Abstract
Background/Aims
Various foods trigger and/or worsen the symptoms of irritable bowel syndrome (IBS). However, Korean food-related gastrointestinal (GI) symptoms in IBS patients have not yet been investigated. This study aims to evaluate the prevalence of self-reported food intolerance in Korean IBS patients and determine the Korean food items and food groups perceived by patients to worsen their GI symptoms.
Methods
We recruited 393 study subjects, comprising 101 IBS patients, 167 symptomatic non-IBS subjects, and 125 control subjects. All participants completed a questionnaire to identify the most problematic foods and assess the occurrence of GI symptoms caused by 119 Korean food items. They also completed the validated Rome III questionnaire for IBS.
Results
The prevalence of self-reported food intolerance in Korean IBS patients was 79.2%, which was significantly higher than that in control subjects (44.8%, P < 0.001). The most problematic foods reported by IBS patients who experienced food intolerance were high-fat foods (25.0%), gluten foods (23.8%), spicy foods (15.0%), and dairy products (15.0%). A total of 63.4% of IBS patients reported GI symptoms related to the consumption of foods high in fermentable oligo-, di-, mono-saccharides, and polyols (FODMAP), while 48.5% of IBS patients reported symptoms associated with high-fat foods. Gas problems and loose stools were the most frequently reported symptoms.
Conclusions
A large proportion of Korean IBS patients complained of intolerance to certain food items, with high-fat and high-FODMAP foods being the main triggers. This study provides a basis for planning food intervention studies for Korean IBS patients.
Keywords: Diet, Food intolerance, Irritable bowel syndrome, Surveys and questionnaires
Introduction
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder characterized by recurrent abdominal pain related to defecation or altered bowel habits.1 IBS affects about 10.0% of the population worldwide; in Korea, the prevalence of IBS has been reported to be 6.6–8.0%.2–5 IBS is a chronic relapsing disease that greatly impairs quality of life (QOL) and imposes a considerable economic burden on society.6,7
Most IBS patients attribute their symptoms to food, and avoid or reduce their consumption of specific foods to minimize their symptoms.8,9 One Swedish study found that self-reported food intolerance is associated with a high symptom burden and reduced QOL.10 Therefore, dietary recommendations and guidelines for IBS, such as those published by the British Dietetic Association and the National Institute for Health and Care Excellence, are widely used in clinical practice despite a lack of solid evidence.11
Diets low in fermentable oligo-, di-, mono-saccharides, and polyols (FODMAP) have emerged as a first-line therapy for patients with IBS. Many randomized controlled trials have reported that low-FODMAP diets efficiently alleviate gastrointestinal (GI) symptoms in about 70% of IBS patients, and this outcome is similar to that of traditional IBS dietary advice.12–16 Low-FODMAP diets are already being used in clinical practice in Western countries. In fact, a smartphone application (the Monash University Low FODMAP diet App) has been developed to help determine the FODMAP content of foods.17,18 However, the applicability and usefulness of low-FODMAP diets may vary according to the dietary patterns of different countries.
Although the pathophysiology of IBS is still not fully understood, it may involve multiple factors, including visceral hypersensitivity, abnormal GI motility, an altered brain-gut axis, psychological disturbances, and low-grade inflammation.19 Multiple putative mechanisms by which foods or their components affect the gut and interact with the pathophysiology of IBS in various ways determine the type and intensity of IBS symptom.20 Food questionnaires are employed in studies to demonstrate the relationship between food and symptoms; however, standardized questionnaires cannot be used because foods widely vary among different countries. To date, almost all studies investigating the association between IBS and food have been conducted in Western countries and with Western foods, and there has been little research on Asian IBS patients, who have very different dietary behaviors from those of the Western population. Likewise, Korean food items that worsen GI symptoms in Korean IBS patients have not been evaluated, and the characteristics of Korean foods, such as diversity, various food components, and complex cooking methods, make this endeavor more difficult. Thus, the evidence required to apply new dietary treatments to Korean IBS patients is still lacking.
Therefore, the aims of this study are to investigate the prevalence of self-reported food intolerance in Korean IBS patients and determine the Korean food items and food groups perceived by patients to worsen their GI symptoms.
Materials and Methods
Study Population
This study included Korean participants aged 18–80 years who visited gastroenterology outpatient clinics at 9 tertiary hospitals in South Korea between January 2016 and February 2017. Asymptomatic adults were also recruited through public advertisements. Subjects were excluded if they had malignant disease, inflammatory bowel disease, had been diagnosed with any organic disease causing GI symptoms within the last 6 months (gastric ulcer, acute gastroenteritis, cholecystitis, cholangitis, pancreatitis, appendicitis, liver abscess, acute hepatitis, etc), had a severe underlying disease, or were unwilling or unable to complete the questionnaire.
