Abstract
The neutrophil enzyme myeloperoxidase (MPO) is a major enzyme made by neutrophils to generate antimicrobial and immunomodulatory compounds, notably hypochlorous acid (HOCl), amplifying their capacity for destroying pathogens and regulating inflammation. Despite its roles in innate immunity, the importance of MPO in preventing infection is unclear, as individuals with MPO deficiency are asymptomatic with the exception of an increased risk of candidiasis. Dysregulation of MPO activity is also linked with inflammatory conditions such as atherosclerosis, emphasising a need to understand the roles of the enzyme in greater detail. Consequently, new tools for investigating granular dynamics in vivo can provide useful insights into how MPO localises within neutrophils, aiding understanding of its role in preventing and exacerbating disease. The zebrafish is a powerful model for investigating the immune system in vivo, as it is genetically tractable, and optically transparent. To visualise MPO activity within zebrafish neutrophils, we created a genetic construct that expresses human MPO as a fusion protein with a C-terminal fluorescent tag, driven by the neutrophil-specific promoter lyz. After introducing the construct into the zebrafish genome by Tol2 transgenesis, we established the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line, and confirmed transgene expression in zebrafish neutrophils. We observed localisation of MPO-mEmerald within a subcellular location resembling neutrophil granules, mirroring MPO in human neutrophils. In Spotless (mpxNL144) larvae—which express a non-functional zebrafish myeloperoxidase—the MPO-mEmerald transgene does not disrupt neutrophil migration to sites of infection or inflammation, suggesting that it is a suitable line for the study of neutrophil granule function. We present a new transgenic line that can be used to investigate neutrophil granule dynamics in vivo without disrupting neutrophil behaviour, with potential applications in studying processing and maturation of MPO during development.
Introduction
The enzyme Myeloperoxidase (MPO) enhances the microbicidal potential of neutrophils by converting hydrogen peroxide (H2O2) into the highly toxic antimicrobial compound hypochlorous acid (HOCl) [1], and by forming radicals by oxidating substrates including phenols, nitrate and tyrosine residues [2]. MPO is located in the primary granules of neutrophils, which deliver MPO and other bactericidal compounds to invading pathogens by fusing with phagocytic vesicles, accelerating pathogen destruction. MPO is the most abundant protein in the primary granules of human neutrophils [3], and consequently neutrophils are able to produce high levels of HOCl to deliver a potent antimicrobial response that is capable of killing a broad variety of major pathogens [4–6]. Importantly, due to the activity of upstream NADPH oxidase, the phagocytic vacuole is thought to be relatively alkaline (~pH 9), and under such conditions MPO activity may be less efficient [7] than other neutrophil enzymes [8]. MPO activity appears to be context-dependent, particularly during phagocytosis of large structures such as fungal hyphae [9] or bacterial biofilms [10]. In these cases, the phagocytic vacuole does not fully close [11], causing MPO to act at the acidic pH of sites of inflammation (~pH 6) [12], at which it can function normally. This observation is supported by the fact that MPO is thought to play a role in the generation of neutrophil extracellular traps (NETs) [13], which are often induced in response to large targets [14]. While the precise site of action of MPO is uncertain, it is clear that it plays a role in antimicrobial defence, as pathogens produce specific virulence factors targeting it [15]. Beyond its role in bolstering the antimicrobial defence, MPO is also an important regulator of inflammation. The arrival of neutrophils at the wound site marks the initial steps of the anti-inflammatory response, as MPO is delivered to the wound site to consume H2O2 and reduce inflammatory signalling [16,17]. There is also a link between aberrant MPO activity and inflammatory conditions: overactivity is associated with cardiovascular disease, multiple sclerosis and glomerulonephritis [18–20], while MPO deficiency has been implicated in pulmonary fibrosis and atherosclerosis [21,22], highlighting its critical role in immune homeostasis. MPO deficiency is a relatively common condition affecting 1 in every 2,000–4,000 people across Europe and North America [23], with no major health risks apart from a susceptibility to Candida albicans infections [24]. This observation is in stark contrast to people with chronic granulomatous disease (CGD), who lack a working NADPH oxidase. Those with CGD are unable to generate an effective phagocytic environment capable of destroying microbes [25,26]. Unlike MPO deficiency, those with CGD experience frequent life-threatening infections from a wide range of pathogens [27], and consequently, the role of MPO is less clear when observed in the context of other oxidative enzymes and compounds. Further studies are required to understand the complex roles of MPO in the immune system.
The zebrafish is a powerful model for studying physiology and pathology in vivo and has been used to model many important conditions ranging from neurodegenerative disorders such as Alzheimer’s disease [28], to cancers including melanoma [29] and leukaemia [30]. They are optically transparent, making them amenable to imaging studies and produce high numbers of offspring, which permits the application of high-throughput approaches. Another major advantage of the zebrafish is their genetic tractability, facilitating the introduction of large genetic constructs into the genome, often expressing fluorescent proteins driven by tissue-specific promoters [31]. Several studies have utilised these features to create transgenic lines labelling macrophages [32] and neutrophils [33] to image the innate immune response during infection [34] and inflammation [35].
MPO can be measured using a variety of cytochemical and cytometry-based approaches [36], however there are relatively few tools that allow granular MPO to be visualised in vivo and in real time. Mouse models that permit imaging of neutrophil granules and MPO do exist [20,37], however murine MPO lacks several transcription factor binding domains [38], and is expressed at 1/10 the level found in human neutrophils [39], raising concerns over whether a murine model can fully represent human MPO.
In this study, we have generated a transgenic zebrafish line expressing fluorescently-labelled human MPO in zebrafish neutrophils, as a tool towards investigating the roles of MPO during infection and inflammation. The transgene for the MPO-mEmerald fusion protein (lyz:Hsa.MPO-mEmerald) was successfully expressed in zebrafish neutrophils and the resulting protein appears to be trafficked to granules, recapitulating expression of MPO in human neutrophils. Additionally, we showed that the MPO-mEmerald enzyme does not disrupt neutrophil recruitment to sites of injury and infection. In the future, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 zebrafish may prove to be a useful tool for investigating MPO and imaging granular dynamics in vivo and in real-time.
Methods
Zebrafish husbandry
Zebrafish (Danio rerio) were raised and maintained under the Animals (Scientific Procedures) Act 1986 using standard protocols [40]. Adult zebrafish were hosted in UK Home Office-approved aquaria at the Bateson Centre, University of Sheffield, and kept under a 14/10 light/dark regime at 28°C.
Cloning the Tg(lyz:Hsa.MPO-mEmerald,cml2:EGFP)sh496 line
The plasmid used for introducing the transgene into the zebrafish genome (pDestTol2CG2 lyz:MPO-mEmerald cmlc2:EGFP) was created by Gateway cloning [31]. Briefly, a gateway vector p5E-MCS containing 6.6kb of the lysozyme C promoter [33] was used to drive neutrophil-specific expression,. The MPO-mEmerald gene was incorporated into an expression vector by first digesting the mEmerald-MPO-N-18 plasmid (Addgene plasmid #54187, Dr. Michael Davidson’s lab), and ligating the MPO-mEmerald fusion protein gene into the multiple cloning site vector pME MCS to generate the middle-entry vector, pME MCS MPO-mEmerald. The final construct was created by an LR reaction combining a 5’ vector containing the lyz promoter, the middle entry vector pME MPO-mEmerald, a 3’ vector containing a polyadenylation site, and the destination vector pDestTol2CG2.
