Abstract
Background/Aims
Current evidence suggests the presence of motility or functional abnormalities in one area of the gastrointestinal tract increases the likelihood of abnormalities in others. However, the relationship of gastroparesis to chronic constipation (slow transit constipation and dyssynergic defecation) has been incompletely evaluated.
Methods
We retrospectively reviewed the records of all patients with chronic dyspeptic symptoms and constipation who underwent both a solid gastric emptying scintigraphy and a high-resolution anorectal manometry at our institution since January 2012. When available, X-ray defecography and radiopaque marker colonic transit studies were also reviewed. Based on the gastric emptying results, patients were classified as gastroparesis or dyspepsia with normal gastric emptying (control group). Differences in anorectal and colonic findings were then compared between groups.
Results
Two hundred and six patients met the inclusion criteria. Patients with gastroparesis had higher prevalence of slow transit constipation by radiopaque marker study compared to those with normal emptying (64.7% vs 28.1%, P = 0.013). Additionally, patients with gastroparesis had higher rates of rectocele (88.9% vs 60.0%, P = 0.008) and intussusception (44.4% vs 12.0%, P = 0.001) compared to patients with normal emptying. There was no difference in the rate of dyssynergic defecation between those with gastroparesis vs normal emptying (41.1% vs 42.1%, P = 0.880), and no differences in anorectal manometry findings.
Conclusions
Patients with gastroparesis had a higher rate of slow transit constipation, but equal rates of dyssynergic defecation compared to patients with normal gastric emptying. These findings argue for investigation of possible delayed colonic transit in patients with gastroparesis and vice versa.
Keywords: Constipation, Dyspepsia, Gastroparesis, Pelvic floor disorders
Introduction
Constipation is common in the general adult population, with an estimated prevalence of 16%.1 Similarly, dyspepsia symptoms, such as nausea, vomiting, pain, bloating, and postprandial discomfort are also common in the general population, with an estimated prevalence of 29.5%.2 Together, these symptoms account for significant morbidity and economic burden.1,3
Constipation is often classified as either normal transit constipation (NTC), slow transit constipation (STC), or dyssynergic defecation (DD).4 The diagnosis of STC vs NTC is made based on delay in colonic motility as assessed by either radiopaque marker testing, whole gut scintigraphy, wireless motility capsule, or colonic manometry.4 DD on the other hand is characterized by inappropriate contraction or failed relaxation of the pelvic floor muscles during defecation.1,5 This may lead to treatment-refractory constipation, incomplete evacuation, excessive straining, and the need for digital disimpaction or enema use.6 The diagnosis is based on the use of multiple modalities, including high-resolution anorectal manometry (HRAM), balloon expulsion test, electromyography, and X-ray or MRI defecography.7 Given their low specificity, at least 2 abnormal methods of testing should be observed before a diagnosis is made.8–10
Several prior studies have shown a significant overlap between dyspeptic and lower gastrointestinal (GI) symptoms, such as constipation and irritable bowel syndrome.11–13 Additionally, prior studies suggest a link between gastroparesis and both esophageal dysmotility14 and small bowel dysmotility.15 These relationships suggest that a common pathophysiology may explain symptoms in multiple areas of the GI tract.
However, the link between constipation and gastroparesis remains incompletely evaluated. One prior study of patients with chronic nausea demonstrated a 45.6% rate of DD. However, this study was limited in its evaluation of gastroparesis, as only 16 of 149 patients had delayed gastric emptying.16 Another study utilizing wireless motility capsule in patients with suspected gastroparesis found no relationship between delayed gastric emptying and both delayed colonic transit time and constipation symptoms. However, this study did not evaluate dyssynergic defecation and anorectal manometry findings.15
This study evaluates the prevalence of STC and DD in patients with gastroparesis using patients with dyspeptic symptoms and normal gastric emptying as controls. We retrospectively identified a cohort of patients who had both gastric emptying scintigraphy (GES) and HRAM performed in the evaluation of dyspeptic symptoms and constipation respectively. When available, X-ray defecography and radiopaque marker colonic transit studies were also evaluated. We hypothesized that STC would be more prevalent in patients with gastroparesis compared with normal gastric emptying given the correlation of motility disorders in other areas of the GI tract. On the other hand, we predicted there would not be a major difference in the prevalence of DD given different neural innervations of the stomach and anorectal area.15,17,18
Materials and Methods
Patient Cohort
The Stanford Translational Research Integrated Database Environment (STRIDE) interface was used to search the electronic medical records to return an appropriate cohort for our study.19 We specifically searched for all patients who had both a solid GES and HRAM, with both occurring between January 2012 and March 2018. All records were reviewed with the aid of the STRIDE interface. Patients were excluded if their studies were incomplete, if they did not have a solid GES, if they had rapid gastric emptying, or if they did not have constipation. The institutional Review Board at Stanford University approved the study (IRB No. 45762).