Subjects were allocated to one of the following 3 groups based on their medical history and Rome III symptom data: control group, healthy subjects not reporting GI symptoms and with no underlying organic disease; symptomatic non-IBS group, patients reporting symptoms of abdominal pain or discomfort during the last 3 months but not meeting the Rome III criteria for IBS and with no underlying organic disease; or IBS group, patients reporting symptoms and fulfilling the Rome III criteria for IBS but with no underlying organic disease.21
The study protocol was approved by the institutional review boards of all participating hospitals: Asan Medical Center (No. 2016-0050), Gyeongsang National University Changwon Hospital (No. 2016-11-003), Samsung Medical Center (No. 2016-05-072), Chung-Ang University College of Medicine (No. 1600-004-253), Wonkwang University College of Medicine (No. WKUH 201607-HR-076), National Medical Center (No. H-1605-066-005), Catholic University of Daegu School of Medicine (No. CR-16-136), Seoul National University College of Medicine (No. 1512-090-728), and Keimyung University School of Medicine (No. 2016-01-008). Informed consent was obtained from all subjects.
Questionnaires
All subjects completed the questionnaire developed for data collection in the present study. The validated Korean Rome III questionnaire was used to diagnose IBS.22 IBS symptom severity was assessed using the IBS Severity Scoring System.23 The overall IBS score ranged from 0 to 500, and patients were divided into 3 groups according to score, as follows: < 175, mild IBS; 175–300, moderate IBS; and > 300, severe IBS.10 The questionnaires evaluated the patients’ demographic and socioeconomic characteristics including age, sex, location, education, employment, income, marital status, alcohol consumption, and smoking history.
The questionnaire designed to assess self-reported food intolerance consisted of 2 question types. The first type had a multiple-choice format in which participants were asked to select the foods that worsen their GI symptoms among a list of 119 different food items included in the Korean food-frequency questionnaire (FFQ; Supplementary Table 1).24 The second type had a short-answer format in which participants were asked whether they thought that food intake affects the occurrence of their GI symptoms and, if so, which food is the “most problematic” and which symptoms occur “most frequently.” They were also asked whether they had “reduced or eliminated” their intake of certain foods to or made any dietary changes minimize their GI symptoms.
Data Analysis
Self-reported food intolerance was defined as present when the subjects reported food-related GI symptoms in the questionnaire. The most common Korean food items causing GI symptoms in IBS patients were evaluated from the multiple-choice questionnaire using the 119 Korean food items included in the Korean FFQ. The most problematic foods causing GI symptoms for each group were evaluated using the short-answer questions.
All 119 Korean food items were categorized either as a “rarely problematic food item” or a “problematic food item” according to the difference in the proportion of subjects with GI symptoms associated with each food item between the control groups and IBS patients (Supplementary Table 1). If the difference was statistically significant (P < 0.05), the food item was classified as “problematic”; otherwise, it was classified as “rarely problematic.”
For the food group-based analysis, some food items included in the Korean FFQ were grouped according to different potentially symptom-causing contents. The list included high-FODMAP foods (36 food items),18,25,26 dairy products (7 food items), high-fat foods (17 food items), gluten foods (10 food items), caffeine-containing foods (7 food items), and alcohol (6 food items) (Supplementary Table 2). All dairy products and gluten foods were included in the high-FODMAP food group.
In IBS patients, the most problematic food-induced GI symptoms and the association between specific food groups and GI symptoms were evaluated using the short-answer questions. The association between self-reported food intolerance and IBS subtype or IBS symptom severity was assessed. The proportions of subjects who made dietary restrictions to minimize GI symptoms were compared between groups.
Statistical Methods
Differences in continuous variables between groups were evaluated using the Kruskal–Wallis test, and differences in categorical variables were evaluated using the χ2 test or Fisher’s exact test. Data analysis was performed using SPSS 20.0 (IBM Corp, Armonk, NY, USA). P-values < 0.05 were considered statistically significant.
Results
Clinical Characteristics of the Study Population
A total of 125 control subjects, 167 symptomatic non-IBS subjects, and 101 IBS patients were enrolled in this study. Of the 101 IBS patients, 10 had IBS with constipation (IBS-C) (9.9%), 44 had IBS with diarrhea (IBS-D) (43.6%), 43 had mixed IBS (42.6%), and 4 had unsubtyped IBS (4.0%). An intergroup comparison of clinical characteristics is shown in Table 1. The proportion of subjects aged > 60 years was slightly higher in the IBS group than in the control and symptomatic non-IBS groups (42.6% vs 28.8% and 31.2%; P = 0.021). The proportion of women was higher in the symptomatic non-IBS and IBS groups than in the control group (71.3% and 62.4% vs 48.8%; P < 0.001). The control group included higher proportions of employed and high-income subjects (P < 0.001 and P = 0.012, respectively). Current drinking and smoking rates were higher in the control group than in subjects with symptoms (P = 0.007 and P = 0.004, respectively). Location, education level, and marital status did not differ significantly between the groups.
Table 1.