Generation of the Tg(lyz:nfsB-mCherry)sh260 line
As for the MPO-mEmerald construct, the lyz promoter in the gateway vector p5E-MCS was used to drive neutrophil-specific expression. For red fluorescence, mCherry was produced as a fusion protein with the nitroreductase gene nfsB (primers F: 5’ GGG GAC AAG TTT GTA CAA AAA AGC AGG CTG CAT GGA TAT CAT TTC TGT CGC CTT, R: 5’ GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC GGT CCA CTT CGG TTA AGG TGA TGT T), which also permits conditional ablation of cells upon addition of metronidazole [41], and cloned into middle entry (pME-nfsB) and 3’ entry vectors (p3E-mCherry). The final lyz:nfsB-mCherry construct was created by recombination of p5E-lyz, pME-nfsB, and p3E-mCherry, and pDestTol2pA2.
Microinjection of lyz:MPO-mEmerald construct DNA
Construct DNA of the donor plasmid pDestTol2CG2 lyz:MPO-mEmerald cmlc2:EGFP or pDestTol2pA2 lyz:nfsB-mCherry was injected into zebrafish embryos at the one-cell stage with 10ng/μl of Tol2 transposase RNA, according to published protocols [40].
TSA staining and colocalisation experiments
3dpf Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 larvae were fixed in ice-cold 4% (w/v) paraformaldehyde (PFA) in PBS-TX (PBS supplemented with 0.5% of Triton X-100) overnight at 4°C. Fixed embryos were washed in PBS-TX twice. Peroxidase activity was detected by incubation in 1:50 Cy5-TSA:amplification reagent (PerkinElmer, Waltham, MA) in the dark for 10 min at 28°C followed by extensive washing in PBS-TX.
Zebrafish tailfin transection
Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 zebrafish at 3 days post-fertilisation were anaesthetised by immersion in E3 supplemented with 4.2% Tricaine and complete transection of the tail was performed with a sterile scalpel. For imaging of larvae, a Nikon custom-build wide-field microscope was used: Nikon Ti-E with a CFI Plan Apochromat λ 10X, N.A.0.45 objective lens, a custom built 500 μm Piezo Z-stage (Mad City Labs, Madison, WI, USA) and using Intensilight fluorescent illumination with ET/sputtered series fluorescent filters 49002 and 49008 (Chroma, Bellow Falls, VT, USA) was used. Analysis was performed using Nikon’s NIS Elements software package.
Bacterial culture preparation
To prepare a liquid overnight culture of S. aureus, 5ml of BHI broth medium (Oxoid) was inoculated with a colony of S. aureus strain USA300, and incubated at 37°C overnight with shaking. To prepare S. aureus for injection, 50ml of BHI media was inoculated with 500μl of overnight culture and incubated for roughly 2 hours at 37°C with shaking. The OD600 of each culture was measured and 40ml of the remaining culture harvested by centrifugation at 4,500g for 15 minutes at 4°C. The pellet was then resuspended in a volume of PBS appropriate to the bacterial dose required. Once the pellets were resuspended they were then kept on ice until required.
Spotless (mpxNL144) fish and sudan black staining
The Spotless (mpxNL144) mutant line contains a C to T mutation at nucleotide 1126 of the mpx RefSeq mRNA sequence (NM_212779), resulting in a premature stop codon [42]. A detailed protocol of Sudan Black B staining can be found in S1 File.
Microscopy of neutrophil granules
Microscopy of neutrophil granules in Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 larvae was performed using a Zeiss Axiovert LSM 880 Airyscan confocal microscope with 63x Plan Apochromat oil objective (NA 1.4). Cells were illuminated with a 488 nm argon laser and/or a 561 nm diode laser. Images were processed using the Zeiss microscope software and analysed using Zen Black.
Statistics
All data were analysed (Prism 7.0, GraphPad Software, San Diego, CA, USA) using a two-way ANOVA with Bonferroni post-test to adjust for multiple comparisons.
Results
Creation of a transgenic zebrafish expressing human myeloperoxidase
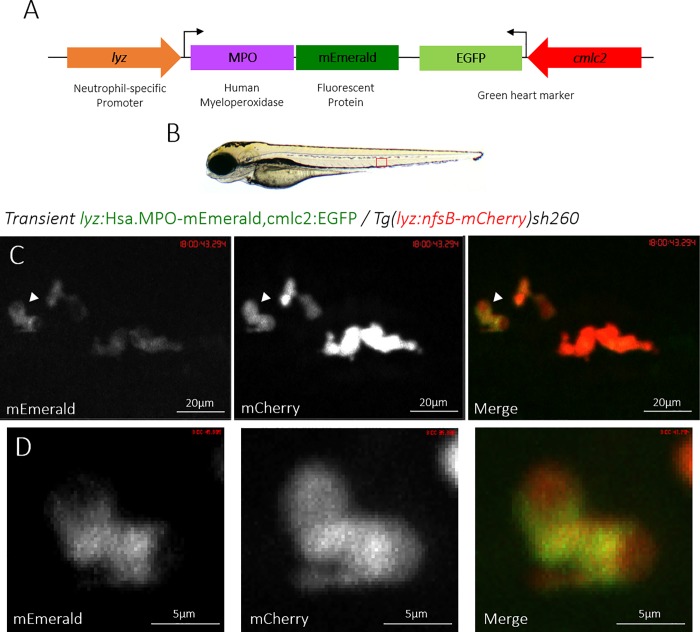
To create a transgenic zebrafish that expresses a fluorescently-tagged human myeloperoxidase (MPO), we created a genetic construct using Gateway cloning that contains the MPO gene with a C-terminal fusion of the fluorescent protein mEmerald, driven by the neutrophil-specific promoter lyz (Fig 1A). After the construct was successfully assembled, it was introduced into the zebrafish genome by Tol2-mediated transgenesis. To verify expression in neutrophils, a second transgenic line was created using a construct expressing mCherry under the lyz promoter, Tg(lyz:nfsB-mCherry)sh260. Successful expression of MPO-mEmerald in zebrafish neutrophils was confirmed by inducing transgenesis in Tg(lyz:nfsB-mCherry)sh260 fish (Fig 1C and 1D). Injected larvae were then screened at 3 days post fertilisation (dpf) for mEmerald expression and colocalisation with mCherry expression. Fig 1C and 1D shows double-transgenic neutrophils expressing both mEmerald and mCherry in the primary haematopoietic tissue of the zebrafish larvae, the caudal haematopoietic tissue (CHT) (indicated in Fig 1B) [43]. This observation confirms that the construct is successfully expressed and suggests that it co-localises with zebrafish neutrophils. We also noted that in double-transgenic neutrophils, there appeared to be a differential subcellular localisation between mEmerald and mCherry signal, with mCherry localised to areas with no visible mEmerald signal (Fig 1D).
Fig 1. Transient expression of the lyz:MPO-mEmerald transgene labels zebrafish neutrophils.
