BDNF) with high time resolution by “caging” a function-blocking mAb against BDNF with a photosensitive protecting compound. Different assays were used to show that this inactivation of the Ab is reversible by UV light. Synaptic potentiation after τ-burst stimulation in the CA1 region of acute hippocampal slices was significantly less when applying the unmodified Ab compared with the caged Ab. Importantly, photoactivation of the caged Ab during the time of induction of synaptic enhancement led to a marked decrease in potentiation. Our experiments therefore strengthen the view that endogenous BDNF has fast effects during induction of synaptic plasticity. The results additionally show that caged Abs can provide a tool for precise spatiotemporal control over endogenous protein levels.
Neurotrophins are signaling molecules known to be crucial for survival and differentiation of peripheral and central neurons (1, 2). Recent evidence, however, has shown that these polypeptide-molecules also subserve other important roles. Given that they are released in an activity-dependent manner (3, 4), it has been suggested that they also convey information during activity-dependent synaptic plasticity (5, 6). Several studies have revealed a significant role of brain-derived neurotrophic factor (BDNF) during physiological changes of synapses such as long-term potentiation (LTP) in the hippocampus (7–9) and cortex (10). Moreover, activity-dependent morphological changes, such as the structural rearrangement of presynaptic axon terminals during the formation of ocular dominance columns or the formation of the dendritic arbors, have been shown to be regulated by BDNF (11–13) (for overview, see refs. 6 and 14).
Different approaches have been used to investigate the role of BDNF during plastic processes, particularly during hippocampal LTP. Initial experiments tested the effects of exogenously applied BDNF on synaptic transmission and synaptic plasticity. BDNF application produced a marked enhancement of synaptic transmission at Schaffer collateral–CA1 synapses in adult hippocampal slices (7). However, application of exogenous neurotrophins differs considerably from physiological release, with respect to spatiotemporal properties as well as absolute values, which often exceed physiological concentrations by several orders of magnitude. Therefore, to better understand the physiological role of neurotrophins, it is essential to study the contribution of endogenous neurotrophins. Evidence for the importance of endogenous BDNF during hippocampal LTP was provided by studies using conventional or conditional knockout techniques of the BDNF gene or its corresponding receptor tyrosine–kinase trkB (8, 15–17).
To investigate more acute effects of neurotrophins, several studies used specific Abs or trkB-IgG fusion bodies to block neurotrophin action (18–20). Acute interference with BDNF signaling in slice preparations of wild-type animals led to a significant reduction in LTP. Compared with genetic approaches using transgenic mice, these experiments allowed a significant increase of the temporal resolution with which endogenous neurotrophin actions were studied. However, even in these experiments, the temporal resolution was not sufficient to determine when and how, exactly, BDNF is needed during synaptic potentiation. Two scenarios are conceivable: either BDNF needs to be present for longer periods of time for LTP to occur, consistent with a slow mechanism that we will call “permissive,” or, alternatively, BDNF might be required during a short defined time window during the induction of synaptic potentiation, consistent with a fast mechanism, for which we will use the term “instructive.”
In principle, function-blocking Abs would be suitable to answer questions about the temporal requirements of BDNF. However, particularly in dense tissue such as slice preparations, slow diffusion makes it impossible to determine the precise onset of the blocking action. In contrast, a timed and localized activation of Abs and their function-blocking activity would allow interference with endogenous proteins at desired timepoints and locations, thus keeping the activation independent of the actual penetration into the tissue. Therefore, the goal of our study was to generate a tool that would allow us to rapidly inhibit endogenous BDNF levels during induction of synaptic potentiation and to use this tool to elucidate further the role of BDNF during synaptic plasticity. To this end, we attempted to generate a “caged Ab” that could be activated at will by irradiation with UV light.
Materials and Methods
Caging of BDNF Abs.
We used function-blocking mouse mAbs (clones nos. 21 or 9, IgG2B) raised against BDNF and previously characterized (21). Abs were incubated at room temperature with biotinylated recombinant Protein G (Sigma) for at least 1 h at concentration ratios of 4:1 or 1:1 (Ab/Protein G). The mixture was then diluted with 0.1 M sodium carbonate buffer (pH 9.5), so that the final concentration of total protein was 0.2 mg/ml. The caging compound 6-nitroveratrylchloroformate (NVOC-Cl) (Fluka) was added at 2% (vol/vol) in dioxane (Fluka) to a final concentration of 0.2 mM NVOC-Cl, and the reaction was allowed to proceed for 30 min at room temperature. Further details of the caging procedure, such as the extent of the caging and processing of the Abs after the caging reaction, are published as supporting information on the PNAS web site (www.pnas.org).
