Abstract
Background
In Mexico, estimates of Chagas disease prevalence and burden vary widely. Updating surveillance data is therefore an important priority to ensure that Chagas disease does not remain a barrier to the development of Mexico's most vulnerable populations.
Methodology/Principal findings
The aim of this systematic review and meta-analysis was to analyze the literature on epidemiological surveys to estimate Chagas disease prevalence and burden in Mexico, during the period 2006 to 2017. A total of 2,764 articles were screened and 36 were retained for the final analysis. Epidemiological surveys have been performed in most of Mexico, but with variable study scale and geographic coverage. Based on studies reporting confirmed cases (i.e. using at least 2 serological tests), and taking into account the differences in sample sizes, the national estimated seroprevalence of Trypanosoma cruzi infection was 3.38% [95%CI 2.59–4.16], suggesting that there are 4.06 million cases in Mexico. Studies focused on pregnant women, which may transmit the parasite to their newborn during pregnancy, reported an estimated seroprevalence of 2.21% [95%CI 1.46–2.96], suggesting that there are 50,675 births from T. cruzi infected pregnant women per year, and 3,193 cases of congenitally infected newborns per year. Children under 18 years had an estimated seropositivity rate of 1.51% [95%CI 0.77–2.25], which indicate ongoing transmission. Cases of T. cruzi infection in blood donors have also been reported in most states, with a national estimated seroprevalence of 0.55% [95%CI 0.43–0.66].
Conclusions/Significance
Our analysis suggests a disease burden for T. cruzi infection higher than previously recognized, highlighting the urgency of establishing Chagas disease surveillance and control as a key national public health priority in Mexico, to ensure that it does not remain a major barrier to the economic and social development of the country's most vulnerable populations.
Author summary
In Mexico, estimates of Chagas disease prevalence and burden vary widely due to the ecology and epidemiology of this disease resulting of many geographical, ecological, biological, and social interactions. Better data are thus urgently needed to help develop appropriate public health programs for disease control and patient care. In this study we performed a meta-analysis from published data on T. cruzi infection seroprevalence in Mexico between 2006 and 2017. This systematic review shows a national estimated seroprevalence of T. cruzi infection of 3.38% [95%CI 2.59–4.16], with over 4.06 million cases in Mexico, which is higher than previously recognized. The presence of T. cruzi infection in specific subpopulations such as pregnant women, children and blood donors also informs on specific risks of infection and calls for the implementation of well-established control interventions. This work confirms the place of Mexico as the country with the largest number of cases, highlighting the urgency of establishing Chagas disease control as a key national public health priority.
Introduction
Chagas disease or American trypanosomiasis is an infection caused by the protozoan parasite Trypanosoma cruzi, which is mainly transmitted to humans and other mammals through the contaminated feces of hematophagous bugs called triatomines (family Reduviidae). However, it can also be spread via non-vectorial routes, such as blood transfusion, congenital transmission, organ transplantation, ingestion of food and beverages contaminated with T. cruzi, or laboratory accidents [1]. Over the years, infection with T. cruzi can cause heart failure or sudden death associated with progressive heart damage [2]. Some patients may also suffer from digestive, neurological or multiple alterations. This disease, classified by the World Health Organization (WHO) within the group of Neglected Tropical Diseases, is a major public health problem in Latin America where it is estimated that 6 to 7 million people are currently infected [1]. Due to human migrations, Chagas disease is emerging in other regions (Europe and United States principally) [3]. Estimates suggest that 80,000 to 120,000 T. cruzi-infected immigrants live in Europe, and 300,000 live in the United States [4], and the disease is a growing concern in these regions [5]. The global economic burden of Chagas disease is more than US$7.2 billion per year, exceeding the costs of other diseases of health impact such as certain cancer (US$6.7 billion for uterine cancer, US$4.7 billion for cervical cancer, and US$5.3 billion for oral cancer) or rotavirus infections (US$2 billion) [6,7].
In Mexico, estimates of Chagas disease prevalence and burden vary widely, which has complicated the establishment of a strong National Chagas Disease Program for vector control as well as for patient detection and care in the country. For the past several years, the Ministry of Health only reports a few hundred cases per year [8], suggesting that the disease has an anecdotal burden in terms of public health. On the other hand, other estimates suggest that there are about 1.1 million individuals infected with T. cruzi in Mexico, and 29.5 million at risk of infection [9,10]. Higher estimates of up to 6 million cases have also been proposed [11]. The annual cost for medical care for patients in the outpatient setting in this country is estimated between US$4,463 and US$9,601, and annual costs for patients admitted via an emergency care unit is between US$6,700 and US$11,838 [12].
There are also important regional differences in prevalence levels or number of cases reported in Mexico. For example, between 1928 and 2004 the states with the highest number of human cases reported were Chiapas, Guerrero, Jalisco, Morelos, Nayarit, Oaxaca and Queretaro. Conversely, few cases were reported in the states of Chihuahua, Coahuila, Guanajuato and Estado de Mexico [11]. It is not clear if such differences in prevalence are reflecting true differences in eco-epidemiological conditions, as Mexico is home to an extensive diversity of triatomine species, habitats, and socioeconomic conditions, or if there are bias in disease surveillance among regions [11].
Such wide discrepancies are important to reconcile to ensure that Chagas disease does not remain a major barrier to the development of Mexico's most vulnerable populations. Updating and improving surveillance data for Chagas disease in Mexico is therefore an important public health priority. In this context, the aim of this systematic review and meta-analysis was to estimate Chagas disease prevalence and burden in Mexico. We focused our study on the period from 2006 to 2017, to define current disease status rather than historical/cumulative burden, but our results are nonetheless compared with past reviews [11,13,14] to shed light on possible temporal trends on the status of Chagas disease in the country.
Methods
The current study was conducted in accordance with the PRISMA statement [15] (Supporting information). Potential data sources were identified and selected in different bibliographic databases. The ISI Web of Science (v5.13.1) was chosen because it incorporates many relevant databases including the SciELO Citation Index from 1997 onwards (provides access to leading journals from Latin America, Portugal, Spain and South Africa) and the Web of Science’s Core Collection from 1980 onwards (https://webofknowledge.com/). A part of the literature was selected from the LILACS database (lilacs.bvsalud.org/en/), which is the most important index of scientific and technical literature of Latin America and the Caribbean. Finally, the BibTri database (https://bibtri.cepave.edu.ar/) was also used because it integrates scientific literature specifically related to Chagas disease.
We restricted our search to the period from January 2006 to December 2017, to obtain information on the current status of Chagas disease in Mexico rather than on its historical/cumulative status, which has been summarized in previous reviews [11,13,14]. Selection was made using the search terms ‘Chagas disease in Mexico/Enfermedad de Chagas en México’ and with the equivalent keywords obtained via Medical Subject Headings (MeSH) website (https://meshb.nlm.nih.gov/search), i. e. American Trypanosomiasis, Chagas' Disease, Trypanosoma cruzi Infection, Trypanosomiasis in South American.