A) Schematic of the lyz:MPO-mEmerald cmlc2:EGFP construct, which includes the neutrophil-specific promoter (lyz), the MPO gene with a C-terminal fluorescent tag (MPO-mEmerald) and a green heart marker to aid optimisation of transgenesis (cmlc2:EGFP). B) A zebrafish larva at 3 days post fertilisation (dpf), with the caudal haematopoietic tissue (CHT) indicated by the red box. C) The CHT of a double-transgenic Transient lyz:MPO-mEmerald,cmlc2:EGFP; Tg(lyz:nfsB-mCherry)sh260 larva with a population of neutrophils expressing both mEmerald and mCherry. The white arrowhead indicates the neutrophil enlarged below. D) Enlarged view of a neutrophil expressing mEmerald and mCherry.
MPO-mEmerald is stably expressed in zebrafish neutrophils
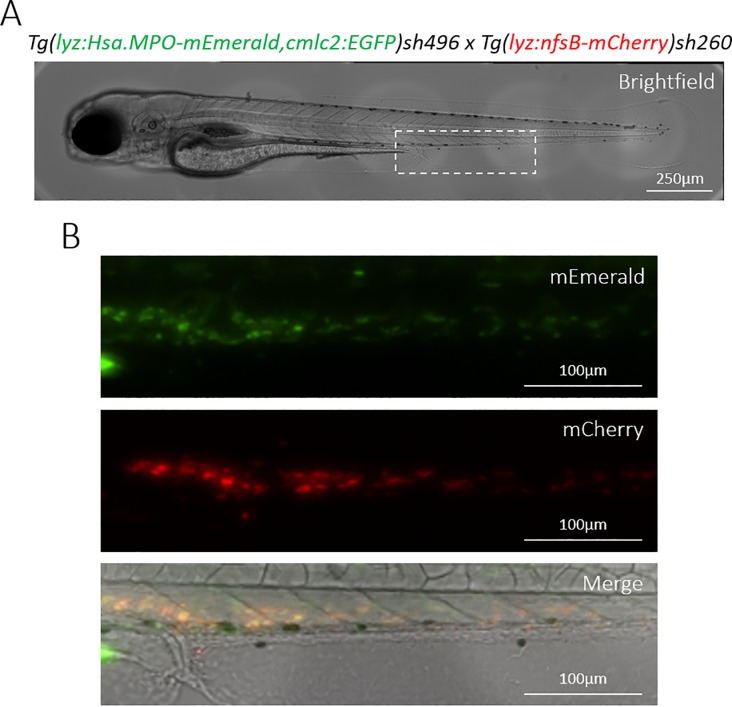
To secure adult zebrafish with stable germline integrations of the lyz:MPO-mEmerald transgene, larvae that transiently expressed the transgene were identified, raised and outcrossed to determine whether the transgene was inherited by their offspring. An adult that produced larvae with a cell population labelled with mEmerald was identified and its progeny raised to produce fish stably expressing the MPO transgene, with the designation Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496. To verify whether the lyz:MPO-mEmerald transgene was expressed in neutrophils of stably transgenic fish, they were crossed to the red neutrophil reporter line Tg(lyz:nfsB-mCherry)sh260, and screened for any co-expression of fluorescent proteins. Both transgenes were expressed in neutrophils throughout the CHT (Fig 2), demonstrating that lyz:MPO-mEmerald is expressed in zebrafish neutrophils in stably transgenic larvae.
Fig 2. Transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 zebrafish stably express the transgene in zebrafish neutrophils.
A) A brightfield view of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 zebrafish larva at 3dpf. The dashed white box indicates the enlarged region shown in B). B) mEmerald and mCherry expression in the CHT of the larva shown in A).
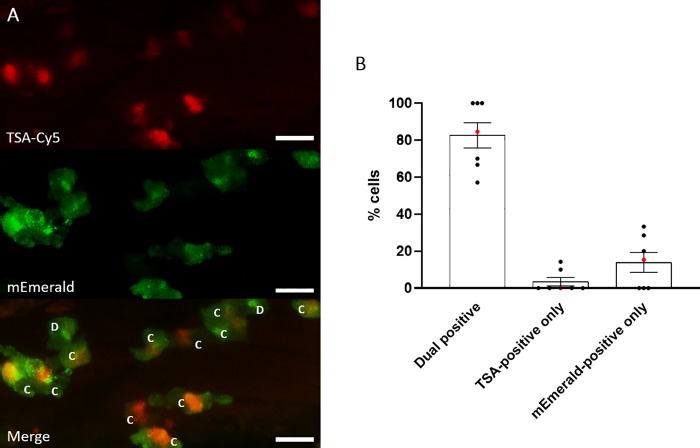
To further confirm that MPO-mEmerald positive cells are neutrophils, we performed TSA-staining on fixed larvae at 3dpf (Fig 3), to stain specific endogenous peroxidase activity in zebrafish neutrophils [44]. An average of 80% of the cells observed in the CHT are positive for both MPO-mEmerald and TSA, suggesting that the transgene specifically labels neutrophils in the larva–the staining of neutrophils with TSA is incomplete accounting for the less than 100% co-staining.
Fig 3. Most MPO-mEmerald-positive neutrophils are TSA-positive.
A) Confocal photomicrographs shown as maximum intensity projections of lyz:MPO-mEmerald larvae fixed at 3 dpf and chemically stained with TSA-Cy5. Dual-positive (C) and mEmerald-positive only (D) cells are indicated; scale bar 10μm. B) Quantification of dual positive, TSA-positive only and mEmerald-positive only cells observed in lyz:MPO-mEmerald larvae fixed at 3 dpf and chemically stained with TSA-Cy5. Red points indicate larva shown in A).
MPO-mEmerald is trafficked to a subcellular location
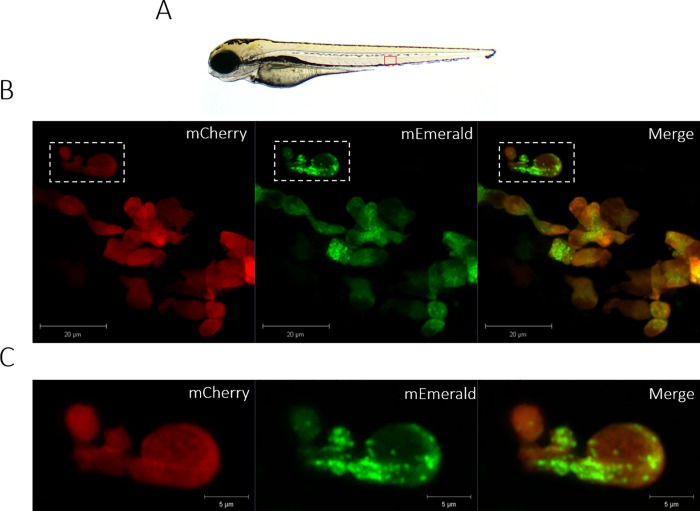
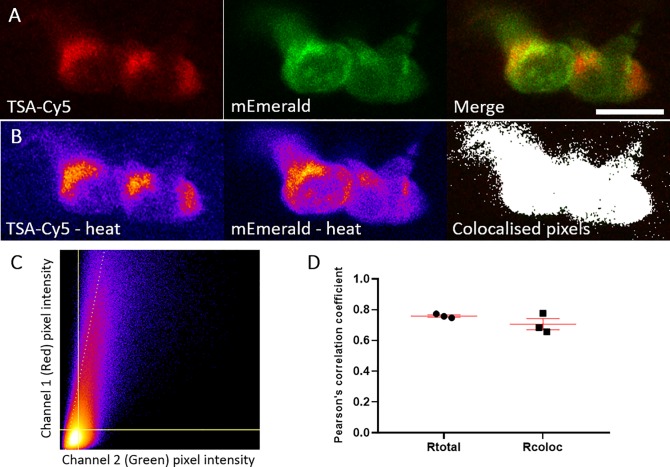
As MPO is located in the primary granules of neutrophils prior to delivery to the phagosome [1], we wished to determine whether the lyz:MPO-mEmerald transgene recapitulates MPO expression in human neutrophils. To investigate the intracellular localisation of the MPO transgene, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 fish were outcrossed to Tg(lyz:nfsB-mCherry)sh260 fish, and at 3dpf the double-transgenic larvae were imaged in high detail using an Airyscan confocal microscope. Both transgenes are expressed in the same cells, with MPO-mEmerald localising with a granular subcellular distribution (Fig 4), suggesting that the MPO-mEmerald fusion protein is trafficked to and packaged within neutrophil granules. High-speed imaging of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 larva shows that the intracellular MPO-mEmerald signal is highly dynamic (S1 Movie), and resembles the Brownian motion that would be observed in primary neutrophil granules. Additionally, we also analysed colocalisation of the MPO-mEmerald signal with the location of TSA histochemical staining in neutrophils (Fig 5). There was 70% colocalisation between MPO-mEmerald and endogenous peroxidase activity (the MPO transgene does not have peroxidase activity–see below). This suggests that MPO-mEmerald is expressed in neutrophil granules, with the differences in co-localisation due to inactive, unprocessed forms of MPO-mEmerald.
