Abstract
Alcohol use disorders (AUDs) cause serious problems in society and few effective treatments are available. Caenorhabditis elegans (C. elegans) is an excellent invertebrate model to study the neurobiological basis of human behavior with a conserved, fully tractable genome, and a short generation time for fast generation of data at a fraction of the cost of other organisms. C. elegans demonstrate movement toward, and concentration-dependent self-exposure to various psychoactive drugs. The discovery of opioid receptors in C. elegans provided the impetus to test the hypothesis that C. elegans may be used as a medications screen to identify new AUD treatments. We tested the effects of naltrexone, an opioid antagonist and effective treatment for AUDs, on EtOH preference in C. elegans. Six-well agar test plates were prepared with EtOH placed in a target zone on one side and water in the opposite target zone of each well. Worms were treated with naltrexone before EtOH preference testing and then placed in the center of each well. Wild-type worms exhibited a concentration-dependent preference for 50, 70 and 95% EtOH. Naltrexone blocked acute EtOH preference, but had no effect on attraction to food or benzaldehyde in wild-type worms. Npr-17 opioid receptor knockout mutants did not display a preference for EtOH. In contrast, npr-17 opioid receptor rescue mutants exhibited significant EtOH preference behavior, which was attenuated by naltrexone. Chronic EtOH exposure induced treatment resistance and compulsive-like behavior. These data indicate that C. elegans can serve as a model system to identify compounds to treat AUDs.
Keywords: alcohol, behavior, preference, reinforcement, nematode
1. Introduction
Addiction represents a major and growing challenge in our society. It takes a massive toll in both direct and indirect human and financial costs. The resulting costs to society are approximately 740 billion dollars annually in the U.S. alone [1]. Clearly, there is an urgent need for effective treatments and prevention strategies developed from an understanding of the mechanisms underlying addictive behavior. Much of what we know about the neurobiology of addiction has been either discovered or enhanced through the use of animal models [2]. This includes the discovery and characterization of some of the basic reward circuitry and the development of behavioral measures to model and study human addiction in animals [3]. Development of pharmacotherapeutics is a promising avenue to reduce the impact of alcohol use disorders (AUDs); however, few effective treatments are currently available.
Recent work has shown that addiction is a phylogenetically ancient process and indicates that many mechanisms that underlie addiction are present in invertebrates. Behavioral models of addiction, using alcohol self-administration, conditioned place preference (CPP), and other tools historically used to study aspects of addictive behavior in vertebrates [4], have also been developed for invertebrates. Crayfish show drug reward, seeking, and withdrawal to cocaine, amphetamines and opiates [5]. Similarly, ethanol (EtOH) self-administration and conditioning paradigms have demonstrated that drosophila melanogaster show conditioned preference responses to cues that had been previously paired with EtOH [6]. Although some might find it surprising that such simple animals can be used to model complex behaviors, behavioral models using invertebrates have played a central role in the discovery of the molecular mechanisms that underlie learning and memory [7].
The nematode Caenorhabditis elegans (C. elegans) is an excellent model organism with conserved neurobiological systems that is often used to model various disease states [8]. It provides the researcher with numerous molecular and genetic tools, including a fully sequenced genome, the availability of thousands of genetic mutants, and the ability to manipulate other genes and their expression through transgenic approaches and RNAi techniques. In addition, a relatively short life cycle and a 3-day generation time from egg to adult can lead to a dramatic increase in the pace of discovery at a fraction of the cost inherent when using higher level organisms. We have discovered that, like mammals and other invertebrates, C. elegans also develops a conditioned preference for cues after previous pairings with methamphetamine or cocaine that is dependent on dopamine neurotransmission [9, 10]. Together, these data indicate that invertebrates, specifically C. elegans, show behavioral responses to addictive drugs that are consistent with those of higher level organisms.
Recent research has established that C. elegans display depressed locomotion and functional tolerance after exposure to EtOH that is mediated, in part, through the BK potassium channel which appears to subserve behavioral responses across multiple species including humans [11, 12]. Importantly, EtOH’s effects on locomotor activity of C. elegans occur when the internal tissue concentration of EtOH reaches levels that correspond to intoxicating blood alcohol levels in humans [13]. Moreover, chronic exposure to EtOH induces adaptive changes that can enhance EtOH preference and self-exposure [14]. These data indicate that C. elegans show a concentration-dependent attraction to EtOH that results in EtOH self-exposure and significant tissue concentrations of EtOH; furthermore, EtOH preference is enhanced after chronic exposure. Recently, researchers have discovered an opioid receptor system in C. elegans [15], therefore, we sought to examine the effect of naltrexone on EtOH preference in C. elegans.
EtOH self-administration and seeking behavior in non-human vertebrates are well established models to study alcohol use disorders (AUDs) [16]. In recent years, attention has turned to the application of these models for use in medications development and the identification of novel targets to treat AUDs [17, 18]. However, the application of these models is limited in the area of medication screening due to the need for relatively large amounts of sometimes expensive and rare test compounds that may only be available in very small quantities. In addition, the costs of these experiments in terms of technician effort and animal per-diem are also significant. Finally, the pace of discovery in these labor-intensive drug studies can be months or longer depending on the protocol. A validated model that employs C. elegans to screen compounds would be a major advancement in the field and provide a much-needed tool to conduct drug screens quickly and economically with the potential of dramatically increasing the pace of medication discovery.
Specifically, the purpose of the present studies was to employ a voluntary EtOH self-exposure chemotaxis assay to examine the effects of naltrexone and/or chronic EtOH exposure on the appetitive properties of EtOH in wild-type and opioid receptor mutant C. elegans. Overall, these studies represent a first step toward the development and validation of a pharmacological drug screening procedure to identify compounds that effectively and selectively reduce the EtOH preference response in C. elegans. This high-throughput C. elegans medications screening model can enable fast and accurate generation of data. The successful implementation of such models could provide powerful and novel tools in the search for new pharmacological treatments for AUDs.
2. MATERIALS AND METHODS
2.1. Materials
All reagents and assay materials were purchased from Sigma-Aldrich and Fisher Scientific, unless indicated otherwise. Fifty and 70 % (v/v) EtOH solutions were prepared with 95% (v/v) EtOH and water for EtOH preference testing. Vehicle (0.97 or 1.94 mM HCl; salt equivalent of 10 and 20 mM naltrexone HCl, respectively), and 10 and 20 mM naltrexone HCl (N-3136; FW 377.9; Sigma-Aldrich) were used to pretreat animals prior to testing. Vehicle (0.97 or 1.94 mM HCl) and naltrexone dosing solutions were adjusted to a pH of 7.2 to 7.4 with NaOH. Benzaldehyde (#418099; 99.5%; Sigma-Aldrich; FW 106.12) was used to test for nonselective effects of naltrexone HCl. 2-nonanone (99%; CAS 821-55-6; FW 142.24; Arcos Organics) was used to show that animals could move away from the drug target zone. All concentrations of drugs include the salt.
2.2. Culture and Maintenance of Strains
The N2 Bristol wild-type (WT) strain was used in all assays. The npr-17 KO mutants [DA2457 npr-17(tm3210) III] lack npr-17, which encodes a protein with sequence similarity to opioid receptors, while the npr-17 rescue mutants [DA2582 npr-17 (tm3210) III], in which the npr-17 gene was rescued (Cheong et al 2015), were used in the acute EtOH preference, benzaldehyde and food assays. The npr-17 KO and rescue mutant strains were obtained directly from Dr. Cheong [15].
