Abstract
Purpose
Oligometastasis is a state in which cancer patients have a limited number of metastatic tumors; patients with oligometastases survive longer than those with polymetastases. Extensive disease (ED)-small cell lung cancer (SCLC) is considered a systemic disease and a poor survival. This study investigated whether the concept of oligometastases is prognostic factor also applicable to patients with ED-SCLC.
Methods
We performed a retrospective study of 141 consecutive patients with ED-SCLC between 2008 and 2016. The patients were divided into four subgroups: group 1; patients with solitary metastatic site in one organ (n = 31), group 2; patients with 2–5 metastatic sites in one organ (n = 18), group 3; patients with over 6 metastases in one organ (n = 15), and group 4; patients with 2 or more metastatic organs (n = 77).
Results
It was identified that 49 patients with ED-SCLC had oligometastases (groups 1 + 2) and 92 had polymetastases (groups 3 + 4). The prognoses of patients with ED-SCLC and oligometastases, defined as ≤5 metastases in a single organ, were significantly superior to those of patients with polymetastases [16.0 (95% CI, 11.0–21.0) months vs. 6.9 (95% CI, 6.0–7.8) months; p<0.001]. 43 of 49 patients with ED-SCLC and oligometastases were relapsed after initial chemotherapy, and 38 (88%) experienced local recurrence.
Conclusions
Patients with ED-SCLC and oligometastases may have improved survival than those with polymetastases. As oligometastatic ED-SCLC tends to recur locally, local therapy combined with systemic chemotherapy may be a treatment option.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide. Small cell lung cancer (SCLC) accounts for 12–15% of all lung cancer cases and is an aggressive disease characterized by widely disseminated metastases and a poor prognosis [1–3], although it is one of the most chemotherapy-sensitive solid tumor types [4–7]. Approximately 60–70% of patients with SCLC present with metastasis beyond a safe radiotherapy field, which is defined as extensive disease (ED). The standard treatment for ED-SCLC is systemic chemotherapy, and the median survival is 8–13 months [5, 6, 8–11].
Oligometastasis is correlated with the management of patients with several solid tumors including non-SCLC. Oligometastasis is defined as having 1–5 metastatic regions; it was shown that patients with oligometastases survive longer than those with polymetastases [12]. The patients with limited metastases such as oligometastases had been aggressively treated with surgical resection and/or radiation therapy [13–16]. International Association for the Study of Lung Cancer proposed the 8th edition of the TNM classification for lung cancer in 2015 [17]. One of the most significant differences between the 7th and 8th editions is the change in the number of M descriptors from three (M0, M1a, and M1b) to four (M0, M1a, M1b, and M1c). It was showed that patients with non-SCLC and single metastasis have a better prognosis than those with polymetastases [17].
Although SCLC is considered a systemic disease, the number of metastatic sites was reported to be associated with the prognosis of patients with ED-SCLC [18, 19]. However, the differences in survival times between oligometastatic and polymetastatic ED-SCLC remain unclear. This retrospective study aimed to analyze whether oligometastases occur in patients with ED-SCLC patients according to the number of metastatic sites at diagnosis, and to examine the efficacy of treatment in these patients.
Materials and methods
Study design and population
This retrospective study enrolled 141 consecutive patients with a pathological diagnosed of SCLC in whom first-line systemic chemotherapy was initiated between January 2008 and December 2016 at the Kitasato University Hospital (Kanagawa, Japan). The results of computed tomography (CT), positron emission tomography (PET), bone scintigraphy, and brain magnetic resonance imaging (MRI) were reviewed to classify the patients using the 8th TNM classification before treatment. Patients with distant metastasis (stage M1a, M1b and M1c) were classified as ED-SCLC.
Subgroups of ED-SCLC patients
The concept of oligometastasis was first proposed in Hellman’s study [12]; oligometastasis is a state in which cancer patients have 1–5 metastatic sites. In this study, ED-SCLC patients were divided into four subgroups: group 1, patients with solitary metastatic site in one organ; group 2, patients with 2–5 metastatic sites in one organ; group 3, patients with over 6 metastases in one organ; and group 4, patients with 2 or more metastatic organs.
Collection of clinical data
For each patient, the following data were extracted in addition to staging: age at diagnosis, sex, smoking status, the Eastern Cooperative Oncology Group performance status (PS), and laboratory data [including levels of albumin, lactate dehydrogenase (LDH), alkaline phosphatase, sodium, neuron-specific enolase, and pro-gastrin-releasing peptide] obtained before initial chemotherapy. We examined the efficacy of cytotoxic chemotherapy and the sites of recurrence in oligometastatic and polymetastatic patients with ED-SCLC. Survival was measured from the date of diagnosis of SCLC to the date of treatment failure (death or disease progression) or last date of follow-up (data cut-off: October 31, 2017). Furthermore, we investigated the efficacy of local radiotherapy on patients with ED-SCLC and oligometastases. The tumor response was classified in accordance with the Response Evaluation Criteria for Solid Tumors (version 1.1), based on the patients’ complete medical histories and results of physical examinations, chest radiography, CT of the chest and abdomen, and other procedures such as brain MRI, PET, and bone scintigraphy.