Fig 4. The lyz:MPO-mEmerald transgene labels intracellular neutrophil granules.
A) 3dpf zebrafish larva, the field of view shown in B) is outlined by the red box. B) An Airyscanner confocal image of neutrophils within the CHT of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 larva at 3dpf. C) An enlarged image of the neutrophil highlighted by the dashed white box in B). Scale bars B) 20μm and C) 5μm.
Fig 5. mEmerald signal colocalises with TSA signal.
A) Representative photomicrograph shown as single focal plane of lyz:MPO-mEmerald (Green) larvae fixed at 3 dpf and chemically stained with TSA-Cy5 (Red). Scale bar 10 μm. B) Pseudocoloured (heat) images of Red and Green channels and image of colocalised pixels above threshold. C) Scatter plot of channel 1 (Red) vs. channel 2 (Green) of the image shown in A and B. The regression line is plotted along with the threshold level for channel 1 (vertical line) and channel 2 (horizontal line). D) Pearson's correlation coefficient for the entire image (Rtotal) or for the pixels above thresholds (Rcoloc) of 3 tested field of views. Mean +/- SEM are indicated in red.
MPO-mEmerald does not disrupt neutrophil migration
In addition to its role in potentiating ROS generation in neutrophils, MPO also influences neutrophil migration to inflammatory stimuli [16]. Accordingly, we sought to determine whether Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 fish exhibit disrupted neutrophil migration to inflammatory and infectious stimuli. Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 fish were crossed to Tg(lyz:nfsB-mCherry)sh260 and at 3dpf their larvae were separated into two groups: non-humanised (lyz:nfsB-mCherry only) and humanised (lyz:MPO-mEmerald; lyz:nfsB-mCherry) to determine how expression of MPO-mEmerald affects these responses.
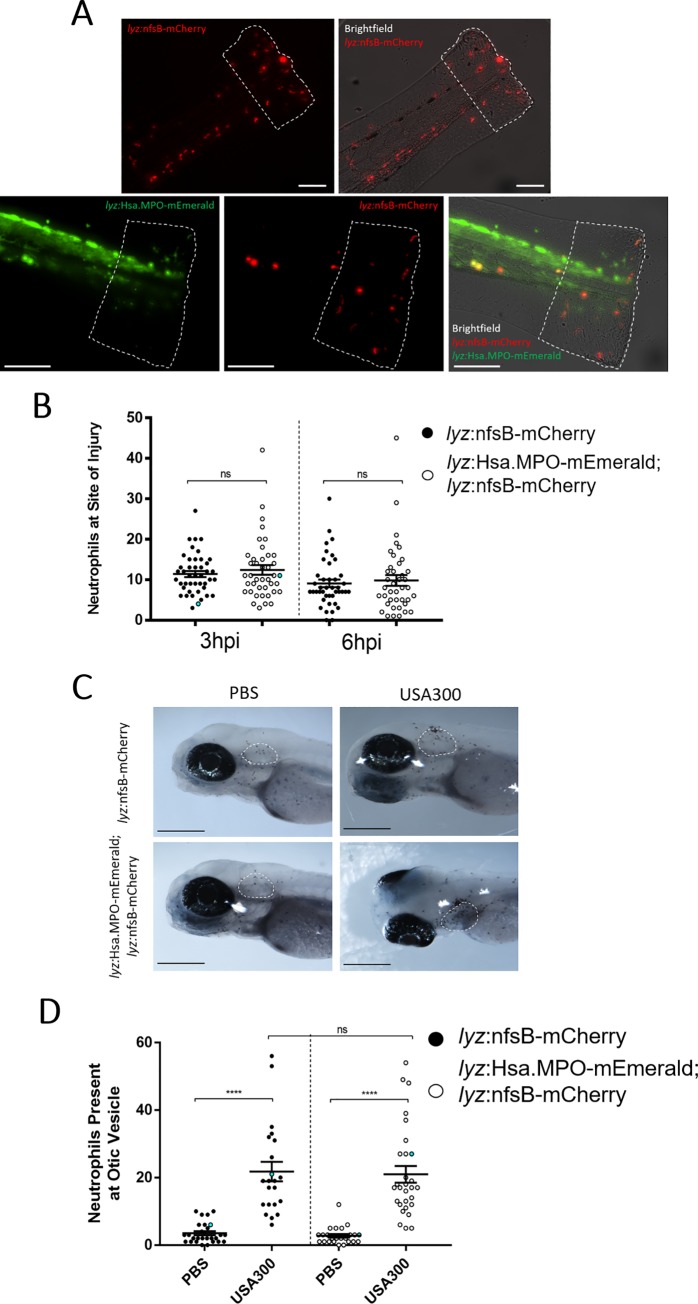
To assess neutrophil migration to inflammatory stimuli, we used a tailfin-transection model that initiates neutrophil recruitment to a vertically transected tailfin injury in zebrafish larvae [35]. Non-humanised and humanised larvae were injured and neutrophil recruitment to the site of injury was imaged at 3 and 6 hours post injury (hpi) (Fig 6A). Both groups exhibited comparable migration of neutrophils to the site of injury at 3 and 6hpi (Fig 6B), suggesting that lyz:MPO-mEmerald does not interfere with neutrophil recruitment to sites of injury.
Fig 6. Transgene expression does not disrupt neutrophil recruitment to sites of injury or infection.
