Abstract
Symplocin A, a linear peptide possessing N-terminal N,N-dimethylisoleucine, statine, and valic acid residues, has been synthesized for the first time employing our previously established ‘one-pot intramolecular tandem protocol’. Moreover, the stereochemistry of natural symplocin A was unambiguously revised through the confirmation by 1D NMR, 2D NMR, and HPLC comparisons with authentic natural product.
Introduction
Secondary metabolites, derived from marine cyanobacteria displaying a variety of physiological activities, including antimicrobial, antimalarial, antifungal, cytotoxic and neurotoxic properties1biological activities, are a promising class of compounds for drug discovery. In particular, the linear depsipeptides, bearing characteristic N-terminal N,N-dimethylamino acid (DMAA) and internal statine residues, have attracted significant attention in recent years;2 for example, dolastatin 10 (1)3 isolated from the sea hare Dolabella auricularia, is a highly cytotoxic compound that inspired design of auristatin as the potent ‘warhead’ component of antibody conjugates (ABC) that have been approved as antineoplastic agents for cancer chemotherapy.4 Grassystatins A, B (2a, 2b) and C, isolated from Lyngbya cf. confervoides collected at Grassy Key and Key Largo, FL, are potent inhibitors of proteases, cathepsin D and E.5
Symplocin A (proposed structure 3a) is a sub-nanomolar cathepsin E inhibitor (IC50 300 pM), isolated by Molinski and co-workers6 in 2012 from the Bahamian cyanobacterium Symploca sp. The planar structure of symplocin A - comprising seven α-amino acid residues, along with valic acid and statine subunits - was secured from spectroscopic analysis, including MS and 2D NMR. The absolute configurations of seven residues, including (3R,4S)-statine, were assigned L-configurations by degradation-Marfey’s analysis.7 An uncommon D-configuration was proposed for both the DMAA and valic acid residues from chiral phase HPLC of the corresponding O-2-naphthacyl esters and comparison with standards. The unexpected finding of a terminal D-DMAA, in contrast to L- for all other known linear peptides of this class, and the potent protease inhibitory properties of symplocin A, provoked sufficient interest to explore the structure in more depth.
As a continuation of our interest in developing divergent syntheses of natural products8 isolated from cyanobacteria and investigating their structure-activity relationships, together with unusual features of the structure, we decided to develop an asymmetric synthesis of symplocin A. Herein, we present the synthesis of symplocin A, along with those of two non-natural diastereomers, and a stereochemical revision of the natural product.
Results and discussion
The retrosynthetic analysis of 3a (Figure 2) converged on five subunits: N,N-dimethyl ester fragment 4, Tyr-OBn (5), N-Boc-Ser (6), N-Boc-(3R,4S)-statine (7), and tetrapeptide 8. Fragment 8 could be prepared by conventional condensations of the constituent amino acids. The γ-amino acid, N-Boc-(3R,4S)-statine (7), could be obtained using our ‘one-pot intramolecular tandem protocol’.9 Importantly, the well-known β,γ-epimerization of statine residues could be avoided through an alternate condensation sequence of the five subunits.
Figure 2.
Retrosynthetic analysis of 3a.
The synthesis of tetrapeptide fragment 8 is outlined in Scheme 1. D-Boc-Phe-OH (9) was N-methylated (MeI, NaH, THF) to provide D-Boc-N-Me-Phe-OH (10) in quantitative yield:10 the latter was directly coupled with L-Pro-OMe (HATU, DIPEA) to produce peptide 11 in 62% overall yield.10 Removal of Boc group in 11 (TFA) and subsequent condensation with Boc-Gly-OH (HATU-DIPEA) afforded tripeptide 12 (80%, two steps). Finally, treatment of compound 12 with TFA and the coupling of the resulting crude salt with L-Boc-Val-OH (HOBt, EDCI, NMM) gave tetrapeptide fragment 8 in 62% overall yield.12
Scheme 1.
Preparation of Tetrapeptide Fragment 8. Reagents and conditions: (a) NaH, MeI, THF; (b) L-Pro-OMe, HATU, DIPEA, DCM, 62% (2 steps); (c) (1) TFA, DCM; (2) Boc-Gly, HATU, DIPEA, DCM, 80% (2 steps); (d) (1) TFA, DCM; (2) Boc-Val-OH, HOBt, EDCI, DMF, 62% (2 steps).
Next, we turned our attention to prepare the key (3R,4S)-statine subunit 7 of 3a (Scheme 2). The most practicable approach to N-Boc-(3R,4S)-statine subunit 7 is the ring-opening of lactam 13, which could be prepared easily according to our previously described one-pot intramolecular tandem protocol.9 Thus, removal of TBS group in N-Boc-lactam 13 (TBAF) gave secondary alcohol 14 (50%). The subsequent ring-opening of 14 (LiOH•H2O, H2O2) generated the 7 in quantitative yield13.
Scheme 2.
Preparation of (3R,4S)-Statine Subunit 7. Reagents and conditions: (a) TBAF, THF, 50%; (b) LiOH•H2O, H2O2, THF/H2O, 100%.
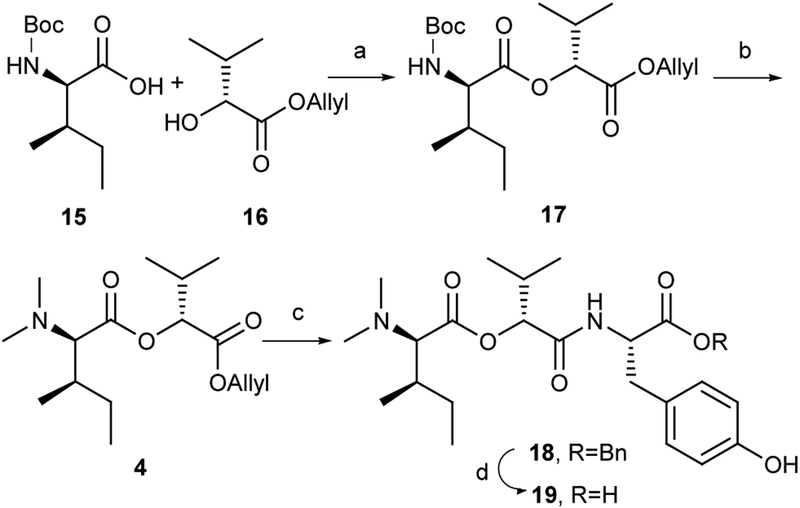
The synthesis of subunit 4 and its subsequent coupling with subunit 5 is shown in Scheme 3. Coupling of D-Boc-Ile-OH (15) and (R)-allyl 2-hydroxy-3-methylbutanoate (16, DCC, DMAP) produced ester 17 (76%). Removal of Boc group in 17 (TFA) and subsequent N,N-dimethylation by reductive amination [40% HCHO aqu., NaBH3(CN)] generated the desired N,N-dimethyl amino ester 4 (64%, two steps).14 The Pd-catalyzed deprotection of the O-allyl group in 4 [Pd(PPh3)4, N-methylaniline, NMA] afforded the corresponding free acid,15 which was used to acylate Tyr-OBn (5, HATU, HOAt, DIPEA) and deliver linear peptide 18 (45%, two steps).10a,d Deprotection of the benzyl ester 18 (H2, 1 atm, Pd/C) gave the free acid 19 in quantitative yield.
Scheme 3.
Preparation of Depsipeptide Fragment 19. Reagents and conditions: (a) DCC, DMAP, DCM, 76%; (b) (1) TFA, DCM; (2) HCHO, NaBH3(CN), CH3CN, 64% (2 steps); (c) (1) Pd(PPh3)4, N-methylaniline, NMA, THF; (2) 5, HATU, HOAt, DIPEA, DCM, 45% (2 steps); (d) H2, 1 atm, Pd/C, MeOH, 100%.