Statistical analysis
The comparison of survival between the different subgroups was performed using the Kaplan–Meier method. Differences in progression-free survival (PFS) and overall survival (OS) between the subgroups based on prognostic factors were compared using the log-rank test. The Cox proportional hazards model was used for univariate and multivariate analyses. A two-tailed p value <0.05 indicated a significant difference for all analyses. The multivariate analysis was performed after adjusting for covariates that included the significant clinical factors in the univariate analyses. All analyses were performed using the SPSS software, version 25.0 (SPSS, Chicago, Illinois, USA).
Ethics
This study was approved by the Kitasato University Medical Ethics Organization (B17-253). The need for patient consent was waived owing to the retrospective design of the study.
Results
Patient characteristics
The baseline clinical characteristics of the patients are shown in Table 1. 16 of 141 patients (82%) were men, and the median age was 70 (range, 42–92) years. A total of 64 patients (45%) had single organ metastasis, whereas 77 (55%) had multiple organ metastases. All 141 patients underwent chest and abdominal CT scans, and 106 (75%), 23 (16%), and 137 (97%) patients underwent PET, bone scintigraphy, and MRI or CT of the brain as part of the initial staging evaluation, respectively. The single organ metastatic sites were the brain (n = 6), lung (n = 3), liver (n = 16), adrenal gland (n = 6), bone (n = 22), and other sites [n = 11; lymph node (n = 9), thyroid (n = 1), and pancreas (n = 1)].
Table 1. Patient characteristics in this study (n = 141).
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| (n = 31) | (n = 18) | (n = 15) | (n = 77) | |
| Age, n | ||||
| Median (range), years | 71 (49–82) | 68 (49–78) | 73 (51–92) | 70 (42–89) |
| <75 years | 25 | 14 | 8 | 54 |
| ≥75 years | 6 | 4 | 7 | 23 |
| Sex, n | ||||
| Male | 27 | 14 | 12 | 63 |
| Female | 4 | 4 | 3 | 14 |
| Smoking status, n | ||||
| Never | 1 | 1 | 2 | 3 |
| Former/current | 29 | 17 | 12 | 73 |
| Unknown | 1 | 0 | 1 | 1 |
| ECOG PS, n | ||||
| 0/1 | 25 | 13 | 9 | 40 |
| 2/3/4 | 6 | 5 | 6 | 37 |
| Metastatic sites, n | ||||
| Brain | 5 | 1 | 0 | - |
| Lung | 1 | 1 | 1 | - |
| Liver | 5 | 5 | 6 | - |
| Adrenal gland | 5 | 1 | 0 | - |
| Bone | 6 | 8 | 8 | - |
| Others | 9 | 2 | 0 | - |
| Blood tests, mean ± SD | ||||
| Albumin, g/dL | 3.8 ± 0.4 | 3.6 ± 0.5 | 3.5 ± 0.6 | 3.6 ± 0.4 |
| LDH, IU/L | 252.3 ± 98.0 | 394.7 ± 315.0 | 473.3 ± 347.6 | 594.8 ± 1015.7 |
| ALP, U/L | 275.9± 88.8 | 339.5 ± 232.0 | 403.8 ± 260.3 | 421.6 ± 317.1 |
| Sodium, mEq/L | 139.1 ± 3.6 | 137.5 ± 5.7 | 137.9 ± 4.4 | 136.5 ± 5.9 |
| NSE, ng/mL | 46.5 ± 54.4 | 123.3 ± 195.7 | 132.8 ± 137.2 | 102.8 ± 119.4 |
| Pro-GRP, ng/mL | 1,627.4 ± 3,577.4 | 1,426.3 ± 1,773.1 | 2,320.9 ± 3,670.4 | 3,798.6±12,424.2 |
Note: ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; NSE, neuron-specific enolase; pro-GRP, pro-gastrin-releasing peptide
Chemotherapy and radiotherapy for patients with ED-SCLC
All patients underwent systemic chemotherapy. The most commonly used first-line chemotherapy regimens were carboplatin-based doublet therapy in 69 patients (49%) and amrubicin monotherapy in 72 patients (51%). A median of 2 (range, 1–8) regimens were received in this study. The treatment details are presented in Table 2. The 141 patients had an OS of 10.0 [95% confidence interval (CI), 8.4–11.6] months, and PFS of 5.5 (95% CI, 4.8–6.3) months after first-line chemotherapy. The numbers of patients who underwent thoracic radiotherapy (TRT) were 6 of 31 (19%) in group 1, 1 of 18 (6%) in group 2, 3 of 15 (20%) in group 3, and 7 of 77 (9%) in group 4. There was no statistically significant survival difference between the oligometastatic and polymetastatic ED-SCLC patients (p = 0.59).