Gastric Emptying Study
Only patients with a solid GES were included. All patients had dyspeptic symptoms at the time the studies were completed and had undergone an appropriate evaluation to rule out other organic causes of dyspepsia. Studies were conducted according to a standard protocol. A standard meal consisting of eggbeater, toast, jam, and water was labeled with Technetium 99m sulphur colloid 0.528 mCi. Subsequent static images were obtained at 1, 2, 3, and 4 hours. Occasionally, the 4-hour time point was not acquired if the 3-hour time point had emptying of > 90%. Experienced staff radiologists interpreted all images. Normal ranges for gastric emptying were defined according to the consensus recommendations of the American Neuro-gastroenterology and Motility Society and the Society of Nuclear Medicine20 and were as follows: at 1 hour 10–70%, at 2 hours 40–100%, at 3 hours 70–100%, and at 4 hours 90–100%. Patients with rapid gastric emptying (1-hour time point with > 70% emptying) were eliminated from our analysis due to small numbers. Patients with a delay at any time point (below the normal range) were classified as having gastroparesis. Patients with no abnormalities in gastric emptying were classified as having dyspepsia with normal gastric emptying.
High-resolution Anorectal Manometry
HRAM was performed using a protocol similar to that recommended by the International Anorectal Physiology Working Group and the International Working Group for Disorders of Gastrointestinal Motility and Function.7 First, mean resting pressure, maximal resting pressure, maximal squeeze pressure, and high-pressure zone length were recorded. Next, the response of the pelvic floor muscles to pseudo-defecation was measured by either anorectal manometry or electromyography. Abnormal pseudo-defecation was defined as either a failure of the pelvic floor to relax, or an increase in activity of the pelvic floor muscles in response to pseudo-defecation. Next, recto-anal inhibitor reflex (RAIR) measurements and rectal sensation testing were performed using slow balloon inflation. An abnormal RAIR was defined as the absence of anal sphincter relaxation at 60 mL of rectal balloon inflation. Finally, the patient was taken to the bathroom and asked to expel the balloon inflated to 60 mL. Failure to do so within 1 minute was defined as an abnormal balloon expulsion test (BET). We defined DD only if at least 2 tests of pelvic floor dysfunction were abnormal (anorectal manometry/electromyography, BET, and defecography).8–10 A staff gastroenterologist or colorectal surgeon trained in anorectal manometry interpreted all studies.
X-ray Defecography
A rectal tube was placed and 180 mL of thin barium contrast was infused, followed by 180 mL of thick barium contrast paste. Fluoroscopic images were then obtained in the neutral state, during contraction of the pelvic floor, during bearing down, and during defecation. Experienced staff radiologists interpreted all studies. Rectal intussusception was defined as invagination of the rectal wall not extending beyond the anal verge. If extension did occur beyond the anal verge, then this was reported as rectal prolapse. Rectocele was defined by the interpreting radiologist as small, medium, or large. Exact measurements were not available in most cases. Incomplete contrast emptying was defined as abnormal contrast retention after defecation. Finally, incomplete pelvic floor relaxation was defined during defecation as either an increase in pelvic floor muscle tone, or a failure of relaxation. Presence of incomplete relaxation or incomplete defecation were used as evidence of dyssynergic defecation.21
Radiopaque Marker Colonic Transit Study
Oral administration of a Sitzmark capsule (Konsyl Pharmaceuticals, Easton, MD, USA) containing 24 individual markers was completed on day 1 of the study. The patient returned on day 5 for an abdominal x-ray. Experienced staff radiologists counted the remaining markers and their location. Studies with greater than 5 markers retained on day 5 were considered to have STC.22,23 Given prior reports suggesting that there is no correlation between colonic marker location and presence of DD assessed by BET, we did not diagnose DD based on radiopaque marker testing.22
Statistical Methods
HRAM, defecography, and radiopaque marker findings were compared between patients with gastroparesis and normal gastric emptying. Continuous variables with a normal distribution were expressed as means and compared using a 2-sample t test. Continuous variables with a non-normal distribution were expressed as medians and compared using the Wilcoxon rank sum test. Binary and categorical variables were expressed as percentages and compared using the chi-square test. If a variable had a significant result at α = 0.05, then further testing with logistic regression was done to evaluate for confounders. The logistic regression model incorporated the following variables: gender, age, body mass index, diabetes, dysautonomia (defined by autonomic testing or presence of autonomic syndromes outside the GI tract), irritable bowel syndrome, Ehlers-Danlos syndrome, presence of incontinence, presence of loose stools, and type of dyspepsia (nausea/emesis, pain/burning, and bloating/early satiety). Backward elimination was used to remove variables that did not significantly contribute to the regression model at a significance cutoff of P ≤ 0.15. All statistical analysis was done using Stata version 13.1 (StataCorp LLC, College Station, TA, USA). In all cases P ≤ 0.05 was considered significant.