A) Non-humanised (lyz:nfsB-mCherry only) and humanised (lyz:MPO-mEmerald; lyz:nfsB-mCherry) 3dpf larvae with tailfins transected to induce neutrophil recruitment; dashed outline represents the area in which neutrophils were counted. Scale bar = 250μm. B) Neutrophils present at the site of injury at 3 and 6 hours post injury (hpi); blue points denote the representative images in A). Error bars shown are mean ± SEM (n = 45 over three independent experiments); groups were analysed using an ordinary two-way ANOVA and adjusted using Bonferroni’s multiple comparisons test; ns, p>0.9999. C) Non-humanised and humanised larvae injected with either a PBS vehicle control or 1,400cfu S. aureus USA300 into the otic vesicle at 3dpf, then fixed in paraformaldehyde at 4 hours post infection (hpi) and stained with Sudan Black B to detect neutrophils; dashed white outline indicates the otic vesicle. D) Neutrophils present at the otic vesicle at 4hpi. Scale bars = 250μm. Error bars shown are mean ± SEM (n = 25 over two independent experiments); groups were analysed using an ordinary two-way ANOVA and adjusted using Bonferroni’s multiple comparisons test. ****, p<0.0001; ns, p>0.9999.
To determine whether the neutrophil response to infection is affected by expression of lyz:MPO-mEmerald, we used an otic vesicle infection model to investigate neutrophil recruitment [45,46]. After separating larvae into non-humanised and humanised groups, they were injected into the otic vesicle with either a PBS vehicle control or S. aureus USA300 at 3dpf. The larvae were then fixed in paraformaldehyde at 4 hours post infection (hpi) and stained with Sudan Black B to detect neutrophils. Injection of S. aureus USA300 induces robust recruitment of neutrophils to the otic vesicle, with comparable numbers recruited between non-humanised and humanised larvae (Fig 6C and 6D). This confirms that expression of the lyz:MPO-mEmerald transgene does not interfere with neutrophil recruitment to sites of infection.
Genotypic and functional identification of myeloperoxidase-null Spotless (mpxNL144) larvae
While the lyz:MPO-mEmerald transgene is expressed in zebrafish neutrophils in a manner that recapitulates expression in human neutrophils, it was still unknown whether MPO-mEmerald is expressed as a functional enzyme. To determine whether MPO was functional, we sought to create a zebrafish that expresses only human MPO by removing expression of the endogenous zebrafish myeloperoxidase (mpx) from the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line. This was achieved using an existing zebrafish line known as Spotless (mpxNL144), which possesses a premature stop codon in the first exon of the mpx gene [42]. Once acquired, we aimed to cross the Spotless line to our Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line to create a line that expresses only human MPO. Before we could create this line, it was necessary to develop a genotyping protocol that could accurately identify Spotless mpxNL144 fish.
The mpxNL144 allele can be identified by PCR amplification of the mutated gene from genomic DNA, followed by restriction digest of the PCR product. The restriction enzyme BtsCI recognises 5’ GG ATG NN 3’ sites in DNA, one of which is present within the mutated mpxNL144 gene (GGA TGA) but not the wild-type mpxwt gene (GGA CGA), allowing the enzyme to determine the presence of a mpxNL144 allele (Fig 7A). The PCR primers were successful in amplifying the region in the mpx gene from mpxwt, mpxNL144 and mpxwt/NL144 groups (Fig 7B), and once digested with BtsCI produced different DNA fragments depending on the mpxNL144 allele of the fish (Fig 7C), confirming BtsCI digestion as an efficient means of identifying the mpxNL144 allele. The accuracy of the restriction digest was confirmed further by sequencing the PCR products, confirming that the fish identified by restriction digest each have the specific basepair in the expected position (Fig 7D). After adults were genotyped, their larvae were then assessed for functional myeloperoxidase expression using the myeloperoxidase-dependent stain Sudan Black B [16] (S1 File), which verified the genotyping results (Fig 7E).
Fig 7. Genotyping and verifying mpx-null zebrafish larvae.
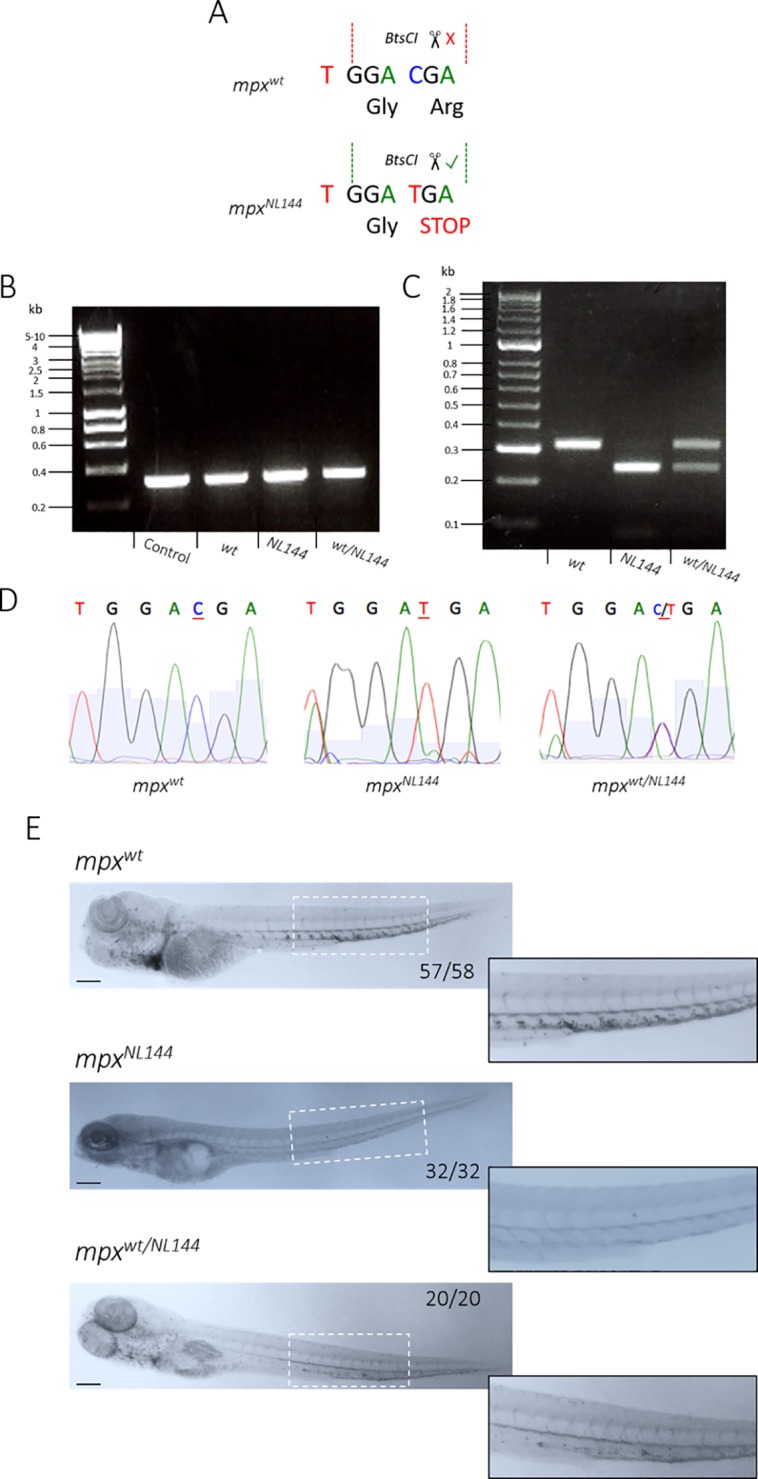
A) Diagram of a WT (mpxwt) and mutated (mpxNL144) gene, showing the BtsCI restriction site cutting only the mutated mpxNL144 gene. B) PCR amplification of the mpx gene from the genomic DNA of mpxwt, mpxwt/NL144 and mpxNL144 fish–fragment 312bp; control DNA is a positive control from a separate genotyping experiment. Hyperladder 1kb. C) Diagnostic digest of the PCR product from mpxwt, mpxwt/NL144 and mpxNL144 fish. Band sizes: mpxwt- 312bp, mpxNL144- 230bp, mpxwt/NL144- 312bp and 230bp. Hyperladder 100bp plus. D) DNA sequencing of the PCR products to confirm the accuracy of the BtsCI digest. E) mpxwt, mpxNL144 and mpxwt/NL144 larvae fixed at 4dpf and stained with Sudan Black B. Larvae with at least one functional mpx allele stained (57/58 mpxwt, 20/20 mpxwt/NL144) and larvae that do not produce Mpx did not stain (32/32 mpxNL144). Inset shows an enlarged view of the region indicated by the dashed white box. Scale bar = 200μm.