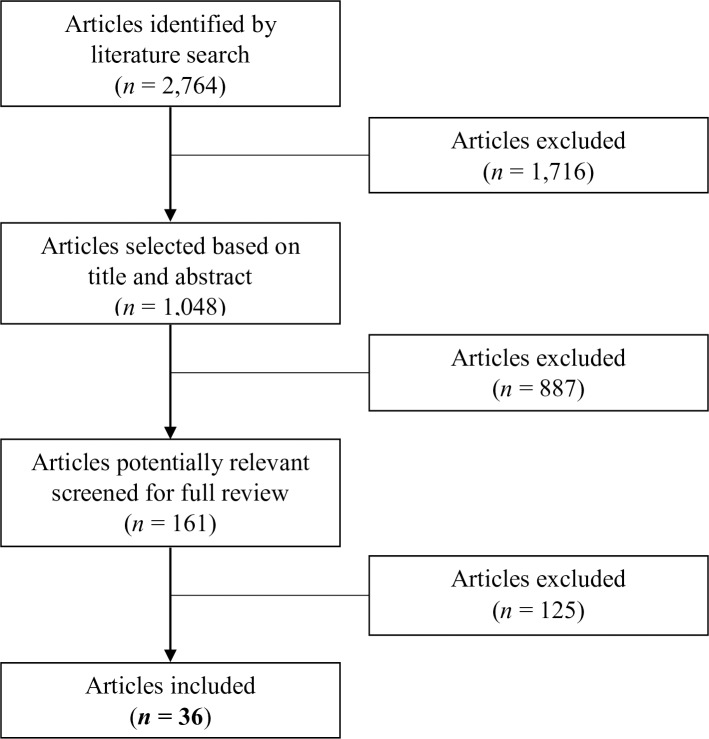
For all these articles, titles and abstracts were screened for any indication that the study contained data related to the seroprevalence of T. cruzi infection in human populations from Mexico. Typically, this excluded studies of, for example, therapeutic options for patients with chronic Chagas disease, molecular studies of lab strains of the parasite, or experimental model developments (Fig 1). In the second step of the process, full text copies were obtained and articles containing quantitative data on T. cruzi infection seroprevalence were retained. Extreme care was taken in cross-validating whether the information contained in each study was unique and not duplicated elsewhere.
Fig 1. Process flow chart for the identification, screening, eligibility, and inclusion of studies.
The ultimate step was to extract the relevant information contained in the selected articles which included 1) publication data (bibliographic information), 2) sampling dates, 3) sampling strategy (archive, random, volunteers, etc…), 4) geographic area covered by the study, 5) studied population (blood donors, patients, pregnant women, newborns, children, random populations), 6) laboratory techniques used (ELISA tests; IHA, PCR…) and the number of laboratory techniques used to validate the cases detected, 7) total sample size, number of human cases. Studied populations were then divided into subgroups to allow for analysis of the seroprevalence of T. cruzi infection at different levels, including the general population (population sampled in different geographic locations), pregnant women, children (under 18 years old), and blood donors (number of patients).
We calculated two general estimated prevalences 1) the first considering all studies, irrespective of the inclusion of confirmatory diagnostic, and 2) the second including only the studies in which at least 2 serological tests were used (as recommended by the WHO for an accurate identification of cases, see results part). We further calculated 95% confidence intervals (95%CI) based on the reported data and sample sizes [16,17]. We assessed the extent of publication bias in the selected studies through a funnel plot. Next, we performed a meta-analysis to calculate the effect size estimate, and the weighted effect size (% weight), based on a random-effects model [18]. For each estimated prevalence, a test for heterogeneity among studies (Q) and the variability of the effect size due to variation between observations (I2) was calculated, and forest plots were elaborated. The estimated prevalence obtained in each population were then compared to the data reported by the Ministry of Health and with other reviews [8].
Results
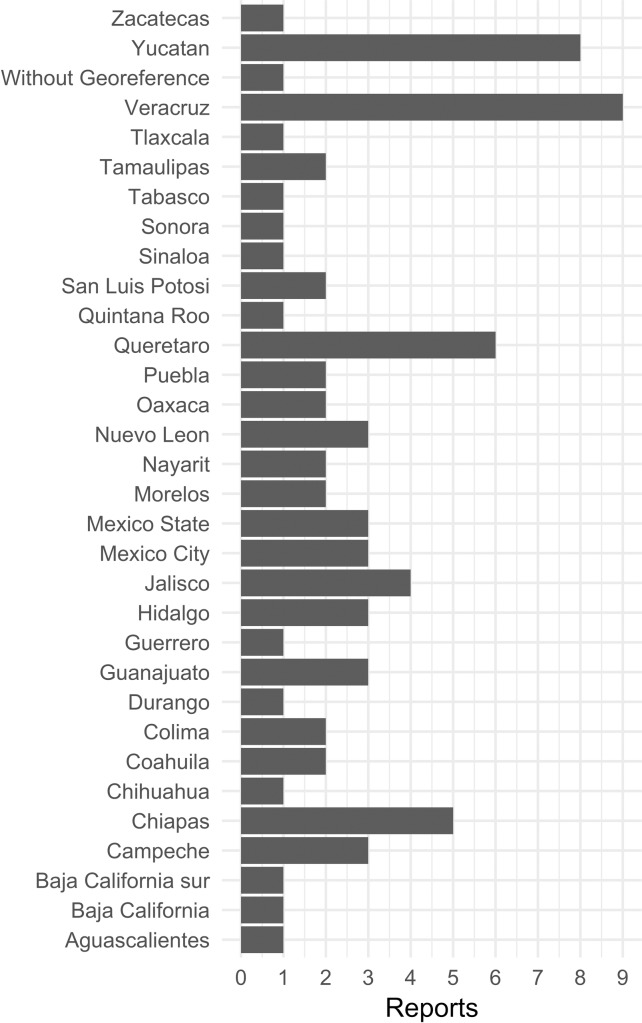
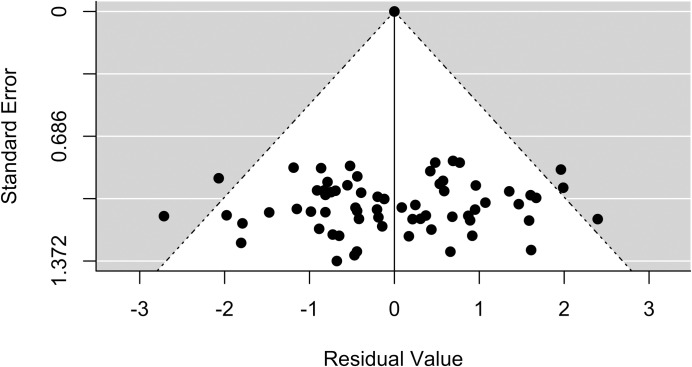
A total of 2,764 articles were screened and 36 were retained for the final analysis (see Fig 1). All the articles included in this study corresponded to serological surveys in different populations and settings, including general or specific populations such as pregnant women or blood donors, published between 2006 and 2017 (Table 1). Research on Chagas disease seroprevalence has been performed in most of the Mexican Republic (Fig 2). The states with more studies were Veracruz, Yucatan, and Queretaro. The extent of publication bias in the selected studies (with a total of 79 observations, each of the 36 studies may have several observations of different states, populations…) was assessed through a funnel plot (Fig 3), and the symmetric distribution of data points indicated a lack of publication bias or systematic heterogeneity of the dataset.