Table 2. Efficacy of Treatment in the ED-SCLC patients (n = 141).
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| (n = 31) | (n = 18) | (n = 15) | (n = 77) | |
| Regimen of initial chemotherapy, n (%) | ||||
| CDDP + CPT | 3 (9.7) | 1 (5.6) | 3 (20.0) | 8 (10.4) |
| CDDP + ETP | 0 (0) | 2 (11.1) | 0 (0) | 4 (5.2) |
| CBDCA + CPT | 1 (3.2) | 1 (5.6) | 0 (0) | 0 (0) |
| CBDCA + ETP | 12 (38.7) | 3 (16.7) | 3 (20.0) | 19 (24.7) |
| AMR | 11 (35.5) | 8 (44.4) | 7 (46.7) | 36 (46.8) |
| CPT + AMR | 3 (9.7) | 1 (5.6) | 2 (13.3) | 4 (5.2) |
| Others* | 1 (3.2) | 2 (11.1) | 0 (0) | 6 (7.8) |
| Number of regimen administered, n (%) | ||||
| 1 | 6 (19.4) | 8 (44.4) | 6 (40.0) | 42 (54.5) |
| 2 | 12 (38.7) | 6 (33.3) | 7 (46.7) | 23 (29.9) |
| ≥3 | 13 (41.9) | 4 (22.3) | 2 (13.3) | 12 (15.6) |
| Response to initial chemotherapy, n (%) | ||||
| Partial response | 24 (77.4) | 10 (55.6) | 8 (53.3) | 49 (63.6) |
| Stable disease | 4 (12.9) | 4 (22.2) | 4 (26.7) | 10 (13.0) |
| Progressive disease | 1 (3.2) | 1 (5.6) | 3 (20.0) | 10 (13.0) |
| Not evaluated | 2 (6.5) | 3 (16.7) | 0 (0) | 8 (10.4) |
| Thoracic radiotherapy, n (%) | ||||
| Yes | 6 (19.4) | 1 (5.6) | 3 (16.7) | 7 (9.1) |
| No | 25 (80.6) | 17 (94.4) | 12 (83.3) | 70 (90.9) |
Note: CDDP, cisplatin; CPT, irinotecan; ETP, etoposide; CBDCA, carboplatin; AMR, amrubicin
Comparison of survival between the subgroups of ED-SCLC patients
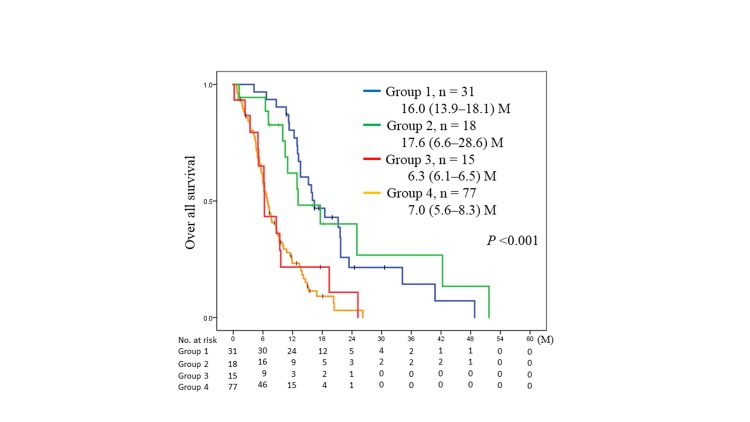
The OS of patients with ED-SCLC in groups 1, 2, 3, and 4 were 16.0 (95% CI, 13.9–18.1), 17.6 (95% CI, 6.6–28.6), 6.3 (95% CI, 6.1–6.5), and 7.0 (95% CI, 5.6–8.4) months, respectively. Of the entire study cohort, the OS of group 1 was similar to that of group 2 (p = 0.74) and superior to that of group 3 (p = 0.001) and group 4 (p <0.001). Moreover, the OS of group 2 was superior to that of the group 3 (p = 0.011) and group 4 (p = 0.001). The OS of group 3 was similar to that of group 4 (p = 0.82) (Fig 1).