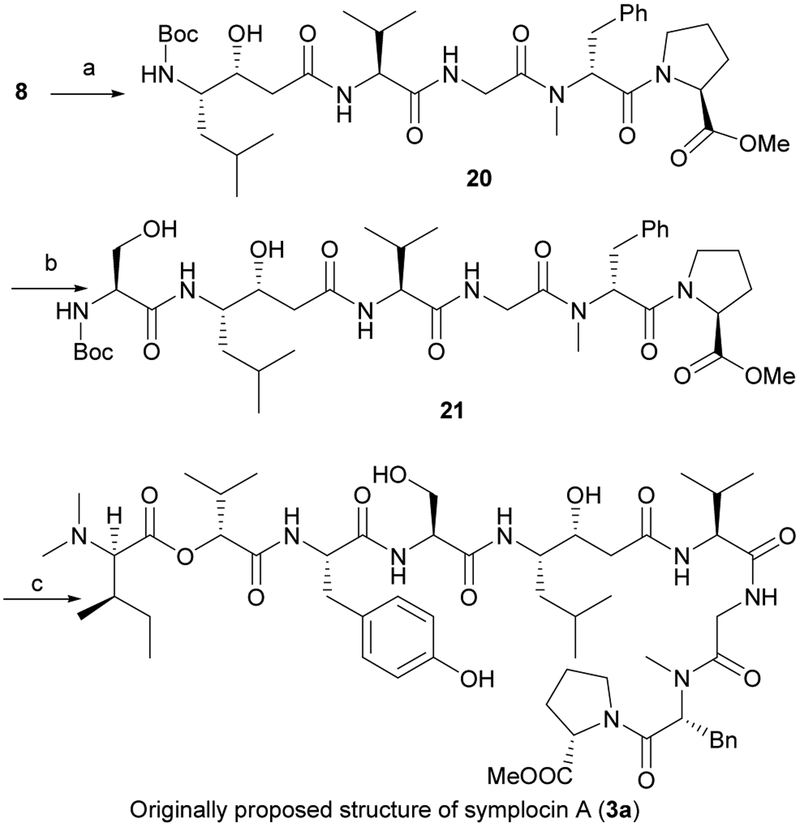
With fragments 8, 7 and 19 in hand, we turned our attention to final assembly of 3a through successive coupling reactions. The crude amine salt, prepared by removal of Boc group in 8 (TFA), was directly coupled with 7 (HATU, HOAt, DIPEA) to give the pentapeptide 20 (59%, two steps). Deprotection of 20 (TFA) afforded a crude amine salt, which, without further purification, was used to acylate 6 (HOBt, EDCI, NMM) and deliver hexapeptide 21 (70%). Finally, removal of the Boc group in 21 (TFA) and direct coupling of the product with the free acid 19 (HOBt, EDCI, NMM), afforded (50R, 51R)-3a {[α]D22.7 +9.6 (c 0.25, MeOH), lit6 symplocin A: [α]D22.5 +16.0 (c 2.18, MeOH)} in 38% overall yield.
To our surprise, the analytical data of optical rotation and NMR spectra generated from synthetic 3a were substantially different from those reported for symplocin A.6 Through comparison of the respective 1H NMR data of 3a with the natural product, we observed perceptible differences in the signals of the DMAA residue, and suspected that the stereochemical assignment of the N,N-dimethylisoleucine residue might be incorrect. The configurational assignment of DMAA residues can be equivocal. An assertion, “that all marine natural products containing an N,N-dimethyl terminal amino acid residue possess the L-configuration at this center”,16a is belied by the finding of both L- and D- variants of N,N,O-trimethyl-Ser in aplyronines A-C.16g
Nevertheless, we suspected the correct configuration of the N,N-dimethylisoleucine residue of symplocin A may indeed be 2S,3S or possibly an allo diastereomer. To test this hypothesis, we synthesized the symplocin A diastereomer 3b with (2S, 3S)-L-N,N-dimethylisoleucine at the N-terminus (Scheme 5). Following the synthetic sequence described above, L-Boc-Ile-OH 22 was converted to its N,N-dimethylamino ester (2S,3S)-4 (Scheme 5) in 37% overall yield. Removal of allyl group in (2S, 3S)-4 [Pd(PPh3)4, NMA] and transformation of the product to (50S,51S)-symplocin A (3b) {[α]D22.7 +21.2 (c 0.25, MeOH)} was achieved in 8% overall yield under conditions similar to those that led to 3a.
Scheme 5.
Preparation of (50S, 51S)-Symplocin A (3b). Reagents and conditions: (a) (1) 16, DCC, DMAP, DCM; (2) TFA, DCM; (3) HCHO, NaBH3(CN), CH3CN, 37% (3 steps); (b) (1) Pd(PPh3)4, N-methylaniline, NMA, THF; (2) 5, HATU, HOAt, DIPEA, DCM, 31% (2 steps); (c) (1) H2, 1 atm, Pd/C; (2) free amine of 21, HOBt, EDCI, NMM, DCM, 26% (2 steps).
Having successfully synthesized (50S, 51S)-symplocin A (3b), we turned our attention to the comparison of their optical rotation and NMR data. Although the 1H NMR spectra of our synthetic 3b showed closer similarity than 3a to natural symplocin A, subtle differences in chemical shifts and multiplicities remained.
Closer comparisons of the 1H and 13C NMR data of synthetic (50R, 51R)-3a and (50S, 51S)-3b with that of the authentic sample revealed fewer differences betweeen authentic symplocin A and (50S,51S)-3b for the region corresponding to C-49 ~ C-56 (N,N-Ile), but larger differences in the domain of C-24 ~ C-31 (statine) (see the Supporting Information). Specifically, the (50S,51S)-3b appeared to be a better match with authentic symplocin A in the DMAA domain, but the smaller chemical shift differences in the statine domain suggested an inversion of one or both of the C-26 and C-27 asymmetric centers. Although it was unlikely the latter stereocenter was in error, given the conditions of hydrolysis-Marfey’s analysis,7 separate SAR studies by indicated that the 3S configuration (statine numbering), associated with pepstatin-derived inhibitors of aspartic proteases, is crucial for tight binding to the enzyme active sites.17 From the latter report, along with the known difficulties associated with assignment of the C-3 stereocenter in statine residues,18,19 we surmised that the native configuration in symplocin A – which potently inhibits cathepsin E6 – may also be 3S,4S; namely a 26S epimer of 3b.
To test this proposal, we synthesized the diastereomeric (26S, 50S, 51S)-symplocin A (3c, Scheme 6). Inversion of the carbinol center in 14 was straight-forward through Swern oxidation20 followed by reduction (NaBH4, MeOH) to afford (2S, 3S)-14 (67%, two steps).8c Hydrolysis of (2S, 3S)-14 (LiOH•H2O, H2O2) gave ring-opened (3S, 4S)-7 in quantitative yield. Following the synthetic sequences described above, diastereomeric (26S, 50S, 51S)-symplocin A (3c) {[α]D21.1 +27.6 (c 1.00, MeOH)} was obtained in 7% overall yield from (3S,4S)-7.
Scheme 6.
Preparation of (26S, 50S, 51S)-Symplocin A (3c). Reagents and conditions: (a) (1) (COCl)2, DMSO, DCM; (2) NaBH4, MeOH, 67% (2 steps); (b) LiOH•H2O, H2O2, THF/H2O, 100%; (c) free amine of 8, HATU, HOAt, DIPEA, DMF, 87%; (d) (1) TFA,DCM; (2) 6, HOBt, EDCI, NMM, DMF, 37% (2 steps); (3) TFA,DCM; (4) (2S,3S)-19, HOBt, EDCI, NMM, DCM, 21% (2 steps).
To our delight, the NMR data of synthetic 3c (see Supporting Information) were in excellent agreement with those reported for natural symplocin A. In analytical reversed phase HPLC (see the Supporting Information), both synthetic 3a and 3b showed different retention times compared to authentic symplocin A, while synthetic 3c and the natural sample were identical by retention time, and co-eluted under two different sets of conditions.
Based on the successful synthesis of 3c, we conclude that the terminal N,N-dimethylisoleucine residue of natural symplocin A should be revised to the L-configuration (50S,51S), and one stereocenter in the statine residue should be changed from 26R to 26S.
Conclusions
In summary, the first total synthesis of symplocin A (3c) and two of its diastereomers, 3a and 3b, has been accomplished using our previously established ‘one-pot intramolecular tandem protocol’. This synthetic strategy avoids tedious protecting group interchanges during the sequential amide condensation reactions of five peptide subunits. In addition, the configuration of the natural product was corrected to 3c,21 and unambiguously confirmed by careful comparisons of all four samples by 1D NMR, 2D NMR and HPLC.
Experimental
General
THF was distilled from sodium/benzophenone. DCM was distilled from phosphorus pentoxide. Reactions were monitored by thin layer chromatography (TLC) on glass plates coated with silica gel with fluorescent indicator. Flash chromatography was performed on silica gel (300–400 mesh). Optical rotations were measured on a polarimeter with a sodium lamp. HRMS were measured on a Thermo Scientific LTQ Orbitrap XL apparatus. IR spectra were recorded using film on a Fourier Transform Infrared Spectrometer. NMR spectra were recorded at 400 MHz or 600 MHz, and chemical shifts are reported in δ (ppm) referenced to the appropriate residual solvent peaks unless otherwise noted.