Results
Baseline Demographics
Two hundred sixteen patients had both a GES and HRAM performed. Seven patients who had rapid gastric emptying, and 3 patients who did not have constipation were eliminated from further analysis. The remaining 206 patients were included in the final analysis. Of those 133 had normal gastric emptying and 73 had gastroparesis. Patients with gastroparesis were slightly younger (mean age 42.2 vs 48.8, P = 0.005) and had higher prevalence of Ehlers-Danlos syndrome (13.7% vs 5.3%, P = 0.035). In patients with gastroparesis, there was a trend to higher prevalence of dysautonomia (26.0% vs 15.8%, P = 0.076) but this did not reach significance. Otherwise both groups were balanced in terms of baseline characteristics (Table 1).
Table 1.
Baseline Demographics
| Variable | Normal gastric emptying (n = 133) | Gastroparesis (n = 73) | P-value |
|---|---|---|---|
| Age (mean ± SE)a | 48.8 ± 1.44 | 42.2 ± 1.65 | 0.005 |
| Female (%) | 84.2 | 91.8 | 0.124 |
| BMI (mean ± SE) | 25.3 ± 0.50 | 25.3 ± 0.86 | 0.973 |
| Race/ethnicity (%) | 0.411 | ||
| White (non-Hispanic) | 65.4 | 58.9 | |
| Black | 3.0 | 1.4 | |
| Hispanic/Latino | 11.3 | 19.2 | |
| Asian | 6.8 | 2.7 | |
| Other | 12.0 | 16.4 | |
| Unknown | 1.5 | 1.4 | |
| Diabetes | 17.3 | 20.6 | 0.565 |
| Dysautonomia | 15.8 | 26.0 | 0.076 |
| IBS | 36.1 | 31.5 | 0.508 |
| Ehlers Danlos syndrome | 5.3 | 13.7 | 0.035 |
| Lower GI symptoms (besides constipation) (%) | |||
| Incontinence | 14.3 | 16.4 | 0.679 |
| Loose stool | 18.8 | 24.7 | 0.322 |
| Predominant dyspeptic symptom (%) | 0.736 | ||
| Bloating/satiety | 36.1 | 39.7 | |
| Nausea/emesis | 25.6 | 27.4 | |
| Pain/burning | 38.4 | 32.9 | |
| Median time between GES and HRAM (IQR) | 70 (16–226) | 83 (14–232) | 0.761 |
| Median time between GES and X-ray defecography (IQR)b | 73 (14–192) | 159 (49–263) | 0.202 |
| Median time between GES and radiopaque marker test (IQR)c | 93 (12–201) | 103 (17–296) | 0.546 |
Age at the time of anorectal manometry.
Only 77 patients had both defecography and gastric emptying scintigraphy (GES).
Only 49 patients had both a radiopaque marker test and GES.
BMI, body mass index; IBS, irritable bowel syndrome; HRAM, high-resolution anorectal manometry; IQR, interquartile range.