MPO-mEmerald is non-functional in zebrafish neutrophils
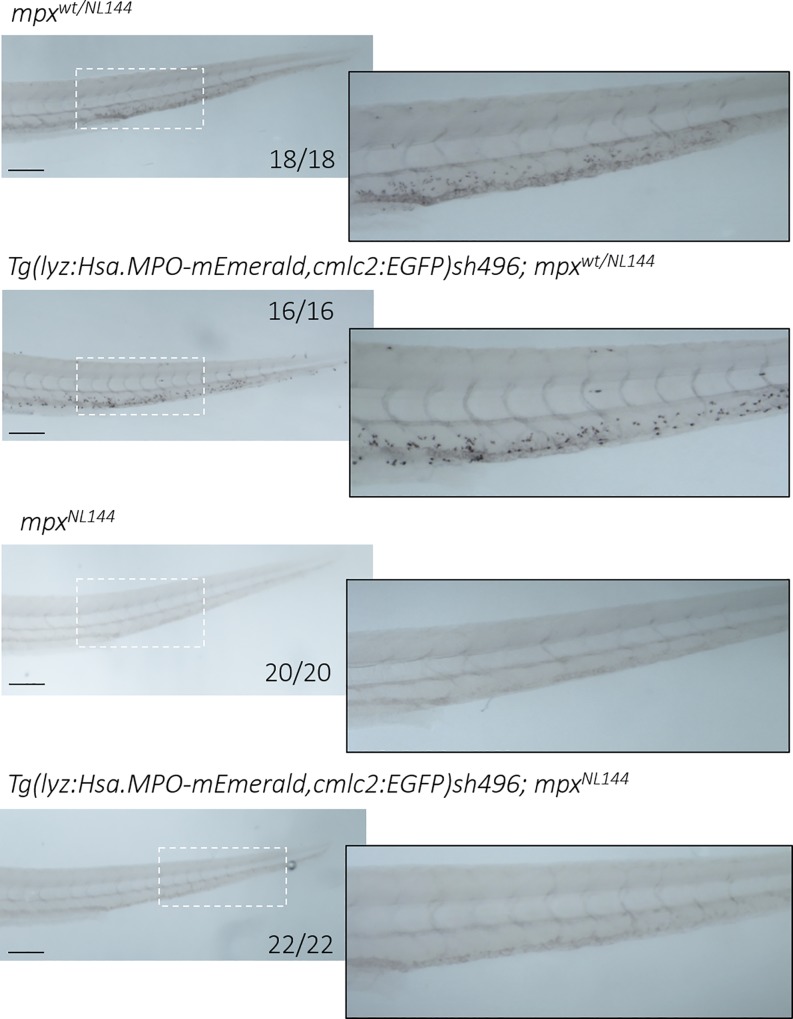
To create zebrafish larvae expressing only human MPO, the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line was crossed to the Spotless line to create zebrafish that express the lyz:MPO-mEmerald transgene and do not produce functional endogenous mpx. Once created, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144 larvae were stained with the myeloperoxidase-dependent stain Sudan Black B to determine whether this conferred staining; these larvae were compared against three sibling control groups–mpxNL144, mpxwt/NL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144. All groups tested containing a functioning mpx allele (mpxwt/NL144, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144) stained with Sudan Black B, indicating that the stain identifies functional endogenous myeloperoxidase (Fig 8). As expected, the negative control group did not stain (mpxNL144), but surprisingly, neither did the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144 human MPO-only larvae, indicating that the lyz:MPO-mEmerald transgene does not produce a functional MPO enzyme (Fig 8).
Fig 8. Larvae expressing only human MPO do not stain with myeloperoxidase-dependent Sudan Black B.
Four groups of larvae were fixed at 4dpf and stained with Sudan Black B: mpxwt/NL144, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144, mpxNL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144. mpxwt/NL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144 stained (18/18, 16/16 respectively); mpxNL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144 did not stain (20/20, 22/22 respectively). Dashed outline indicates the enlarged region shown adjacent. Scale bar = 200μm.
Discussion
In this study, we created a transgenic line expressing a fluorescently-tagged human myeloperoxidase in zebrafish neutrophils. Expression in neutrophils was determined by observing expression of lyz:MPO-mEmerald in the fluorescent red neutrophil line, Tg(lyz:nfsB-mCherry)sh260, which expresses mCherry in the cytoplasm of zebrafish neutrophils. Both transgenes were expressed within the same cells (Figs 1 and 2), and TSA staining showed that the majority of MPO-mEmerald cells produced active peroxidase that colocalises with MPO-mEmerald signal (Fig 3) confirming that the lyz:MPO-mEmerald transgene labels neutrophils. However, as MPO localises with the primary granules of neutrophils, it was essential that the fluorescent signal observed in the lyz:MPO-mEmerald line should differ from the cytoplasmic signal observed in the Tg(lyz:nfsB-mCherry)sh260 line. This was observed in several instances; in double transgenic neutrophils, distinct areas of the cell remain unlabelled with mEmerald (Figs 1 and 4) suggesting that MPO is translated and trafficked to a subcellular location that is distinct from the cytoplasm. This observation is also evident in Airyscan confocal imaging (Fig 4C), where a large unlabelled area of a double-transgenic neutrophil is visible in the mEmerald channel. This is likely to be a region of the cell that is inaccessible to the primary granules, for example the nucleus, and could be verified using a fluorescent nuclear probe.
In addition to the lyz:MPO-mEmerald and lyz:nfsB-mCherry signals being distinct, double-transgenic neutrophils contain small intracellular foci of mEmerald signal (Fig 4), suggesting that MPO-mEmerald might be targeted to the primary granules. Using high-speed imaging, we found that these foci are highly dynamic, resembling the Brownian motion exhibited by neutrophil granules (S1 Movie). Despite these observations, we found that an average of 80% of cells expressing MPO-mEmerald stain with peroxidase-sensitive TSA, and 70% of mEmerald signal colocalises with TSA signal (Fig 5). It is important to note that not all labelled MPO would localise with active peroxidase, as immature MPO present in the endoplasmic reticulum and trans-golgi network prior to dimerisation would also be visible in MPO-mEmerald fish [47]. Additionally, it is likely that neutrophils at different stages of development would contain different levels of functional MPO, which may explain incomplete staining with TSA.