All animals were maintained at 22°C, and all general culturing techniques have been described previously by Nass and Hamza [19]. Worms were grown with E. coli strain NA22 as a food source on maintenance plates, produced by filling 60-mm petri dishes with 10-ml regular NGM agar (25g bactoagar, 20g bactopeptone, 3g NaCI, 1L H20, 1ml cholesterol (5mg/ml 95% ethanol), 1ml 1M CaCI2, 1ml 1M MgSO4, and 25ml of potassium phosphate buffer). The potassium phosphate buffer contained 5g of K2HPO4 dibasic/anhydrous, 30g of KH2PO4 monobasic, and 500ml of H20, pH adjusted to 6.0 [20].
Adult worms were used for all experiments to control for any effects of different sensitivities and responses to drugs at varying developmental stages. Worms were age synchronized by lysing gravid adults with bleach and sodium hydroxide, allowing eggs to be released into solution and hatched in M9 buffer [20]. After 18 hours, hatched L1 larvae were washed three times with water, plated, and maintained on NGM plates with NA22 E. coli bacterial lawns until reaching adulthood. Testing began approximately 72 hr after plating the L1 larvae, when worms were adults.
6-well Costar™ cell culture plates were used to determine drug preference (Fisher cat. no. 07-200-80). Clear templates were taped to the bottom of each 6-well plate to create two 1.2 cm diameter circular target zones within the 3.5 cm diameter of each well. Test plates were produced by filling each well of the plates with 3.8ml of NaCI free agar(17g bactoagar, 2.5g bactopeptone, 1L H20, 1ml 1M CaCI2, 1ml 1M MgSO4, and 25ml of potassium phosphate buffer). Cholesterol was not included in the salt-free agar in order to obtain clearer images of worms during testing. Chemotaxis assays are typically performed in single well 60 mm or 100 mm plates. However, in the present studies we sought to create higher throughput for testing by scaling down the standard experimental parameters used in 60 and 100 mm plates, in order to perform chemotaxis assays in 6 well plates containing 35 mm wells.
2.3. Naltrexone Pretreatment
Worms were washed off maintenance plates with 15 ml of water and transferred to 15 ml centrifuge tubes. Adults were allowed to settle on the bottom of each tube for 5 min and then the supernatant was removed. This was repeated two more times with 10 ml of water to remove the majority of bacteria from the worms. After the final removal of the supernatant, approximately 0.3 to 0.5 ml of worms were transferred to a 5 ml eppendorf tube and 3 ml of vehicle (0.97 or 1.94 mM HCI) or naltrexone HCI (10 or 20 mM; dose selected from Cheong et al., 2015 [15]) was added to each tube. These tubes were placed on a nutator for 30 min prior to EtOH preference testing. Following vehicle or drug treatment, tubes were taken off nutator and worms were allowed to settle to the bottom of each tube for approximately 3 min. The supernatant was removed to a point were worms were diluted to a ratio of approximately 1 part worms to 2 parts vehicle or drug solution. Then, 4 μl aliquots, containing approximately 40 to 80 worms, were pipetted into the center of each well of a 6-well testing plate and excess liquid was removed from the worms using a Kimwipe. Pictures of each well were taken 30 min after placing worms on test plates (see Fig. 1 for schematic).
Fig. 1. Drug Preference Testing Paradigm.

Worms were exposed to either vehicle (0.97 and 1.94 mM HCl) or naltrexone HCl (10 and 20 mM) in a centrifuge tube for 30 min prior to the ethanol (EtOH) preference testing. Animals were then pipetted into the center of each well of a 6-well agar test plate containing two target zones; one spotted with EtOH and the other with vehicle. Images were taken 30 min after plating worms to determine an EtOH preference index for each well.
2.4. EtOH Preference Testing Procedure
In general, 4 μl of vehicle (water) and an EtOH solution were applied to the center of the 1.2 cm target zones of each well. These spotting solutions were allowed to absorb into agar for 30 min prior to testing. EtOH preference was tested on plates spotted with 0, 50, 70, or 95% (v/v) EtOH concentrations in one target zone and vehicle in the other. Vehicle (water) and drug solutions were prepared fresh, prior to each day of testing. The use of the term “concentration dependent” is an operational definition defined by this procedure, in which different concentrations of EtOH were spotted in 4ul volumes to create EtOH target zones. When spotting the center of a target zone, these small volumes of EtOH are absorbed into the agar, diluting the EtOH and creating a concentration gradient from the center to the edge of the target zone. Thus, it is worth noting that worms were exposed to much lower concentrations of EtOH than what was used to spot the agar.
2.5. Food Preference
1 μl of water (control) or food (NA22 bacterial solution) was spotted to the two target zones of each well 30 min before testing. Images were taken at 30 min. In order to determine if naltrexone pretreatment produced nonselective effects on EtOH preference behavior, concentrations and volumes of food and benzaldehyde were selected to produce similar preference responses to those observed with EtOH.
2.6. Benzaldehyde Preference
2 μl of a 1 %(v/v) benzaldehyde solution dissolved in 25%(v/v) EtOH was spotted in one target zone, while 25% EtOH was spotted in the opposite target zone, 30 min before testing. Images were taken at 30 min. Typically, 95% EtOH is typically the diluent used for benzaldehyde. Since 95% EtOH was a preferred concentration of EtOH in our studies, we dissolved 1% benzaldehyde in 25% ethanol, since preliminary studies determined that 25% EtOH by itself was not associated with EtOH preference in our assay (data not shown).
2.7. Nonanone Aversion
In order to determine if animals were capable of moving away from the EtOH target zones and were not rendered ataxic by EtOH, 2 μl of 10%(v/v) nonanone (an aversive compound) was spotted to the outer edge of the EtOH target zone of each well (i.e., between the edge of the EtOH target zone and the outer edge of the well) immediately after taking 30 min images for EtOH preference experiments. Therefore, images were taken immediately before and 10 min after placing nonanone into each well. Pre- and post-nonanone Pls (as described below under EtOH preference testing) were calculated for each well in order to quantitate the change in preference from the EtOH target zone in response to nonanone. It should be noted that if animals display significant EtOH preference, then nonanone can be effective in reducing EtOH preference (i.e., moving them away from the EtOH target zone). In contrast, if animals do not display significant EtOH preference, then one may not be able to observe significant movement away from the EtOH target zone, since there is no significant EtOH preference to reduce (i.e., a floor effect). We have observed this repeatedly throughout our testing (data not shown).
2.8. Body Bend Assay
The body bend assay used here was adapted from Hart, 2006 [21]. After 30 min pretreatment with vehicle (0.97 mM HCI) or naltrexone HCI (10 and 20 mM) (as described above in the naltrexone pretreatment section), 2 μl of worms diluted in a ratio of 1 part worms to 2 parts vehicle or drug were placed on a microscope slide on the stage of a microscope (Bausch & Lomb ASZ45L3 45×). After selecting a single worm to track, the number of times the worm’s pharynx crossed this midline and extended to about a 45-90 degree arc over a 20-sec period was recorded. Only instances where the midline was completely crossed were counted. Investigators were blind to the treatment group associated with a particular worm, in order to avoid biasing body bend data.