Radiopaque Marker Colonic Transit Study
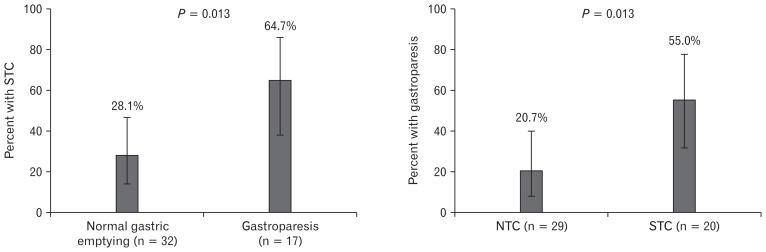
Forty-nine of 206 patients had a radiopaque marker study performed during the study period. The baseline characteristics and prevalence of gastroparesis in this cohort were similar to those who did not undergo radiopaque marker testing (data not shown). Patients with gastroparesis were more likely to have STC than those with normal gastric emptying (64.7% vs 28.1%, P = 0.013) (Fig. 1). Multiple logistic regression did not reveal any significant confounding variables, and STC remained a significant predictor of gastroparesis after adjustment (odds ratio [OR], 5.30; 95% confidence interval [95% CI], 1.28–21.9) (Table 2). Ehlers-Danlos syndrome was also an independent predictor of gastroparesis after adjustment (OR, 13.7; 95% CI, 1.26–150) (Table 3). Conversely, patients with STC were more likely to have gastroparesis than normal gastric emptying (55.0% vs 20.7%, P = 0.013). Gastroparesis remained an independent predictor of STC using multiple logistic regression (OR, 4.69; 95% CI, 1.33–16.5) (data not shown). No other variables in this logistic regression model were predictive of STC.
Figure 1.
Overlap between gastroparesis and slow transit constipation. Of the 206 total study patients, 49 had radiopaque marker colonic motility studies performed. Slow transit constipation (STC) was defined as greater than 5 markers retained on day 5. Otherwise patients were defined as normal transit constipation (NTC). Rates of STC in patients with normal gastric emptying and gastroparesis were compared using the chi square test (left panel). Likewise rates of gastroparesis in patients with NTC and STC were compared using the chi square test (right panel). Error bars represent 95% confidence intervals.
Table 2.
Predictors of Gastroparesis by Multiple Logistic Regression
| Predictors of gastroparesis | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| Radiopaque marker colonic transit test | ||||
| Delayed transit constipation | 4.69 (1.33–16.5) | 0.016 | 5.30 (1.28–21.9) | 0.021 |
| Ehlers Danlos syndrome | 12.9 (1.37–122) | 0.026 | 13.7 (1.26–150) | 0.032 |
| Rectal intussusception (defecography) | ||||
| Rectal intussusception | 5.87 (1.87–18.3) | 0.002 | 5.88 (1.82–19.0) | 0.003 |
| Ehlers Danlos syndrome | 4.47 (1.02–19.6) | 0.047 | 4.50 (0.94–21.6) | 0.060 |
| Rectocele (defecography) | ||||
| Rectocele | 5.33 (1.41–20.1) | 0.013 | 5.44 (1.38–21.3) | 0.015 |
| Ehlers Danlos syndrome | 4.47 (1.02–19.6) | 0.047 | 4.63 (0.95–22.5) | 0.057 |
Three separate logistic regression models were run for the 3 predictor variables of interest (slow transit constipation, rectal intussusception, and rectocele). Other variables included in all models were as follows: gender, age, body mass index, diabetes, dysautonomia, irritable bowel syndrome, Ehlers-Danlos syndrome, presence of incontinence, presence of loose stools, and type of dyspepsia (nausea/emesis, pain/burning, and bloating/early satiety). Variables that were not predictive were removed from the model using backward elimination at a significance level of 0.15. Unadjusted odds ratios (ORs) were calculated without adjustment for these variables. Adjusted ORs were calculated after taking the effects of these variables into account.
Table 3.