Table 1. Characteristics of included studies.
| Reference | Year | Population | State | Category Person | Num test validity* | Sample size | Num of cases* |
|---|---|---|---|---|---|---|---|
| Balan et al. [19] | 2011 | General population | Campeche | Adults/Children | 2 | 2,800 | 3 |
| Becerril-Flores et al. [20] | 2007 | General population | Hidalgo | Adults/Children | 2 | 175 | 6 |
| Becerril-Flores et al. [20] | 2007 | General population | Querétaro | Adults/Children | 2 | 39 | 2 |
| Benière et al. [21] | 2007 | General population | Jalisco | Adults | 1 | 242 | 6 |
| Buekens et al. [22] | 2018 | Pregnant woman | Yucatan | Adults | 3 | 12,160 | 32 |
| Campos-Valdez et al. [23] | 2016 | Pregnant woman | Chiapas | Adults | 2 | 1,125 | 23 |
| Cenalmor-Aparicio [24] | 2013 | General population | Chiapas | Adults | 2 | 129 | 25 |
| Dhiman et al. [25] | 2009 | General population | Chiapas | Adults | 2 | 1,481 | 121 |
| Escamilla-Guerrero et al. [26] | 2012 | Blood donor | Mexico City | Adults | 2 | 37,333 | 64 |
| Estrada-Franco et al. [27] | 2006 | General population | Mexico State | Children | 2 | 356 | 22 |
| Galavíz-Silva et al. [28] | 2009 | Blood donor | Coahuila | Adults | 2 | 65 | 2 |
| Galavíz-Silva et al. [28] | 2009 | Blood donor | Nuevo Léon | Adults | 2 | 809 | 21 |
| Galavíz-Silva et al. [28] | 2009 | Blood donor | Tamaulipas | Adults | 2 | 126 | 5 |
| Gamboa-León et al. [29] | 2014 | General population | Yucatan | Adults | 2 | 390 | 9 |
| Gamboa-León et al. [29] | 2014 | General population | Yucatan | Children | 2 | 685 | 3 |
| Gamboa-León et al. [30] | 2011 | Pregnant woman | Guanajuato | Adults | 2 | 488 | 2 |
| Gamboa-León et al. [30] | 2011 | Pregnant woman | Yucatan | Adults | 2 | 500 | 3 |
| García-Montalvo [31] | 2011 | Blood donor | Yucatan | Adults | 2 | 86,343 | 607 |
| Guzman-Gómez et al. [32] | 2015 | General population | Veracruz | Adults/Children | 2 | 184 | 62 |
| Hernández-Romano et al. [33] | 2015 | Blood donor | Veracruz | Adults | 2 | 87,232 | 438 |
| Jiménez-Cardoso et al. [34] | 2012 | Pregnant woman | Jalisco | Adults | 3 | 558 | 67 |
| Jiménez-Cardoso et al. [34] | 2012 | Pregnant woman | Mexico City | Adults | 3 | 97 | 4 |
| Jiménez-Cardoso et al. [34] | 2012 | Pregnant woman | Oaxaca | Adults | 3 | 794 | 35 |
| Jiménez-Coello et al. [35] | 2010 | General population | Yucatan | Adults | 3 | 60 | 4 |
| Jiménez-Coello et al. [35] | 2010 | General population | Yucatan | Children | 3 | 15 | 2 |
| Juarez-Tobias et al. [36] | 2009 | General population | Hidalgo | Adults/Children | 2 | 51 | 1 |
| Juarez-Tobias et al. [36] | 2009 | General population | San Luis Potosi | Adults/Children | 2 | 933 | 62 |
| Juarez-Tobias et al. [36] | 2009 | General population | Veracruz | Adults/Children | 2 | 15 | 2 |
| Kirchhoff et al. [37] | 2006 | Blood donor | Jalisco | Adults | 3 | 5,183 | 41 |
| Kirchhoff et al. [37] | 2006 | Blood donor | Nayarit | Adults | 3 | 2,113 | 14 |
| López-Céspedes et al. [38] | 2012 | General population | Querétaro | Adults | 2 | 258 | 28 |
| Martínez-Tovar et al. [39] | 2014 | Blood donor | Mexico State | Adults | 2 | 1,615 | 5 |
| Molina-Garza et al. [40] | 2014 | General population | Nuevo Léon | Adults | 2 | 2,688 | 52 |
| Monteon et al. [41] | 2013 | General population | Campeche | Adults | 2 | 128 | 3 |
| Montes-Rincón et al. [42] | 2016 | Pregnant woman | Guanajuato | Adults | 2 | 520 | 20 |
| Newton-Sánchez et al. [43] | 2017 | General population | Colima | Adults/Children | 1 | 925 | 22 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Aguascalientes | Adults | 2 | 7,187 | 1 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Baja California | Adults | 2 | 6,932 | 16 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Baja California sur | Adults | 2 | 375 | 0 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Campeche | Adults | 2 | 1,530 | 20 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Chiapas | Adults | 2 | 3,873 | 12 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Chihuahua | Adults | 2 | 6,012 | 26 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Coahuila | Adults | 2 | 4,611 | 10 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Colima | Adults | 2 | 636 | 5 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Durango | Adults | 2 | 1,151 | 4 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Guanajuato | Adults | 2 | 6,286 | 8 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Guerrero | Adults | 2 | 4,480 | 20 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Hidalgo | Adults | 2 | 4,117 | 30 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Jalisco | Adults | 2 | 7,150 | 14 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Mexico City | Adults | 2 | 5,055 | 15 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Mexico State | Adults | 2 | 54,514 | 140 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Morelos | Adults | 2 | 3,403 | 26 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Nayarit | Adults | 2 | 3,194 | 50 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Nuevo Léon | Adults | 2 | 36,441 | 79 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Oaxaca | Adults | 2 | 1,554 | 4 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Puebla | Adults | 2 | 7,988 | 11 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Querétaro | Adults | 2 | 3,870 | 22 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Quintana Roo | Adults | 2 | 2,768 | 55 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | San Luis Potosi | Adults | 2 | 1,532 | 4 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Sinaloa | Adults | 2 | 1,946 | 0 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Sonora | Adults | 2 | 8,947 | 12 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Tabasco | Adults | 2 | 1,893 | 34 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Tamaulipas | Adults | 2 | 8,263 | 53 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Tlaxcala | Adults | 2 | 532 | 3 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Veracruz | Adults | 2 | 19,599 | 185 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Yucatan | Adults | 2 | 13,045 | 76 |
| Novelo-Garza et al. [44] | 2010 | Blood donor | Zacatecas | Adults | 2 | 1,190 | 0 |
| Olivera-Mar et al. [45] | 2006 | Pregnant woman | Chiapas | Adults | 2 | 60 | 3 |
| Olivera-Mar et al. [45] | 2006 | Pregnant woman | Veracruz | Adults | 2 | 85 | 3 |
| Portugal-García et al. [46] | 2011 | General population | Morelos | Adults/Children | 2 | 233 | 3 |
| Ramos-Ligonio et al. [47] | 2006 | Blood donor | Veracruz | Adults | 3 | 420 | 2 |
| Ramos-Ligonio et al. [47] | 2010 | General population | Veracruz | Adults | 2 | 654 | 110 |
| Ruiz et al. [48] | 2011 | Pregnant woman | Veracruz | Adults | 2 | 4,851 | 20 |
| Salazar et al. [49] | 2007 | General population | Veracruz | Children | 3 | 150 | 5 |
| Salazar-Schettino et al. [50] | 2009 | General population | Querétaro | Children | 2 | 826 | 11 |
| Salazar-Schettino et al. [51] | 2016 | General population | NA | Children | 2 | 3,327 | 37 |
| Sánchez-Guillén et al. [52] | 2006 | Blood donor | Puebla | Adults | 2 | 2,140 | 166 |
| Villagrán et al. [53] | 2009 | General population | Querétaro | Adults/Children | 2 | 1,029 | 68 |
| Villagrán-Herrera et al. [54] | 2014 | General population | Querétaro | Adults/Children | 2 | 3 | 3 |
*Num test validity (number of laboratory techniques used), Num of cases (number of human cases detected). I2 = 99.54%; Q = 1,948.
Fig 2. Number of publications which report human cases of T. cruzi seropositivity from states of Mexico, 2006–2017.
Each publication can cover several states.
Fig 3. Funnel plot for the examination of study bias.
The plot is based on 79 observations from 36 publications which report human cases of T. cruzi seropositivity from states of Mexico, 2006–2017.