2.9. Chronic EtOH Exposure
To create 300mM EtOH agar plates for chronic exposure, maintenance plates with bacteria were incubated at 37° C with lids off for 1.5 hrs. After incubation, the weight of each plate was recorded and an appropriate volume of 95%(v/v) EtOH, based on the weight of the agar in the plate, was pipetted around the edges of the bacterial lawn. For example, if the volume of agar in a given plate was 10.0 ml, then 184.32 μl of 95% EtOH was added to the surface of this agar plate to create a final EtOH concentration of 300mM. The EtOH was allowed to absorb into the agar for at least 1 hr, then plates were sealed with parafilm and stored at 4° C until ready to use. Control plates were created using the same procedure, except that water was pipetted around the edges of the bacterial lawn instead of EtOH. Synchronized N2 wild-type worms in the L4 larval stage were transferred from EtOH-free maintenance plates to either 300mM EtOH or control plates the day before testing and were allowed to develop to adults on the plates prior to experimental testing. This procedure was adapted from Davies et al., 2015 [22].
2.10. Imaging and Worm Counting
Worms were imaged by taking pictures with an Olympus 770sw digital camera positioned on top of a light box, which emitted light indirectly and underneath each 6-well plate. Images were analyzed using ImageJ software to count the number of worms in the target zones of the test plates. Using ImageJ, the target zone was cropped from each photo and the color threshold of the image was adjusted. Specifically, threshold color was set to red, color space was set to RGB, and color threshold was adjusted so worms were highlighted in red. Particles were analyzed with a pixel size of 80 to infinity. The number of worms counted in each target zone was recorded and analyzed in Microsoft Excel.
A chemotaxic preference index (PI) for each EtOH concentration was then calculated by dividing the number of worms in the EtOH target zone by the total sum of worms counted in both the EtOH and vehicle zones converted to a percentage. Food and benzaldehyde preference were also determined using this same calculation.
2.11. Determination of Internal EtOH Concentrations
N2 gravid adult worms were used. As indicated in the Naltrexone Pretreatment and EtOH Preference Testing Procedure sections of the Methods, 4ul aliquots of worms were placed in the center of each well of a 6 well plate, previously spotted with 4ul of water in one target zone and 70% EtOH in the opposing target zone of each well. Worms were allowed to move in each well for 30 min. 6 well plates were placed on top of ice to immobilize worms to pick them from target zones. Individual worms were picked out of the water or 70% EtOH target zones using a platinum wire and placed in 0.5 ml eppendorf tubes containing 40 μl of H20. The number of worms picked form the target zones was recorded for each sample and ranged from 74 to 120 worms per tube. Tubes were stored at −80°C until analysis. Samples were thawed on ice and ground in the tube with a sonicator for 5 to 10 sec (Branson Sonifier 350 Cell Disruptor, Danbury, CT, USA). Sonicated worm homogenates were stored on ice prior to analysis.
2.12. Gas Chromatography EtOH Concentration Analysis
Internal worm EtOH concentrations were measured by gas chromatography (GC) with flame-ionization detection (FID) using a Shimadzu GC-2010 Plus gas chromatograph using a Phenomenex ZB-BAC2 column (30 m × 0.32 mm) and a HS-20 head space autosampler. Thirty μl samples of worm homogenates were added to a 10 ml glass GC head-space sampling vial containing 100 μl of saturated NaCI and sealed with a crimp-cap septum seal. Samples were heated to gaseous state and injected on to the gas chromatography system and detected with FID, as previously conducted [23]. The output was analyzed using LabSolutions chromatography analysis software and EtOH levels in samples were determined by comparison with a standard curve.
2.13. Calculation of Internal EtOH Concentration
The entire body volume of an N2 adult C. elegans was determined to be 5.5 nl/worm and since 80% of this volume has been determined to be water, the average internal body volume of an N2 adult C. elegans was estimated to be 4.4 nl/worm [24]. Therefore, we used the following calculations to determine the concentration of EtOH in a single worm from a sample containing 100 adult N2 worms in 40 μl of H20, for example: (1) nl/worm × 100 worms = 440 nl of worms (internal volume), and (2) 440 nl of worms (internal volume)/40,000 nl sample volume = 90.9(X) dilution for this sample.
2.14. Statistical analyses
Data were analyzed using one, two, or three-way ANOVAs, followed by decomposition of factors and Bonferroni post hoc tests, as appropriate and previously conducted [9]. The statistical software used to perform these analyses was IBM® SPSS® Statics (version 25) for Mac.
3. RESULTS
3.1. Acute EtOH Preference in N2 strain
A two-way ANOVA found a main effect of pretreatment (vehicle vs. naltrexone) [F(2, 412) = 20.3, p < 0.001, ηp2 = .092], a main effect of EtOH concentration (0, 50, 70 and 95% EtOH) [F(3, 412) = 5.0, p < 0.003, ηp2 = .036], and a significant interaction between pretreatment and EtOH concentration [F(6, 412) = 3.4, p < 0.004, ηp2 = .048] on EtOH preference (Fig. 2).
Figure 2. Pretreatment with 10 and 20 mM naltrexone decreased EtOH preference in N2 C. elegans.

Vehicle pretreated worms displayed significant (p < 0.05) preference indices for 50, 70 and 95% EtOH compared to water, as indicated by *. 10 mM naltrexone significantly (p < 0.05) decreased 70% and 95% EtOH preference compared to vehicle pretreatment, as indicated by +. Similarly, 20 mM naltrexone significantly (p < 0.05) decreased 70% and 95% EtOH preference compared to vehicle pretreatment, as indicated by #. The number of wells analyzed for the vehicle treated groups was 62 (0%), 63 (50%), 66 (70%), and 64 (95%), for the 10 mM naltrexone treated group was 15 (0%), 18 (50%), 18 (70%), and 17 (95%), and for the 20 mM naltrexone treated group was 18 (0%), 24 (50%), 24 (70%), and 24 (95%).
For vehicle pretreated worms, a one-way ANOVA revealed a significant effect of EtOH concentration on EtOH preference [F(3, 254) = 11.9; p < 0.001], and Bonferroni post hoc tests revealed significant (p < 0.007) preference indices for 50, 70 and 95% EtOH compared to 0% EtOH. For 10 mM naltrexone pretreated worms, a one-way ANOVA found no effect of EtOH concentration on EtOH preference [F(3,68) = 1.3; ns]. For 20 mM naltrexone pretreated worms, a one-way ANOVA found a main effect of EtOH concentration on EtOH preference [F(3,90) = 3.4; p < 0.03]. For the 20 mM naltrexone treated group, Bonferroni post hoc tests found a significant (p < 0.02) increase in EtOH preference for 50% EtOH compared to 0%.
One-way ANOVAs examining differences between vehicle and naltrexone pretreatment groups for each EtOH concentration found no main effect of pretreatment on 0% EtOH preference [F(2,94) = 2.4, ns] or 50% EtOH [F(2,104) = 0.8, ns]. However, naltrexone pretreatment significantly decreased 70% EtOH preference compared to vehicle [F(2,107) = 24.2, p < 0.001] and Bonferroni post hoc tests found that 10 and 20 mM naltrexone significantly (p < 0.001) decreased 70% EtOH preference compared to vehicle treatment. Naltrexone treatment significantly decreased 95% EtOH preference compared to vehicle [F(2,104) = 7.2, p < 0.002] and Bonferroni post hoc tests revealed that the 10 and 20 mM naltrexone significantly (p < 0.05) decreased 95% EtOH preference compared to vehicle treatment.