Irradiation of the Abs used for ELISAs or bioassays was done with a 6-W hand-held 365-nm UV lamp at volumes of 4–10 μl. The low power of the hand-held lamp necessitated relatively long uncaging times for complete reactivation of the Ab (e.g., 3 h in Fig. 1B). Further details about the irradiation levels are published as supporting information on the PNAS web site (www.pnas.org).
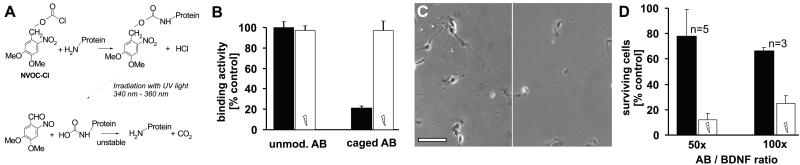
Figure 1.
Reversible inactivation of a function-blocking mAb against the neurotrophin BDNF. (A) Illustration of the caging reaction with NVOC-Cl: free amino groups of lysines on the Ab are modified by reaction with the amine-reactive caging compound. A chemically stable but photolabile carbamate is formed when the nitrogen of a free amine attacks the carbonyl carbon of NVOC-Cl to displace the chloride. Irradiation causes an internal redox reaction to generate a nitrosobenzyl and an unstable carbamic acid which spontaneously decarboxylates to regenerate the free amine. (B) ELISA demonstrating the reduced binding of the Ab to BDNF after modification with the caging group and its successful reactivation by UV light. The remaining signal of the caged Ab (21%) reflects unspecific binding (see text). Filled bars show the binding activity of the unirradiated Ab; open bars show the activity after irradiation. (C) Bioassay to test the blocking activity of the caged or uncaged Ab. (Bar = 100 μm.) (Left) Chick nodose ganglion neurons cultured in the presence of BDNF and caged Ab. (Right) Parallel preparation after adding the irradiated Ab. (D) Quantification of the altered function-blocking properties. Filled bars: percentage of surviving cells with unirradiated Ab; open bars, percentage of surviving cells after adding the irradiated Ab.
ELISA–BDNF Immunoassay.
ELISAs were done according to standard procedures with Dynex (Chantilly, VA) microfluor 2 plates by using 50 ng of BDNF bound per well. To test the affinity of the caged or uncaged Abs to BDNF, the entire solution containing the protein G/Ab complexes was added to the wells at Ab concentrations of 100 ng/well (diluted in 1% BSA) and incubated overnight at 4°C. Ab/Protein G–biotin complexes were detected with avidin-β-galactosidase (Sigma) by using 4-methylumbelliferyl-β-galactoside (Sigma) as a fluorescent substrate and were analyzed with a Fluoroskan II reader (Titertek, Huntsville, AL).
Modification of the Ab with NVOC-Caged Rhodamine Green.
NVOC-caged Rhodamine green (Molecular Probes) was coupled to BDNF Abs according to the manufacturer's instructions. Ab solution (10 mg/ml) in 0.1 M sodium carbonate (pH 9) was reacted with 1/10 volume of 10 mg/ml of NVOC-caged Rhodamine green in DMSO for 1 h at room temperature. The reaction mixture was briefly centrifuged and the supernatant washed twice with Centricon C-30 in PBS. To quantify fluorescence, the mean fluorescence was measured in the irradiated spot and in the surrounding unirradiated area before and after photoactivation. Fluorescence was corrected for background values, normalized to the maximal fluorescence, and averaged over all experiments. The rationale for using this compound to measure photoactivation as well as diffusional properties is spelled out in detail in the supporting information (www.pnas.org).
Neuronal Cultures for Bioassays.
Sensory neurons were obtained from nodose ganglia of chick embryos at embryonic day 7/8 (22). Ganglia were dissociated with trypsin and cultured on polyornithine/laminin coated 48-well plates (Costar) with 10% horse serum. Function-blocking BDNF Abs were added at different concentrations together with a fixed BDNF concentration (0.5 ng/ml). Survival rate was defined as (N − Nbaseline)/(Nmax − Nbaseline), where N is the number of counted cells, Nbaseline the number of cells which survived in presence of the unmodified Ab, and Nmax the maximal survival observed with only BDNF present (i.e., with no Ab). At least two wells were used and tested per experimental condition in each experiment.