Anorectal Manometry Findings Among Patients With Gastroparesis and Normal Gastric Emptying
| Variable | Normal gastric emptying (n = 133) | Gastroparesis (n = 73) | P-value |
|---|---|---|---|
| Mean resting anal pressure (mean ± SE, mmHg) | 69.5 ± 2.3 | 70.7 ± 3.2 | 0.747 |
| Maximal resting anal pressure (mean ± SE, mmHg) | 78.9 ± 2.6 | 84.1 ± 5.7 | 0.344 |
| Anal squeeze pressure (mean ± SE, mmHg) | 155.4 ± 5.6 | 150.6 ± 6.9 | 0.600 |
| HPZ length (mean ± SE, cm) | 3.24 ± 0.09 | 3.16 ± 0.14 | 0.607 |
| First sensation (median [IQR], mL) | 40 (20–60) | 30 (20–55) | 0.211 |
| First urge (median [IQR], mL) | 80 (60–100) | 60 (45–100) | 0.180 |
| First pain (median [IQR], mL) | 115 (80–170) | 110 (75–148) | 0.132 |
| RAIR abnormal (%)a | 16.8 | 15.5 | 0.811 |
| Pseudodefecation abnormal (%)b | 75.6 | 81.7 | 0.318 |
| BET abnormal (%)c | 38.0 | 39.4 | 0.840 |
| Diagnosis of dyssynergic defecation (%)d | 42.1 | 41.1 | 0.888 |
Abnormal if not present at 60 mL of balloon inflation.
Abnormal if either failed relaxation or increased contraction of the pelvic floor muscles during pseudo-defecation.
Abnormal if failed expulsion of balloon inflated to 60 mL within 1 minute.
Diagnosed if at least 2 tests of pelvic floor dysfunction were abnormal (anorectal manometry/electromyography, balloon expulsion test, and defecography).
HPZ, high pressure zone; IQR, interquartile range; RAIR, rectoanal inhibitory reflex; BET, balloon expulsion test.
High-resolution Anorectal Manometry
All 206 patients had both a GES and an HRAM. There were no differences in HRAM findings between patients with gastroparesis and normal gastric emptying (Table 3). There was no difference in rate of DD (defined as an abnormality on at last 2 modes of testing) between patients with gastroparesis (41.1%) and normal gastric emptying (42.1%), P = 0.880. Similarly, there was no difference in the rate of gastroparesis among patients with DD (34.9%) and without DD (35.8%), P = 0.880.
We obtained similar results when restricting the analysis to patients who had an HRAM and GES within 90 days of each other (data not shown). Additionally, in an analysis of patients who had a radiopaque marker study, there were no differences in HRAM findings when stratified by NTC and STC (Table 4).
Table 4.
Anorectal Manometry Findings Among Patients With Normal and Delayed Transit Constipation
| Variable | Normal transit constipation (n = 29) | Delayed transit constipation (n = 20) | P-value |
|---|---|---|---|
| Mean resting anal pressure (mean ± SE, mmHg) | 71.6 ± 4.05 | 72.4 ± 7.35 | 0.926 |
| Maximal resting anal pressure (mean ± SE, mmHg) | 81.5± 5.2 | 93.7 ± 5.5 | 0.424 |
| Anal squeeze pressure (mean ± SE, mmHg) | 158 ± 14.1 | 169.8 ± 11.3 | 0.556 |
| HPZ length (mean ± SE, cm) | 2.85 ± 0.17 | 3.31 ± 0.24 | 0.116 |
| First sensation (median [IQR], mL) | 30 (20–50) | 30 (20–50) | 0.668 |
| First urge (median [IQR], mL) | 70 (60–120) | 60 (50–120) | 0.865 |
| First pain (median [IQR], mL) | 130(90–180) | 110 (85–170) | 0.453 |
| RAIR abnormal (%)a | 10.7 | 21.1 | 0.329 |
| Pseudodefecation abnormal (%)b | 72.4 | 85.0 | 0.299 |
| BET abnormal (%)c | 37.9 | 50.0 | 0.401 |
| Diagnosis of dyssynergic defecation (%)d | 51.7 | 50.0 | 0.906 |
Abnormal if not present at 60 mL of balloon inflation.
Abnormal if either failed relaxation or increased contraction of the pelvic floor muscles during pseudo-defecation.
Abnormal if failed expulsion of balloon inflated to 60 mL within 1 minute.
Diagnosed if at least 2 tests of pelvic floor dysfunction were abnormal (anorectal manometry/electromyography, balloon expulsion test, and defecography).
HPZ, high pressure zone; IQR, interquartile range; RAIR, rectoanal inhibitory reflex; BET, balloon expulsion test.