The number of wells analyzed for the vehicle treated groups was 62 (0%), 63 (50%), 66 (70%), and 64 (95%), for the 10 mM naltrexone treated group was 15 (0%), 18 (50%), 18 (70%), and 17 (95%), and for the 20 mM naltrexone treated group was 18 (0%), 24 (50%), 24 (70%), and 24 (95%).
3.2. Acute EtOH Preference after Nonanone in the N2 Strain
A three-way repeated measures ANOVA on pre- vs post-nonanone EtOH preference found a main effect of nonanone on EtOH preference [F(1, 36) = 71.7; p < 0.001, ηp2 = .666], with no significant effects of naltrexone treatment [F(1, 36) = 2.5; ns, ηp2 = .064] or EtOH concentration [F(1, 36) = 3.4; ns, ηp2 = .087]. In addition, no significant interactions were found on pre- vs post-nonanone EtOH preference. Bonferroni post hoc tests found a significant (p < 0.001) decrease in EtOH preference (collapsed across treatment and EtOH concentration) after nonanone presentation [pre- (61.9 ± 3.3 %; n=40) vs post-nonanone (27.8 ± 4.5 %; n = 40) EtOH preference; data not shown].
3.3. N2 Internal EtOH Concentrations
The mean (± SEM) EtOH concentrations for worms picked from the 70% EtOH target zones were 115.5 (± 15.0) mg% (n = 6 samples), and from the water target zones were 2.5 (± 0.9) mg% (n = 4 samples).
3.4. Control Experiments in the N2 Strain
For vehicle, 10 and 20 mM naltrexone pretreatment, food preference was 79.6 ± 4.7 (n = 24), 78.7 ± 2.9 (n = 18), and 71.8 ± 3.2 % (n = 21), respectively (Fig. 3A). A one-way ANOVA found no main effect of naltrexone on food preference [F(2,62) = 1.2; ns, ηp2 = .039].
Figure 3. Pretreatment with 10 or 20 mM naltrexone does not alter food (NA22) or benzaldehyde preference in N2 C. elegans.
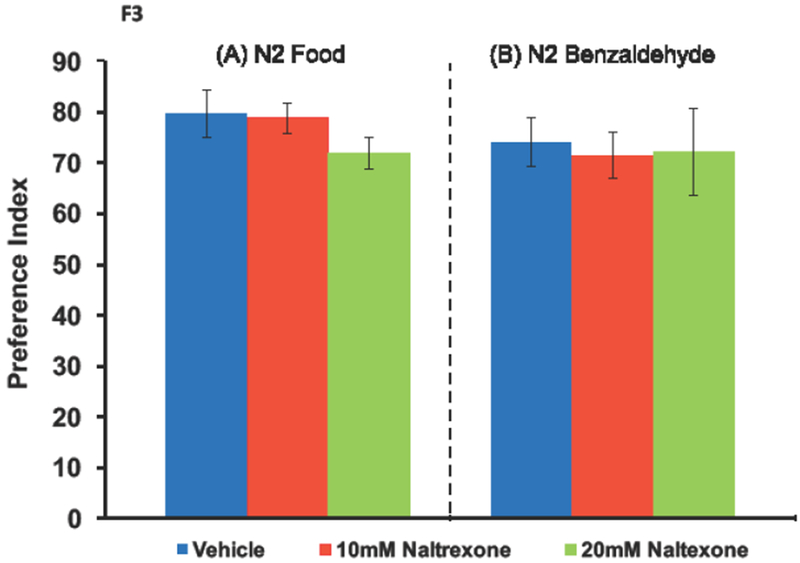
Worms were pretreated with vehicle, 10, or 20 mM naltrexone for 30 min and subsequently tested for food or benzaldehyde preference. Panel A: 1 μl of water or food was spotted to the two target zones of each well. Naltrexone had no significant effect on food preference compared to vehicle pretreatment. The number of wells for the vehicle, 10 and 20 mM naltrexone groups was 24, 18 and 21, respectively. Panel B: 2 μl of a 1% (v/v) benzaldehyde solution dissolved in 25% (v/v) EtOH was spotted in one target zone, while 25% EtOH was spotted in the opposite target zone. No statistically significant differences were found between treatment groups. The number of wells analyzed for the vehicle, 10 and 20 mM naltrexone conditions were 35, 24 and 12, respectively.
A one-way ANOVA found that naltrexone pretreatment (10 and 20 mM) had no significant effect on 1% (v/v) benzaldehyde preference [F(2,70) = 0.7; ns, ηp2 = .002]. Mean benzaldehyde PI’s were 74.0 ± 4.9 % (n = 35), 71.4 ± 4.5 % (n = 24), and 72.1 ± 8.6 % (n = 12), for the 0 (vehicle), 10 and 20mM naltrexone treatments, respectively (Fig. 3B).
Exposure to 10 and 20 mM naltrexone for 30 min prior to testing had no significant effect on locomotor activity compared to vehicle exposure [F(2,46) = 0.71; ns, ηp2 = .031]. Specifically, the number of body bends in 20 sec for vehicle, 10 and 20 mM naltrexone treatments were 52 ± 3 (n = 19), 49 ± 3 (n = 16), and 46 ± 4 (n = 12), respectively (data not shown).
3.5. Acute EtOH Preference in npr-17 KO and npr-17 Rescue Strains
A one-way ANOVA did not reveal a significant effect of EtOH concentration [F(3, 178) = 0.5, ns, ηp2 = .009], on EtOH preference in npr-17 KO mutants (Fig. 4). The number of wells analyzed were 47 (0%), 36 (50%), 48 (70%), and 48 (95%).
Figure 4. EtOH preference in npr-17 KO mutant C. elegans.

A one-way ANOVA did not reveal a main effect of EtOH concentration on EtOH preference [F(3, 178) = 0.5; ns] npr-17 KO mutants. The number of wells analyzed for npr-17 KO mutants were 47 (0%), 36 (50%), 48 (70%), and 48 (95%).
A two-way ANOVA found a main effect of pretreatment (vehicle vs. 10 mM naltrexone) [F(1,283) = 16.4, p < 0.001, ηp2 = .056], a main effect of EtOH concentration (water, 50, 70 and 95% EtOH) [F(3,283) = 7.0, p < 0.001, ηp2 = .070], and no interaction between pretreatment and EtOH concentration [F(3, 283) = 0.6, ns, ηp2 = .006] on EtOH preference in npr-17 rescue mutants (Fig. 5). The number of wells analyzed for the vehicle treated rescue mutant groups was 36 (0%), 36 (50%), 36 (70%), and 36 (95%), and for the 10 mM naltrexone treated group was 32 (0%), 36 (50%), 36 (70%), and 36 (95%).
Figure 5. EtOH preference in npr-17 Rescue mutant C. elegans.

A two-way ANOVA found a main effect of pretreatment (vehicle vs. 10 mM naltrexone) [F(1,283) = 16.4, p < 0.001], a main effect of EtOH concentration (water, 50, 70 and 95% EtOH) [F(3,283) = 7.0, p < 0.001], and no interaction between pretreatment and EtOH concentration [F(3, 283) = 0.6, ns] on EtOH preference in npr-17 rescue mutants (Fig. 5). * indicates a significant effect of EtOH concentration, while + indicates that naltrexone significantly decreased EtOH preference compared to the vehicle condition. The number of wells analyzed for the vehicle treated rescue mutant groups was 36 (0%), 36 (50%), 36 (70%), and 36 (95%), and for the 10 mM naltrexone treated group was 32 (0%), 36 (50%), 36 (70%), and 36 (95%).