Demographic and Socioeconomic Characteristics of the Study Population
| Variables | Control (n = 125) | Symptomatic non-IBS (n = 167) | IBS (n = 101) | P-value |
|---|---|---|---|---|
| Age (yr) | 0.021 | |||
| 20–29 | 12 (9.6) | 32 (19.2) | 9 (8.9) | |
| 30–39 | 31 (24.8) | 23 (13.8) | 18 (17.8) | |
| 40–49 | 26 (20.8) | 29 (17.4) | 16 (15.8) | |
| 50–59 | 20 (16.0) | 31 (18.6) | 15 (14.9) | |
| 60–69 | 22 (17.6) | 33 (19.8) | 34 (33.7) | |
| 70–79 | 14 (11.2) | 19 (11.4) | 9 (8.9) | |
| Sex | < 0.001 | |||
| Male | 64 (51.2) | 48 (28.7) | 38 (37.6) | |
| Female | 61 (48.8) | 119 (71.3) | 63 (62.4) | |
| Location | 0.570 | |||
| Urban | 96 (76.8) | 130 (77.8) | 85 (84.2) | |
| Rural | 25 (20.0) | 34 (20.4) | 16 (15.8) | |
| Missing | 4 (3.2) | 3 (1.8) | 0 (0.0) | |
| Education | 0.334 | |||
| Elementary school | 15 (12.0) | 10 (6.0) | 12 (11.9) | |
| Middle school | 9 (7.2) | 13 (7.8) | 7 (6.9) | |
| High school | 25 (20.0) | 35 (21.0) | 28 (27.7) | |
| ≥ College | 75 (60.0) | 107 (64.1) | 53 (52.5) | |
| Missing | 1 (0.8) | 2 (1.2) | 1 (1.0) | |
| Employment | < 0.001 | |||
| Employed/student | 100 (80.0) | 109 (65.3) | 58 (57.4) | |
| Unemployed/retired | 8 (6.4) | 6 (3.6) | 5 (5.0) | |
| Household professional | 17 (13.6) | 51 (30.5) | 37 (36.6) | |
| Missing | 0 (0.0) | 1 (0.6) | 1 (1.0) | |
| Household income (10 000 KRW) | 0.012 | |||
| < 150 | 23 (18.4) | 37 (22.2) | 18 (17.8) | |
| 150–300 | 19 (15.2) | 39 (23.4) | 31 (30.7) | |
| 300–500 | 45 (36.0) | 36 (21.6) | 30 (29.7) | |
| ≥ 500 | 32 (25.6) | 45 (26.9) | 15 (14.9) | |
| Missing | 6 (4.8) | 10 (6.0) | 7 (6.9) | |
| Marital status | 0.252 | |||
| Never married | 22 (17.6) | 42 (25.1) | 29 (28.7) | |
| Married | 92 (73.6) | 113 (67.7) | 62 (61.4) | |
| Separated/divorced/widowed | 8 (6.4) | 12 (7.2) | 10 (9.9) | |
| Missing | 3 (2.4) | 0 (0.0) | 0 (0.0) | |
| Alcohol | 0.007 | |||
| None/ex-drinker | 50 (40.0) | 67 (40.1) | 59 (58.4) | |
| Current drinker | 74 (59.2) | 99 (59.3) | 42 (41.6) | |
| Missing | 1 (0.8) | 1 (0.6) | 0 (0.0) | |
| Smoking | 0.004 | |||
| Non-smoker | 75 (60.0) | 133 (79.6) | 73 (72.3) | |
| Ex-smoker | 28 (22.4) | 23 (13.8) | 17 (16.8) | |
| Current smoker | 22 (17.6) | 11 (6.6) | 9 (8.9) | |
| Missing | 0 (0.0) | 0 (0.0) | 2 (2.0) |
IBS, irritable bowel syndrome; KRW, South Korean Won.
Values are presented as n (%).
Self-reported Food Intolerance and Korean Food
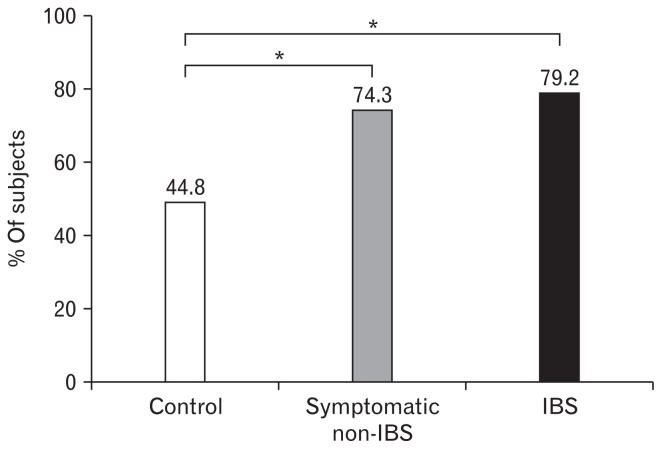
The prevalence of self-reported food intolerances was significantly higher in the IBS and symptomatic non-IBS groups than in the control group (79.2% and 74.3% vs 44.8%, P < 0.001; Fig. 1). The prevalence of self-reported food intolerance in IBS patients was not related to age, sex, education, employment, income, or marital status (P = 0.366, P = 0.117, P = 0.107, P = 0.329, P = 0.527, and P = 0.473, respectively).
Figure 1.
Frequency of self-reported food intolerance. The prevalence of self-reported food intolerances was significantly higher in the irritable bowel syndrome (IBS) and symptomatic non-IBS groups than in the control group (*P < 0.001).
The most common Korean food item causing GI symptoms in IBS patients was whole milk (34.7%), followed by instant ramen (27.7%), black bean-sauce noodles/Chinese-style noodles with vegetables and seafood (24.8%), pork belly (24.8%), and pizza/hamburger (23.8%). For all food items listed in Table 2, a greater number of IBS patients than controls reported food-induced GI symptoms.
Table 2.