Fig 1. Kaplan–Meier analysis of overall survival (OS) in group 1 (blue) vs. group 2 (green) vs. group 3 (red) vs. group 4 (yellow).
P values were determined using the log-rank test; the number of individuals in each group and median survival [95% confidence interval (CI)] are indicated. Group 1 included patients with a solitary metastatic site in one organ, group 2 patients with 2–5 metastatic sites in one organ, group 3 those with ≥6 metastases in one organ, and group 4 those with ≥2 organs with metastases. M; months.
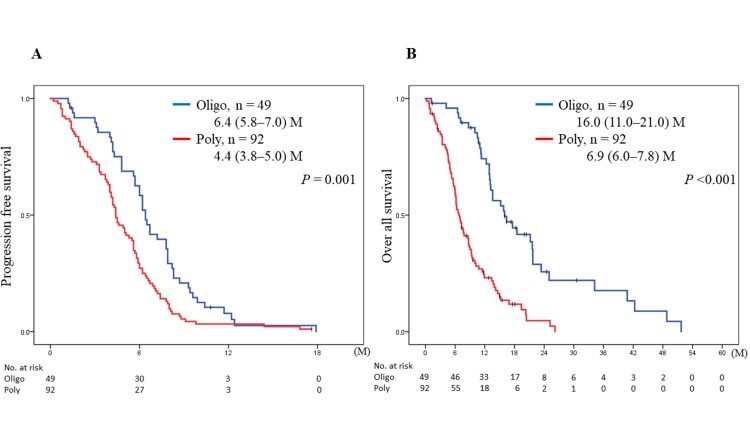
The PFS after the first-line chemotherapy was significantly different between patients with 1–5 metastases in one organ (groups 1 and 2; oligometastases) and those with ≥6 metastases in one organ or metastases in multiple organs (groups 3 and 4; polymetastases) [6.4 (95% CI, 5.8–7.0) vs. 4.4 (95% CI, 3.8–5.0) months; p = 0.001; Fig 2A]. The OS of patients with ED-SCLC and oligometastases was also significantly better than that of patients with polymetastases [16.0 (95% CI, 11.0–21.0) vs. 6.9 (95% CI, 6.0–7.8) months; p <0.001; Fig 2B].
Fig 2.
Kaplan–Meier analyses of progression-free survival (PFS) (A) and overall survival (OS) (B) of patients with oligometastases (blue: group 1 and 2) vs. patients with polymetastases (red: group 3 and 4) treated with chemotherapy. P values were determined using the log-rank test; the number of individuals in each group and median survival (95% CI) are indicated. M; months, Oligo; oligometastases, Poly; polymetastases.
In the univariate survival analysis of patients with ED-SCLC who received chemotherapy, patients with polymetastases had an unfavorable prognosis [hazard ratio (HR) 1.83; 95% CI, 1.48–2.27; p <0.001] in addition to those with known prognostic factors such as older age, lower PS, and serum levels of LDH and sodium. In the multivariate analysis, having polymetastases was significantly associated with an unfavorable prognosis when compared to having oligometastases (HR 1.76; 95% CI, 1.41–2.21; p = <0.001) (Table 3).
Table 3. Univariate and multivariate analyses for overall survival in the ED-SCLC patients (n = 141).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.83 | 1.23–2.74 | 0.003 | 1.89 | 1.25–2.86 | 0.003 |
| ≥75 vs. <75 | ||||||
| Sex | 1.09 | 0.68–1.74 | 0.72 | |||
| Female vs. male | ||||||
| Smoking status | 1.30 | 0.53–3.20 | 0.57 | |||
| Former/Current vs. never | ||||||
| ECOG PS | 1.62 | 1.11–2.37 | 0.01 | 1.31 | 0.88–1.95 | 0.18 |
| 2–4 vs. 0–1 | ||||||
| Pleural effusion | 1.00 | 0.69–1.45 | 1.00 | |||
| Yes vs. no | ||||||
| Albumin | 1.23 | 0.78–1.93 | 0.37 | |||
| Low vs. normal | ||||||
| LDH | 2.14 | 1.34–3.42 | 0.002 | 1.56 | 0.96–2.54 | 0.076 |
| High vs. normal | ||||||
| ALP | 1.36 | 0.94–1.97 | 0.10 | |||
| High vs. low | ||||||
| Sodium | 1.88 | 1.28–2.78 | 0.001 | 1.65 | 1.11–2.44 | 0.013 |
| Low vs. normal | ||||||
| Platinum-based chemotherapy | 0.71 | 0.49–1.02 | 0.03 | |||
| Yes vs. No | ||||||
| Metastases | 1.83 | 1.48–2.27 | <0.001 | 1.76 | 1.41–2.21 | <0.001 |
| Oligo- vs. polymetastases | ||||||
Note: HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; ALP, alkaline phosphatase
Patterns of progression in oligometastatic ED-SCLC
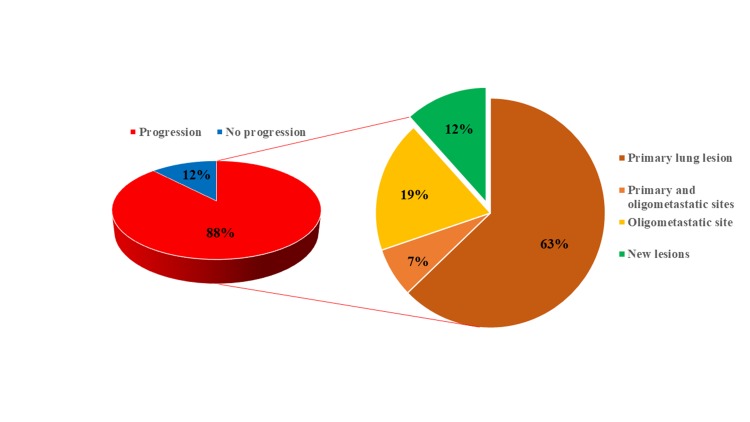
We then investigated the patterns of recurrence in patients with ED-SCLC and oligometastases. In 43 of 49 (88%) patients with oligometastases, relapses occurred in the primary lung tumor in 27 (63%) patients, metastatic site in 8 (19%) patients and 3 (7%) patients experiencing both (Fig 3). In 35 of 43 (81%) patients, the primary tumor had progressed. In all patients with brain, lung, or adrenal gland metastases, relapse occurred in both the primary tumor and metastatic organs before chemotherapy.