A three-way repeated measures ANOVA on pre- vs post-nonanone EtOH preference in npr-17 rescue mutants found a main effect of nonanone [F(1,210) = 7.9; p < 0.007, ηp2 = .036], with no significant effects of 10 mM naltrexone treatment [F(1,210) = 3.1; ns, ηp2 = .014] or EtOH concentration [F(2, 210) = 1.2; ns, ηp2 = .012], In addition, a significant interaction was found between pre- vs post-nonanone EtOH preference and treatment F(1, 210) = 19.7; p < 0.001, ηp2 = .002]. Therefore, the data were collapsed across EtOH concentration and one-way ANOVAs were performed on pre- vs post-nonanone EtOH preference; these analyses revealed that vehicle-treated rescue mutants displayed significant decreases in EtOH preference after nonanone presentation [F(1, 107) = 22.0; p < 0.001], while naltrexone-treated rescue mutants did not exhibit significant changes in EtOH preference after nonanone presentation [F(1, 107) = 1.7; ns]. Furthermore, pre-nonanone EtOH preference was significantly greater for vehicle- versus naltrexone-treated rescue mutants [F(1,215) = 18.9; p < 0.001], and post-nonanone EtOH preference was not significantly different between vehicle and naltrexone treated rescue mutants [F(1,215) = 2.0; ns]. Fig. 6 shows pre- vs post-nonanone EtOFI preference data collapsed across EtOH concentrations (50, 70 and 95%) for vehicle- and naltrexone-treated npr-17 rescue mutants.
Figure 6. Pre- vs post-nonanone EtOH preference data collapsed across EtOH concentrations (50, 70 and 95%) for vehicle- and naltrexone-treated npr-17 rescue mutants.
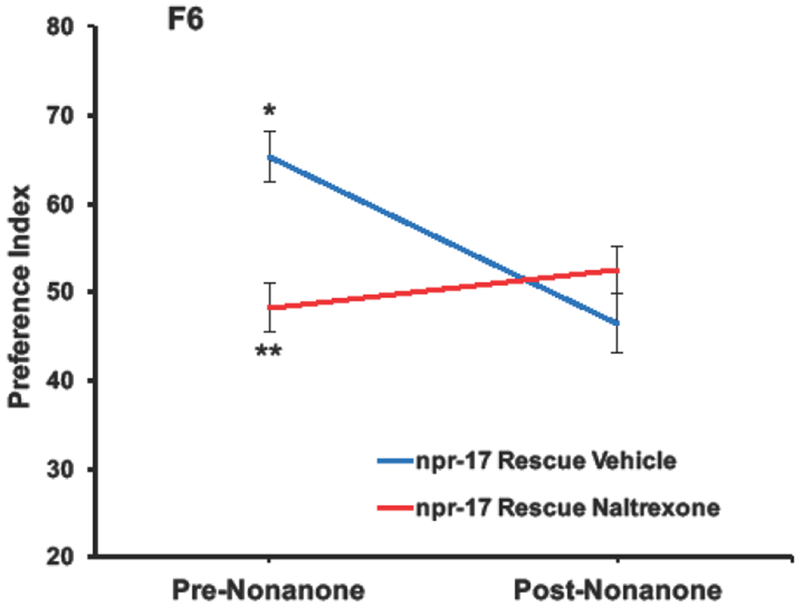
A three-way repeated measures ANOVA on pre- vs post-nonanone EtOH preference in npr-17 rescue mutants found a main effect of nonanone [F(1,210) = 7.9; p < 0.007], with no significant effects of 10 mM naltrexone treatment [F(1,210) = 3.1; ns] or EtOH concentration [F(2, 210) = 1.2; ns]. A significant interaction was also found between pre- vs post-nonanone EtOH preference and treatment F(1,210) = 19.7; p < 0.001]. Therefore, the data was collapsed across EtOH concentration and a one-way ANOVAs was performed on pre- vs post-nonanone EtOH preference. Vehicle-treated rescue mutants displayed significant decreases in EtOH preference after nonanone presentation [F(1, 107) = 22.0; p < 0.001] as indicated by *, while naltrexone-treated rescue mutants did not exhibit significant changes in EtOH preference after nonanone presentation [F(1, 107) = 1.7; ns]. Furthermore, pre-nonanone EtOH preference was significantly greater for vehicle- versus naltrexone-treated rescue mutants [F(1,215) = 18.9; p < 0.001] as indicated by **, and post-nonanone EtOH preference was not significantly different between vehicle and naltrexone treated rescue mutants [F(1,215) = 2.0; ns].
3.6. Food and Benzaldehyde Preference in N2, npr-17 KO and npr-17 Rescue Strains
Comparing NA22 food preference in vehicle treated N2, KO and rescue strains, a one-way ANOVA found a main effect of strain on food preference [F(2,71) = 4.4; p < 0.02, ηp2 = .113]. Bonferroni post hoc tests found that N2 strain had significantly (p < 0.02) lower food preference than npr-17 rescue mutants. Mean (± SEM) food preference indices were 79.6 ± 4.7 (n = 24), 87.3 ± 2.6 (n = 24), 93.7 ± 2.1% (n = 24) for N2, npr-17 KO and rescue strains, respectively (Table 1).
Table 1.
Mean (± SEM) Food and Benzaldehyde Preference in N2, npr-17 KO and Rescue Mutants
| Strain | Food | Benzaldehyde |
|---|---|---|
| N2 (wild-type) | *79.6 ± 4.7 % (n = 24) | 74.0 ± 4.8 % (n = 35) |
| npr-17 KO | 87.3 ± 2.6 % (n = 24) | 80.0 ± 3.4 % (n = 20) |
| npr-17 Rescue | 93.7 ± 2.1 % (n = 24) | 82.7 ± 2.6 % (n = 24) |
Post hoc tests found that N2 food preference was significant higher in npr-17 rescue mutants compared to N2 worms. No other significant differences were observed in N2, npr-17 KO, and npr-17 rescue mutants, in terms of food or benzaldehyde preference.
Comparing 1% benzaldehyde preference in vehicle treated N2, KO and rescue strains, a one-way ANOVA found no effect of strain on benzaldehyde preference [F(2,78) = 1.2; ns, ηp2 = .032], Mean (± SEM) benzaldehyde preference indices were 74.0 ± 4.8 (n = 35), 80.0 ± 3.4 (n = 20), 82.7 ± 2.6% (n = 24) for N2, npr-17 KO and rescue strains, respectively (Table 1).
3.6. Chronic EtOH Exposure and EtOH Preference in the N2 Strain
In chronic EtOH exposed wild-type worms, a two-way ANOVA found a main effect of EtOH concentration on EtOH preference [F(2,407 = 11.9; p < 0.001, ηp2 = .056], However, naltrexone treatment had no effect on EtOH preference [F(2,407 = 1.8; ns, ηp2 = .009], No significant interaction was observed between naltrexone treatment and EtOH concentration [F(4,407) = 11.9; ns, ηp2 = .014]. Bonferroni post hoc tests found that significant (p < 0.001) increases in EtOH preference were observed for 50% and 70% EtOH compared to 0% EtOH (Fig. 7).
Figure 7. Effects of Chronic EtOH exposure and Naltrexone on EtOH preference in N2 C. elegans.