X-ray Defecography
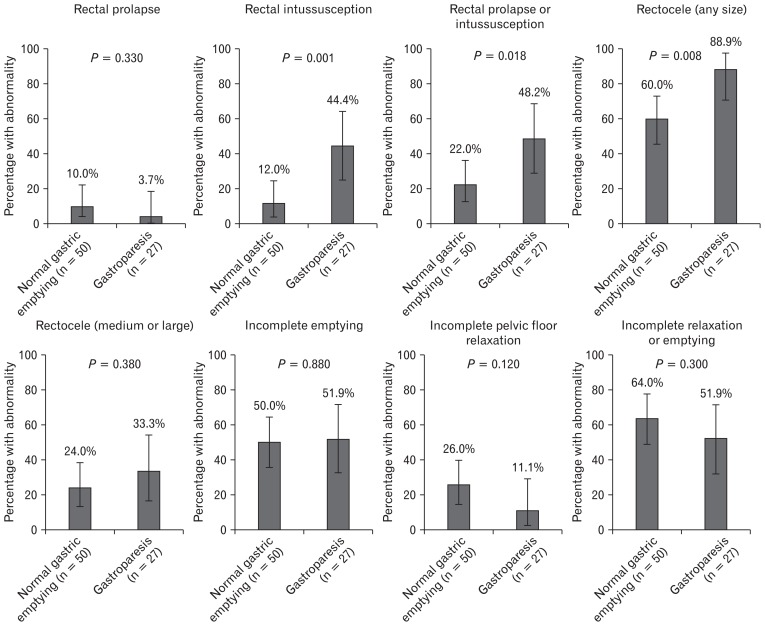
Seventy-seven of the 206 patients underwent defecography. Patients undergoing defecography were more likely to be female (96.1% vs 81.4% P = 0.002) and white (76.6% vs 55.0%, P = 0.009) than those not undergoing defecography. Otherwise baseline characteristics were similar (data not shown). Rectal intussusception (defined as rectal invagination not extending beyond the anal canal) was higher in patients with gastroparesis compared to those with normal gastric emptying (44.4% vs 12.0%, P = 0.001). When including patients who had rectal prolapse as well, the result remained significant (22.0% vs 48.2%, P = 0.018) (Fig. 2). Multiple logistic regression did not reveal any significant confounding variables, and intussusception remained a significant predictor of gastroparesis after adjustment (OR, 5.88; 95% CI, 1.82–19.0) (Table 2). The presence of a rectocele of any size was also higher in those who had gastroparesis vs normal gastric emptying (88.9% vs 60.0%, P = 0.008). However, there was no difference when limiting the analysis to medium/large rectoceles (33.3% vs 24.0%, P = 0.380) (Fig. 2). Multiple logistic regression did not reveal any significant confounding variables, and presence of any sized rectocele remained a significant predictor of gastroparesis after adjustment (OR, 5.44; 95% CI, 1.39–21.3) (Table 2). Incomplete pelvic floor relaxation and incomplete rectal emptying rates were similar between patients with normal gastric emptying and gastroparesis (Fig. 2).
Figure 2.
X-ray defecography findings in patients with gastroparesis and normal gastric emptying. Of the 206 total study patients, 77 had defecography performed. Defecography was performed in the standard fashion with standard interpretations as described in the methods section. Findings on defecography were compared between patients with gastroparesis and normal gastric emptying using the chi-square test. Error bars represent 95% confidence intervals.
Discussion
Common pathogenic mechanisms may underlie dysfunction in different areas of the GI lumen. As such, it is important to consider potential involvement of multiple GI regions when treating a specific set of symptoms. Studies show a high prevalence of lower GI symptoms in those with upper GI symptoms and vice versa.11–13 Patients with gastric dysmotility are more likely to have esophageal dysmotility,14 while patients with gastroparesis are more likely to have delayed small bowel transit, as assessed by wireless motility capsule.15 Additionally, patients with chronic intestinal pseudo-obstruction have a high prevalence of motility abnormalities in other areas of the GI tract and an abnormal esophageal manometry is predictive of poor outcomes.24
Our study documented that patients with gastroparesis are more likely to have STC (assessed by radiopaque marker testing) than patients with dyspeptic symptoms and normal gastric emptying. This fits with the general principle that the presence of dysmotility in one area of the GI tract increases the chances for dysmotility in another. This correlation argues for the use of colonic motility testing or aggressive constipation treatment in patients with refractory gastroparesis symptoms, as such symptoms may be related to delayed colonic transit as opposed to delayed gastric emptying. Conversely, testing for delayed gastric emptying should also be considered under certain circumstances in those with refractory constipation, as recommended in the 2013 AGA guideline on constipation treatment.8
Our study is contrary to a recent study utilizing the wireless motility capsule system which showed that patients with delayed gastric emptying were not more likely to have delayed colonic transit compared with normal gastric emptying.15 The discordance of results may be due to different testing modalities, as concordance between radiopaque marker testing and wireless capsule motility is 64–87%, and concordance between wireless motility capsule and GES is 35–81%.25 Since radiopaque markers do not necessarily measure just colonic delay, it is possible that a delay in small intestinal or gastric transit times could also partially explain marker retention. However, this contribution is likely less important as the highest component of whole gut transit time is colonic.26 It is currently unclear which technologies for assessing motility are superior, as there is no clear gold standard. However, radiopaque marker testing has a long track record of use, and is a simple, relatively low-cost test to administer.