Fig 3. Pattern of recurrence in the oligometastatic ED-SCLC patients (n = 49).
43 of 49 (88%) ED-SCLC patients had relapsed after initial chemotherapy. Of the patients who relapsed, 27 (63%) exacerbated primary lung lesion (red), 8 (19%) relapsed at oligometastatic sites (orange) and 3 (7%) relapsed in both the primary and the oligometastatic sites (light orange). 5 (12%) patients had recurrence into new lesions (green).
Next, we evaluated the efficacy of local radiotherapy in patients with oligometastatic ED-SCLC. Patients with oligometastatic ED-SCLC who underwent thoracic radiotherapy (TRT; n = 7) tended to have better survival time than those who did not underwent TRT (n = 42), although there was no significant difference [23.4 (95% CI, 10.1–36.7) months vs. 15.8 (95% CI, 12.7–18.9) months; p = 0.42; S1 Fig].
Discussion
This study suggests that oligometastatic ED-SCLC has a better prognosis than polymetastatic disease and is characterized by local recurrence. Some studies evaluated the revised M descriptors of the 8th edition of TNM classification in patients with ED-SCLC [20, 21]. In this study, the OS of patients with ED-SCLC and a single metastasis in one organ (group 1), categorized as the M1b stage based on the 8th edition of TNM classification, was 16.0 (95% CI, 11.4–21.0) months. Furthermore, the OS of patients with ED-SCLC and 2–5 metastases in one organ (group 2) was not inferior to that of group 1 patients and was superior to that of patients with ≥6 metastases in one organ (group 3) or ≥2 metastatic organs (group 4). Oligometastases means that patients have a limited number of metastases and organ site(s) and may have a more indolent biology and progression at existing sites without widespread metastases. These patients have a better prognosis than those who have widespread metastases [12]. Based on the concept of oligometastasis, patients with ED-SCLC and 1–5 metastases in one organ were defined as having “oligometastatic” ED-SCLC.
In this study, we observed a significant difference in the outcomes of patients with ED-SCLC patients and oligometastases depending on the site of oligometastatic organs (S2 Fig). The survival of patients with oligometastases in the brain [n = 6; 40.8 (95% CI, 10.6–71.0) months] or adrenal gland [n = 6; 34.2 (95% CI, 3.4–65.0) months] metastases was better than that of those with metastases in other organs [n = 37; 13.6 (95% CI, 10.7–16.5) months]. It was reported that only 27% of patients with asymptomatic brain metastases responded to systemic chemotherapy [22]. Four of six patients (67%) with brain metastases had recurrence in the brain after initial chemotherapy. Among patients with non-SCLC, the prognosis of single brain metastasis has been shown to be considerably better than that of multiple brain metastases; furthermore, patients with non-SCLC and single brain metastasis benefit from locally aggressive and ablative treatments such as stereotactic irradiation or surgery [23–25]. Local radiation therapy for brain metastases is also considered to be effective in SCLC patients [26]. In this study, 6 patients with ≤5 brain metastases underwent local treatments; one patient underwent brain tumor excision before initiating chemotherapy, all patients underwent whole-brain radiation therapy, and 3 of 6 patients underwent a cyber knife treatment for exacerbation of brain metastases after WBRT. Brain irradiation underwent somewhere during treatment after chemotherapy. This finding suggests that local brain irradiation may be beneficial in patients with systemic disease in ED-SCLC patients with oligometastases.