In chronic EtOH exposed wild-type worms, a two-way ANOVA found a main effect of EtOH concentration on EtOH preference [F(2,407 = 11.9; p < 0.001) ]. However, 10 and 20 mM naltrexone treatments had no effect on EtOH preference [F(2,407 = 1.8; ns)]. Bonferroni post hoc tests found that significant (p < 0.001) increases in EtOH preference were observed for 50% and 70% EtOH compared to 0% EtOH as indicated by *. The number of wells analyzed for the vehicle treated groups was 45 (0%), 46 (50%), and 48 (70%), for the 10 mM naltrexone treated group was 46 (0%), 43 (50%), and 44 (70%), and for the 20 mM naltrexone treated group was 48 (0%), 46 (50%), and 42 (70%).
It should be noted that the data from Figs. 7 and 8 were derived from the same animals. Specifically, after animals were treated with vehicle or naltrexone to determine EtOH preference (Fig. 7), nonanone was applied to these same animals to determine their response to nonanone (Fig. 8). Thus, animals were only treated with naltrexone on one occasion. A three-way repeated measures ANOVA in EtOH exposed worms found a significant main effect of nonanone [F(1,252) = 18.8; p < 0.001, ηp2 = .069] and naltrexone treatment on pre- versus post-nonanone EtOH preference [F(2,252) = 5.0; p < 0.008, ηp2 = .038]. No significant interactions were observed in the three-way ANOVA. Bonferroni post hoc tests found that pre- versus post-nonanone EtOH preference, collapsed across EtOH concentrations (50 and 70%), was significantly (p < 0.004) reduced by 20 mM naltrexone compared to vehicle treatment (Fig. 8).
Figure 8. Effects of Chronic EtOH exposure and Naltrexone on response to nonanone in N2 C. elegans.

A three-way repeated measures ANOVA in EtOH exposed worms found a significant main effect of nonanone [F(1,252 = 18.8; p < 0.001)] and naltrexone treatment on pre- versus post-nonanone EtOH preference [F(2,252 = 5.0; p < 0.008)]. Since there was no significant effect of EtOH concentration, the data was collapsed across the 50 and 70% EtOH concentrations. Bonferroni post hoc tests found that pre- versus post-nonanone EtOH preference was significantly (p < 0.004) reduced by 20 mM naltrexone compared to vehicle treatment, as indicated by *.
The number of wells analyzed for the vehicle treated groups was 45 (0%), 46 (50%), and 48 (70%), for the 10 mM naltrexone treated group was 46 (0%), 43 (50%), and 44 (70%), and for the 20 mM naltrexone treated group was 48 (0%), 46 (50%), and 42 (70%).
4. Discussion
We have shown that C. elegans demonstrate movement toward, and concentration-dependent self-exposure to EtOH, which has been characterized as a “preference response” [14]. The recent discovery of opioid receptors in C. elegans provided the impetus to test the hypothesis that C. elegans can be used as a medications screen to identify new treatments for AUDs. We tested the effects of naltrexone, an opioid antagonist and effective treatment for AUDs and other addictions, on EtOH preference in C. elegans. Naltrexone treatment blocked acute EtOH preference, but had no effect on motor activity or attraction to food or benzaldehyde, a volatile attractant. Opioid receptor knockout (KO) mutants did not display a significant preference response for EtOH. However, opioid receptor rescue mutants displayed EtOH preference behavior, which was attenuated by naltrexone, similar to findings observed in wild-type C. elegans. Chronic EtOH exposure in wild-type worms EtOH preference induced treatment resistance and aversion resistant behavior, as evidenced by the attenuated response to naltrexone and sustained self-exposure to EtOH in the presence of an aversive stimulus, nonanone. Together these data indicate that C. elegans have the potential to serve as a model system to identify compounds to treat AUDs and other addictive disorders. However, additional studies are needed to fully characterize the model and confirm that the phenomena observed thus far are consistent with efficacy of treatments for AUDs (i.e., verify predictive validity). Thus, future experiments will test compounds, previously shown to reduce EtOH drinking and/or seeking in vertebrate models, in the C. elegans EtOH preference test in acute and chronic models, to characterize the selectivity of the response.
4.1. The EtOH Preference Assay
A well-characterized and popular method to assess how C. elegans respond behaviorally to a chemical or substance is the simple chemotaxis assay [25, 26] that, in fact, is a type of voluntary self-exposure paradigm. Well described in previous studiesb are findings of significant tissue EtOH concentrations after C. elegans are placed on agar plates that contain EtOH. The EtOH from the agar enters and accumulates in C. elegans to produce replicable behavioral responses, including locomotor inhibition [27] at internal tissue levels that produce similar responses in humans [13], and results in tolerance with extended periods of exposure [28]. The ingestion of substances by C. elegans in the absence of food is described as “drinking” behavior [29]. Such comprehensive studies under set conditions have led to the identification of genes that mediate the locomotor depressant effects of EtOH and tolerance; many of these genes are orthologs associated with AUDs in humans [27] (see [30] for review).
The current findings build on these studies with some essential new methods to evaluate EtOH behavioral responses dependent on choice behavior as opposed to conditions of forced EtOH exposure. C. elegans show responses to various drugs of abuse, including stimulants and EtOH [9, 14, 31–33]. C. elegans have long been known to show chemotaxis toward EtOH [14]. Utilizing 6-well plates with 35mm testing areas (Fig. 1), we have shown clear, replicable concentration-dependent behavioral preference (PI significantly > 50% and significantly greater than control) responses for EtOH (Fig 2, vehicle). Importantly, we have shown that animals entering the target zone containing 70% EtOH quickly attain internal tissue EtOH concentrations of 25 mM (i.e., 115 mg%). These levels are in agreement with previous work [13, 27], indicating that this is an EtOH self-exposure paradigm resulting in physiological internal EtOH concentrations, adding face validity to the model. Of note, the exogenous concentrations of all drugs used in the proposed studies are consistent with previous C. elegans work, and reflect the well-described barrier to entry associated with the cuticle [15, 34, 35]. Our data indicates that in the acute model (i.e., EtOH naive), addition of the aversive compound nonanone, near the target zone after the preference response has been established, causes the animals to immediately move away from the EtOH target zone, inducing a measurable aversive response. This indicates that EtOH is not simply functioning as a locomotor anesthetic or paralytic agent. From these data, we developed the hypothesis that C. elegans can be a viable model system to screen potential candidate drugs to treat AUDs.
4.2. Findings with naltrexone
Recently, C. elegans were found to have functional opioid-like receptors [15]. Thus, to determine the predictive validity of the model, we tested the effectiveness of naltrexone, one of the very few compounds shown consistently to reduce EtOH drinking behavior in animal models as well as humans [36]. In addition, naltrexone has been demonstrated to reduce opioid intake in animal models and humans [37], and has recently been shown to decrease cannabis self-administration and subjective effects in chronic cannabis users [38].
In C. elegans, we found that exposure to naltrexone had no effect on locomotor activity (i.e., body bend assay), but significantly reduced the EtOH preference response (Fig. 2). This effect of naltrexone was not observed if food or benzaldehyde (Fig. 3) was used as the attractant in the place of EtOH. These data are consistent with rodent data showing that naltrexone can inhibit EtOH intake at doses that do not affect sucrose intake or body weight [39]. In most instances, little or no prior work has been published to determine if other treatments have effects on drug preference responses in C. elegans. However, varenicline pre-exposure has been shown to reduce chemotaxis to nicotine in C. elegans [31, 33].