Preparation and Perfusion of Slices.
Acute hippocampal transverse slices (400 μm thick) were prepared from female WT mice of the NMRI/SV129 strain (4–6 weeks old) by using techniques described previously (20). Slices were maintained under standard conditions in artificial cerebrospinal fluid (ACSF): 124 mM NaCl/3 mM KCl/1.25 mM KH2PO4/2 mM MgSO4/26 mM NaHCO3/2.5 mM CaCl2/10 mM glucose; room temperature, oxygenation with 95% O2 and 5% CO2. Siliconized tubing and beakers were used, and BSA (0.2 mg/ml final concentration) was added to the ACSF to reduce unspecific binding. Caged Abs were prepared freshly each day and then diluted in ACSF. Slices were incubated in the Ab/BSA solution for 1–2 h (total volume 5 ml, without perfusion) before the recordings. In closed-loop experiments, a total volume of 30 ml of ACSF was used. The ACSF was kept in an ice-water bath to reduce deterioration of the medium over time. Initially, we used a closed-loop system and added Abs or control solutions to the perfused ACSF (perfusion rate 2 ml/min). Later, we stopped adding the Abs to the perfusate to reduce the amount of Ab required and used only incubated slices and an open-loop perfusion system.
Electrophysiology.
Hippocampal slices were placed in a submerged-type recording chamber (32°C), and excitatory postsynaptic field potentials (EPSPs) were recorded in the CA1 dendritic region while stimulating the Schaffer collaterals. Recordings were done at a depth of at least 200 μm below the slice surface to be close to the side of the objective and UV illumination. Data were collected, stored, and analyzed with custom-made labview software (National Instruments, Austin, TX). Experiments were done and analyzed blindly. The initial slope of field EPSPs was measured over time, normalized to baseline (mean response before TBS), and plotted as average ± SEM. As a statistical test, the unpaired one-tailed Student's t test (assuming unequal variances) was used on values averaged for the 5-min period preceding the actual time point. On each single experimental day, both conditions of the respective experimental series were measured. We excluded unstable recordings and experiments in which a large population spike after induction of potentiation produced artifactually large LTP.
Photoactivation in Slices.
For irradiation of the Ab in hippocampal slices, we used a 100-W mercury lamp (Zeiss) mounted to the epifluorescent path of an inverted microscope. The irradiation beam was centered and focused on the tip of the recording electrode. The same diaphragm opening was used for all electrophysiological experiments, resulting in a spot size of about 800-μm diameter. Before and after TBS, irradiation was applied for 2 min every 0.5 s, with a duration of 50 ms. During TBS, a stronger paradigm was used (duration, 250 ms; interval, 1 s). After each experiment, the focus and position of the objective relative to the recording electrode were verified by applying intense irradiation (duration, 2 s; interval, 5 s for 1.5 min). This procedure led to a fast drop in amplitude and slope when objective and electrode were properly centered and aligned.
Results
Developing a Caging Protocol to Reversibly Inactivate Function-Blocking Abs Against BDNF.
Initially, we optimized the reaction conditions for caging the function-blocking BDNF Ab. We used an Ab against BDNF that previously had been successfully applied in experiments studying the specific role and contribution of endogenous BDNF during synaptic potentiation (20). The Ab was reacted with the photosensitive protecting compound NVOC-Cl (6-nitroveratrylchloroformate) to inactivate the Ab in a controlled fashion and to later regain its function-blocking activity by irradiation-induced photolysis of the caging compounds (23–25). NVOC-Cl preferentially reacts with free amino groups at high pH, thereby almost exclusively targeting lysines within proteins (Fig. 1A). The goal was to randomly modify enough sites of the Ab variable regions critical for binding to BDNF with the caging group. The reaction conditions were worked out by testing the Abs in a one-sided ELISA with BDNF as antigen. Indeed, detection of BDNF with the caged Ab revealed a reduced signal. However, this decrease could have been caused by either the reduced affinity of the primary Ab to BDNF or a decreased binding of the secondary Ab to the modified primary BDNF Ab (26). To ensure that the decrease of the measured signal derived solely from caging the BDNF-binding site, Abs were incubated with biotinylated Protein G before any chemical modifications. Detection of the biotin–Protein G/Ab complex with avidin-β-galactosidase then allowed us to unambiguously reveal any change in BDNF affinity, because biotin is not modified with NVOC-Cl. Initially, we tested qualitatively whether it is possible to recover binding activity of the caged Ab by irradiation. Samples of a few microliters were exposed to unfocussed UV light from a low-intensity hand-held UV lamp, necessitating relatively long irradiation times. The results of such an experiment are shown in Fig. 1B. ELISA readings were normalized to the unmodified control. Reaction of the Ab with the caging compound blocked its binding to BDNF. The remaining ELISA signal was caused by background activity, because unirradiated caged control c-myc or luciferase Abs each produced a similar unspecific signal (data not shown). This result indicates that binding of the BDNF Ab to its antigen could be prevented by modification with caging compounds. More importantly, however, the reduction could be completely reversed by irradiation of the modified Ab with UV light (λ = 365 nm), which restored the binding activity to original levels (see Fig. 1B, open bars).