Regarding the occurrence of oligometastases in adrenal glands, initial staging should be carefully performed as the presence of a tumor does not necessarily represent metastasis but could indicate a benign adenoma (2–9% of cases) [27]. Although CT, PET, and MRI are useful tools for the detection of adrenal metastases, it should be noted these non-invasive modalities have some limitations [28–33]. For distinguishing adrenal metastases, PET-CT with a mean attenuation ≥10 HU and SUV max ≥3.1 had 97.3% sensitivity and 86.2% specificity [34]. Six patients with adrenal metastasis in this study met the condition of PET-CT. Regarding the cause of death of the six patients with adrenal metastases, three had exacerbations of the primary tumor, one had brain metastases, and the remaining had disease progression while managing adverse events, febrile neutropenia, and renal failure due to chemotherapy. None of the patients received treatment for local adrenal metastasis, and none died from adrenal metastasis.
In previous studies, 75–90% of patients with ED-SCLC had residual intrathoracic disease, 90% developed intrathoracic progression after chemotherapy [35], and most patients with ED-SCLC died of thoracic progression and associated complications [36]. In this study, of the 38 patients with 1–5 metastases (excluding those who survived or whose cause of death was unknown), 27 (71%) died due to exacerbation of the primary lesions. The patients with oligometastatic ED-SCLC who underwent TRT (n = 7) trended to have a better prognosis than those who did not undergo TRT (n = 42). A recent study showed that the introduction of high-dose radiation therapy to chemotherapy was an effective treatment strategy for patients with ED-SCLC as it significantly improved patients’ survival [37]. Furthermore, patients with 1–4 extracranial metastases who underwent consolidative radiation therapy had a 50% reduction in intrathoracic recurrence than those who did not (80% vs. 44%, respectively; p = 0.001) [38]. In a previous report, TRT in ED-SCLC patients with 0–2 distant organ metastases significantly improved PFS (n = 61; HR = 2.02; p = 0.003) [39]. Thus, we suggested that patients with oligometastatic ED-SCLC, who had mainly intrathoracic progression, should be indicated for intrathoracic irradiation.
Our study had several limitations. This study was a retrospective study conducted at a single institution; therefore, our results cannot be regarded as definitive. The sample size may not have been sufficient. Some of the patients received local radiotherapy, but others did not. Patients who received high- or low-dose radiotherapy and the period when they received the treatment were not identified. To determine which patients will most likely benefit from escalated doses of radiotherapy, a large prospective study is needed to corroborate our findings.
Conclusion
Patients with oligometastatic ED-SCLC may have a better prognosis than those with polymetastatic disease. Patients with oligometastatic ED-SCLC patients tend to experience local recurrence. For oligo- and polymetastatic ED-SCLC patients, treatment strategies should be developed separately. Local treatment combined with systemic chemotherapy may be a treatment option for the oligometastatic ED-SCLC patients, especially in patients with cerebral or adrenal grand oligometastases.
Supporting information
Kaplan–Meier analysis of OS for the patients with oligometastases (blue) vs. the patients with polymetastases (red) treated with TRT. P values were determined using the log-rank test; the number of individuals in each group and median survival (95% confidence interval) are indicated. M; months.
(TIF)
Kaplan–Meier analysis of OS in patients with oligometastases for each oligometastatic site: adrenal gland (blue), brain (green), liver (black), lung (yellow), bone metastases (gray), the others (red). The number of individuals in each group and median survival (95% confidence interval) are indicated. M; months.
(TIF)
(XLSX)
Acknowledgments
The authors would like to thank Editage (https://www.editage.jp/) for English language review.