4.3. Findings in Mutants
The opioid receptor knockout mutant C. elegans, npr-17 KO, did not exhibit a preference for EtOH (Fig. 4). However, the opioid receptor rescue mutant C. elegans (npr-17 rescue) did display EtOH preference behavior, which was significantly reduced by naltrexone pretreatment (Fig. 5). These findings in rescue mutants were similar to those observed in wild-type C. elegans (Fig. 2). In agreement with the present findings, mu-opioid receptor knockout mice showed no evidence of EtOH self-administration in operant EtOH self-administration and two bottle-choice EtOH drinking paradigms [40]. Additional studies found decreased EtOH drinking in mu-opioid receptor knockout mice compared to wild-type mice [41–43]. In agreement with the aforementioned preclinical findings in C. elegans and mice, human studies support a role for polymorphisms in genes coding for MOR1, DOR1, KOR1, and other opioid receptors in clinical populations of alcoholics [44]. Overall, these findings suggest that opioid receptors play a critical role in behavior associated with EtOH self-exposure and self-administration, which is conserved across several different species, including worms, mice and humans.
4.4. Chronic Exposure Model
Chronic 300 mM EtOH exposure from the L4 larval stage to adulthood in N2 worms: (1) resulted in similar EtOH preference behavior to that observed in untreated N2 worms (Fig. 7), (2) imparted resistance to the effect of naltrexone to reduce preference (Fig. 7), which is in agreement with the effects of chronic EtOH exposure in rats [45]; and (3) reduced the effect of the aversive stimulus nonanone to move them away from EtOH (Fig. 8). This type of aversion-resistant EtOH self-exposure is consistent with models of compulsive EtOH self-administration in rats [46]. Interestingly, naltrexone treatment enabled the normal aversive response to nonanone from both the 50 and 70% EtOH target zones (Fig. 8).
With regard to acute versus chronic EtOH exposure models, some of the most promising therapeutics compounds for the treatment of AUDs do not modify acute EtOH reward and/or reinforcement, but instead target neuroadaptations produced after chronic EtOH exposure that regulate maladaptive behavior in mammalian models [47, 48], and similar distinctions may be observed in C. elegans. It is, therefore, important to examine effects after chronic EtOH exposure, previously shown to enhance EtOH preference in C. elegans [14]. The EtOH exposure model utilized here can differentiate the effects of potential treatments on acute vs. chronic effects of EtOH and is able to identify compounds that may only show significant effects in models of chronic exposure. Additionally, the attenuated response of naltrexone to reduce EtOH preference in chronic vs. acute exposure for some inhibitors might model treatment resistance in EtOH-adapted subjects and/or have differential effects on compulsive self-exposure as observed with naltrexone in the current findings, consistent with reports in rats [45].
C. elegans has a simple nervous system that lacks the complex neurocircuitry of mammals involved in addiction [3]. However, C. elegans phenotypes are highly conserved functionally and clear parallels are observed in neurobiology, pharmacology, and molecular systems compared to vertebrates. The similarities in responses to substances of abuse between C. elegans and mammals suggests that the behavioral responses to drugs of abuse may depend more on functional similarities rather than complexities in the neuroanatomy. This seems particularly relevant in terms of how substances of abuse affect systems that mediate survival in the active search for food. However, it is possible that differences in neurotransmitter receptor systems and molecular pharmacology in C. elegans could also be advantageous in terms of providing a unique perspective concerning how some classes of potential treatments affect the appetitive properties of substances of abuse. For example, one potential treatment for AUDs that has been identified is topiramate [49]. Several cellular targets have been proposed to be involved in the therapeutic activity of topiramate and there are several possible molecular mechanisms by which topiramate may reduce EtOH reinforced behaviors. One possible mechanism could be through topiramate’s ability to blockade voltage-sensitive sodium channels [49], which are absent in C. elegans [50]. So, if topiramate were found to be ineffective in modifying EtOH preference behavior in C. elegans, this would suggest that sodium channels may have a role in reducing the appetitive properties of EtOH in vertebrates. Thus, cross-species findings could be assessed with respect to molecular homology of the mechanisms proposed to be mediating the appetitive or reinforcing properties of EtOH. Overall, these types of studies will help to characterize the molecular and pharmacological foundations of the effects of these compounds, regardless of whether the findings are consistent with the anticipated results.
In summary, our data indicate that C. elegans exhibit a concentration-dependent attraction to EtOH, which previous work shows results in self-exposure and elevations of internal concentrations of EtOH to levels associated with intoxication in humans. As observed in the EtOH naïve worms, the EtOH preference response in worms chronically exposed to EtOH was not due to an anesthetic or paralytic effect. In addition, we found that naltrexone could selectively reduce EtOH self-exposure in this paradigm. Moreover, chronic EtOH exposure induces treatment resistance and aversion resistant behavior as evidenced by naltrexone’s inability to modify EtOH preference and sustained self-exposure to EtOH in the presence of the aversive stimulus nonanone. These data are consistent with the efficacy of naltrexone in vertebrate animal models and humans, providing face and predictive validity for the model.
In the present studies, we establish and provide support for a high-throughput C. elegans behavioral medications screening model to enable fast and accurate generation of data. Although naltrexone has effects on the acute and rewarding properties of EtOH, as well as the relapse-inducing effects of chronic EtOH [17], some promising new agents only show efficacy in chronic models [2, 47]. Therefore, future studies will test compounds in both acute and chronic exposure models to compare effects with those observed in vertebrate models. The further development and application of this model may provide the field with a new and powerful tool to discover novel targets and treatments for AUDs.
ACKNOWLEDGEMENTS
The authors would like to thank colleagues Drs. J.C. Bettinger and L. Avery from VCU for enabling, along with co-author Dr. Cheong for graciously providing, the npr-17 KO and rescue mutant strains used in the present studies. We would also like to thank Dr. C.R. Goodlett for his expertise and guidance on the statistical analyses used in this manuscript. Some strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs [P40 OD010440]. This work was supported by the National Institutes of Health grants to Dr. Eric A. Engleman [AA024891, P60 007611, DA035468].
Support: These studies were supported by the NIH grants DA035468 and AA024891 to Dr. Eric A. Engleman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].NIDA, Trends & Statistics, 2017. https://www.drugabuse.gov/related-topics/trendsstatistics.