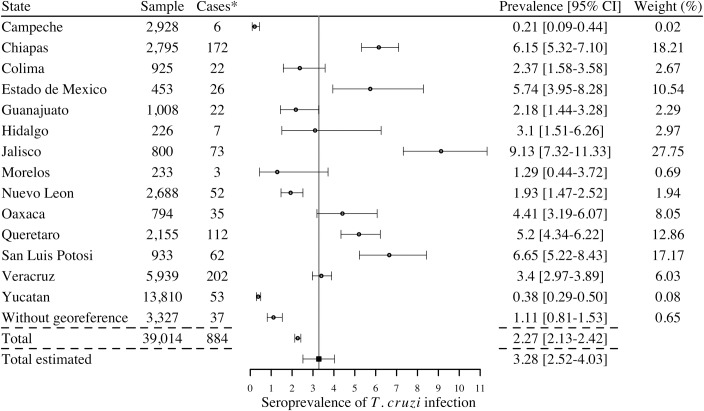
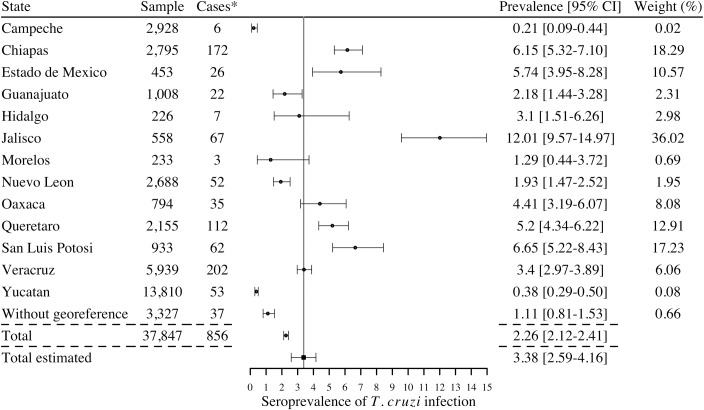
We first considered the studies in the general population (population sampled in different geographic locations) and in pregnant women (which can be considered as highly representative of the general population as well [55]) to obtain a national estimate. When considering all studies, irrespective of the inclusion of confirmatory diagnostic (i.e. based on a single serological test, 28 studies), the total number of human cases reported in the literature during the period 2006–2017 was 884 with a national estimated prevalence (calculated according to the sample size between studies) of 3.28% [95%CI 2.52–4.03] (Fig 4). The seroprevalence of infection varied between 0.21% and 9.13% depending on the state. Only two studies (in Jalisco and Colima) were based on a single test and when considering only the studies in which at least 2 serological tests had been performed (26 studies), hence cases had been confirmed as currently recommended by the WHO for an accurate identification of cases, the national estimated seroprevalence was 3.38% [95%CI 2.59–4.16], with seroprevalences varying between 0.21% and 12.01% depending on the state (Fig 5). The highest seroprevalence levels were reported in the states of Jalisco, San Luis Potosi, Chiapas, Estado de Mexico, Queretaro, and Oaxaca. Based on a national population of nearly 120 million (National census of 2015), this seroprevalence level would correspond to 4.06 million cases in the country [95%CI 2.45–4.50 million]. On the other hand, the number of cases of T. cruzi infection reported by the national program of epidemiologic surveillance of the Ministry of Health during 2006–2017 period reached 8,687 ([8] and Table 2), with a regular increase in the number of cases detected with time.
Fig 4. Cases of Trypanososma cruzi infection detected in serological surveys of general populations and pregnant women during 2006–2017 (28 studies).
*Cases are reported using 1 or more serological tests to determine the infection. I2 = 98.56%; Q = 625.
Fig 5. Confirmed cases of Trypanososma cruzi seropositivity detected in serological surveys of general populations and pregnant women during 2006–2017 (26 studies).
*Cases are reported using 2 or more serological tests to determine the infection. I2 = 98.52%; Q = 610.
Table 2. Number of new cases of Chagas disease per years, reported by the Ministry of Health, in all the states of Mexico [8].
| Years | Sample |
|---|---|
| 2006 | 400 |
| 2007 | 392 |
| 2008 | 679 |
| 2009 | 613 |
| 2010 | 528 |
| 2011 | 801 |
| 2012 | 830 |
| 2013 | 762 |
| 2014 | 735 |
| 2015 | 1,095 |
| 2016 | 994 |
| 2017 | 856 |
| Total | 8,687 |
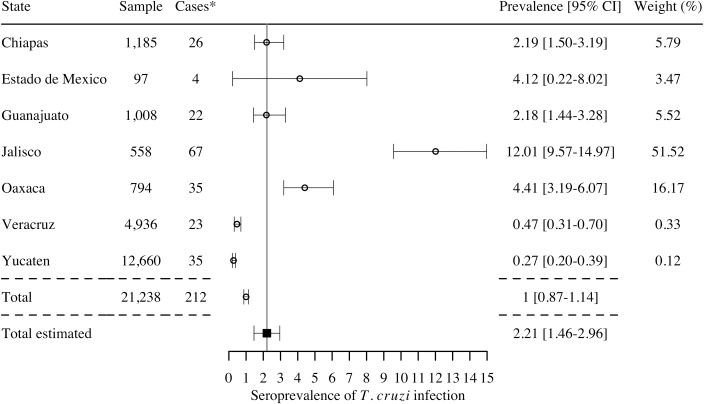
A few of these studies (7 studies) focused on pregnant women, which may transmit the parasite to their newborn during pregnancy [56]. While these studies only covered 7 states (Fig 6), a total of 212 T. cruzi-infected pregnant women were detected, for a global estimated seroprevalence of T. cruzi infection of 2.21% [95%CI 1.46–2.96] in this specific population. The highest seroprevalence levels in pregnant women were reported in the states of Jalisco, Oaxaca, and Estado de Mexico. Based on current birth rate in Mexico (2,293,000 births in 2016), this would correspond to 50,675 births from T. cruzi infected pregnant women per year. With a congenital transmission rate of 6.3% [22], there may be 3,193 cases of congenitally infected newborns per year in the country.
Fig 6. Prevalence of seropositive pregnant women by state, 2006–2017 (7 studies).
*Cases are reported using 2 or more serological tests to determine the infection. I2 = 93.27%; Q = 134.
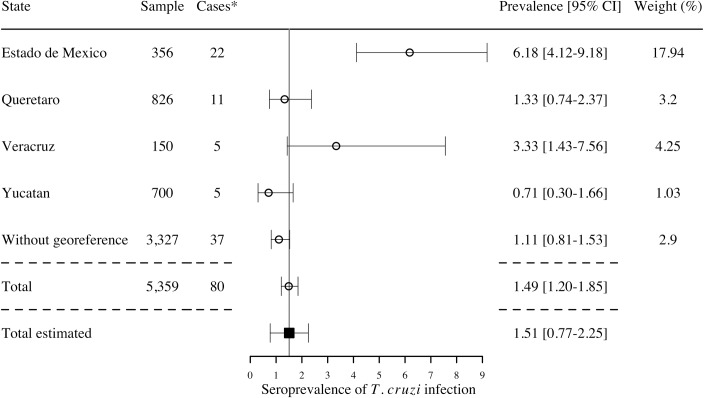
Some studies also focused on or included data on children under 18 years (6 studies), which may indicate more recent transmission. These covered only 4 states (Fig 7), with a global estimated seroprevalence of T. cruzi infection of 1.51% [95%CI 0.77–2.25].
Fig 7. Prevalence of seropositive children under 18 years by state, 2006–2017 (6 studies).
*Cases are reported using 2 or more serological tests to determine the infection. I2 = 52.18%; Q = 19.