Data Availability
All relevant data is within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet (London, England). 2011;378(9804):1741–55. Epub 2011/05/14. 10.1016/s0140-6736(11)60165-7 . [DOI] [PubMed] [Google Scholar]
- 2.Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e400S–e19S. Epub 2013/05/10. 10.1378/chest.12-2363 . [DOI] [PubMed] [Google Scholar]
- 3.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nature reviews Cancer. 2017;17(12):725–37. Epub 2017/10/28. 10.1038/nrc.2017.87 . [DOI] [PubMed] [Google Scholar]
- 4.Morita T. A statistical study of lung cancer in the annual of pathological autopsy cases in Japan, from 1958 to 1997, with reference to time trends of lung cancer in the world. Japanese journal of cancer research: Gann. 2002;93(1):15–23. Epub 2002/01/23. 10.1111/j.1349-7006.2002.tb01195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. The New England journal of medicine. 2002;346(2):85–91. Epub 2002/01/11. 10.1056/NEJMoa003034 . [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Bunn PA, Jr., Langer C, Einhorn L, Guthrie T, Jr., Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(13):2038–43. Epub 2006/05/02. 10.1200/jco.2005.04.8595 . [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(24):4665–72. Epub 2002/12/19. 10.1200/jco.2002.12.111 . [DOI] [PubMed] [Google Scholar]
- 8.Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM Jr., Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115(12):2721–31. Epub 2009/04/30. 10.1002/cncr.24314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(28):4539–44. Epub 2006/09/30. 10.1200/jco.2005.04.4859 . [DOI] [PubMed] [Google Scholar]
- 10.Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. British journal of cancer. 2007;97(2):162–9. Epub 2007/06/21. 10.1038/sj.bjc.6603810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puglisi M, Dolly S, Faria A, Myerson JS, Popat S, O'Brien ME. Treatment options for small cell lung cancer—do we have more choice? British journal of cancer. 2010;102(4):629–38. Epub 2010/01/28. 10.1038/sj.bjc.6605527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellman S, Weichselbaum RR. Oligometastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1995;13(1):8–10. Epub 1995/01/01. 10.1200/jco.1995.13.1.8 . [DOI] [PubMed] [Google Scholar]
- 13.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands). 2010;69(3):251–8. Epub 2010/06/12. 10.1016/j.lungcan.2010.05.003 . [DOI] [PubMed] [Google Scholar]
- 14.Plones T, Osei-Agyemang T, Krohn A, Passlick B. Surgical Treatment of Extrapulmonary Oligometastatic Non-small Cell Lung Cancer. The Indian journal of surgery. 2015;77(Suppl 2):216–20. Epub 2016/01/06. 10.1007/s12262-012-0771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung cancer (Amsterdam, Netherlands). 2013;82(2):197–203. Epub 2013/09/21. 10.1016/j.lungcan.2013.07.026 . [DOI] [PubMed] [Google Scholar]
- 16.Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clinical lung cancer. 2014;15(5):346–55. Epub 2014/06/05. 10.1016/j.cllc.2014.04.003 . [DOI] [PubMed] [Google Scholar]
- 17.Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10(11):1515–22. Epub 2015/11/05. 10.1097/jto.0000000000000673 . [DOI] [PubMed] [Google Scholar]
- 18.Albain KS, Crowley JJ, Livingston RB. Long-term survival and toxicity in small cell lung cancer. Expanded Southwest Oncology Group experience. Chest. 1991;99(6):1425–32. Epub 1991/06/01. . [DOI] [PubMed] [Google Scholar]
- 19.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1990;8(9):1563–74. Epub 1990/09/01. 10.1200/jco.1990.8.9.1563 . [DOI] [PubMed] [Google Scholar]
- 20.Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11(3):300–11. Epub 2016/01/03. 10.1016/j.jtho.2015.10.008 . [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhao Y, Li C, Zhu L, Liu C, Liu L. The revision of 8th edition TNM stage criteria is more accurate in prediction postoperative survival for SCLC patients. International journal of surgery (London, England). 2017;48:83–5. Epub 2017/10/11. 10.1016/j.ijsu.2017.09.072 . [DOI] [PubMed] [Google Scholar]
- 22.Seute T, Leffers P, Wilmink JT, ten Velde GP, Twijnstra A. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(13):2079–83. Epub 2006/05/02. 10.1200/jco.2005.03.2946 . [DOI] [PubMed] [Google Scholar]
- 23.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Practical radiation oncology. 2012;2(3):210–25. Epub 2012/07/01. 10.1016/j.prro.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. International journal of radiation oncology, biology, physics. 2005;63(1):37–46. Epub 2005/08/23. 10.1016/j.ijrobp.2005.05.023 . [DOI] [PubMed] [Google Scholar]