- [2].Edwards S, Koob GF, Experimental Psychiatric Illness and Drug Abuse Models: From Human to Animal, an Overview, Methods Mol Biol 829 (2012) 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koob GF, Volkow ND, Neurocircuitry of addiction, Neuropsychopharmacology 35(1) (2010) 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tzschentke TM, Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade, Addict Biol 12(3–4) (2007) 227–462. [DOI] [PubMed] [Google Scholar]
- [5].Huber R, Panksepp JB, Nathaniel T, Alcaro A, Panksepp J, Drug-sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal, Neurosci Biobehav Rev 35(9) (2011) 1847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U, A Drosophila model for alcohol reward, Nat Neurosci 14(5) (2011) 612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kandel ER, The molecular biology of memory storage: a dialogue between genes and synapses, Science 294(5544) (2001) 1030–8. [DOI] [PubMed] [Google Scholar]
- [8].Hulme SE, Whitesides GM, Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research, Angew Chem Int Ed Engl 50(21) (2011) 4774–807. [DOI] [PubMed] [Google Scholar]
- [9].Musselman HN, Neal-Beliveau B, Nass R, Engleman EA, Chemosensory cue conditioning with stimulants in a Caenorhabditis elegans animal model of addiction, Behav Neurosci 126(3) (2012) 445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Katner SN, Neal-Beliveau BS, Engleman EA, Embryonic Methamphetamine Exposure Inhibits Methamphetamine Cue Conditioning and Reduces Dopamine Concentrations in Adult N2 Caenorhabditis elegans, Dev Neurosci 38(2) (2016) 139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bettinger JC, Davies AG, The role of the BK channel in ethanol response behaviors: evidence from model organism and human studies, Front Physiol 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davis SJ, Scott LL, Hu K, Pierce-Shimomura JT, Conserved single residue in the BK potassium channel required for activation by alcohol and intoxication in C. elegans, J Neurosci 34(29) (2014) 9562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alaimo JT, Davis SJ, Song SS, Burnette CR, Grotewiel M, Shelton KL, Pierce-Shimomura JT, Davies AG, Bettinger JC, Ethanol metabolism and osmolarity modify behavioral responses to ethanol in C. elegans, Alcohol Clin Exp Res 36(11) (2012) 1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee J, Jee C, McIntire SL, Ethanol preference in C. elegans, Genes Brain Behav 8(6) (2009) 578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheong MC, Artyukhin AB, You YJ, Avery L, An opioid-like system regulating feeding behavior in C. elegans, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McBride WJ, Li TK, Animal models of alcoholism: neurobiology of high alcoholdrinking behavior in rodents, Crit Rev Neurobiol 12(4) (1998) 339–69. [DOI] [PubMed] [Google Scholar]
- [17].Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A, Medications development to treat alcohol dependence: a vision for the next decade, Addict Biol 17(3) (2012) 513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bell RL, Sable HJK, Colombo G, Hyytia P, Rodd ZA, Lumeng L, Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity, Pharmacol Biochem Be 103(1) (2012) 119–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nass R, Hamza I, The nematode C. elegans as an animal model to explore toxicology in vivo: solid and axenic growth culture conditions and compound exposure parameters, Curr Protoc Toxicol Chapter 1 (2007) Unit 1 9. [DOI] [PubMed] [Google Scholar]
- [20].Bianchi L, Driscoll M, Culture of embryonic C. elegans cells for electrophysiological and pharmacological analyses, WormBook (2006) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hart AC, Behavior, WormBook, 2006. [Google Scholar]
- [22].Davies AG, Blackwell GG, Raabe RC, Bettinger JC, An Assay for Measuring the Effects of Ethanol on the Locomotion Speed of Caenorhabditis elegans, J Vis Exp (98) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Westland JL, Dorman FL, Comparison of SPME and static headspace analysis of blood alcohol concentration utilizing two novel chromatographic stationary phases, Forensic Sci Int 231(1–3) (2013) e50–6. [DOI] [PubMed] [Google Scholar]
- [24].McCulloch D, Gems D, Body size, insulin/IGF signaling and aging in the nematode Caenorhabditis elegans, Exp Gerontol 38(1–2) (2003) 129–36. [DOI] [PubMed] [Google Scholar]
- [25].Bargmann CI, Horvitz HR, Control of larval development by chemosensory neurons in Caenorhabditis elegans, Science 251(4998) (1991) 1243–6. [DOI] [PubMed] [Google Scholar]
- [26].Bargmann CI, Chemosensation in C. elegans, WormBook (2006) 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McIntire SL, Ethanol, in: Maricq AV (Ed.), WormBook 2010, pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL, Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans, Neuron 42(5) (2004) 731–43. [DOI] [PubMed] [Google Scholar]
- [29].Vidal-Gadea AG, Davis S, Becker L, Pierce-Shimomura JT, Coordination of behavioral hierarchies during environmental transitions in, Worm 1(1) (2012) 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Engleman EA, Katner SN, Neal-Beliveau BS, Caenorhabditis elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction, Prog Mol Biol Transl Sci 137 (2016) 229–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sellings L, Pereira S, Qian C, Dixon-McDougall T, Nowak C, Zhao B, Tyndale RF, van der Kooy D, Nicotine-motivated behavior in Caenorhabditis elegans requires the nicotinic acetylcholine receptor subunits acr-5 and acr-15, Eur J Neurosci 37(5) (2013) 743–56. [DOI] [PubMed] [Google Scholar]
- [32].Ward A, Walker VJ, Feng Z, Xu XZ, Cocaine modulates locomotion behavior in C. elegans, PLoS One 4(6) (2009) e5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Engleman EA, Steagall KB, Bredhold KE, Breach M, Kline HL, Bell RL, Katner SN, Neal-Beliveau BS, Caenorhabditis elegans Show Preference for Stimulants and Potential as a Model Organism for Medications Screening, Front Physiol 9(1200) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Epstein HF, Caenorhabditis elegans: modern biological analysis of an organism, Academic Press; 1995. [Google Scholar]
- [35].Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL, A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans, Cell 115(6) (2003) 655–66. [DOI] [PubMed] [Google Scholar]
- [36].Heilig M, Egli M, Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms, Pharmacol Ther 111(3) (2006) 855–76. [DOI] [PubMed] [Google Scholar]
- [37].Negus SS, Banks ML, Medications development for opioid abuse, Cold Spring Harb Perspect Med 3(1) (2013) a012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD, Naltrexone Maintenance Decreases Cannabis Self-Administration and Subjective Effects in Daily Cannabis Smokers, Neuropsychopharmacology 40(11) (2015) 2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Henderson-Redmond A, Czachowski C, Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats, Psychopharmacology (Berl) 231(22) (2014) 4309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH, mu-Opioid receptor knockout mice do not self-administer alcohol, J Pharmacol Exp Ther 293(3) (2000) 1002–8. [PubMed] [Google Scholar]
- [41].Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V, Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice, Naunyn Schmiedebergs Arch Pharmacol 365(4) (2002) 296–302. [DOI] [PubMed] [Google Scholar]
- [42].Contet C, Kim A, Le D, Iyengar SK, Kotzebue RW, Yuan CJ, Kieffer BL, Mandyam CD, mu-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal neurogenic niche, Addict Biol 19(5) (2014) 770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hall FS, Sora I, Uhl GR, Ethanol consumption and reward are decreased in muopiate receptor knockout mice, Psychopharmacology (Berl) 154(1) (2001) 43–9. [DOI] [PubMed] [Google Scholar]
- [44].Levran O, Yuferov V, Kreek MJ, The genetics of the opioid system and specific drug addictions, Hum Genet 131(6) (2012) 823–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F, Reinstatement of ethanolseeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone, Psychopharmacology (Berl) 168(1–2) (2003) 208–15. [DOI] [PubMed] [Google Scholar]
- [46].Leao RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, Hope BT, Koob GF, George O, Chronic nicotine activates stress/rewardrelated brain regions and facilitates the transition to compulsive alcohol drinking, J Neurosci 35(15) (2015) 6241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats, J Neurosci 32(22) (2012) 7563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lehtonen M, Reisner K, Auriola S, Wong G, Callaway JC, Mass-spectrometric identification of anandamide and 2-arachidonoylglycerol in nematodes, Chem Biodivers 5(11) (2008) 2431–41. [DOI] [PubMed] [Google Scholar]
- [49].Johnson BA, Progress in the development of topiramate for treating alcohol dependence: from a hypothesis to a proof-of-concept study, Alcohol Clin Exp Res 28(8) (2004) 1137–44. [DOI] [PubMed] [Google Scholar]
- [50].Bargmann CI, Neurobiology of the Caenorhabditis elegans genome, Science 282(5396) (1998) 2028–33. [DOI] [PubMed] [Google Scholar]