In addition to the role of MPO in antibacterial defence, it is also an important enzyme regulating the migration of neutrophils to sites of infection and inflammation, primarily by mediating H2O2 flux [16]. Using a combination of approaches for studying neutrophil migration, we found that expression of the lyz:MPO-mEmerald transgene does not interfere with neutrophil recruitment to sites of infection and inflammation (Fig 6). One disadvantage of the line is the dim fluorescent signal and fast photobleaching of MPO-mEmerald. High levels of autofluorescent green signal are visible in Fig 6 as a result of the high exposure required for visualisation, and S1 Movie suggests that the mEmerald signal bleaches quickly. Despite these drawbacks, neutrophils are clearly distinguished on higher power systems using this line (Figs 3–5). Currently, there are no existing tools for visualising neutrophil granules in vivo, and measuring MPO is limited to cytochemical and cytometry-based approaches [36]. Therefore, the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 transgenic line may be used to study granule dynamics in vivo without disrupting neutrophil function.
In order to determine whether MPO-mEmerald is produced as a functional enzyme, it was necessary to produce zebrafish that do not express endogenous zebrafish myeloperoxidase. We describe here a genotyping protocol that can be used to identify Spotless (mpxNL144) fish, an mpx-null mutant line created in a separate study [42]. This was accomplished by amplifying a region present in the first exon of the mpx gene, followed by restriction digest with BtsCI (Fig 7B–7D); this was then functionally verified using the myeloperoxidase-dependent stain Sudan Black B [16] (Fig 7E). We believe this to be a useful and robust method for identifying Spotless fish, and may be useful in future studies.
Once a robust method for identifying Spotless fish was established, the Spotless line was crossed to the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line to generate a line that expresses human MPO, and does not express zebrafish Mpx (known as Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144). By comparing staining with Sudan Black B with sibling controls, we found that MPO-mEmerald is not produced as a functional enzyme, as lyz:MPO-mEmerald expression does not complement staining in the mpxNL144 background. It is unclear why the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line does not produce functional MPO, however it is important to note that MPO is a complex glycoprotein enzyme that undergoes numerous tightly regulated post-translational modifications. Before mature MPO is produced, the peptide associates with calreticulin and calnexin in the endoplasmic reticulum before undergoing a series of proteolytic events leading to insertion of a haem group and dimerisation of the enzyme, followed by glycosylation and ending with granule targeting [47]. The importance of each step in producing a functional enzyme is unclear, however studies of myeloperoxidase-deficient individuals suggest that targeting to the primary granules universally correlates with functional MPO [23,48–50], and in vitro studies show that dimerisation is not required for enzyme function [15,51,52]. Additionally, the discrepancy is unlikely to lie with calnexin and calreticulin, as they possess roughly 70% amino acid identity with the human chaperones, and are important during development of the zebrafish lateral line [53]. Differences at any other stages may lead to incomplete MPO maturation and function in the zebrafish and consequently, the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line may also be useful in investigating how MPO is processed and targeted to the granules during development.
Conclusion
We have generated a transgenic zebrafish line expressing fluorescently labelled human MPO within its neutrophils. The enzyme is non-functional and does not interfere with neutrophil recruitment to sites of infection or inflammation, suggesting that it may be used to study granule dynamics in vivo without disrupting neutrophil behaviour. Additionally, the Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 line may be used to investigate processing and targeting of MPO during development, which is currently uncharacterised in vivo. Lastly, we provide a protocol for genotyping endogenous myeloperoxidase-null Spotless (mpxNL144) fish, which will prove useful in future studies investigating myeloperoxidase in the zebrafish.
Supporting information
An airyscanner confocal timelapse of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 larva. Maximum intensity projection of 60 slices with a total width of 10μm, 1 minute per timepoint, 10 frames per second. The timelapse shows numerous neutrophils in the caudal haematopoietic tissue of a 3dpf larva.
(AVI)
(DOCX)
Acknowledgments
K.D.B performed experiments with assistance from T.K.P, N.V.O, M.v.G, N.W.M.d.J, and J.K. S.A.R, J.A.G.v.S and S.J.F conceived the study and designed experiments. T.K.P performed TSA-staining and colocalisation experiments. N.V.O generated the Tg(lyz:nfsB-mCherry)sh260 line K.D.B and S.A.R wrote the manuscript with significant input from all authors. Thank you to Annemarie Meijer for providing the Spotless (mpxNL144) line, to the Bateson Centre aquarium staff for all their help, and to the Wolfson Light Microscopy Facility.
Data Availability
Manuscript and raw data have been uploaded to the preprint server biorxiv, available at https://www.biorxiv.org/content/10.1101/456541v2, DOI: https://doi.org/10.1101/456541.
Funding Statement
This study was supported by S.A.R MRNO2995X/1 Medical Research Council, https://mrc.ukri.org/; and S.A.R GR077544AIA The Wellcome Trust, https://wellcome.ac.uk/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klebanoff SJ, Kettle a. J, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. Journal of Leukocyte Biology. 2012. pp. 185–198. 10.1189/jlb.0712349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3: 3–15. 10.1080/13510002.1997.11747085 [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89: 3503–21. Available: http://www.ncbi.nlm.nih.gov/pubmed/9160655 [PubMed] [Google Scholar]
- 4.Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leukoc Biol. 2006;79: 87–94. 10.1189/jlb.0605338 [DOI] [PubMed] [Google Scholar]
- 5.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Critical Role of Myeloperoxidase and Nicotinamide Adenine Dinucleotide Phosphate–Oxidase in High-Burden Systemic Infection of Mice with Candida albicans. J Infect Dis. 2002; 1833–1837. 10.1086/340635 [DOI] [PubMed] [Google Scholar]
- 6.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol. 2005;174: 1557–65. 10.4049/jimmunol.174.3.1557 [DOI] [PubMed] [Google Scholar]
- 7.Levine AP, Duchen MR, de Villiers S, Rich PR, Segal AW. Alkalinity of Neutrophil Phagocytic Vacuoles Is Modulated by HVCN1 and Has Consequences for Myeloperoxidase Activity. Gaggar A, editor. PLoS One. 2015;10: e0125906 10.1371/journal.pone.0125906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416: 291–7. 10.1038/416291a [DOI] [PubMed] [Google Scholar]
- 9.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8: 668–76. 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 10.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186: 6585–96. 10.4049/jimmunol.1002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmesser M. Role of neutrophils in invasive aspergillosis. Infect Immun. 2006;74: 6514–6. 10.1128/IAI.01551-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69: 522–30. 10.1189/jlb.69.4.522 [DOI] [PubMed] [Google Scholar]
- 13.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191: 677–91. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15: 1017–25. 10.1038/ni.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong NWM, Ramyar KX, Guerra FE, Nijland R, Fevre C, Voyich JM, et al. Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc Natl Acad Sci. 2017;114: 9439–9444. 10.1073/pnas.1707032114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pase L, Layton JE, Wittmann C, Ellett F, Nowell CJ, Reyes-Aldasoro CC, et al. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr Biol. Elsevier Ltd; 2012;22: 1818–1824. 10.1016/j.cub.2012.07.060 [DOI] [PubMed] [Google Scholar]