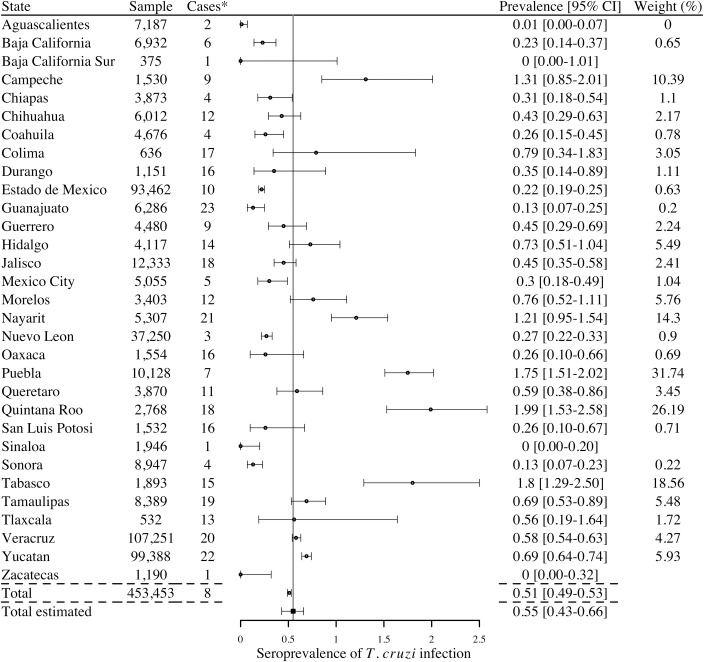
Several additional studies also evaluated T. cruzi infection in blood donors (9 studies), and seropositive human cases were detected in every state of the Mexican Republic, except for Baja California Sur, Sinaloa, and Zacatecas (Fig 8). The total number of blood donor cases reported was 2,300 corresponding to a national estimated seroprevalence of 0.55% [95% CI 0.43–0.66]. The highest seroprevalence was observed in the states of Quintana Roo, Tabasco, Puebla, Campeche and Nayarit.
Fig 8. Prevalence of positive serology in blood banks by state, 2006–2017 (9 studies).
*Cases are reported using 2 or more serological tests to determine the infection. I2 = 99.19%; Q = 1,114.
Finally, we also examined the type of serological test performed in these studies. The most widely used tests were indirect hemagglutination, followed by Chagatest ELISA from Wiener lab, and immunofluorescence assays (Table 3), which represented 67.8% of all tests used. Several other commercial ELISA tests were also used (24.7% of tests), and in-house tests including ELISA, and western blot represented 3.7% of the tests used.
Table 3. Type of diagnostic tests used in epidemiological studies in Mexico.
| Type of test | Sample | Cases |
|---|---|---|
| IHA, Indirect hemagglutination | 391,148 | 2,005 |
| Chagatest Elisa (v. 3.0, 4.0 or unspecified), Wiener Lab | 234,282 | 1,234 |
| IFA, Immunofluorescence assays | 103,396 | 962 |
| BioElisa Chagas, Biokit/Werfen | 89,021 | 448 |
| Chagas Recombinant Micro-Elisa Kit, Accutrack | 88,787 | 558 |
| Elisa Kit, Bioschile | 87,342 | 672 |
| In-House Elisa with Antigenic Extract | 34,832 | 521 |
| Chagas STAT-PAK rapid test, Chembio | 18,208 | 293 |
| Abbott Chagas' EIA Kit, Abbott Lab Meridian | 7,296 | 55 |
| Chagas Elisa Kit, Meridian Biosci. | 7,296 | 55 |
| RIPA, Radioimmunoprecipitation | 7,296 | 55 |
| WB, Western blot | 5,379 | 216 |
| Enzyme Chagas Kit, Interbiol | 214 | 8 |
| Chagatek Elisa, Lomos-Biomerieux | 184 | 62 |
| Total | 1,074,681 | 7,144 |
Discussion
Chagas disease remains one of the most relevant parasitic disease in the Americas, but its epidemiology in Mexico is still poorly understood. Better data are thus urgently needed to help develop appropriate public health programs for disease control and patient care. In this study we analyzed published data on T. cruzi seroprevalence of infection in Mexico between 2006 and 2017. A total of 36 studies were identified, covering most of the country with the notable exception of the state of Michoacán. To take into account the sample size heterogeneity among studies, we analyzed the data based on meta-analysis techniques using a random-effects model. Due to discrepancies in previous studies often attributed to diagnostic methods and uncertainties about the confirmation of cases [57], current recommendations of health agencies request a minimum of 2 serological techniques for accurate diagnostic [10]. Based on this criterion, we found a national estimated seroprevalence of T. cruzi infection of 3.38%, corresponding to 4.06 million cases in the country. Only a few studies were discarded for lack of confirmatory testing, indicating that most of recent studies followed current guidelines for the accurate diagnostic of cases. This seroprevalence level can thus be considered rather conservative, but it is much higher than previous estimates. For example, the Pan American Health Organization (PAHO) estimated that 1,100,000 individuals were infected with T. cruzi in Mexico in 2006, and 29,500,000 were at risk of infection [58], and a national prevalence of 0.65% (with 733,333 cases) was established in 2010 by the Mexican Ministry of Health [59]. The most recent estimates from the WHO based on 2010 data reports 876,458 cases [10], corresponding to a national prevalence of 0.78%. Our analysis of data from the last decade thus suggests that the magnitude of T. cruzi infection in Mexico may have been underestimated in these previous reports. Based on ours data, annual cost for medical care in the outpatient setting was estimated between US$18 and US$39 billion, and annual costs in emergency care unit is between US$27 and US$48 billion [12]. In addition, recent studies pointing out a low sensitivity of commercial serological tests for T. cruzi diagnostic [22,32,60], some of which are widely used in Mexico (Table 3) also raise concerns that the seroprevalence of T. cruzi infection may even be higher than currently detected. Improvements in serological tests are thus urgently needed for a more reliable disease surveillance [61]. Our analysis nonetheless places Mexico as the country with the largest number of cases of T. cruzi infection as previously estimated [10], and highlights the urgency of establishing national priorities for the control of parasite transmission and patient care as well as improved epidemiologic surveillance.
Our results also point out to some regional differences in T. cruzi infection seroprevalence among states. The ecology and epidemiology of Chagas disease are the result of many geographical, ecological, biological, and social interactions [62], which may explain some of these differences. High seroprevalence levels have been previously reported for several states including Jalisco, Chiapas, Queretaro, Oaxaca, Veracruz, and Morelos [11], suggesting a well-established endemicity in these states. States with seroprevalence levels higher than previously reported also emerged through our study, in spite of limited sample sizes. These include San Luis Potosi, Estado de Mexico, Hidalgo and Guanajuato.
T. cruzi infection is also present at a significant estimated seroprevalence in pregnant women in Mexico. Despite the limited information available for this specific population, we could estimate that there are 50,675 births from T. cruzi infected pregnant women per year, corresponding to 3,193 cases of congenitally infected newborns per year in the country. This prevalence is again higher than previous estimates [63], strengthening the urgency of addressing congenital Chagas disease in the country. Because infected newborns can be effectively treated, the lack of specific screening programs to identify them is a missed opportunity for the control of the disease. Indeed, a recent health economic study in the US evidenced the large benefits of maternal screening for T. cruzi infection, as lifetime societal savings due to screening and treatment was estimated at $634 million saved for every birth year cohort [64].
Very limited information is available on T. cruzi infection in children. Nonetheless, we were able to identify a few studies in children up to 18 years, who presented an average prevalence of 1.51%. This may indicate more recent and active transmission compared to data on adult populations, and suggests that the incidence of T. cruzi infection has been fairly stable over time. Therefore, effective vector control programs tailored to the extensive diversity of triatomine species present in Mexico [65] are urgently needed to reduce vectorial T. cruzi transmission to human populations [66,67].