(S)-Methyl 1-((R)-2-(tert-butoxycarbonyl)-3-phenylpropanoyl)pyrrolidine-2-carboxylate (11)
To a stirred solution of D-Boc-Phe-OH (31.83 g, 120.00 mmol) and MeI (74.70 mL, 1.20 mol) in THF (250 mL) under argon atmosphere, was slowly added NaH (60% in mineral oil; 24.00 g, 600.00 mmol) in portions over a period of 2 h at 0 °C. After being stirred for overnight at room temperature, ice was added to quench the reaction. After removal of THF in vacuo, the residue was dissolved in H2O (100 mL) and extracted with EtOAc (50 mL). The aqueous layer was acidified to pH = 3 with an aqueous solution of 1N HCl and extracted with EtOAc (50 mL × 3). The combined organic layers were washed with saturated Na2S2O3 followed by brine, then dried over MgSO4, filtered and concentrated to give 10 as a colorless oil (32.20 g, quant.), which was used for the next step without further purification. To a stirred solution of 10 (30.73 g, 110.0 mmol), Pro-OMe (14.21 g, 110.0 mmol) and HATU (62.74 g, 165.0 mmol) in DCM (450 mL) under argon atmosphere, was added DIPEA (57.5 mL, 330 mmol) at 0 °C. After being stirred overnight at room temperature, saturated NH4Cl (100 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (100 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc = 1:1) to give 11 as a white solid (26.63 g, 62%). [α]D22.5 +74.25 (c 2.00, CHCl3); IR (film) νmax 2976, 2933, 2868, 1748, 1696, 1653, 1436, 1388, 1170 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32–7.10 (m, 5H), 5.25–5.10 (m, 0.5H), 4.95–4.70 (m, 0.5H), 4.60–4.35 (m, 1H), 3.73 (s, 3H), 3.58–3.36 (m, 2H), 3.25–3.16 (m, 0.5H), 3.15–3.07 (m, 0.5H), 3.03–2.81 (m, 4H), 2.30–2.10 (m, 1H), 2.05–1.75 (m, 3H), 1.40–1.31 (m, 4.5H), 1.25–1.16 (m, 4.5H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.6, 172.2, 168.9, 168.6, 155.2, 154.5, 138.0, 137.5, 129.4, 129.3, 128.2, 127.9, 126.2, 126.0, 79.8, 79.7, 59.3, 59.1, 58.8, 57.4, 52.0, 51.9, 46.3, 46.0, 34.65, 34.57, 29.4, 28.7, 28.0, 27.8, 25.0 ppm; HRMS (ESI) m/z calcd for C21H31N2O5+ (M+H)+ 391.2228, found 391.2227.
(S)-Methyl 1-((R)-2-(2-(tert-butoxycarbonyl)-N-methylacetamido)-3-phenylpropanoyl) pyrrolidine-2-carboxylate (12)
To a stirred solution of 11 (22.7 g, 58.0 mmol) in DCM (116 mL) was added TFA (58 mL) at 0 oC, and the mixture stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (5 mL) and evaporated. Repeatition of this process three times gave the corresponding crude amine TFA salt, which was treated with Boc-Gly-OH (12.19 g, 69.60 mmol) and HATU (28.7 g, 75.4 mmol) in DCM (200 mL) and DIPEA (50.5 mL, 290 mmol) at 0 °C under an argon atmosphere. After being stirred for overnight at room temperature, saturated NH4Cl (50 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (50 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, the residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc = 1:1) to give 12 as a colorless oil (20.8 g, 80%). [α]D22.7 +63.20 (c 2.00, MeOH); IR (film) νmax 3363, 2982, 1750, 1712, 1648, 1431, 1165 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.34–7.11 (m, 5H), 5.65–5.48 (m, 1H), 4.42–4.31 (m, 1H), 3.97 (d, J = 17.1 Hz, 1H), 3.74 (d, J = 17.1 Hz, 1H). 3.71 (s, 3H), 3.52–3.33 (m, 2H), 3.25–3.12 (m, 1H), 3.01 (s, 3H), 2.88–2.77 (m, 1H), 2.26–2.12 (m, 1H), 1.98–1.77 (m, 3H), 1.43 (s, 9H) ppm; 13C NMR (100 MHz, CD3OD) δ 173.9, 171.3, 170.2, 158.3, 138.6, 130.5, 129.4, 127.6, 80.5, 60.6, 57.8, 52.6, 48.3, 43.0, 35.7, 30.4, 29.9, 28.7, 25.9 ppm; HRMS (ESI) m/z calcd for C23H34N3O6+ (M+H)+ 448.2442, found 448.2443.
(S)-Methyl 1-((R)-2-(2-((S)-2-(tert-butoxycarbonyl)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl) pyrrolidine-2-carboxylate (8)
To a stirred solution of 12 (2.60 g, 5.80 mmol) in DCM (12 mL) was added TFA (6 mL) at 0 oC and stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (3 mL) and evaporated. This process was repeated three times to give a crude amine, which was dissolved in DMF and treated, sequentially, with a solution of L-Boc-Val-OH (1.12 g, 6.38 mmol) in DMF (20 mL) and HOBt (862 mg, 6.38 mmol) at −15 °C under an argon atmosphere. The mixture was and stirred for 30 min at the same temperature before adding EDCI (1.22 g, 6.38 mmol) and stirring an additional 1.5h at −15 °C before adding NMM (1.28 mL, 11.6 mmol). After being stirred for overnight at room temperature, saturated NH4Cl (30 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (30 mL × 3). The combined organic layers were washed with saturated NaHCO3 followed by brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (DCM/MeOH = 40:1) to give 8 as a white foam (1.96 g, 62%). [α]D22.8 +34.3 (c 2.00, MeOH); IR (film) νmax, 3315, 2958, 1750, 1717, 1641, 1451, 1370, 1175 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.30–7.23 (m, 3H), 7.22–7.10 (m, 2H), 6.86 (br s, 1H), 5.62–5.45 (m, 1H), 5.14–5.00 (m, 1H), 4.46–4.38 (m, 1H), 4.19–4.08 (m, 1H), 4.07–3.99 (m, 1H), 3.94–3.86 (m, 1H), 3.73 (s, 3H), 3.44–3.32 (m, 2H), 3.31–3.24 (m, 1H), 3.00 (s, 3H), 2.88–2.80 (m, 1H), 2.21–2.11 (m, 2H), 2.08–2.04 (m, 1H), 1.97–1.87 (m, 2H), 1.44 (s, 9H), 0.97–0.93 (m, 3H), 0.91–0.86 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.6, 171.6, 168.01, 167.97, 155.9, 137.1, 129.5, 128.5, 126.8, 80.0, 59.8, 59.1, 56.4, 52.4, 46.9, 41.3, 35.1, 31.2, 29.8, 28.9, 28.4, 25.2, 19.4, 17.5 ppm; HRMS (ESI) m/z calcd for C28H43N4O7+ (M+H)+ 547.3126, found 547.3126.
(2S,3R)-tert-Butyl 3-hydroxy-2-isobutyl-5-oxopyrrolidine-1-carboxylate (14)
To a stirred solution of 13 (372 mg, 1.00 mmol) in THF (10 mL) was added TBAF (1.0 M in THF, 2.00 mL, 2.00 mmol), dropwise, at 0 oC, and the mixture allowed to stir for 2 h while warming to room temperature. Saturated NH4Cl (5 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc = 2:1) to give 12 as colorless crystals (129 mg, 50 %). Mp 114 – 116 oC, [α]D22.9 +56 (c 0.50, MeOH); IR (film) νmax 3415, 2955, 2933, 2870, 1770, 1717, 1367, 1290, 1152, 1042, 1022 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.16 (d, J = 5.1 Hz, 1H), 4.07 (dd, J = 10.9, 3.1 Hz, 1H), 2.84 (dd, J = 18.1, 5.3 Hz, 1H), 2.77 (brs, 1H), 2.44 (d, J = 18.1 Hz, 1H), 1.76–1.65 (m, 1H), 1.53 (s, 9H), 1.52–1.46 (m, 1H), 1.28 (ddd, J = 13.5, 11.0, 4.3 Hz, 1H), 1.04–0.93 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 173.0, 149.9, 83.2, 67.8, 66.1, 41.1, 41.0, 28.2, 25.5, 23.8, 21.9 ppm; HRMS (ESI) m/z calcd for C13H24NO4+ (M+H)+ 258.1700, found 258.1695.
(3R,4S)-4-(tert-Butoxycarbonyl)-3-hydroxy-6-methylheptanoic acid (7)
To a stirred solution of 14 (103 mg, 0.40 mmol) in THF/H2O (4:1) (6 mL) at room temperature, aqueous H2O2 (30% v/v, 0.1 mL) and LiOH.H2O (50 mg, 1.20 mmol) were added sequentially. Stirring was continued at room temperature for 12 h. After removal of THF in vacuo, the basic aqueous residue was acidified by the addition of 10% citric acid (to pH = 4), and the mixture extracted with EtOAc (10 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated to give 7 as a white powder (110 mg, quant.), which was used for the next step without further purification.