Korean Food Items Causing Gastrointestinal Symptoms in Korean Patients With Irritable Bowel Syndrome
| Ranking | Korean food items | Food group | Patient number (%) | P-valuea |
|---|---|---|---|---|
| 1st | Whole milk | High-FODMAP foods | 35 (34.7) | < 0.001 |
| 2nd | Instant ramen | High-FODMAP foods and high-fat foods | 28 (27.7) | < 0.001 |
| 3rd | Black bean-sauce noodles/Chinese-style noodles with vegetables and seafood | High-FODMAP foods and high-fat foods | 25 (24.8) | < 0.001 |
| 3rd | Pork belly | High-fat foods | 25 (24.8) | < 0.001 |
| 5th | Pizza/hamburger | High-FODMAP foods and high-fat foods | 24 (23.8) | < 0.001 |
| 6th | Noodle soup | High-FODMAP foods | 21 (20.8) | < 0.001 |
| 6th | Bread | High-FODMAP foods | 21 (20.8) | < 0.001 |
| 8th | Processed meat | High-fat foods | 18 (17.8) | < 0.001 |
Pearson’s chi-square test was used to examine the differences between controls and patients with irritable bowel syndrome.
FODMAP, fermentable oligo-, di-, mono-saccharides, and polyols.
The responses to short-answer questions proved that the “most problematic foods” causing GI symptoms among subjects reporting food intolerances were high-fat foods for the IBS and symptomatic non-IBS groups (25.0% and 21.0%, respectively) and gluten foods for the control group (28.6%; Table 3A).
Table 3.
Food Groups Reported to Cause Gastrointestinal Symptoms
| (A) Foods Reported by Subjects With Self-reported Food Intolerance as Being the “Most Problematic” in Causing Gastrointestinal Symptoms (Results Obtained From the Short-answer Questions) | ||||
|---|---|---|---|---|
|
| ||||
| Most problematic food | Control (n = 56) | Symptomatic non-IBS (n = 124) | IBS (n = 80) | Total (n = 260) |
| High-fat foods | 9 (16.1) | 26 (21.0) | 20 (25.0) | 56 (21.5) |
| Gluten foods | 16 (28.6) | 34 (27.4) | 19 (23.8) | 69 (26.5) |
| Spicy foods | 11 (19.6) | 19 (15.3) | 12 (15.0) | 42 (16.2) |
| Dairy products | 4 (7.1) | 14 (11.3) | 12 (15.0) | 31 (11.9) |
| Others | 16 (28.6) | 31 (25.0) | 17 (21.3) | 62 (23.8) |
IBS, irritable bowel syndrome.
Values are presented as n (%).
| (B) Proportion of Subjects With Gastrointestinal Symptoms Related to the Intake of a Specific Food Group (Results Obtained From the Multiple-choice Questions) | ||||
|---|---|---|---|---|
|
| ||||
| Food group | Control (n = 125) | Symptomatic non-IBS (n = 167) | IBS (n = 101) | P-value |
| High-FODMAP foods | 35 (28.0) | 92 (55.1) | 64 (63.4) | < 0.001 |
| High-fat foods | 27 (21.6) | 72 (43.1) | 49 (48.5) | < 0.001 |
| Gluten foods | 19 (15.2) | 68 (40.7) | 44 (43.6) | < 0.001 |
| Dairy products | 15 (12.0) | 49 (29.3) | 41 (40.6) | < 0.001 |
| Caffeine | 12 (9.6) | 30 (18.0) | 18 (17.8) | 0.103 |
| Alcohol | 11 (8.8) | 31 (18.6) | 12 (11.9) | 0.046 |
IBS, irritable bowel syndrome; FODMAP, fermentable oligo-, di-, mono-saccharides, and polyols.
Values are presented as n (%).
The responses to multiple-choice questions proved that the proportions of subjects with GI symptoms were related to the intake of 6 specific food groups (Table 3B). For all food groups except caffeine, more subjects in the IBS and symptomatic non-IBS groups than in the control group reported symptoms. Among the IBS patients, 63.4% reported GI symptoms related to at least one of the high-FODMAP foods. High-fat foods were also frequently perceived to be associated with GI symptoms by Korean IBS patients (48.5%).
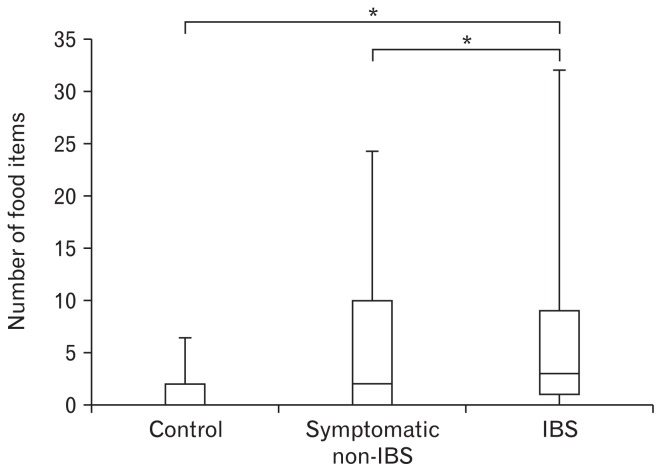
Of all the 119 Korean food items, the 55 items cited as problematic by a significantly greater number of IBS patients than control subjects were categorized as “problematic food items,” whereas the remaining 64 food items were categorized as “rarely problematic food items” (Supplementary Table 1). IBS patients reported GI symptoms for a median of 3 food items (interquartile range [IQR], 1–9), which was more than that reported by the control group (median value 0; IQR, 0–2) or the symptomatic non-IBS group (median value 2; IQR, 0–10) (P < 0.001; Fig. 2).