- 25.Modi A, Vohra HA, Weeden DF. Does surgery for primary non-small cell lung cancer and cerebral metastasis have any impact on survival? Interactive cardiovascular and thoracic surgery. 2009;8(4):467–73. Epub 2009/01/22. 10.1510/icvts.2008.195776 . [DOI] [PubMed] [Google Scholar]
- 26.Bernhardt D, Adeberg S, Bozorgmehr F, Opfermann N, Horner-Rieber J, Konig L, et al. Outcome and prognostic factors in single brain metastases from small-cell lung cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al. ]. 2018;194(2):98–106. Epub 2017/11/01. 10.1007/s00066-017-1228-4 . [DOI] [PubMed] [Google Scholar]
- 27.McNicholas MM, Lee MJ, Mayo-Smith WW, Hahn PF, Boland GW, Mueller PR. An imaging algorithm for the differential diagnosis of adrenal adenomas and metastases. AJR American journal of roentgenology. 1995;165(6):1453–9. Epub 1995/12/01. 10.2214/ajr.165.6.7484585 . [DOI] [PubMed] [Google Scholar]
- 28.Burt M, Heelan RT, Coit D, McCormack PM, Bains MS, Martini N, et al. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. Impact of magnetic resonance imaging. The Journal of thoracic and cardiovascular surgery. 1994;107(2):584–8; discussion 8–9. Epub 1994/02/01. . [PubMed] [Google Scholar]
- 29.Schwartz LH, Ginsberg MS, Burt ME, Brown KT, Getrajdman GI, Panicek DM. MRI as an alternative to CT-guided biopsy of adrenal masses in patients with lung cancer. The Annals of thoracic surgery. 1998;65(1):193–7. Epub 1998/02/10. . [DOI] [PubMed] [Google Scholar]
- 30.Korobkin M, Giordano TJ, Brodeur FJ, Francis IR, Siegelman ES, Quint LE, et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200(3):743–7. Epub 1996/09/01. 10.1148/radiology.200.3.8756925 . [DOI] [PubMed] [Google Scholar]
- 31.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR American journal of roentgenology. 1998;171(1):201–4. Epub 1998/07/02. 10.2214/ajr.171.1.9648789 . [DOI] [PubMed] [Google Scholar]
- 32.Maurea S, Mainolfi C, Bazzicalupo L, Panico MR, Imparato C, Alfano B, et al. Imaging of adrenal tumors using FDG PET: comparison of benign and malignant lesions. AJR American journal of roentgenology. 1999;173(1):25–9. Epub 1999/07/09. 10.2214/ajr.173.1.10397094 . [DOI] [PubMed] [Google Scholar]
- 33.Kim HK, Choi YS, Kim K, Kim J, Shim YM. Preoperative evaluation of adrenal lesions based on imaging studies and laparoscopic adrenalectomy in patients with otherwise operable lung cancer. Lung cancer (Amsterdam, Netherlands). 2007;58(3):342–7. Epub 2007/09/11. 10.1016/j.lungcan.2007.07.001 . [DOI] [PubMed] [Google Scholar]
- 34.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250(2):523–30. Epub 2009/02/04. 10.1148/radiol.2502080219 . [DOI] [PubMed] [Google Scholar]
- 35.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. The New England journal of medicine. 2007;357(7):664–72. Epub 2007/08/19. 10.1056/NEJMoa071780 . [DOI] [PubMed] [Google Scholar]
- 36.Postmus PE, Haaxma-Reiche H, Smit EF, Groen HJ, Karnicka H, Lewinski T, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(19):3400–8. Epub 2000/10/03. 10.1200/jco.2000.18.19.3400 . [DOI] [PubMed] [Google Scholar]
- 37.Li-Ming X, Zhao LJ, Simone CB 2nd, Cheng C, Kang M, Wang X, et al. Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2017;125(2):331–7. Epub 2017/10/29. 10.1016/j.radonc.2017.10.005 . [DOI] [PubMed] [Google Scholar]
- 38.Gore EM, Hu C, Sun AY, Grimm DF, Ramalingam SS, Dunlap NE, et al. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017;12(10):1561–70. Epub 2017/06/27. 10.1016/j.jtho.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slotman BJ, Faivre-Finn C, van Tinteren H, Keijser A, Praag J, Knegjens J, et al. Which patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: A secondary analysis of the Phase III CREST trial. Lung cancer (Amsterdam, Netherlands). 2017;108:150–3. Epub 2017/06/20. 10.1016/j.lungcan.2017.03.007 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan–Meier analysis of OS for the patients with oligometastases (blue) vs. the patients with polymetastases (red) treated with TRT. P values were determined using the log-rank test; the number of individuals in each group and median survival (95% confidence interval) are indicated. M; months.
(TIF)
Kaplan–Meier analysis of OS in patients with oligometastases for each oligometastatic site: adrenal gland (blue), brain (green), liver (black), lung (yellow), bone metastases (gray), the others (red). The number of individuals in each group and median survival (95% confidence interval) are indicated. M; months.
(TIF)
(XLSX)
Data Availability Statement
All relevant data is within the paper and its Supporting Information files.