(2R,3R)-((R)-1-(Allyloxy)-3-methyl-1-oxobutan-2-yl) 2-(tert-butoxycarbonyl)-3-methylpentanoate (17)
To a stirred solution of D-Boc-Ile-OH (925 mg, 4.00 mmol) and 15 (791 mg, 5.00 mmol) in dry DCM (8 mL) were added DCC (2.47 g, 12.00 mmol) followed by DMAP (489 mg, 4.00 mmol) at 0 °C, and the reaction mixture was stirred at room temperature overnight. The reaction mixture was diluted with EtOAc and then filtrated, and the filtrate was washed with saturated NH4Cl, NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, the residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc = 20/1) to give 17 as a colorless oil (1.13 g, 76%). [α]D22.6 +13.50 (c 2.00, CHCl3); IR (film) νmax 2970, 2934, 2880, 1748, 1719, 1502, 1367, 1159, 1020 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.96–5.80 (m, 1H), 5.37–5.29 (m, 1H), 5.27–5.21 (m, 1H), 4.97 (d, J = 9.4 Hz, 1H), 4.86 (d, J = 4.3 Hz, 1H), 4.64–4.60 (m, 2H), 4.46 (dd, J = 9.4, 3.4 Hz, 1H), 2.30–2.20 (m, 1H), 2.08–1.98 (m, 1H), 1.51–1.45 (m, 1H), 1.43 (s, 9H), 1.30–1.21 (m, 1H), 1.03–0.99 (m, 3H), 0.99–0.93 (m, 6H), 0.91–0.88 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.7, 169.1, 155.9, 131.6, 119.1, 79.9, 77.4, 65.9, 56.5, 37.8, 30.2, 28.4, 26.3, 18.9, 17.3, 14.3, 11.9 ppm; HRMS (ESI) m/z calcd for C19H34NO6+ (M+H)+ 372.2381, found 372.2381.
(2R,3R)-((R)-1-(Allyloxy)-3-methyl-1-oxobutan-2-yl) 2-(dimethylamino)-3-methylpentanoate (4)
To a stirred solution of 17 (929 mg, 2.50 mmol) in DCM (10 mL) was added TFA (5 mL) at 0 oC and stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (3 mL) and evaporated. Repeated this process three times to give a crude amine. To a stirred solution of the above amine and aqueous HCHO (5 ml, 40%) in MeCN (10 mL) was added Na(BH3)CN (471 mg, 7.50 mmol) at 0 °C. The mixture was stirred for 1 h at room temperature, and then AcOH (0.2 mL) was added dropwise until the solution was neutral. Stirring was continued for overnight, AcOH being added occasionally to maintain the pH near neutrality. The solvent was removed in vacuo and then the residue was dissolved in EtOAc. The solvent was washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, and the residue purified by flash chromatography on silica gel (petroleum ether/EtOAc = 15:1) to give 4 as a colorless oil (479 mg, 64%). [α]D22 +37.3 (c 1.00, CHCl3); IR (film) νmax 2967, 2935, 2877, 1758, 1735, 1455, 1273, 1196, 1124, 1020 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.98–5.85 (m, 1H), 5.38–5.31 (m, 1H), 5.28–5.23 (m, 1H), 4.85 (d, J = 4.4 Hz, 1H), 4.67–4.63 (m, 2H), 2.94 (d, J = 10.8 Hz, 1H), 2.34 (s, 6H), 2.30–2.23 (m, 1H), 1.88–1.79 (m, 1H), 1.51–1.41 (m, 1H), 1.15–1.06 (m, 1H), 1.04–1.01 (m, 3H), 1.01–0.96 (m, 6H), 0.94–0.88 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 171.5, 169.5, 131.7, 119.1, 76.8, 73.0, 65.9, 41.3, 34.0, 30.1, 26.3, 19.2, 17.5, 15.3, 11.2 ppm; HRMS (ESI) m/z calcd for C16H30NO4+ (M+H)+ 300.2169, found 300.2167.
(2R,3R)-((R)-1-((S)-1-(Benzyloxy)-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-methyl-1-oxobutan-2-yl) 2-(dimethylamino)-3-methylpentanoate (18)
To a stirred solution of 4 (359 mg, 1.20 mmol) in THF (24 mL) containing Pd(PPh3)4 (116 mg, 0.12 mmol) under argon atmosphere was dropped N-Methylaniline (0.32 mL, 3.00 mmol) at room temperature under. After being stirred for 2 h, the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (DCM/MeOH = 10/1) to give a free acid. To a stirred solution of the acid, Tyr-OBn (390 mg, 1.44 mmol) and HATU (821 mg, 2.16 mmol) in DCM (10 mL) under argon atmosphere, was added DIPEA (1.25 mL, 7.20 mmol) at 0 °C. After being stirred for overnight at room temperature, saturated NH4Cl (10 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (10 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, and the residue purified by flash chromatography on silica gel (petroleum ether/EtOAc/NH4OH = 4/1/0.025) to give 18 as a colorless oil (277 mg, 45%). [α]D22 +32.50 (c 1.00, CHCl3); IR (film) νmax 3344, 2958, 2925, 2877, 1736, 1660, 1512, 1455, 1213, 1165, 1113, 1003 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.41–7.25 (m, 5H), 6.86–6.81 (m, 2H), 6.67–6.61 (m, 3H), 5.21–5.15 (m, 1H), 5.13–5.07 (m, 2H), 4.88–4.80 (m, 1H), 3.13–3.04 (m, 1H), 3.02–2.94 (m, 1H), 2.88 (d, J = 10.4Hz, 1H), 2.29 (s, 6H), 2.28–2.22 (m, 1H), 1.84–1.75 (m, 1H), 1.32–1.27 (m, 0.7H), 1.24–1.20 (m, 0.3H), 1.04–0.97 (m, 1H), 0.96–0.91 (m, 9H), 0.86–0.80 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 171.3, 170.9, 169.4, 155.3, 135.0, 130.5, 128.8, 128.7, 127.0, 115.6, 77.9, 73.4, 67.5, 53.5, 41.6, 37.1, 34.3, 30.8, 26.5, 19.1, 17.1, 15.4, 11.4 ppm; HRMS (ESI) m/z calcd for C29H41N2O6+ (M+H)+ 513.2959, found 513.2959.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3R,4S)-4-(tert-butoxycarbonyl)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl)pyrrolidine-2-carboxylate (20)
To a stirred solution of 8 (219 mg, 0.40 mmol) in DCM (4 mL) was added TFA (2 mL) at 0 oC and stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (1 mL) and evaporated. Repeated this process three times to give a crude amine. To a stirred solution of the amine, (3R,4S)-Boc-statine 7 (110 mg, 0.40 mmol), HATU (304 mg, 0.80 mmol) and HOAt (65 mg, 0.48 mmol) in DMF (2 mL) under argon atmosphere, was added DIPEA (0.35 mL, 2.00 mmol) at 0 °C. After being stirred for overnight at room temperature, saturated NH4Cl (10 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (10 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, and the residue purified by flash chromatography on silica gel (DCM/MeOH = 30:1) to give 20 as a colorless oil (165 mg, 59%). [α]D22.8 +14.40 (c 1.00, MeOH); IR (film) νmax 3424, 1631, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.27–7.18 (m, 5H), 5.51–5.09 (m, 1H), 4.80–4.60 (m, 2H), 4.44–4.38 (m, 1H), 4.34–4.24 (m, 1H), 4.14–4.04 (m, 1H), 3.98–3.88 (m, 2H), 3.73 (s, 3H), 3.71–3.66 (m, 1H), 3.43–3.31 (m, 2H), 3.30–3.23 (m, 1H), 2.93 (s, 3H), 2.90–2.82 (m, 1H), 2.49–2.41 (m, 3H), 2.39–2.35 (m, 1H), 2.30–2.22 (m, 1H), 2.19–2.12 (m, 1H), 2.03–1.93 (m, 1H), 1.93–1.87 (m, 1H), 1.86–1.79 (m, 1H), 1.72–1.62 (m, 1H), 1.43 (s, 9H), 1.35–1.29 (m, 1H), 1.01–0.96 (m, 3H), 0.95–0.92 (m, 6H), 0.92–0.89 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 173.4, 172.9, 172.0, 168.9, 168.2, 156.9, 137.0, 129.5, 128.6, 126.9, 80.0, 72.4, 59.3, 53.2, 52.6, 47.1, 41.3, 39.6, 35.0, 29.9, 28.9, 28.5, 25.2, 24.9, 23.8, 21.7, 19.5, 17.7 ppm; HRMS (ESI) m/z calcd for C36H58N5O9+ (M+H)+ 704.4229, found 704.4228.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3R,4S)-4-((S)-2-(tert-butoxycarbonyl)-3-hydroxypropanamido)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl) pyrrolidine-2-carboxylate (21)