Figure 2.
Comparison of the number of food items causing gastrointestinal symptoms among the 3 groups. Irritable bowel syndrome (IBS) patients reported more food items causing gastrointestinal symptoms than control group and symptomatic non-IBS group (whiskers: 10th-90th percentiles; *P < 0.001).
Self-reported Food Intolerance and Associated Gastrointestinal Symptoms
The most problematic food-induced GI symptoms reported by IBS patients with food intolerance (n = 80; 79.2% of the IBS group) were loose stool/diarrhea (36.3%) and gas distension/bloating (28.8%; Table 4). In IBS patients with self-reported food intolerance, concerning the association between specific food groups and GI symptoms, spicy foods were the most commonly reported for abdominal pain (41.7%), gluten foods for gas distension/bloating (47.4%), and high-fat foods for loose stool/diarrhea (50.0%).
Table 4.
Most Problematic Food-induced Gastrointestinal Symptoms Reported by Irritable Bowel Syndrome Patients With Self-reported Food Intolerance (n = 80)
| Most problematic symptoms | Most problematic foods | ||||
|---|---|---|---|---|---|
|
| |||||
| Gluten foods (n = 19) | Spicy foods (n = 12) | High-fat foods (n = 20) | Dairy products (n = 12) | Others (n = 17) | |
| Loose stool/diarrhea (29 [36.3%]) | 3 (15.8) | 4 (33.3) | 10 (50.0) | 7 (58.3) | 5 (29.4) |
| Gas distension/bloating (23 [28.8%]) | 9 (47.4) | 3 (25.0) | 2 (10.0) | 3 (25.0) | 6 (35.3) |
| Abdominal pain (12 [15.0%]) | 1 (5.3) | 5 (41.7) | 2 (10.0) | 2 (16.7) | 2 (11.8) |
| Epigastric fullness (10 [12.5%]) | 4 (21.1) | 0 (0.0) | 4 (20.0) | 0 (0.0) | 2 (11.8) |
| Others (4 [5.0%]) | 1 (5.3) | 0 (0.0) | 2 (10.0) | 0 (0.0) | 1 (5.9) |
| Missing (2 [2.5%]) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) |
Values are presented as n (%).
Self-reported Food Intolerance With Respect to Irritable Bowel Syndrome Subtype and Severity
IBS subtypes were not associated with food-related GI symptoms except that abdominal pain was more prevalent in patients with IBS-C (Table 5). The frequency of self-reported food intolerance was similar among groups according to IBS symptom severity (Table 6). However, IBS patients with higher symptom severity reported a higher number of food items causing GI symptoms (P = 0.020).
Table 5.
Self-reported Food Intolerance by Irritable Bowel Syndrome Subtype
| Items | IBS-C (n = 10) | IBS-D (n = 44) | IBS-M (n = 43) | IBS-U (n = 4) | P-value |
|---|---|---|---|---|---|
| Self-reported food intolerance | 9 (90.0) | 35 (79.5) | 32 (74.4) | 4 (100.0) | 0.501 |
| Number of food items causing GI symptoms | 2 (1–7) | 3 (0–8) | 3 (1–11) | 15 (2–35) | 0.616 |
| Food-induced symptoms | NA | ||||
| Abdominal pain | 3 (33.3) | 6 (17.1) | 3 (9.4) | 0 (0.0) | |
| Gas distension/bloating | 3 (33.3) | 7 (20.0) | 10 (31.3) | 3 (75.0) | |
| Loose stool/diarrhea | 0 (0.0) | 17 (48.6) | 12 (37.5) | 0 (0.0) | |
| Epigastric fullness | 2 (22.2) | 2 (5.7) | 5 (15.6) | 1 (25.0) | |
| Others | 1 (11.1) | 2 (5.7) | 1 (3.1) | 0 (0.0) | |
| Missing | 0 (0.0) | 1 (2.9) | 1 (3.1) | 0 (0.0) |
IBS, irritable bowel syndrome; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-M, mixed IBS; IBS-U, unsubtyped IBS; GI, gastrointestinal; IQR, interquartile range; NA, not applicable.
Values are presented as n (%) or median (IQR).
Table 6.
Self-reported Food Intolerance by Irritable Bowel Syndrome Severity
| Items | Mild (n = 31) | Moderate (n = 34) | Severe (n = 29) | P-valuea |
|---|---|---|---|---|
| Self-reported food intolerance | 21 (67.7) | 29 (85.3) | 25 (86.2) | 0.124 |
| Number of food items causing GI symptoms | 2 (0–6) | 3 (0–11) | 6 (2–31) | 0.020 |
| Food-induced symptoms | ||||
| Abdominal pain | 1 (4.8) | 6 (20.7) | 4 (16.0) | |
| Gas distension/bloating | 4 (19.0) | 11 (37.9) | 6 (24.0) | |
| Loose stool/diarrhea | 8 (38.1) | 9 (31.0) | 10 (40.0) | |
| Epigastric fullness | 6 (28.6) | 1 (3.4) | 3 (12.0) | |
| Others | 1 (4.8) | 2 (6.9) | 1 (4.0) | |
| Missing | 1 (4.8) | 0 (0.0) | 1 (4.0) | |
Seven irritable bowel syndrome patients without symptom severity score were excluded from this analysis. GI, gastrointestinal; IQR, interquartile range.