To confirm these results in a biological system, we tested the Abs in a survival assay of dissociated neurons of the chick nodose ganglion. These neurons require the presence of BDNF for survival and neurite outgrowth (22) and hence provide a sensitive assay for biologically active BDNF. We cultured neurons in the presence of BDNF with either modified or unmodified Ab added at concentrations known to effectively block BDNF activity. Fig. 1 C and D show that most neurons survive in the presence of the caged Ab (Fig. 1C Left). However, photoactivation of the caged Ab results in the death of most neurons (Fig. 1C Right), indicating that the Ab's blocking activity has been restored to levels comparable to the unmodified Ab (P < 0.02; paired t test for 50×). The results obtained from two different assay systems therefore demonstrate that a reversible modification of the BDNF Ab with a photolabile group is possible, and that this Ab can inhibit the biological action of BDNF.
Photoactivation of Abs in Hippocampal Slice Tissue.
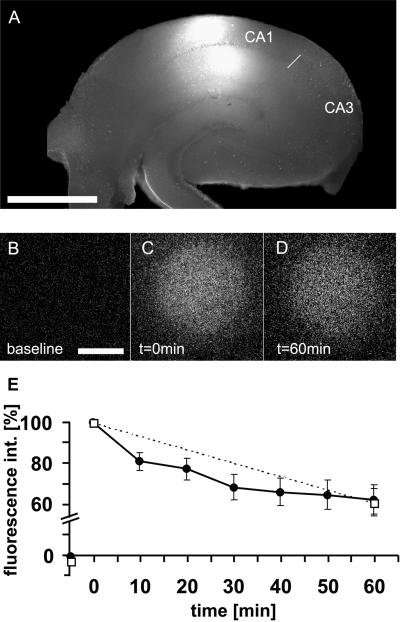
These results showed that our approach should in principle be feasible, but the goal of the study was to work in acute brain slices. Thus, it was important to evaluate in compact tissue the efficiency of uncaging by UV irradiation and the diffusion properties of the photoactivated Ab, as rapid diffusion could potentially decrease its effective concentration. Fig. 2A shows the size of an irradiated spot in a hippocampal slice. The spot had a diameter of about 800 μm and encompassed both the dendritic and somatic CA1 regions but did not reach the cell bodies in CA3. To visualize photoactivation and diffusion, we used NVOC-caged Rhodamine green, a dye modified with the same caging group, and coupled it to BDNF Abs to ensure similar diffusion properties. Fig. 2 B and C show how the fluorescence of the Rhodamine green appeared after pulsed light (λ = 365 nm) was applied for a total irradiation time of 18 sec. Within 1 h after irradiation, the intensity of the spot slowly decreased to 62% of the initial value (Fig. 2 B–E). This decrease was caused by diffusion and not photobleaching, because an identical decrease in fluorescence was obtained when the number of exposures was reduced to only two (Fig. 2E, □). The amount of fluorescence after 1 h predicts for the BDNF-blocking experiments that ≈60% of the original Ab concentration can be expected 1 h after uncaging. This result therefore indicated that diffusion is not a major obstacle, and that the method should be useful for investigating the temporal requirements of BDNF action during synaptic plasticity with the required temporal resolution.
Figure 2.
Photoactivation in hippocampal slices. (A) Illustration of the setup for the physiological experiments. The irradiation spot (produced with a 32× objective) was placed in the CA1 area. (Bar = 750 μm.) (B–E) Diffusion of the Ab in slices assessed by NVOC-caged Rhodamine green coupled to BDNF Abs. The photoactivation paradigm was identical to that used in the LTP experiments. (B–D) Examples of photoactivation within the slice. (B) Area of irradiation before photoactivation, (C) immediately after irradiation, and (D) 60 min after irradiation. (Bar = 125 μm.) (E) Average time course of relative fluorescence within the photoactivated spot. The slow decrease in fluorescence over time shows the decrease in concentration of photoactivated molecules caused by diffusion. Fluorescence intensity was measured in 10-min intervals (●, n = 7) or only once after 60 min (□, n = 7) to demonstrate that bleaching is not the cause of the decay in fluorescence.