Blood transfusion has been considered the second most important mode of transmission of Chagas disease in Mexico [68]. In 1998, the screening of almost 65,000 blood donors from 18 government-run transfusion centers showed a 1.5% prevalence of anti-T. cruzi antibodies in blood donors [69]. The highest prevalence was detected in the states of Hidalgo, Tlaxcala, Puebla, Chiapas y Yucatan, as expected from previous reports, whereas the northern states of Nuevo Leon and Chihuahua, had the lowest seroprevalence in blood donors. For the period between 1978–2004, Cruz-Reyes et al. defined a national prevalence of positive serology in blood banks of 2.03% [11]. In our study, the national estimated prevalence detected in blood donors was lower with 0.55%. These differences can be explained by the increased reliability of serologic screening of blood donors with the passing of legislation making screening mandatory in the year 2000 [13,70]. The addition of a pre-screening questionnaire to exclude high-risk individuals may also have led to a lower prevalence in screened donors. The highest prevalence of 1.99% is detected in the state of Quintana Roo and the lowest in Baja California Sur, Sinaloa and Zacatecas (with a prevalence of 0%).
Strengths and limitations
A major strength of our analysis was to consider the sample size heterogeneity among studies and the reliability of serological testing performed, and to ensure it followed WHO recommendations for confirmation of cases using at least a second test. Hence, our estimates of seroprevalence are robust and conservative. On the other hand, there are some limitations. First, some heterogeneity among study designs and particularly sampling strategies and recruitment of subjects may have generated some bias. For example, the difference in the number of studies performed per state can lead to over- or under-estimation of the prevalences. Also, while we did not detect major publication bias, there was an uneven coverage of the different states by research studies, which may be a confounding factor affecting differences in T. cruzi infection prevalence among states. This highlights the need for much improved nationwide disease surveillance to clearly identify geographic heterogeneities in T. cruzi transmission and Chagas disease epidemiology. Finally, the small number of studies/sample sizes for some of the subgroup analysis also add uncertainties to our estimates of T. cruzi estimated seroprevalence in specific subpopulations.
Conclusion
In conclusion, our systematic review and meta-analysis estimates a national seroprevalence of T. cruzi infection of 3.38%, with 4.06 million cases in Mexico, which is higher than previously recognized. It places Mexico as the country with the largest number of cases, highlighting the urgency of establishing Chagas disease control as a key national public health priority, to ensure that it does not remain a major barrier to the economic and social development of Mexico's most vulnerable populations. It remains essential to strengthen effective surveillance for Chagas disease in all the country to obtain more precise data. The presence of T. cruzi infection in specific subpopulations such as pregnant women, children and blood donors also informs on specific risks of infection, and calls for the implementation of well-established control interventions [56,67,71]. Finally, while our estimates are conservative and based on confirmed cases, the lack of sensitivity of current serological tests observed in Mexico suggest that the true magnitude of Chagas disease in the country may still be underestimated, and the development of more reliable diagnostic tests will be key for an effective identification of cases as well as improved patient care [61].
Supporting information
(PDF)
(PDF)
Acknowledgments
We thank Dr. Pierre Buekens for his helpful comments on a draft version of the manuscript.
Data Availability
All the data may be found directly in the Fig 1 of the article.
Funding Statement
This work was partially funded by grant #187714 from the Carlos Slim Foundation, grant #632083 from Tulane University School of Public Health and Tropical Medicine. CONACYT Basic Science (ID CB2015-258752) and National Problems (PN2015-893) grants awarded to EW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Chagas disease (American trypanosomiasis), Fact sheet N°340. Available at http://www.who.int/mediacentre/factsheets/fs340/en/. 2017.
- 2.Rassi AJ, Rassi A, Marin-Neto JA. Chagas disease. Lancet. Elsevier Ltd; 2010;375: 1388–1402. 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 3.Schmunis GA, Yadon ZE. Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. Elsevier B.V.; 2010;115: 14–21. 10.1016/j.actatropica.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Soriano-Arandes A, Angheben A, Serre-Delcor N, Treviño-Maruri B, Gómez I Prat J, Jackson Y. Control and management of congenital Chagas disease in Europe and other non-endemic countries: Current policies and practices. Trop Med Int Heal. 2016;21: 590–596. 10.1111/tmi.12687 [DOI] [PubMed] [Google Scholar]
- 5.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138: e169–e209. 10.1161/CIR.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 6.Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13: 342–348. 10.1016/S1473-3099(13)70002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385: 117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirección General de Epidemiología. Anuarios de morbilidad, información de epidemiológica Secretaría de Salud 1984–2017. 2018. Available at http://www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html. [Google Scholar]
- 9.Hotez PJ, Dumonteil E, Betancourt Cravioto M, Bottazzi ME, Tapia-Conyer R, Meymandi S, et al. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis. 2013;7: e-2300 10.1371/journal.pntd.0002300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90: 33–43. 10.2147/IBPC.S70402 [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years-A review. Mem Inst Oswaldo Cruz. 2006;101: 345–354. 10.1590/S0074-02762006000400001 [DOI] [PubMed] [Google Scholar]
- 12.Ramsey JM, Elizondo-Cano M, Sanchez-González G, Peña-Nieves A, Figueroa-Lara A. Opportunity cost for early treatment of Chagas disease in Mexico. PLoS Negl Trop Dis. 2014;8: e2776 10.1371/journal.pntd.0002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman-Bracho C. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol. 2001;17: 372–376. [DOI] [PubMed] [Google Scholar]
- 14.Dumonteil E. Update on Chagas’ disease in Mexico. Salud Publica Mex. 1999;41: 322–327. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6: e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998; [DOI] [PubMed] [Google Scholar]
- 17.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927; 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 18.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5: 52 10.1186/1756-0500-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balan LU, Yerbes IM, Piña MAN, Balmes J, Pascual A, Hernández O, et al. Higher seroprevalence of Trypanosoma cruzi infection in dogs than in Humans in an urban area of Campeche, Mexico. Vector-Borne Zoonotic Dis. 2011;11: 843–844. 10.1089/vbz.2010.0039 [DOI] [PubMed] [Google Scholar]
- 20.Becerril-Flores M a, Rangel-Flores E, Imbert-Palafox JL, Gómez-Gómez JV, Figueroa-Gutiérrez AH. Human infection and risk of transmission of Chagas disease in Hidalgo state, Mexico. Am J Trop Med Hyg. 2007;76: 318–23. Available: http://www.ncbi.nlm.nih.gov/pubmed/17297042 [PubMed] [Google Scholar]