Despite differences in colonic motility, there were no differences in HRAM findings and DD when comparing gastroparesis to normal gastric emptying. DD was present in gastroparesis patients at a rate of 41.1%, and normal gastric emptying at a rate of 42.1%. These numbers are similar to another study of patients presenting for evaluation of chronic nausea, where 45.6% of patients had DD.16 This study did not report rates for gastroparesis and normal gastric emptying patients separately. The reason for a concordance between gastroparesis and slowed colonic motility but not DD may be related to differential innervation patterns. Sacral nerve roots provide much of the extrinsic innervation to the pelvic floor and distal colon, and the pelvic floor contains a high degree of skeletal muscle under voluntary control.27 In contrast, the extrinsic innervation to the rest of the GI tract and colon is largely from the autonomic nervous system via the vagus and thoracolumbar nerves.17,18 This may fit with the argument of a strong central/learned component to dyssynergic defecation,28 as opposed to underlying disordered motility.
We also found a higher rate of rectocele and intussusception in patients with gastroparesis compared to those with normal gastric emptying. However, medium and large rectocele prevalence was no different between the two groups. The significance of these findings is unclear. Small rectoceles were present in 93% of asymptomatic females in one study (but 0% of males).29 Current thought suggests that only larger or highly retentive rectoceles are of clinical importance, with size cutoffs of greater than 2–4 cm cited in the literature.7,21 Likewise, rectal intussusception was present in 20% of healthy subjects in one study, implying a benign condition.29 Though smaller rectoceles and intussusception may not cause DD per se, they may be a consequence of DD.30 However, our study did not show a difference in DD as assessed by any method, so the reason for these radiological differences remains unclear. It is possible that a systemic process that causes the neuromuscular dysfunction in gastroparesis may also be at play in the rectum.
The strengths of our study include the large sample size, the novelty of the reported findings, and the ability to look for potential confounding variables. All patients had a gastric emptying study, so we were able to separate those who had gastroparesis and normal gastric emptying. The study is limited largely by its retrospective nature. Patient selection was not random, and only patients with both upper and lower GI symptoms who underwent both GES and HRAM were captured. Despite this, our cohort may represent a realistic sample seen in an outpatient motility clinic, where patients are unlikely to get a GES or HRAM if they are asymptomatic. Not all patients in our cohort underwent defecography and radiopaque marker studies, which may have introduced bias into those analyses. However, baseline characteristics were similar between those who had such studies performed, and those who did not. We were also unable to control for the use of narcotics in our study, as this was not always accurately recorded in the medical records. However, patients at our institution generally have narcotics stopped before motility testing. Finally, GI evaluations were not necessarily obtained at the same visit, and it is possible that medical changes occurred in the interim.
In conclusion, our study demonstrates a higher rate of STC in patients with gastroparesis than those with normal gastric emptying. However, there were no differences in the prevalence of DD or HRAM findings. Differential innervation patterns of the pelvic floor/rectum and remainder of the GI tract may explain these findings. In the proper clinical context, these findings argue for evaluation of delayed colonic transit in patients with gastroparesis and vice versa. Additional testing may lead to additional diagnoses and treatments, and improve overall symptom burden.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Thomas A Zikos, George Triadafilopoulos, John O Clarke, and Linda A Nguyen: conception/design, data-analysis drafting/revising, and final approval; and Afrin N Kamal, Leila Neshatian, Monica Nandwani: data-analysis drafting/revising and final approval.