Effects of the Caged BDNF Ab on Synaptic Potentiation in the Hippocampal CA3-CA1 Pathway After Photoactivation.
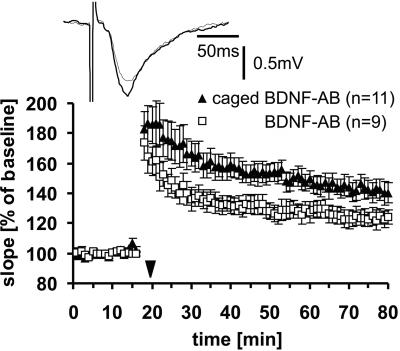
Subsequently, we tested whether and under which circumstances LTP would be affected by uncaging of the modified BDNF Ab. Extracellular recordings were done in the CA1 region of mouse hippocampal slices that had been incubated for 1–2 h with the Abs. Synaptic potentials elicited by stimulation of the Schaffer collaterals were monitored before and after TBS. Because we had previously shown that function-blocking Abs against BDNF led to a reduction of LTP (20), we first tested whether we could observe the same reduction in synaptic potentiation in the presence of the unmodified active versus the modified unirradiated and therefore presumably inactive Ab. To obtain an optimal differential effect between the unmodified and modified Ab, we tested three different Ab concentrations. In agreement with the results obtained in the bioassay and the ELISA (data not shown), we found that low (1 μg/ml) and high (4 μg/ml) concentrations produced only a small or no difference in LTP, whereas at an intermediate concentration of 2 μg/ml, there was a significant difference. LTP in slices that had been incubated with 2 μg/ml of the caged Ab was significantly larger than in slices incubated with the unmodified Ab (Fig. 3; 60 min after TBS: modified Ab, 143 ± 6%, n = 11; unmodified Ab, 123 ± 6%, n = 9; P < 0.05). This concentration effect is most likely because of the caged Ab having a residual binding activity, which becomes apparent only at high concentrations, whereas at low concentrations, too little Ab is present to exert a measurable effect.
Figure 3.
LTP in the CA1–Schaffer collateral pathway of hippocampal slices in the presence of caged (▴) or unmodified (□) BDNF-Ab (concentration 2 μg/ml). After TBS (indicated by the ▾ symbol), synaptic potentiation was significantly larger in slices treated with caged Ab compared with unmodified Ab. Inset depicts two EPSP traces for the caged Ab experiment, 10 min before (thin trace) and 60 min after (thick trace) TBS.
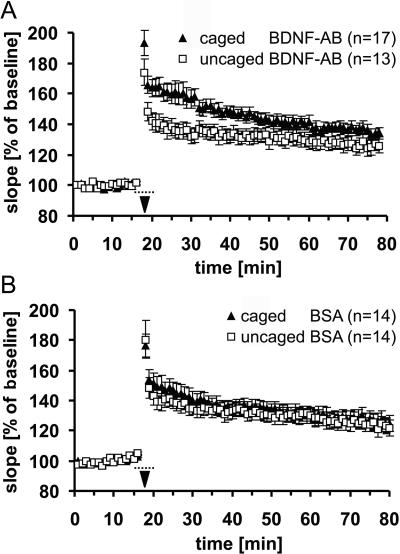
Next, we photoactivated the caged Ab within the slice around the time of TBS to test whether rapid and immediate inhibition of BDNF would lead to any changes in synaptic potentiation. Intermittent pulsed stimulation [interflash interval (IFI) 0.5 s, flash duration (d) 50 ms] was chosen to reduce potential phototoxic damage. This irradiation paradigm showed no effects on baseline transmission (data not shown). Irradiation, started 2 min before TBS to allow sufficient photoactivated Ab to accumulate, was continued during TBS with more intense irradiation IFI = 1 s, d = 250 ms) and extended (IFI = 0.5 s, d = 50 ms) for an additional 2 min after TBS. Both groups of slices were incubated with modified BDNF Ab, but only one group was irradiated at the time of potentiation, according to the paradigm described above. Fig. 4A demonstrates that synaptic potentiation of the photoactivated group is significantly reduced compared with the unirradiated controls during the first 50 min after TBS (50 min after TBS: caged Ab, 138 ± 3%, n = 17; uncaged Ab, 129 ± 4%, n = 13; P < 0.05). This shows that blocking BDNF activity for a short period around LTP induction is sufficient to affect potentiation significantly, supporting a fast instructive effect of BDNF.