- 21.Brenière SF, Bosseno MF, Magallón-Gastelúm E, Castillo Ruvalcaba EG, Gutierrez MS, Montaño Luna EC, et al. Peridomestic colonization of Triatoma longipennis (Hemiptera, Reduviidae) and Triatoma barberi (Hemiptera, Reduviidae) in a rural community with active transmission of Trypanosoma cruzi in Jalisco state, Mexico. Acta Trop. 2007;101: 249–257. 10.1016/j.actatropica.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Buekens P, Cafferata ML, Alger J, Althabe F, Belizán JM, Bustamante N, et al. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: An observational prospective study. Am J Trop Med Hyg. 2018;98: 478–485. 10.4269/ajtmh.17-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos-Valdez G, Canseco-Ávila LM, González-Noriega F, Alfaro-Zebadua O, Nava-Medecigo IY, Jiménez-Cardoso E. Transmisión materno-fetal de Trypanosoma cruzi, un problema de salud poco estudiado en México: Caso Chiapas. Salud Publica Mex. 2016;58: 378–384. 10.21149/spm.v58i3.7898 [DOI] [PubMed] [Google Scholar]
- 24.Cenalmor-Aparicio C, Ballinas-Verdugo M, Reyes PA. Tripanosomosis americana (enfermedad de Chagas) en el municipio de Chilón, Chiapas: una encuesta clínicoepidemiológica en 2011. Salud Publica Mex. 2013;55: 149–150. [DOI] [PubMed] [Google Scholar]
- 25.Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, et al. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol. 2009;16: 660–666. 10.1128/CVI.00019-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escamilla-Guerrero G, Martínez-Gordillo MN, Riverõn-Negrete L, Aguilar-Escobar DV, Bravo-Lindoro A, Cob-Sosa C, et al. Trypanosoma cruzi: Seroprevalence detected in the blood bank of the Instituto Nacional de Pediatría, Mexico City, in the period 2004 through 2009. Transfusion. 2012;52: 595–600. 10.1111/j.1537-2995.2011.03322.x [DOI] [PubMed] [Google Scholar]
- 27.Estrada-Franco JG, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbabosa A, Vazquez-Chagoyan JC, et al. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg Infect Dis. 2006;12: 624–630. 10.3201/eid1204.050450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galavíz-Silva L, Molina-Garza DP, González-Santos MA, Mercado-Hernández R, González-Galavíz JR, Rosales-Encina JL, et al. Update on seroprevalence of anti-Trypanosoma cruzi antibodies among blood donors in Northeast Mexico. Am J Trop Med Hyg. 2009;81: 404–406. 81/3/404 [pii] [PubMed] [Google Scholar]
- 29.Gamboa-León R, Ramirez-Gonzalez C, Pacheco-Tucuch FS, O’Shea M, Rosecrans K, Pippitt J, et al. Seroprevalence of Trypanosoma cruzi among mothers and children in rural Mayan communities and associated reproductive outcomes. Am J Trop Med Hyg. 2014;91: 348–353. 10.4269/ajtmh.13-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamboa-León R, Gonzalez-Ramirez C, Padilla-Raygoza N, Sosa-Estani S, Caamal-Kantun A, Buekens P, et al. Do commercial serologic tests for Trypanosoma cruzi infection detect Mexican strains in women and newborns? J Parasitol. 2011;97: 338–343. 10.1645/GE-2545.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Montalvo B. Trypanosoma cruzi antibodies in blood donors in Yucatan state, Mexico. Rev Med Inst Mex Seguro Soc. 2011;97: 338–343. [PubMed] [Google Scholar]
- 32.Guzmán-Gómez D, López-Monteon A, De La Soledad Lagunes-Castro M, Álvarez-Martínez C, Hernández-Lutzon MJ, Dumonteil E, et al. Highly discordant serology against Trypanosoma cruzi in central Veracruz, Mexico: Role of the antigen used for diagnostic. Parasites and Vectors. 2015;8: 466 10.1186/s13071-015-1072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernández-Romano P, Cámara-Contreras M, Bravo-Sarmiento E, Lõpez-Balderas N. Prevalence of Trypanosoma cruzi antibodies in blood donors from Veracruz State, Mexico. Transfusion. 2015;55: 647–656. 10.1111/trf.12860 [DOI] [PubMed] [Google Scholar]
- 34.Jiménez Cardoso E, Campos Valdéz G, Cortes Campos A, de la Luz Sanchez R, Riviera Mendoza C, Plascencia Hernández A, et al. Maternal fetal transmission of Trypanosoma cruzi: A problem of public health little studied in Mexico. Exp Parasitol. 2012;131: 425–432. 10.1016/j.exppara.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 35.Jiménez-Coello M, Guzmán-Marín E, Ortega-Pacheco A, Acosta-Viana KY. Serological survey of American trypanosomiasis in dogs and their owners from an urban area of Merida Yucatan, Mexico. Transbound Emerg Dis. 2010;57: 33–36. 10.1111/j.1865-1682.2010.01130.x [DOI] [PubMed] [Google Scholar]
- 36.Juarez-Tobias S, Vaughan G, Torres-Montoya A, Escobar-Gutierrez A. Short report: Seroprevalence of Trypanosoma cruzi among Teenek Amerindian residents of the Huasteca region in San Luis Potosi, Mexico. Am J Trop Med Hyg. 2009;81: 219–222. 81/2/219 [pii] [PubMed] [Google Scholar]
- 37.Kirchhoff L V., Paredes P, Lomelí-Guerrero A, Paredes-Espinoza M, Ron-Guerrero CS, Delgado-Mejía M, et al. Transfusion-associated Chagas disease (American trypanosomiasis) in Mexico: implications for transfusion medicine in the United States. Transfusion. 2006;46: 298–304. 10.1111/j.1537-2995.2006.00715.x [DOI] [PubMed] [Google Scholar]
- 38.López-Céspedes Á, Villagrán E, Briceño Álvarez K, De Diego JA, Hernández-Montiel HL, Saldaña C, et al. Trypanosoma cruzi: seroprevalence detection in suburban population of Santiago de Querétaro (Mexico). Sci World J. 2012;2012: 8–15. 10.1100/2012/914129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Tovar JG, Rebollar-Téllez EA, Fernández Salas I. Seroprevalence of T. cruzi infection in blood donors and Chagas cardiomyopathy in patients from the coal mining region of Coahuila, Mexico. Rev Inst Med Trop Sao Paulo. 2014;56: 169–174. 10.1590/S0036-46652014000200014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina-Garza ZJ, Rosales-Encina JL, Mercado-Hernández R, Molina-Garza DP, Gomez-Flores R, Galaviz-Silva L. Association of Trypanosoma cruzi infection with risk factors and electrocardiographic abnormalities in northeast Mexico. BMC Infect Dis. 2014;14: 117 10.1186/1471-2334-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteon V, Alducin C, Hernández J, Ramos-Ligonio A, Lopez R. High frequency of human blood in Triatoma dimidiata captured inside dwellings in a rural community in the Yucatan Peninsula, Mexico, but low antibody seroprevalence and electrocardiographic findings compatible with Chagas disease in humans. Am J Trop Med Hyg. 2013;88: 566–571. 10.4269/ajtmh.12-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montes-Rincón LM, Galaviz-Silva L, González-Bravo FE, Molina-Garza ZJ. Trypanosoma cruzi seroprevalence in pregnant women and screening by PCR and microhaematocrit in newborns from Guanajuato, Mexico. Acta Trop. Elsevier B.V.; 2016;164: 100–106. 10.1016/j.actatropica.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 43.Newton-Sanchez OA, Espinoza-Gomez F, Melnikov V, Delgado-Enciso I, Rojas-Larios F, Dumonteil E, et al. Seroprevalence of Trypanosoma cruzi (TC) and risk factors in Colima, Mexico. Gac Med Mex. 2017;153: 179–184. [PubMed] [Google Scholar]
- 44.Novelo.-Garza BA, Benítez-Arvizu G, Peña-Benítez A, Galván-Cervantes J, Morales-Rojas A. Detección de Trypanosoma cruzi en donadores de sangre. Rev Medica del IMSS. 2010;48: 139–144. Available: http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=82512272&lang=es&site=ehost-live [PubMed] [Google Scholar]