- 17.Schürmann N, Forrer P, Casse O, Li J, Felmy B, Burgener A, et al. Myeloperoxidase targets oxidative host attacks to Salmonella and prevents collateral tissue damage. Nat Microbiol. Nature Publishing Group; 2017;2: 16268 10.1038/nmicrobiol.2016.268 [DOI] [PubMed] [Google Scholar]
- 18.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of Total and Subtotal Myeloperoxidase Deficiency: Risk or Benefit? Acta Haematol. 2000;104: 10–15. 10.1159/000041062 [DOI] [PubMed] [Google Scholar]
- 19.Yang JJ, Pendergraft WF, Alcorta DA, Nachman PH, Hogan SL, Thomas RP, et al. Circumvention of normal constraints on granule protein gene expression in peripheral blood neutrophils and monocytes of patients with antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. J Am Soc Nephrol. 2004;15: 2103–14. 10.1097/01.ASN.0000135058.46193.72 [DOI] [PubMed] [Google Scholar]
- 20.Chen JW, Breckwoldt MO, Aikawa E, Chiang G, Weissleder R. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain. 2008;131: 1123–1133. 10.1093/brain/awn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan M-L, Anderson MM, Shih DM, Qu X-D, Wang X, Mehta AC, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107: 419–430. 10.1172/JCI8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. Mukhopadhyay P, editor. PLoS One. 2012;7: e30923 10.1371/journal.pone.0030923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLeo FR, Goedken M, McCormick SJ, Nauseef WM. A novel form of hereditary myeloperoxidase deficiency linked to endoplasmic reticulum/proteasome degradation. J Clin Invest. 1998;101: 2900–9. 10.1172/JCI2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969;48: 1478–1488. 10.1172/JCI106114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnani A, Brosselin P, Beauté J, de Vergnes N, Mouy R, Debré M, et al. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol. 2014;134: 655–662.e8. 10.1016/j.jaci.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 26.Levine AP, Segal AW. The NADPH Oxidase and Microbial Killing by Neutrophils, With a Particular Emphasis on the Proposed Antimicrobial Role of Myeloperoxidase within the Phagocytic Vacuole. Microbiol Spectr. 2016;4: 1557–64. 10.1128/microbiolspec.MCHD-0018-2015 [DOI] [PubMed] [Google Scholar]
- 27.Assari T. Chronic Granulomatous Disease; fundamental stages in our understanding of CGD. Med Immunol. 2006;5: 4 10.1186/1476-9433-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paquet D, Bhat R, Sydow A, Mandelkow E-M, Berg S, Hellberg S, et al. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest. 2009;119: 1382–95. 10.1172/JCI37537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9: 139–51. 10.1007/s10456-006-9040-2 [DOI] [PubMed] [Google Scholar]
- 30.Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299: 887–90. 10.1126/science.1080280 [DOI] [PubMed] [Google Scholar]
- 31.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, et al. The Tol2kit: A multisite gateway-based construction Kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236: 3088–3099. 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- 32.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117: e49–56. 10.1182/blood-2010-10-314120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7: 42 10.1186/1471-213X-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elks PM, Brizee S, van der Vaart M, Walmsley SR, van Eeden FJ, Renshaw S a, et al. Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS Pathog. 2013;9: e1003789 10.1371/journal.ppat.1003789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renshaw SA, Loynes CA, Trushell DMI, Elworthy S, Ingham PW, Whyte MKB. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108: 3976–3978. 10.1182/blood-2006-05-024075 [DOI] [PubMed] [Google Scholar]
- 36.Pulli B, Ali M, Forghani R, Schob S, Hsieh KLC, Wojtkiewicz G, et al. Measuring myeloperoxidase activity in biological samples. Johnson R, editor. PLoS One. 2013;8: e67976 10.1371/journal.pone.0067976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikushima K, Kita S, Higuchi H. A non-invasive imaging for the in vivo tracking of high-speed vesicle transport in mouse neutrophils. Sci Rep. 2013;3: 1913 10.1038/srep01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nauseef WM. The proper study of mankind. J Clin Invest. 2001;107: 401–3. 10.1172/JCI11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rausch PG, Moore TG. Granule enzymes of polymorphonuclear neutrophils: A phylogenetic comparison. Blood. 1975;46: 913–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/173439 [PubMed] [Google Scholar]
- 40.Nüsslein-Volhard C, Dahm R. Zebrafish: A Practical Approach. Practical. Oxford; New York: Oxford University Press, c2002.; 2002. [Google Scholar]
- 41.Okuda KS, Misa JP, Oehlers SH, Hall CJ, Ellett F, Alasmari S, et al. A zebrafish model of inflammatory lymphangiogenesis. Biol Open. 2015;4: 1270–1280. 10.1242/bio.013540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elks PM, van der Vaart M, van Hensbergen V, Schutz E, Redd MJ, Murayama E, et al. Mycobacteria counteract a TLR-mediated nitrosative defense mechanism in a zebrafish infection model. Foulkes NS, editor. PLoS One. 2014;9: e100928 10.1371/journal.pone.0100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin H-F, et al. Tracing Hematopoietic Precursor Migration to Successive Hematopoietic Organs during Zebrafish Development. Immunity. 2006;25: 963–975. 10.1016/j.immuni.2006.10.015 [DOI] [PubMed] [Google Scholar]
- 44.Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, et al. A Zebrafish Compound Screen Reveals Modulation of Neutrophil Reverse Migration as an Anti-Inflammatory Mechanism. Sci Transl Med. 2014;6: 225ra29–225ra29. 10.1126/scitranslmed.3007672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benard EL, van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH. Infection of zebrafish embryos with intracellular bacterial pathogens. J Vis Exp. 2012; 1–8. 10.3791/3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng Q, Sarris M, Bennin D a, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J Leukoc Biol. 2013;93: 761–9. 10.1189/jlb.1012534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys. 2006;445: 214–24. 10.1016/j.abb.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 48.Nauseef WM. Lessons from MPO deficiency about functionally important structural features. Jpn J Infect Dis. 2004;57: 4–5. [PubMed] [Google Scholar]
- 49.Nauseef WM, Cogley M, McCormick S. Effect of the R569W missense mutation on the biosynthesis of myeloperoxidase. J Biol Chem. 1996;271: 9546–9549. 10.1074/jbc.271.16.9546 [DOI] [PubMed] [Google Scholar]
- 50.Nauseef WM, McCormick S, Goedken M. Impact of missense mutations on biosynthesis of myeloperoxidase. Redox Rep. 2000;5: 197–206. 10.1179/135100000101535753 [DOI] [PubMed] [Google Scholar]
- 51.Moguilevsky N, Garcia-Quintana L, Jacquet A, Tournay C, Fabry L, Piérard L, et al. Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem. 1991;197: 605–14. Available: http://www.ncbi.nlm.nih.gov/pubmed/1851479 [DOI] [PubMed] [Google Scholar]
- 52.Andrews PC, Krinsky NI. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981;256: 4211–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/6260790 [PubMed] [Google Scholar]
- 53.Hung I-C, Cherng B-W, Hsu W-M, Lee S-J. Calnexin is required for zebrafish posterior lateral line development. Int J Dev Biol. 2013;57: 427–38. 10.1387/ijdb.120166sl [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An airyscanner confocal timelapse of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 larva. Maximum intensity projection of 60 slices with a total width of 10μm, 1 minute per timepoint, 10 frames per second. The timelapse shows numerous neutrophils in the caudal haematopoietic tissue of a 3dpf larva.
(AVI)
(DOCX)
Data Availability Statement
Manuscript and raw data have been uploaded to the preprint server biorxiv, available at https://www.biorxiv.org/content/10.1101/456541v2, DOI: https://doi.org/10.1101/456541.