Figure 4.
Effect of photoactivation of the Ab during the time of TBS. Irradiation was started 2 min before and stopped 2 min after TBS (indicated by the dotted line). (A) Uncaging of the BDNF Ab (□) significantly reduced the degree of synaptic potentiation up to 50 min after TBS compared with experiments without irradiation (▴). (B) Control experiments show no significant difference between uncaged (□) and caged (▴) BSA.
To control for unspecific effects caused by the irradiation itself, the same experiment was repeated with caged BSA as a control protein (Fig. 4B). Comparison between irradiated and unirradiated slices shows that both groups almost completely overlap, with the irradiated group not being significantly smaller in its potentiation than the unirradiated group (60 min after TBS: caged BSA, 130 ± 6%, n = 14; uncaged BSA, 125 ± 5% n = 14; P = 0.6). The small difference shortly after TBS might reflect some minor toxic effects of the irradiation itself or of the photoactivation reaction side product. This control indicates that the effect observed with the caged BDNF-Ab was specific to the Ab and was not caused by the irradiation as such.
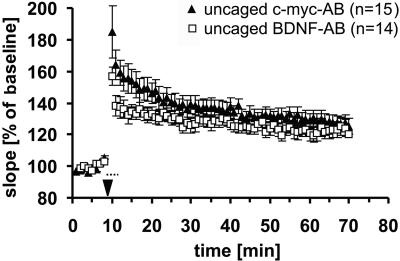
In a second experiment, we further increased the temporal resolution and tested the effects of a shorter irradiation paradigm starting only immediately before TBS (7 s; interflash interval, 500 ms; d, 50 ms) and continued during and after TBS as before. In this case, two caged Abs, one against c-myc and one against BDNF, were both photoactivated and compared with one another. Fig. 5 shows that, again, synaptic responses in the presence of photoactivated BDNF Ab are less potentiated compared with the c-myc control Ab (10 min after TBS: myc-Ab, 149 ± 7%, n = 15; BDNF Ab, 133 ± 5%, n = 14; P < 0.05). However, the effect was significantly smaller than in the previous paradigm and lasted only for the first 10–15 min after TBS (60 min after TBS: myc-Ab, 127 ± 4%, n = 15; BDNF Ab, 123 ± 6%, n = 14; P = 0.3). This reduction can easily be explained by the shortening of the photoactivation paradigm, with a total of only 700 ms of activation time before TBS compared with 12 s in the first experiment, necessarily leading to smaller amounts of activated Ab. Thus, increased temporal resolution by less irradiation is paid for with a reduced effect. Still, because these experiments, as all others, were done blindly, this paradigm also reveals a significant albeit small effect.
Figure 5.
Experiments similar to those in Fig. 4, with photoactivation starting only 7 s before TBS. In this case, a modified c-myc Ab (against the human c-myc 9E10 epitope) was used as control. Uncaging of the BDNF-Ab (□) reduced potentiation during the first 15 min compared with the control group (▴).
Discussion
Previous studies concerning the temporal requirements of BDNF during synaptic potentiation have either lacked the temporal resolution or have shown the involvement of BDNF only after TBS (19). Our method provides a way to precisely time the inactivation of endogenous BDNF, thereby allowing us to focus on the role of BDNF during TBS. The experiments reported here demonstrate that interfering with BDNF action within a narrow time window around the time of LTP induction is sufficient to significantly reduce the level of synaptic potentiation. These results strongly suggest that endogenous BDNF released during TBS is a pivotal component of synaptic potentiation. Evidently, our experiments do not rule out that BDNF additionally may have permissive effects, but they provide the first demonstration, to our knowledge, that endogenous BDNF has a fast-acting instructive role during synaptic plasticity. The use of the uncaging method also allowed us to address, at least to a limited extent, the spatial requirements of BDNF during potentiation. The restricted photoactivation in the CA1 region excluded BDNF action on somatic or dendritic compartments of CA3 cells.