To a stirred solution of 20 (134 mg, 0.19 mmol) in DCM (2 mL) was added TFA (1 mL) at 0 oC and stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (1 mL) and evaporated. Repeated this process three times to give a crude amine. To a solution of Boc-Ser-OH 6 (47 mg, 0.23 mmol) in DMF (2 mL) was added HOBt (36 mg, 0.27 mmol) at −15 °C under argon atmosphere and stirred for 30 min at the same temperature. EDCI (51 mg, 0.27 mmol) was added and stirred at −15 °C for 1.5 h, then NMM (0.084 mL, 0.76 mmol) and a solution of the above amine in DMF was added. After being stirred for overnight at room temperature, saturated NH4Cl (10 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (10 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, and the residue purified by flash chromatography on silica gel (DCM/MeOH = 10:1) to give 21 as a colorless oil (105 mg, 70%). [α]D22.8 +7.40 (c 1.00, MeOH); IR (film) νmax 3301, 2949, 1646, 1531, 1455, 1360, 1175, 1070 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.51–7.42 (m, 1H), 7.27–7.10 (m, 5H), 7.03–6.87 (m, 1H), 5.78–5.72 (m, 1H), 5.54–5.43 (m, 1H), 4.88–4.77 (m, 1H), 4.46–4.33 (m, 2H), 4.22–4.14 (m, 1H), 4.14–4.08 (m, 1H), 4.02–3.95 (m, 1H), 3.94–3.87 (m, 2H), 3.87–3.80 (m, 1H), 3.73 (s, 3H), 3.48–3.35 (m, 1H), 3.33–3.24 (m, 1H), 3.19–3.10 (m, 1H), 3.07 (s, 3H), 2.89–2.78 (m, 1H), 2.52–2.39 (m, 2H), 2.39–2.30 (m, 1H), 2.24–2.19 (m, 3H), 2.11–2.03 (m, 1H), 1.97–1.81 (m, 2H), 1.80–1.70 (m, 1H), 1.67–1.51 (m, 2H), 1.43 (s, 9H), 0.98–0.85 (m, 12H) ppm; 13C NMR (100 MHz, CDCl3) δ 173.4, 172.8, 171.8, 171.7, 169.2, 168.5, 156.3, 136.9, 129.5, 128.6, 126.9, 80.6, 72.1, 62.5, 58.9, 58.5, 56.4, 55.4, 52.5, 52.4, 46.9, 41.3, 39.6, 38.4, 35.4, 30.3, 30.0, 29.0, 28.4, 25.0, 24.9, 23.8, 21.6, 19.6, 17.4 ppm; HRMS (ESI) m/z calcd for C39H63N6O11+ (M+H)+ 791.4549, found 791.4549.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3R,4S)-4-((S)-2-((S)-2-((R)-2-((2R,3R)-2-(dimethylamino)-3-methylpentanoyloxy)-3-methylbutanamido)-3-(4-hydroxyphenyl)propanamido)-3-hydroxypropanamido)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl)pyrrolidine-2-carboxylate (3a)
To a stirred solution of 21 (103 mg, 0.13 mmol) in DCM (2 mL) was added TFA (1 mL) at 0 oC and stirred for 3 h at room temperature. After removal of the solvent in vacuo, the residue was dissolved in toluene (1 mL) and evaporated. Repeated this process three times to give a crude amine. Compound 18 (87 mg, 0.17 mmol), 10% Pd/C (9 mg) were stirred in MeOH (20 mL) at room temperature under H2 atmosphere (1 atm). After being stirred for 3 h, the reaction mixture was filtered carefully, and the filtrate was concentrated in vacuo to give a crude acid. The crude acid was suspended in anhydrous DCM (3 mL) and cooled to −15 °C under argon atmosphere, HOBt (27 mg, 0.20 mmol) was added and stirred for 30 min at the same temperature. EDCI (38 mg, 0.20 mmol) was added and stirred at −15 °C for 1.5 h, then NMM (0.057 mL, 0.52 mmol) and a solution of the above amine in DCM was added. After being stirred for overnight at room temperature, saturated NH4Cl (5 mL) was added to quench the reaction, and the mixture was extracted with EtOAc (5 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (DCM/MeOH = 10:1), and then by semi-preparative reversed-phase HPLC (C18, 2 mL/min, gradient, 40:60 to 100:0 (CH3CN + 0.1% TFA(aq.)) − (H2O + 0.1% TFA(aq.)) over 40 min) to give 3a as a colorless amorphous solid (54 mg, 38%). [α]D22.7 +9.60 (c 0.25, MeOH); IR (film) νmax 3386, 1669, 1436, 1203, 1141, 1037 cm−1; 1H NMR (600 MHz, Acetonitrile-d3) δ 8.16 – 8.10 (m, 1H), 8.10–8.04 (m, 1H), 7.68–7.55 (m, 1H), 7.55–7.45 (m, 1H), 7.25–7.19 (m, 4H), 7.19–7.16 (m, 1H), 7.06 (d, J = 8.4 Hz, 2H), 6.84–6.76 (m, 1H), 6.70 (d, J = 8.4 Hz, 2H), 5.42 (t, J = 7.2 Hz, 1H), 4.89 (d, J = 4.2 Hz, 1H), 4.61–4.55 (m, 1H), 4.29 (s, 1H), 4.27 (dd, J = 8.2, 5.9 Hz, 1H), 4.16–4.09 (m, 1H), 4.08–4.02 (m, 1H), 4.01–3.95 (m, 1H), 3.95–3.89 (m, 1H), 3.84–3.80 (m, 1H), 3.78–3.75 (m, 2H), 3.74–3.69 (m, 1H), 3.66 (s, 3H), 3.38–3.33 (m, 1H), 3.33–3.27 (m, 1H), 3.22–3.17 (m, 1H), 3.15–3.10 (m, 1H), 2.94 (s, 3H), 2.86 (s, 6H), 2.81–2.75 (m, 2H), 2.41–2.37 (m, 2H), 2.15–2.07 (m, 3H), 2.03–1.98 (m, 1H), 1.89–1.86 (m, 1H), 1.81–1.78 (m, 1H), 1.78–1.74 (m, 1H), 1.62–1.55 (m, 1H), 1.52–1.47 (m, 1H), 1.44–1.33 (m, 2H), 1.15–1.10 (m, 1H), 1.09 (d, J = 6.8 Hz, 3H), 0.94–0.87 (m, 12H), 0.87–0.82 (m, 6H), 0.68 (d, J = 6.7 Hz, 3H) ppm; 13C NMR (600 MHz, Acetonitrile-d3) δ 174.0, 173.5, 173.0, 172.5, 171.7, 169.9, 169.4, 168.7, 167.4, 156.5, 138.5, 131.0, 130.2, 128.9, 128.9, 127.1, 115.8, 81.0, 73.2, 72.2, 62.5, 60.3, 59.9, 57.6, 57.0, 56.1, 52.4, 52.4, 47.3, 42.1, 41.4, 39.9, 38.8, 37.1, 35.1, 34.0, 31.0, 30.7, 30.5, 29.3, 25.5, 25.2, 24.4, 23.7, 21.6, 19.3, 18.7, 18.1, 16.6, 15.4, 11.1 ppm; HRMS (ESI) m/z calcd for C56H87N8O14+ (M+H)+ 1095.6336, found 1095.6314.
(2S,3S)-((R)-1-(Allyloxy)-3-methyl-1-oxobutan-2-yl) 2-(tert-butoxycarbonyl)-3-methylpentanoate ((2S,3S)-17)
Following the same procedure used for the preparation of 17 as described above, L-Boc-Ile-OH 22 (1.16 g, 5.00 mmol), instead of D-Boc-Ile-OH, was employed to produce the desired compound as a colorless oil (873 mg, 47%). [α]D22 +18.30 (c 1.00, CHCl3); IR (film) νmax 2968, 2935, 2873, 1741, 1707, 1508, 1365, 1156, 1013 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.00–5.80 (m, 1H), 5.40–5.30 (m, 1H), 5.30–5.25 (m, 1H), 5.03 (d, J = 8.8 Hz, 1H), 4.88 (d, J = 4.4 Hz, 1H), 4.70–4.60 (m, 2H), 4.39 (dd, J = 8.9, 4.6 Hz, 1H), 2.32–2.23 (m, 1H), 1.99–1.90 (m, 1H), 1.53–1.47 (m, 1H), 1.45 (s, 9H), 1.23–1.14 (m, 1H), 1.03–1.00 (m, 3H), 1.00–0.96 (m, 6H), 0.96–0.91 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.0, 169.1, 155.5, 131.6, 119.1, 79.9, 77.5, 65.9, 58.2, 38.1, 30.3, 28.5, 25.0, 19.0, 17.3, 15.6, 11.8 ppm; HRMS (ESI) m/z calcd for C19H34NO6+ (M+H)+ 372.2381, found 372.2383.