- 45.Olivera Mar A, Ortega FG, Vidal SC, Hernández-Becerril N, Galdamez EP, Concepción GC, et al. Serological and parasitological screening of Trypanosoma cruzi infection in mothers and newborns living in two chagasic areas of Mexico. Arch Med Res. 2006;37: 774–777. 10.1016/j.arcmed.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 46.Portugal-García C, García-Vázquez Z, Monteón-Padilla V, Chávez-López V, Olamendi-Portugal M, Ramos C. Anticuerpos contra Trypanosoma cruzi en humanos y perros y presencia del parásito en Meccus pallidipennis en la localidad de Puente Pantitlán, Morelos, México. Biomedica. 2011;22: 67–75. [Google Scholar]
- 47.Ramos-Ligonio A, Ramírez-Sánchez ME, González-Hernández JC, Rosales-Encina JL, López-Monteon A. Prevalencia de anticuerpos contra Trypanosoma cruzi en donadores de sangre del IMSS, Orizaba, Veracruz, México. Salud Publica Mex. 2006;48: 13–21. 10.1590/S0036-36342006000100004 [DOI] [PubMed] [Google Scholar]
- 48.Ruiz A, Salazar PM, Rojas G, Guevara Y, Torres E, Gutiérrez M, et al. Seguimiento serológico de hijos de madres seropositivas a Trypanosoma cruzi en la jurisdicción sanitaria de Tuxpan, Veracruz, México. Biomédica. 2011;31: 23–205. [Google Scholar]
- 49.Salazar PM, Rojas G, Bucio M, Cabrera M, García G, Ruiz A, et al. Seroprevalencia de anticuerpos contra Trypanosoma cruzi y su asociación con factores de riesgo en menores de 18 años de Veracruz, México. Rev Panam Salud Pública. 2007;22: 75–82. 10.1590/S1020-49892007000700001 [DOI] [PubMed] [Google Scholar]
- 50.Salazar-Schettino PM, Perera R, Ruiz-Hernandez AL, Bucio Torres MI, Zamora-Gonzalez C, Cabrera-Bravo M, et al. Chagas disease as a cause of symptomatic chronic myocardopathy in mexican children. Pediatr Infect Dis J. 2009;28: 1011–1013. 10.1097/INF.0b013e3181ad8425 [DOI] [PubMed] [Google Scholar]
- 51.Salazar-Schettino PM, Cabrera-Bravo M, Vazquez-Antona C, Zenteno E, Alba-Alvarado M De, Gutierrez ET, et al. Chagas disease in Mexico: report of 14 cases of chagasic cardiomyopathy in children. Tohoku J Exp Med. 2016;240: 243–249. 10.1620/tjem.240.243 [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-Guillén M del C, López-Colombo A, Ordóñez-Toquero G, Gomez-Albino I, Ramos-Jimenez J, Torres-Rasgado E, et al. Clinical forms of Trypanosoma cruzi infected individuals in the chronic phase of Chagas disease in Puebla, Mexico. Mem Inst Oswaldo Cruz. 2006;101: 733–740. 10.1590/S0074-02762006000700005 [DOI] [PubMed] [Google Scholar]
- 53.Villagrán ME, Sánchez-Moreno M, Marín C, Uribe M, de la Cruz JJ, de Diego JA. Seroprevalence to Trypanosoma cruzi in rural communities of the state of Querétaro (Mexico). Statistical evaluation of tests. Clin Biochem. The Canadian Society of Clinical Chemists; 2009;42: 12–16. 10.1016/j.clinbiochem.2008.09.122 [DOI] [PubMed] [Google Scholar]
- 54.Villagrán-Herrera ME, Sánchez-Moreno M, Rodríguez-Méndez AJ, Hernández-Montiel HL, Dávila-Esquivel F de J, González-Pérez G, et al. Comparative serology techniques for the diagnosis of Trypanosoma cruzi infection in a rural population from the state of Querétaro, Mexico. Mem Inst Oswaldo Cruz. 2014;109: 967–972. 10.1590/0074-0276130413 [DOI] [PubMed] [Google Scholar]
- 55.Eaton JW, Rehle TM, Jooste S, Nkambule R, Kim AA, Mahy M, et al. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS. 2014;28: S507–S514. 10.1097/QAD.0000000000000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlier Y, Sosa-Estani S, Luquetti AO, Buekens P. Congenital Chagas disease: An update. Mem Inst Oswaldo Cruz. 2015;110: 363–368. 10.1590/0074-02760140405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moure Z, Angheben A, Molina I, Gobbi F, Espasa M, Anselmi M, et al. Serodiscordance in chronic Chagas disease diagnosis: a real problem in non-endemic countries. Clin Microbiol Infect. 2016;22: 788–792. 10.1016/j.cmi.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 58.Organización Panamericana de la Salud and Organización Mundial de la Salud (OPS & OMS). Estimación cuantitativa de la enfermedad de Chagas en las Américas. by Jean, J Roberto, S. 2006;OPS: Montevideo, Uruguay. OPS/HDM/CD/425-06
- 59.Manne JM, Snively CS, Ramsey JM, Salgado MO, Bärnighausen T, Reich MR. Barriers to treatment access for Chagas disease in Mexico. PLoS Negl Trop Dis. 2013;7 10.1371/journal.pntd.0002488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ballinas-Verdugo MA, Mejía-Domínguez AM, Sánchez-Guerrero SA, Lerma C, Martínez-Cruz M, Álvarez-Manilla-Toquero E, et al. The type of Trypanosoma cruzi strain (native or non-native) used as substrate for immunoassays influences the ability of screening asymptomatic blood donors. Rev Invest Clin. 2016;68: 286–291. [PubMed] [Google Scholar]
- 61.Dumonteil E, Herrera C. Ten years of Chagas disease research: looking back to achievements, looking ahead to challenges. PLoS Negl Trop Dis. 2017;11: e0005422 10.1371/journal.pntd.0005422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenthal J. Climate change and the geographic distribution of infectious diseases. Ecohealth. 2009;6: 489–495. 10.1007/s10393-010-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, et al. Mother-to-child transmission of Chagas’ disease in North America: Why don’t we do more? Matern Child Health J. 2008;12: 283–286. 10.1007/s10995-007-0246-8 [DOI] [PubMed] [Google Scholar]
- 64.Stillwaggon E, Perez-Zetune V, Bialek SR, Montgomery SP. Congenital chagas disease in the United States: cost savings through maternal screening. Am J Trop Med Hyg. 2018;98: 1733–1742. 10.4269/ajtmh.17-0818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramsey JM, Townsend Peterson A, Carmona-Castro O, Moo-Llanes DA, Nakazawa Y, Butrick M, et al. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Mem Inst Oswaldo Cruz. 2015;110: 339–352. 10.1590/0074-02760140404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gourbière S, Dorn P, Tripet F, Dumonteil E. Genetics and evolution of triatomines: From phylogeny to vector control. Heredity (Edinb). 2012;108: 190–202. 10.1038/hdy.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waleckx E, Gourbière S, Dumonteil E. Intrusive versus domiciliated triatomines and the challenge of adapting vector control practices against chagas disease. Mem Inst Oswaldo Cruz. 2015;110: 324–338. 10.1590/0074-02760140409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernández-Becerril N, Mejía AM, Ballinas-Verdugo MA, Garza-Murillo V, Manilla-Toquero E, López R, et al. Blood transfusion and iatrogenic risks in Mexico city. Anti-Trypanosoma cruzi seroprevalence in 43,048 blood donors, evaluation of parasitemia, and electrocardiogram findings in seropositive. Mem Inst Oswaldo Cruz. 2005;100: 111–116. 10.1590/S0074-02762005000200002 [DOI] [PubMed] [Google Scholar]
- 69.Bracho Guzmán, Carmen García García Lourdes Floriani Verdugo, Jorge Guerrero Martínez Sandra Torres Cosme, Mario Ramírez Melgar, Carmen Velasco Castrejón O. Riesgo de transmisión de Trypanosoma cruzi por transfusión de sangre en México. Rev Panam Salud Publica. 1998;4: 94–99. 10.1590/S1020-49891998000800004. [DOI] [PubMed] [Google Scholar]
- 70.Willoquet JMR. Chagas disease transmission in Mexico: a case for translational research, while waiting to take disease burden seriously. Salud Publica Mex. 2007;49: e291. [Google Scholar]
- 71.Schmunis GA, Cruz JR. Safety of the blood supply in Latin America. Clin Microbiol Rev. 2005;18: 12–29. 10.1128/CMR.18.1.12-29.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All the data may be found directly in the Fig 1 of the article.