Values are presented as n (%) or median (IQR).
Dietary Changes
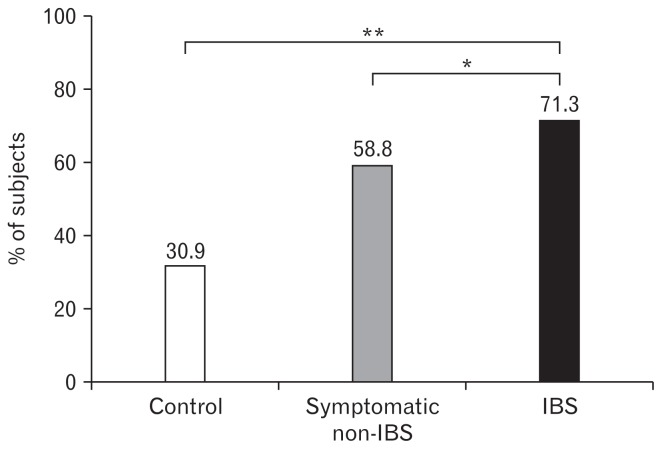
To minimize symptoms, 71.3% of IBS patients reported reducing or eliminating specific foods; this proportion was significantly higher than that in the symptomatic non-IBS (58.8%, P = 0.040) and control groups (30.9%, P < 0.001; Fig. 3). IBS patients also attempted the following changes to their diets: increasing fiber intake (11.9%), eating less (10.9%), eating a liquid diet (5.9%), taking probiotics (5.9%), adopting elimination diets (5.0%), avoiding cold foods (5.0%), eating yogurt (3.0%), eating poached vegetables (2.0%), and other dietary changes (7.9%).
Figure 3.
Frequency of dietary restriction to minimize gastrointestinal symptoms in control, symptomatic non-irritable bowel syndrome (IBS) and IBS group (*P = 0.040 and **P < 0.001).
Discussion
About 80% of Korean IBS patients reported food intolerance. Among the study subjects, the prevalence of food intolerance was the highest in the IBS group, followed by the symptomatic non-IBS group and then the control group. High-fat, high-FODMAP, and spicy foods were considered by many IBS patients to induce their GI symptoms, especially gas problems and loose stools. These features of self-reported food intolerance in Korean IBS patients are comparable to those reported in studies from Western countries. We described in detail the Korean foods perceived by IBS patients to worsen their GI symptoms compared with the control group. Our findings provide a clinical basis for planning food intervention studies for Korean IBS patients.
Food is a well-known trigger of IBS symptom, with 63–90% of IBS patients reporting that their symptoms are related to food.8–10,27,28 The prevalence of self-reported food intolerance in Korean IBS patients in the present study is similar to that reported in previous studies.8–10,28 IBS patients reported a greater number of food items causing GI symptoms than that reported by the control group, and patients with more severe IBS reported a greater number of food items causing GI symptoms, suggesting that self-reported food intolerance may be associated with a high symptom burden. Böhn et al10 interpreted such results as being related to increased severity of somatic symptoms and reduced QOL, suggesting that self-reported food intolerance may be associated with patients’ underlying psychological issues and over-monitoring of diets.
The most problematic foods for Korean IBS patients were high-fat foods, high- FODMAP foods, including dairy products and gluten foods, and spicy foods, which were also the most often implicated foods in previous studies. High-fat foods were the most problematic food items reported by Korean IBS patients, and about half of the patients complained of fat-associated GI symptoms. Of the top 8 Korean food items causing GI symptoms, 5 were high-fat foods. Intraluminal lipids normally affect gut motility and sensitivity, and these effects are exaggerated in IBS patients.29 Two duodenal lipid infusion studies reported inhibited gut motility and increased visceral sensitivity in IBS patients, with these responses contributing to IBS symptoms such as gas distension and abdominal pain.30,31
FODMAPs are major dietary contributors to the exacerbation of IBS symptoms. The exacerbation is related to the capacity of FODMAPs to increase small intestinal water volume through their high osmotic action, increase colonic gas production by promoting bacterial fermentation, and to their ability to alter intestinal motility.32 In the present study, 63.4% of Korean IBS patients reported GI symptoms related to at least one of the food items with high FODMAP content, which is comparable to the proportion of 70.0% reported in a previous study.10 Randomized controlled trials demonstrated that a diet low in FODMAP effectively reduced global IBS symptom scores and bloating.12 Moreover, low-FODMAP diets have shown outcomes that are similar to or even better than those of traditional IBS dietary advice in IBS treatment.15,16 A significant benefit of low-FODMAP diet on health-related QOL, as well as on improving GI symptoms, has also been demonstrated in a randomized controlled study.33 Meanwhile, a recent placebo-controlled dietary advice trial, which made excellent efforts to maintain blinding, has shown that the difference in symptom relief between a low-FODMAP diet and a “sham diet” was borderline significant in the intention-to-treat analysis.34 Although there is increasing evidence supporting the use of low-FODMAP diets as an IBS treatment, clinical data supporting the application of this strategy to Korean IBS patients are lacking. Our findings may provide a clinical clue for the application of low-FODMAP diets in Korean IBS patients. Further studies are warranted to elucidate the FODMAP composition of frequently consumed Korean food items.