(2S,3S)-((R)-1-(Allyloxy)-3-methyl-1-oxobutan-2-yl) 2-(dimethylamino)-3-methylpentanoate ((2S,3S)-4)
Following the same procedure used for the preparation of 4 as described above, (2S,3S)-17 (743 mg, 2 mmol), instead of 17, was employed to produce the desired compound (2S,3S)-4 as a colorless oil (470 mg, 78%). [α]D22 +15.10 (c 2.00, CHCl3); IR (film) νmax 2966, 2936, 2876, 1757, 1736, 1456, 1272, 1195, 1125, 1015 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.98–5.83 (m, 1H), 5.39–5.31 (m, 1H), 5.28–5.23 (m, 1H), 4.86 (d, J = 4.8 Hz, 1H), 4.67–4.63 (m, 2H), 2.94 (d, J = 10.2 Hz, 1H), 2.35 (s, 6H), 2.32–2.22 (m, 1H), 1.91–1.82 (m, 1H), 1.70–1.62 (m, 1H), 1.20–1.12 (m, 1H), 1.04–1.01 (m, 3H), 1.01–0.98 (m, 3H), 0.93–0.90 (m, 3H), 0.90–0.87 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 171.8, 169.6, 131.7, 119.1, 76.7, 72.4, 72.4, 65.8, 41.6, 33.4, 30.2, 25.2, 19.1, 17.5, 15.8, 10.6 ppm; HRMS (ESI) m/z calcd for C16H30NO4+ (M+H)+ 300.2169, found 300.2167.
(2S,3S)-((R)-1-((S)-1-(Benzyloxy)-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-methyl-1-oxobutan-2-yl) 2-(dimethylamino)-3-methylpentanoate ((2S,3S)-18)
Following the same procedure used for the preparation of 18 as described above, (2S,3S)-4 (449 mg, 1.50 mmol), instead of 4, was employed to produce the desired compound (2S,3S)-18 as a colorless oil (110 mg, 31%). [α]D22 +24.90 (c 1.00, CHCl3); IR (film) νmax 3320, 2968, 2930, 2873, 1731, 1660, 1512, 1451, 1175, 1118, 1037 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48–7.22 (m, 5H), 6.88–6.76 (m, 2H), 6.72–6.56 (m, 3H), 5.26–5.07 (m, 3H), 4.87–4.80 (m, 1H), 3.19–3.10 (m, 1H), 3.04–2.96 (m, 1H), 2.90 (d, J = 10.0 Hz, 1H), 2.33–2.24 (m, 1H), 2.19 (s, 6H), 1.88–1.77 (m, 1H), 1.69–1.59 (m, 1H), 1.18–1.09 (m, 1H), 0.96–0.91 (m, 6H), 0.91–0.86 (m, 3H), 0.84–0.78 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 171.3, 170.9, 169.4, 155.3, 135.0, 130.5, 128.80, 128.78, 127.1, 115.7, 77.7, 72.5, 67.6, 53.6, 41.5, 36.8, 33.5, 30.9, 25.3, 19.0, 17.1, 16.0, 10.6 ppm; HRMS (ESI) m/z calcd for C29H41N2O6+ (M+H)+ 513.2959, found 513.2959.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3R,4S)-4-((S)-2-((S)-2-((R)-2-((2S,3S)-2-(dimethylamino)-3-methylpentanoyloxy)-3-methylbutanamido)-3-(4-hydroxyphenyl)propanamido)-3-hydroxypropanamido)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl)pyrrolidine-2-carboxylate (3b)
Following the same procedure used for the preparation of 3a as described above, (2S,3S)-18 (90 mg, 0.18 mmol), instead of 18, was employed to produce the desired compound 3b as a colorless amorphous solid (17 mg, 26%). [α]D22.7 +21.20 (c 0.25, MeOH); IR (film) νmax 3367, 1636, 1403, 1037 cm−1; 1H NMR (600 MHz, Acetonitrile-d3) δ 7.95–7.87 (m, 1H), 7.87–7.78 (m, 1H), 7.40–7.32 (m, 1H), 7.32–7.26 (m, 1H), 7.26–7.21 (m, 4H), 7.19–7.14 (m, 1H), 7.06 (d, J = 8.4 Hz, 2H), 6.95–6.82 (m, 1H), 6.70 (d, J = 8.4 Hz, 2H), 5.44 (t, J = 7.6 Hz, 1H), 4.85 (d, J = 4.4 Hz, 1H), 4.56 (m, 1H), 4.35–4.29 (m, 1H), 4.27 (dd, J = 8.4, 5.9 Hz, 1H), 4.15 (t, J = 6.5 Hz, 1H), 4.09–4.03 (m, 1H), 4.00–3.93 (m, 1H), 3.91 (d, J = 4.3 Hz, 1H), 3.89–3.85 (m, 1H), 3.85–3.79 (m, 1H), 3.79–3.69 (m, 2H), 3.65 (s, 3H), 3.38–3.32 (m, 1H), 3.33–3.26 (m, 1H), 3.22–3.16 (m, 1H), 3.16–3.11 (m, 1H), 2.94 (s, 3H), 2.82 (s, 6H), 2.81–2.78 (m, 1H), 2.78–2.74 (m, 1H), 2.43–2.36 (m, 2H), 2.17–2.09 (m, 3H), 2.01 (m, 1H), 1.90–1.86 (m, 1H), 1.81–1.73 (m, 2H), 1.64–1.55 (m, 1H), 1.53–1.47 (m, 1H), 1.45–1.36 (m, 2H), 1.36–1.31 (m, 1H), 0.99–0.88 (m, 15H), 0.84 (d, J = 6.8 Hz, 6H), 0.71 (d, J = 6.7 Hz, 3H) ppm; 13C NMR (600 MHz, Acetonitrile-d3) δ 173.9, 173.4, 172.6, 172.3, 171.6, 169.7, 169.4, 168.7, 168.3, 156.6, 138.6, 130.9, 130.2, 128.9, 128.9, 127.1, 115.9, 81.2, 72.3, 71.7, 62.6, 59.9, 59.8, 57.4, 56.7, 56.0, 52.7, 52.3, 47.3, 42.7, 41.4, 39.9, 39.1, 36.7, 35.1, 33.9, 30.9, 30.6, 30.5, 29.4, 27.3, 25.5, 25.2, 23.7, 21.6, 19.3, 18.8, 17.9, 16.7, 14.1, 11.5 ppm; HRMS (ESI) m/z calcd for C56H87N8O14+ (M+H)+ 1095.6336, found 1095.6326.
(2S,3S)-tert-Butyl 3-hydroxy-2-isobutyl-5-oxopyrrolidine-1-carboxylate ((2S,3S)-14)
To a stirred solution of oxalyl chloride (0.43 mL, 5.00 mmol) in dry DCM (10 mL) was dropped DMSO (0.71 mL, 10.00 mmol) over 15 min at −78 oC under argon atmosphere, and stirred for 1 h. A solution of 14 (643 mg, 2.50 mmol) in dry DCM (5.0 mL) was dropped and the mixture was stirred for another 2 h, then TEA (2.08 mL, 15.00 mmol) was dropped and the mixture was allowed to warm to room temperature. Saturated NH4Cl (20 mL) was added to quench the reaction, and the mixture was extracted with DCM (15 mL × 3). The combined organic layers were washed with saturated NaHCO3, followed by brine, dried over MgSO4, filtered and concentrated to give a crude product as a colorless oil which was used in the next step without further purification. The crude mixture was dissolved in MeOH (15 mL) and cooled to 0 oC, then NaBH4 (236 mg, 6.25 mmol) was added in three portions. After being stirred for 3 h at 0 oC to room temperature, the reaction was quenched with saturated NaHCO3 and extracted three times with DCM. The combined organic layers were washed with brine and dried over MgSO4. Filtered and concentrated, the residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc = 2:1) to give (2S, 3S)-14 as colorless crystals (431 mg, 1.67 mmol, 67%). Mp 89 – 91 oC, [α]D23.2 +65.50 (c 1.00, MeOH); IR (film) νmax 3445, 2957, 2929, 2866, 1773, 1720, 1358, 1295, 1256, 1154 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.60–4.50 (m, 1H), 4.25–4.15 (m, 1H), 2.69 (dd, J = 17.1, 7.3 Hz, 1H), 2.59 (dd, J = 17.1, 8.0 Hz, 1H), 1.87–1.73 (m, 2H), 1.53 (s, 9H), 1.50–1.43 (m, 1H), 1.01–0.94 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.2, 150.0, 83.4, 65.7, 59.9, 40.4, 37.3, 28.2, 25.2, 23.6, 22.6 ppm; HRMS (ESI) m/z calcd for C13H24NO4+ (M+H)+ 258.1700, found 258.1700.