A major unresolved issue concerning BDNF action during synaptic plasticity and potentiation relates to its temporal role. In light of the fact that BDNF is important for synaptic potentiation and plasticity, its activity-dependent release (3, 4) has led to the suggestion that BDNF might act as a fast messenger, which is instructive in the sense that it contributes to the initiation of the observed changes of synaptic strength. So far, most experiments could not differentiate between fast and slow effects of endogenous BDNF. The only, albeit rather indirect, experimental support favoring a fast effect of BDNF has been provided by experiments by using exogenously applied BDNF to induce rapid physiological changes (27). In principle, these changes can occur within a few minutes. An even faster effect of BDNF in the millisecond range was recently described by Kafitz et al. (28), who found that local and fast application of BDNF to hippocampal neurons can induce rapid depolarizations of neurons, comparable to the excitatory neurotransmitter glutamate. To determine whether endogenous BDNF can, in fact, have equally rapid effects on synaptic potentiation, it is crucial to acutely block BDNF activity within seconds. With the temporal resolution provided by our approach, the need for BDNF activity during synaptic potentiation can be evaluated at the critical time point, namely during TBS. Taking advantage of this method to block BDNF activity for only minutes around the time of TBS (compared with hours in previous experiments), we still observed a significant reduction in potentiation. Moreover, the reduction was seen immediately, starting with the first time point after TBS, further highlighting the evidence for a fast role of BDNF and supporting the idea of fast release of BDNF.
Although a direct proof for BDNF release during TBS is still missing, it is reasonable to assume that this strong repetitive stimulation used to elicit potentiation can trigger the release of BDNF, similar to that described for strong depolarizing stimuli such as KCl or glutamate (3, 4, 29). A different, and in our view less likely, explanation is that θ-burst activity leads to increased sensitivity to BDNF. Some studies have, in fact, provided evidence that the cellular response to neurotrophins can depend on the level of neuronal activity (30, 31). However, this effect so far has been demonstrated only in experiments using exogenous BDNF combined with relatively long exposure times. In any case, the latter possibility is also consistent with fast actions of BDNF during synaptic plasticity
Temporal Resolution of the Experiment.
One major advantage of our method compared with previous approaches is that it allows the control of the onset of BDNF blockade with high precision. Initially, we were concerned that the activated Abs would quickly diffuse away from the irradiated zone, thereby preventing the necessary buildup of sufficient levels of function-blocking Abs. Photoactivated fluorescence of caged Rhodamine green coupled to BDNF Abs provided a reasonable estimate of the diffusion properties of the Ab in hippocampal tissue, thereby demonstrating that the concentration of the activated Abs appears to be relatively stable during the time of TBS and shortly thereafter.
It is difficult to get a good estimate of the overall concentration of photoactivated Abs compared with endogenous BDNF. Decreasing the time of irradiation by omitting 2 min of irradiation before TBS (see Fig. 5) clearly reduced the effect on potentiation. This observation suggests that, in this experiment, the level of function-blocking Abs was nonsaturating. The paradigm with 2-min pre- and postactivation was originally chosen to activate a sufficient number of Abs, while trying to retain an acceptable temporal resolution. Longer pulses and shorter interpulse intervals could not be used, because they were found to be phototoxic due to cumulative UV exposure. Presumably the limited activation of Abs accounts for the modest difference between the two curves in Fig. 4A, particularly at later time points.
Even though the onset of blocking in our experiments is temporally well defined, its offset is much less well defined because of a variety of reasons. These include (i) unknown binding constants of the Ab and unknown concentrations of the Ab compared with BDNF, (ii) diffusion mechanisms, which continuously decrease the number of photoactivated Abs, and that (iii) it is not clear how much of the Ab is necessary to impair potentiation. It is possible that our method also affects BDNF levels after irradiation; therefore, we cannot rule out that BDNF might additionally be needed after induction of LTP, as suggested by previous studies (19). In any case, the sharp onset of BDNF inactivation and its immediate effect on potentiation are consistent with a fast action of BDNF during the induction of potentiation.
Caging of Abs.
Our experiments show that modified Abs display a decreased binding to BDNF. Although the modification with the NVOC-Cl caging group significantly reduced binding to BDNF, the blocking effect was not complete. The use of 4 μg/ml of Ab in electrophysiological experiments produced curves that were statistically not different when modified Abs were compared with unmodified controls. This observation can be explained by residual binding activity, which, at higher concentrations, became substantial enough to inhibit synaptic potentiation. This interpretation is corroborated by data from ELISA and survival bioassay experiments (data not shown). Two possible scenarios may account for the residual binding activity. Either the extent of modification with the caging group is more or less identical for all Ab molecules, causing an equal reduction in affinity of each single Ab molecule for the BDNF antigen, or, alternatively, the pool of Abs could be composed of Abs with different affinities toward BDNF. Irrespective of the mechanism, NVOC-Cl caging not only transforms a positively charged side chain into a nonpolar one, but it may also introduce a steric hindrance because of the mere presence of the extra aromatic moiety. Therefore, the impact of the caging reaction largely depends on the presence and contribution of lysine residues to Ab–antigen binding.