References
- 1.Bharucha AE, Pemberton JH, Locke GR., 3rd American gastroenterological association technical review on constipation. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373:1853–1863. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 4.Sbahi H, Cash BD. Chronic constipation: a review of current literature. Curr Gastroenterol Rep. 2015;17:47. doi: 10.1007/s11894-015-0471-z. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 7.Carrington EV, Scott SM, Bharucha A, et al. Expert consensus document: advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol. 2018;15:309–323. doi: 10.1038/nrgastro.2018.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Gastroenterological Association. Bharucha AE, Dorn SD, Lembo A, Pressman A. American gastroenterological association medical position statement on constipation. Gastroenterology. 2013;144:211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 10.Rao SS, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology. doi: 10.1053/j.gastro.2016.02.009. Published Online First: 25 Mar 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW, Crowell MD. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010;8:401–409. doi: 10.1016/j.cgh.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki J, Suzuki H, Asakura K, et al. Classification of functional dyspepsia based on concomitant bowel symptoms. Neurogastroenterol Motil. 2012;24:325–e164. doi: 10.1111/j.1365-2982.2011.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zikos TA, Clarke JO, Triadafilopoulos G, et al. A positive correlation between gastric and esophageal dysmotility suggests common causality. Dig Dis Sci. 2018;63:3417–3424. doi: 10.1007/s10620-018-5175-4. [DOI] [PubMed] [Google Scholar]
- 15.Hasler WL, May KP, Wilson LA, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil. :30. doi: 10.1111/nmo.13196. Published Online First: 5 Sep 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolar GJ, Camilleri M, Burton D, Nadeau A, Zinsmeister AR. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil. 2014;26:131–138. doi: 10.1111/nmo.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 19.Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE--An integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–395. [PMC free article] [PubMed] [Google Scholar]
- 20.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American neurogastroenterology and motility society and the society of nuclear medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim NY, Kim DH, Pickhardt PJ, Carchman EH, Wald A, Robbins JB. Defecography: an overview of technique, interpretation, and impact on patient care. Gastroenterol Clin North Am. 2018;47:553–568. doi: 10.1016/j.gtc.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Staller K, Barshop K, Ananthakrishnan AN, Kuo B. Rectosigmoid localization of radiopaque markers does not correlate with prolonged balloon expulsion in chronic constipation: results from a multicenter cohort. Am J Gastroenterol. 2015;110:1049–1055. doi: 10.1038/ajg.2015.140. [DOI] [PubMed] [Google Scholar]
- 23.Hinton JM, Lennard-Jones JE, Young AC. A ne method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amiot A, Joly F, Cazals-Hatem D, et al. Prognostic yield of esophageal manometry in chronic intestinal pseudo-obstruction: a retrospective cohort of 116 adult patients. Neurogastroenterol Motil. 2012;24:1008–e542. doi: 10.1111/j.1365-2982.2012.01973.x. [DOI] [PubMed] [Google Scholar]
- 25.Stein E, Berger Z, Hutfless S, et al. Wireless motility capsule versus other diagnostic technologies for evaluating gastroparesis and constipation: a comparative effectiveness review. Rockville (MD): 2013. [PubMed] [Google Scholar]
- 26.Diaz Tartera HO, Webb DL, Al-Saffar AK, et al. Validation of Smart-Pill® wireless motility capsule for gastrointestinal transit time: intra-subject variability, software accuracy and comparison with video capsule endoscopy. Neurogastroenterol Motil. 2017;29:1–9. doi: 10.1111/nmo.13107. [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Kim NK. Essential anatomy of the anorectum for colorectal surgeons focused on the gross anatomy and histologic findings. Ann Coloproctol. 2018;34:59–71. doi: 10.3393/ac.2017.12.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neshatian L. The assessment and management of defecatory dysfunction: a critical appraisal. Curr Opin Gastroenterol. 2018;34:31–37. doi: 10.1097/MOG.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 29.Palit S, Bhan C, Lunniss PJ, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Dis. 2014;16:538–546. doi: 10.1111/codi.12595. [DOI] [PubMed] [Google Scholar]
- 30.Felt-Bersma RJ, Tiersma ES, Cuesta MA. Rectal prolapse, rectal intussusception, rectocele, solitary rectal ulcer syndrome, and enterocele. Gastroenterol Clin North Am. 2008;37:645–668. ix. doi: 10.1016/j.gtc.2008.06.001. [DOI] [PubMed] [Google Scholar]