(3S,4S)-4-(tert-Butoxycarbonyl)-3-hydroxy-6-methylheptanoic acid ((3S,4S)-7)
Following the same procedure used for the preparation of 7 as described above, (2S,3S)-14 (335 mg, 1.30 mmol), instead of 14, was employed to produce the desired compound (3S,4S)-7 as a white powder (358 mg, quant.), which was used for the next step without further purification.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3S,4S)-4-(tert-butoxycarbonyl)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl)pyrrolidine-2-carboxylate ((3S,4S)-20)
Following the same procedure used for the preparation of 20 as described above, (3S,4S)-7 (358 mg, 1.30 mmol), instead of 7, was employed to produce the desired compound (3S,4S)-20 as a colorless oil (795 mg, 87%). [α]D22.8 +7.40 (c 1.00, MeOH); IR (film) νmax 3323, 2957, 2929, 2866, 1745, 1639, 1525, 1436, 1366, 1278, 1173, 1046 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.27–7.09 (m, 5H), 6.91–6.73 (m, 1H), 6.65–6.52 (m, 1H), 5.61–5.52 (m, 1H), 4.85–4.79 (m, 1H), 4.46–4.39 (m, 1H), 4.38–4.32 (m, 1H), 4.19–4.14 (m, 1H), 4.14–4.07 (m, 1H), 4.02–3.94 (m, 1H), 3.94–3.85 (m, 1H), 3.73 (s, 3H), 3.65–3.55 (m, 1H), 3.40–3.32 (m, 2H), 3.32–3.24 (m, 1H), 3.00 (s, 3H), 2.87–2.78 (m, 1H), 2.53–2.44 (m, 1H), 2.43–2.34 (m, 1H), 2.21–2.08 (m, 2H), 1.99–1.90 (m, 1H), 1.87–1.78 (m, 1H), 1.71–1.61 (m, 1H), 1.60–1.50 (m, 1H), 1.44 (s, 9H), 1.39–1.29 (m, 1H), 0.96–0.90 (m, 12H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.9, 172.6, 171.0, 168.01, 167.96, 156.5, 137.0, 129.5, 128.5, 126.9, 79.4, 70.6, 59.1, 58.4, 56.4, 52.42, 52.38, 46.9, 41.7, 41.3, 40.4, 35.1, 31.1, 29.9, 29.0, 28.5, 25.2, 24.9, 23.2, 22.3, 19.4, 18.0 ppm; HRMS (ESI) m/z calcd for C36H58N5O9+ (M+H)+ 704.4229, found 704.4229.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3S,4S)-4-((S)-2-(tert-butoxycarbonyl)-3-hydroxypropanamido)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-N-methylacetamido)-3-phenylpropanoyl) pyrrolidine-2-carboxylate ((3S,4S)-21)
Following the same procedure used for the preparation of 21 as described above, (3S,4S)-20 (704 mg, 1.00 mmol), instead of 20, was employed to produce the desired compound (3S,4S)-21 as a colorless oil (290 mg, 37%). [α]D23.0 +14.50 (c 1.00, MeOH); IR (film) νmax 3442, 2958, 1748, 1645, 1533, 1454, 1367, 1173, 1056 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.30 (m, 1H), 7.26–7.17 (m, 5H), 7.15–7.08 (m, 1H), 5.79–5.72 (m, 1H), 5.58–5.49 (m, 1H), 4.60–4.49 (m, 1H), 4.43–4.38 (m, 1H), 4.38–4.32 (m, 1H), 4.22–3.87 (m, 6H), 3.73 (s, 3H), 3.68–3.59 (m, 1H), 3.42–3.34 (m, 1H), 3.33–3.26 (m, 1H), 3.04 (s, 3H), 2.88–2.76 (m, 1H), 2.65–2.53 (m, 1H), 2.43–2.34 (m, 1H), 2.25–2.16 (m, 1H), 2.14–2.06 (m, 2H), 1.98–1.76 (m, 3H), 1.75–1.56 (m, 2H), 1.43 (s, 9H), 0.98–0.86 (m, 12H) ppm; 13C NMR (100 MHz, CDCl3) δ 172.7, 172.3, 172.2, 171.9, 168.5, 168.1, 156.2, 137.0, 129.5, 128.5, 126.9, 80.6, 70.6, 62.9, 59.0, 58.7, 56.5, 56.0, 52.4, 52.0, 46.9, 41.3, 41.0, 40.1, 35.2, 30.7, 30.1, 29.0, 28.4, 25.1, 24.9, 23.3, 22.2, 19.5, 18.0 ppm; HRMS (ESI) m/z calcd for C39H63N6O11+ (M+H)+ 791.4549, found 791.4550.
(S)-Methyl 1-((R)-2-(2-((S)-2-((3S,4S)-4-((S)-2-((S)-2-((R)-2-((2S,3S)-2-(dimethylamino)-3-methylpentanoyloxy)-3-methylbutanamido)-3-(4-hydroxyphenyl)propanamido)-3-hydroxypropanamido)-3-hydroxy-6-methylheptanamido)-3-methylbutanamido)-Nethylacetamido)-3-phenylpropanoyl)pyrrolidine-2-carboxylate (3c)
Following the same procedure used for the preparation of 3a as described above, (2S,3S)-18 (187 mg, 0.36 mmol), instead of 18, and (3S,4S)-20 (240 mg, 0.30 mmol), instead of 20, were employed to produce the desired compound 3c as a colorless amorphous solid (70 mg, 21%). [α]D21.1 +27.6 (c 1.00, MeOH); IR (film) νmax 3450, 2965, 1745, 1654, 1545, 1444, 1203, 1132, 1052 cm−1; 1H NMR (600 MHz, Acetonitrile-d3) δ 7.69–7.62 (m, 1H), 7.62–7.58 (m, 1H), 7.32–7.26 (m, 1H), 7.26–7.19 (m, 5H), 7.19–7.14 (m, 1H), 7.05 (d, J = 8.2 Hz, 2H), 7.02–6.94 (m, 1H), 6.70 (d, J = 8.2 Hz, 2H), 5.47 (t, J = 7.4 Hz, 1H), 4.82 (d, J = 4.5 Hz, 1H), 4.65–4.58 (m, 1H), 4.36–4.30 (m, 1H), 4.27 (dd, J = 8.5, 5.7 Hz, 1H), 4.22–4.15 (m, 1H), 4.07–4.01 (m, 1H), 3.98–3.89 (m, 3H), 3.87–3.79 (m, 2H), 3.78–3.69 (m, 1H), 3.65 (s, 3H), 3.36–3.32 (m, 1H), 3.32–3.27 (m, 1H), 3.21–3.17 (m, 1H), 3.17–3.12 (m, 1H), 2.93 (s, 3H), 2.85 (s, 6H), 2.83–2.78 (m, 1H), 2.78–2.74 (m, 1H), 2.42–2.31 (m, 2H), 2.20–2.07 (m, 3H), 2.03–1.97 (m, 1H), 1.89–1.84 (m, 1H), 1.82–1.73 (m, 2H), 1.64–1.56 (m, 1H), 1.54–1.45 (m, 2H), 1.39–1.28 (m, 2H), 1.00–0.94 (m, 6H), 0.93–0.85 (m, 12H), 0.83 (d, J = 6.8 Hz, 3H), 0.72 (d, J = 6.7 Hz, 3H) ppm; 13C NMR (600 MHz, Acetonitrile-d3) δ 173.3, 172.4, 172.4, 172.2, 171.6, 169.4, 169.3, 168.8, 168.0, 156.5, 138.6, 130.9, 130.1, 128.9, 128.8, 127.0, 115.9, 81.2, 71.6, 71, 62.7, 59.8, 59.5, 57.1, 56.7, 55.7, 52.3, 52.3, 47.4, 42.8, 41.5, 41.1, 40.7, 36.7, 35.1, 33.9, 31.0, 30.9, 30.3, 29.4, 27.3, 25.5, 25.2, 23.3, 22.1, 19.4, 18.8, 17.9, 16.8, 14.0, 11.5 ppm; HRMS (ESI) m/z calcd for C56H87N8O14+ (M+H)+ 1095.6336, found 1095.6340.
Supplementary Material
Figure 1.
The Structures of DMAA Linear Peptides.
Scheme 4.