Thus far, reports about caged proteins that were used in biological systems have been limited (25, 32). For example, NVOC-Cl was used to reversibly block polymerization activity of G-actin (24), to control the transcriptional activity of GAL4VP16 in living Drosophila embryos (33), or to study the kinetics of intracellular signaling pathways by using caged protein kinases or their inhibitors (34). Caging of Abs was recently achieved by producing changes in binding strength between Abs and antigen or Abs and Protein A (26); however, the caged Abs were not used in further experiments. Our results demonstrate the applicability of Abs modified with a photolabile caging group as a means to inhibit specific endogenous proteins in biological systems at desired timepoints and locations. Here we show that BDNF has an immediate, possibly instructive, role in synaptic plasticity. Moreover, this method should make it possible to study also other biologically active polypeptide molecules in different systems with hitherto unattained spatiotemperal control.
Supplementary Material
Acknowledgments
We thank Y.-A. Barde, B. Cürten, and M. Korte for comments on earlier versions of the manuscript and Y.-A. Barde (Max Planck Institute for Neurobiology) for generously providing the BDNF and c-myc Abs. This research was supported by the Max-Planck-Gesellschaft, the Deutsche Forschungsgemeinschaft (SFB 391), and the European Community Biotech Program. S.B.C. was supported by the European Molecular Biology Organization and the National Science Foundation–North Atlantic Treaty Organization postdoctoral fellowships.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- EPSP
excitatory postsynaptic potential
- LTP
long-term potentiation
- TBS
τ-burst stimulation
- NVOC-Cl
6-nitroveratrylchloroformate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lewin G R, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.Davies A M. Trends Neurosci. 1994;17:195–199. doi: 10.1016/0166-2236(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 3.Blöchl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 4.Griesbeck O, Canossa M, Campana G, Gärtner A, Hoener M C, Nawa H, Kolbeck R, Thoenen H. Microsc Res Technol. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 5.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 6.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Kang H J, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 8.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 10.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 12.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 13.McAllister A K, Katz L C, Lo D C. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 14.Cellerino A, Maffei L. Prog Neurobiol. 1996;49:53–71. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 15.Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp H P, Bonhoeffer T, Klein R. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 16.Pozzo-Miller L D, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z H, Lu B. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu B, Gottschalk W, Chow A, Wilson R I, Schnell E, Zang K, Wang D, Nicoll R A, Lu B, Reichardt L F. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Kolbeck R, Barde Y-A, Bonhoeffer T, Kossel A. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolbeck R, Bartke I, Eberle W, Barde Y-A. J Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 22.Hofer M M, Barde Y-A. Nature (London) 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- 23.Adams S R, Tsien R Y. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 24.Marriott G. Biochemistry. 1994;33:9092–9097. doi: 10.1021/bi00197a010. [DOI] [PubMed] [Google Scholar]
- 25.Marriott G, Walker J W. Trends Plant Sci. 1999;4:330–334. doi: 10.1016/s1360-1385(99)01452-1. [DOI] [PubMed] [Google Scholar]
- 26.Self C H, Thompson S. Nat Med. 1996;2:817–820. doi: 10.1038/nm0796-817. [DOI] [PubMed] [Google Scholar]
- 27.Berninger B, Poo M M. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 28.Kafitz K W, Rose C R, Thoenen H, Konnerth A. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 29.Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H. EMBO J. 2001;7:1640–1650. doi: 10.1093/emboj/20.7.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAllister A K, Katz L C, Lo D C. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 31.Gottschalk W, Pozzo-Miller L D, Figurov A, Lu B. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curley K, Lawrence D S. Curr Opin Chem Biol. 1999;3:84–88. doi: 10.1016/s1367-5931(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 33.Cambridge S B, Davis R L, Minden J S. Science. 1997;277:825–828. doi: 10.1126/science.277.5327.825. [DOI] [PubMed] [Google Scholar]
- 34.Curley K, Lawrence D S. Pharmacol Ther. 1999;82:347–354. doi: 10.1016/s0163-7258(98)00055-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.