Gluten foods were also frequently perceived to be related to GI symptoms by many Korean IBS patients (43.6%). Of the top 8 Korean food items causing GI symptoms, 4 were gluten foods. Gluten may act on IBS symptoms via altered gut permeability, tight junctional biology, and enhanced systemic immune responses.35 However, the role of gluten in IBS symptoms remains controversial. Two randomized controlled studies have shown that gluten causes IBS symptoms in patients with IBS despite an absence of celiac disease and that a gluten-free diet reduces IBS symptoms in patients with IBS-D.35,36 However, gluten-containing grains also have high fructan content and are classified as high-FODMAP foods. One trial showed no gluten-specific effects in IBS patients on low-FODMAP diets, suggesting that the effect of gluten-free diets in IBS patients is probably due to the reduced intake of FODMAP.37
Whole milk was the most frequently cited (34.7%) problematic food item among Korean IBS patients. Lactose is a disaccharide that is broken down during the digestive process into the monosaccharides glucose and galactose and classified as a FODMAP. Lactose intolerance is known to be more common in Asians than in Europeans; however, the prevalence of lactose intolerance in Koreans has not been clarified.38 In contrast with our expectations, 40.7% of the patients complained of dairy product-associated GI symptoms, which is similar to the proportion of 24–49% reported in previous studies.9,10
Spicy food was reported as the “most problematic food” by 15% of Korean IBS patients in the short-answer questions. Previous studies reported that 28–45% of IBS patients cited spicy food as problematic with respect to their IBS symptoms.8–10 A randomized controlled trial showed that chili ingestion exacerbates abdominal pain and burning in IBS-D patients.39 Capsaicin-induced visceral hypersensitivity and pain in IBS patients results in sensitization of transient reporter potential channel V1, which is one of the nociceptors responsive to capsaicin, mediated by histamine receptor H1.40
Gas distension/bloating and loose stool/diarrhea were the most problematic food-induced GI symptoms. Constipation-associated symptoms were rarely reported; one of the reasons for this may be that the IBS-C patients represented < 10% of the enrolled IBS patients, although similar results were reported in a previous study comprising 25% IBS-C patients.8 The small number of IBS-C patients is one of the limitations of our study, and this should be considered when interpreting our data, although previous studies reported that the IBS subgroup is unrelated to food-associated symptoms and to the number of food items that worsen GI symptoms.8,10
The relationships between specific symptoms and foods have not been well evaluated, and study results have been inconsistent.8,9 In the present study, spicy foods were markedly associated with abdominal pain whereas most other foods were associated with gas problems and loose stools. Therefore, Korean IBS patients whose main complaint is abdominal pain may be advised to particularly avoid spicy foods.
More than two-thirds of IBS patients in our study avoided numerous food items to minimize their symptoms, which potentially places them at a risk of developing nutritional deficiencies. The results of published studies on the adequacy of nutrient intake in IBS patients have been inconsistent.28,41 Further studies are needed to evaluate the dietary intake and nutritional adequacy of Korean IBS patients.
An interesting finding of this study was that the symptomatic non-IBS group showed a similar pattern of self-reported food intolerance to the IBS group. Our questionnaire focused on IBS symptoms and, unfortunately, did not investigate all symptoms of other functional GI disorders; therefore, we could not describe the pattern of GI symptoms in detail. The symptomatic non-IBS group might have included subjects with heterogeneous characteristics, including subjects with abdominal pain-related functional disorders such as functional dyspepsia or asymptomatic migraine, subjects who have not yet met the criterion related to duration of symptoms but are likely to be diagnosed with IBS in the future, subjects who have not yet met the criterion related to frequency of symptoms, or subjects who have experienced nonspecific or transient abdominal pain. Further investigations are required to clearly characterize the “symptomatic non-IBS group” of our study, and to evaluate the association between food intolerance and other functional GI disorders, rather than only IBS, in Korean patients.
Our study has several limitations. The questionnaire used to assess food intolerance was non-validated and developed based on a literature review to clarify the characteristics of self-reported food intolerance. The questionnaire could not assess the temperature (hot or cold), spiciness, or fiber content of foods. The food group classification was based on the literature and the opinions of clinical nutritionists because a database on the composition of Korean food items was lacking. Psychological co-morbidity was not considered in our study, which affects symptom reporting.42 Future investigations including the assessment of psychological co-morbidity would provide a more accurate analysis.10 Moreover, QOL was not evaluated. Our study was performed in a secondary/tertiary-care setting, making it difficult to generalize our findings to the general IBS population. Finally, organic diseases could not be completely excluded.
In conclusion, a large proportion of Korean IBS patients complained of intolerances to certain food items, with foods containing fat and incompletely absorbed carbohydrates being the main triggers. Our study results provide a clinical basis for planning food intervention studies for Korean IBS patients. Further prospective interventional studies are warranted to develop and apply evidence-based dietary advice for Korean IBS patients.