Preparation of Symplocin A (3a). Reagents and conditions: (a) (1) TFA, DCM; (2) 7, HATU, HOAt, DIPEA, DMF, 59% (2 steps); (b) (1) TFA, DCM; (2) 6, HOBt, EDCI, NMM, DMF, 70% (2 steps); (c) (1) TFA, DCM; (2) 19, HOBt, EDCI, NMM, DCM, 38% (2 steps).
Acknowledgements
We thank the National Natural Science Foundation of China (21472022, 21272041 to B.-G. Wei), Project funded by China Postdoctoral Science Foundation (KLF301012 to C.-M. Si) and National Institutes of Health, USA (AI100776 to TFM) for financial support. The authors thank X. Wang (UCSD) for some NMR data, and Dr. Han-Qing Dong (Arvinas, Inc.) for helpful suggestions.
Footnotes
† Footnotes relating to the title and/or authors should appear here.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.(a) Hamada Y and Shioiri T, Chem. Rev. (Washington, DC, U. S.), 2005, 105, 4441; [DOI] [PubMed] [Google Scholar]; (b) Bionda N and Cudic P, Croat. Chem. Acta, 2011, 84, 315; [Google Scholar]; (c) Salvador-Reyes LA and Luesch H, Nat. Prod. Rep, 2015, 32, 478; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vijayakumar S, Manogar P and Prabhu S, Biomed. Pharmacother, 2016, 83, 362. [DOI] [PubMed] [Google Scholar]
- 2.(a) Simmons TL, McPhail KL, Ortega-Barria E, Mooberry SL and Gerwick WH, Tetrahedron Lett, 2006, 47, 3387; [Google Scholar]; (b) Maderna A and Leverett CA, Mol. Pharmaceutics, 2015, 12, 1798; [DOI] [PubMed] [Google Scholar]; (c) Gunasekera SP, Imperial L, Garst C, Ratnayake R, Dang LH, Paul VJ and Luesch H, J. Nat. Prod, 2016, 79, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luesch H, Moore RE, Paul VJ, Mooberry SL and Corbett TH, J. Nat. Prod, 2001, 64, 907. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gerber H-P, Koehn FE, Abraham RT, Nat. Prod. Rep, 2013, 30, 625; [DOI] [PubMed] [Google Scholar]; (b) Perez EA, Hillman DW, Fishkin PA, Krook JE, Tan WW, Kuriakose PA, Alberts SR and Dakhil SR, Investigational New Drugs, 2005, 23, 257; [DOI] [PubMed] [Google Scholar]; (c) Kindler HL, Tothy PK, Wolff R, McCormack RA, Abbruzzese JL, Mani S, Wade-Oliver KT and Vokes EE, Investigational New Drugs, 2005, 23, 489. [DOI] [PubMed] [Google Scholar]
- 5.Kwan JC, Eksioglu EA, Liu C, Paul VJ and Luesch H, J Med Chem, 2009, 52, 5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinski TF, Reynolds KA and Morinaka BI, J. Nat. Prod, 2012, 75, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marfey P, Carlsberg Res. Commun, 1984, 49, 591. [Google Scholar]
- 8.(a) Ma J-Y, Xu L-F, Huang W-F, Wei B-G and Lin G-Q, Synlett, 2009, 1307; [Google Scholar]; (b) Ma J-Y, Huang W and Wei B-G, Tetrahedron Lett, 2011, 52, 4598; [Google Scholar]; (c) Huang W, Ren R-G, Dong H-Q, Wei B-G and Lin G-Q, J. Org. Chem, 2013, 78, 10747; [DOI] [PubMed] [Google Scholar]; (d) Ren R-G, Ma J-Y, Mao Z-Y, Liu Y-W and Wei B-G, Chin. Chem. Lett, 2015, 26, 1209; [Google Scholar]; (e) Mao Z-Y, Si C-M, Liu Y-W, Dong H-Q, Wei B-G and Lin G-Q, J. Org. Chem, 2016, 81, 9903; [DOI] [PubMed] [Google Scholar]; (f) Huang X, Huang W, Li L, Sun X, Song S, Xu Q, Zhang L, Wei B-G and Deng X, Mol. Pharmaceutics, 2016, 13, 3756. [DOI] [PubMed] [Google Scholar]
- 9.(a) Si C-M, Huang W, Du Z-T, Wei B-G and Lin G-Q, Org. Lett, 2014, 16, 4328; [DOI] [PubMed] [Google Scholar]; (b) Si C-M, Mao Z-Y, Dong H-Q, Du Z-T, Wei B-G and Lin G-Q, J. Org. Chem, 2015, 80, 5824; [DOI] [PubMed] [Google Scholar]; (c) Si C-M, Mao Z-Y, Liu Y-W, Du Z-T, Wei B-G and Lin G-Q, Org. Chem. Front, 2015, 2, 1485; [Google Scholar]; (d) Liu YW, Han P, Zhou W, Mao ZY, Si CM and Wei BG, Org Biomol Chem, 2016, 14, 10714. [DOI] [PubMed] [Google Scholar]
- 10.Cheung ST, Benoiton NL, Can. J. Chem, 1977, 55, 906. [Google Scholar]
- 11.(a) Carpino LA, J. Am. Chem. Soc, 1993, 115, 4397; [Google Scholar]; (b) Carpino LA, El-Faham A, Albericio F, Tetrahedron Lett, 1994, 35, 2279; [Google Scholar]; (c) Carpino LA, El-Faham A, J. Org. Chem, 1994, 59, 695; [Google Scholar]; (d) Carpino LA, El-Faham A, Albericio F, J. Org. Chem, 1995, 60, 3561. [Google Scholar]
- 12.König W, Geiger R, Chem. Ber, 1970, 103, 788. [DOI] [PubMed] [Google Scholar]
- 13.Kende AS, Dong H-Q, Mazur AW, Ebetino FH, Tetrahedron Lett, 2001, 42, 6015. [Google Scholar]
- 14.Jentoft N, Dearborn DG, Methods Enzymol, 1983, 91, 570. [DOI] [PubMed] [Google Scholar]
- 15.(a) Jeffrey PD, McCombie SW, J. Org. Chem, 1982, 47, 587; [Google Scholar]; (b) Mastalerz H, Vinet V, J. Chem. Soc., Chem. Commun 1987, 1283. [Google Scholar]
- 16.(a) Harrigan GG, Luesch H, Yoshida WY, Moore RE, Nagle DG, Paul VJ, Mooberry SL, Corbett TH and Valeriote FA, J. Nat. Prod, 1998, 61, 1075; [DOI] [PubMed] [Google Scholar]; (b) Harrigan GG, Luesch H, Yoshida WY, Moore RE, Nagle DG and Paul VJ, J. Nat. Prod, 1999, 62, 655; [DOI] [PubMed] [Google Scholar]; (c) Luesch H, Yoshida WY, Moore RE, Paul VJ, Mooberry SL and Corbett TH, J. Nat. Prod, 2002, 65, 16; [DOI] [PubMed] [Google Scholar]; (d) Conroy T, Guo J-T, Hunt NH and Payne RJ, Org. Lett, 2010, 12, 5576; [DOI] [PubMed] [Google Scholar]; (e) Linington RG, Clark BR, Trimble EE, Almanza A, Ureña L-D, Kyle DE, Gerwick WH, J. Nat. Prod, 2009, 72, 14; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Conroy T, Guo JT, Linington RG, Hunt NH and Payne RJ, Chem. - Eur. J, 2011, 17, 13544; [DOI] [PubMed] [Google Scholar]; (g) Yamada K, Ojika M, Kigoshi H, Suenaga K, Nat. Prod. Rep, 2009, 26, 27. [DOI] [PubMed] [Google Scholar]
- 17.Rich DH, J. Med. Chem, 1985, 28, 263. [DOI] [PubMed] [Google Scholar]
- 18.(a) Preciado A and Williams PG, J. Org. Chem, 2008, 73, 9228; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liang Z, Sorribas A, Sulzmaier FJ, Jimenez JI, Wang X, Sauvage T, Yoshida WY, Wang G, Ramos JW, Williams PG, J. Org. Chem, 2011, 76, 3635; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rinehart KL, Sakai R, Kishore V, Sullins DW and Li KM, J. Org. Chem, 1992, 57, 3007. [Google Scholar]
- 19.Matsunaga shows evidence of fragmentation of statine residues by retroaldol reaction under acid hydrolysis.Sun Y, Takada K, Nogi Y, Okada S and Matsunaga S, J Nat Prod, 2014, 77, 1749..
- 20.(a) Omura K, Swern D, Tetrahedron, 1978, 34, 1651; [Google Scholar]; (b) Mancuso AJ and Swern D, Synthesis, 1981, 165. [Google Scholar]
- 21.The errors in stereoassignment of symplocin A, specifically pertaining to the HPLC analysis of O-(2’-naphthacyl)-α-DMAA esters, is under investigation in one of our laboratories (TFM).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.