Supplementary Information
Footnotes
Supplementary Materials
Note: To access the supplementary tables mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm18125.
Financial support: This work was funded by the Korean Society of Neurogastroenterology and Motility, Korea (2016).
Conflicts of interests: None.
Author contributions: Hyo Jeong Lee analyzed the data and wrote the manuscript; Hyun Jin Kim collected the data and wrote the manuscript; Kyoung Sup Hong and Kyung Sik Park designed the study, obtained the funding, collected the data, and revised the manuscript; Eun Hee Kang analyzed and interpreted the data; and Kee Wook Jung, Seung-Jae Myung, Yang Won Min, Chang Hwan Choi, Han Seung Ryu, Jong Kyoung Choi, and Joong Goo Kwon collected the data and revised the manuscript. All authors have approved the final draft submitted.
References
- 1.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376:2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Han SH, Lee OY, Bae SC, et al. Prevalence of irritable bowel syndrome in Korea: population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006;21:1687–1692. doi: 10.1111/j.1440-1746.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 5.Park DW, Lee OY, Shim SG, et al. The differences in prevalence and sociodemographic characteristics of irritable bowel syndrome according to Rome II and Rome III. J Neurogastroenterol Motil. 2010;16:186–193. doi: 10.5056/jnm.2010.16.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal N, Spiegel BM. The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am. 2011;40:11–19. doi: 10.1016/j.gtc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265–269. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 8.Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 9.Hayes P, Corish C, O’Mahony E, Quigley EM. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27(suppl 2):36–47. doi: 10.1111/jhn.12114. [DOI] [PubMed] [Google Scholar]
- 10.Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie YA, Bowyer RK, Leach H, et al. British dietetic association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet. 2016;29:549–575. doi: 10.1111/jhn.12385. [DOI] [PubMed] [Google Scholar]
- 12.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 14.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 15.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111:1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 16.Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407. e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 17.Gibson PR. The evidence base for efficacy of the low FODMAP diet in irritable bowel syndrome: is it ready for prime time as a first-line therapy? J Gastroenterol Hepatol. 2017;32(suppl 1):32–35. doi: 10.1111/jgh.13693. [DOI] [PubMed] [Google Scholar]
- 18.University. M. [accessed 19 Jan 2019];The Monash Uni low FODMAP diet app. Available from URL: http://www.med.monash.edu.au/cecs/gastro/fodmap/iphone-app.html.
- 19.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 20.Gibson PR, Varney J, Malakar S, Muir JG. Food components and irritable bowel syndrome. Gastroenterology. 2015;148:1158–1174. e4. doi: 10.1053/j.gastro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Pinto-Sanchez MI, Ford AC, Avila CA, et al. Anxiety and depression increase in a stepwise manner in parallel with multiple FGIDs and symptom severity and frequency. Am J Gastroenterol. 2015;110:1038–1048. doi: 10.1038/ajg.2015.128. [DOI] [PubMed] [Google Scholar]
- 22.Song KH, Jung HK, Min BH, et al. Development and validation of the Korean Rome III questionnaire for diagnosis of functional gastrointestinal disorders. J Neurogastroenterol Motil. 2013;19:509–515. doi: 10.5056/jnm.2013.19.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 24.Oh SY, Shin MH, Lee SH, et al. The development of food frequency questionnaire for nutrition assessment in adults [Abstract]. Proceedings of 2007 Conference of The Korean Society of Health Promotion; Seoul, Korea. pp. 67–72. [Google Scholar]
- 25.Iacovou M, Tan V, Muir JG, Gibson PR. The low FODMAP diet and its application in east and southeast Asia. J Neurogastroenterol Motil. 2015;21:459–470. doi: 10.5056/jnm15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–666. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 27.Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (NY) 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 28.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome--etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 29.Feinle-Bisset C, Azpiroz F. Dietary lipids and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:737–747. doi: 10.1038/ajg.2013.76. [DOI] [PubMed] [Google Scholar]
- 30.Caldarella MP, Milano A, Laterza F, et al. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am J Gastroenterol. 2005;100:383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 31.Serra J, Salvioli B, Azpiroz F, Malagelada JR. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology. 2002;123:700–706. doi: 10.1053/gast.2002.35394. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–717. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 33.Eswaran S, Chey WD, Jackson K, Pillai S, Chey SW, Han-Markey T. A diet low in fermentable oligo-, di-, and monosaccharides and polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin Gastroenterol Hepatol. 2017;15:1890–1899. e3. doi: 10.1016/j.cgh.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Staudacher HM, Lomer MCE, Farquharson FM, et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153:936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911. e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 37.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328. e1–e3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Swagerty DL, Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845–1850. [PubMed] [Google Scholar]
- 39.Gonlachanvit S, Mahayosnond A, Kullavanijaya P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol Motil. 2009;21:23–32. doi: 10.1111/j.1365-2982.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 40.Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150:875–887. e9. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Williams EA, Nai X, Corfe BM. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterol. 2011;11:9. doi: 10.1186/1471-230X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vu J, Kushnir V, Cassell B, Gyawali CP, Sayuk GS. The impact of psychiatric and extraintestinal comorbidity on quality of life and bowel symptom burden in functional GI disorders. Neurogastroenterol Motil. 2014;26:1323–1332. doi: 10.1111/nmo.12396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.