Abstract
We describe incident human immunodeficiency virus (HIV) and syphilis trends in men who have sex with men (MSM) and transgender women (TGW) presenting for HIV voluntary counseling and testing (VCT) services and sexually transmitted infection (STI) management at the Silom Community Clinic, Bangkok, Thailand. Clients underwent rapid HIV testing and syphilis rapid plasma reagin (RPR) testing. For incidence analysis, we included clients with >1 follow-up visit. Initial negative HIV with subsequent positive HIV defined incident HIV infection; incident syphilis infection was defined as negative RPR followed by positive RPR (titer ≥1:8) and confirmatory anti-Treponema pallidum antibodies. Calculation of incidence using Poisson regression assumed a uniform probability distribution throughout the serocon-version interval. From 15 September 2005 to 31 December 2015, we tested 10,158 clients for HIV and 10,324 for syphilis. Overall, 7109 clients tested HIV-seronegative and contributed 7157 person-years (PY). Three-hundred forty-seven incident HIV infections resulted in an incidence rate of 4.8 per 100 PY (95% confidence interval [CI] 4.4–5.4). We found an inverted U-shape trend of HIV incidence over time with a peak of 6.4 per 100 PY in quarter 2/2011 (p < 0.01) (Poisson with RCS function, p = 0.001). Overall, 8713 clients tested seronegative for syphilis and contributed 8623 PY. The incidence of syphilis infection was 4.4 per 100 PY (95% CI 3.9–4.8). Despite an apparent decline in HIV incidence among MSM and TGW attending VCT services, syphilis incidence rose and remained high. Evaluating temporal trends of HIV and syphilis incidence provides an opportunity to evaluate epidemic trajectories and target limited program funding. We recommend focused HIV and STI prevention interventions for MSM in Bangkok.
Keywords: Men who have sex with men, HIV, syphilis, epidemiology
Introduction
Despite successful control of the human immunodeficiency virus (HIV) epidemic among heterosexual men in Thailand by the late 1990s,1 and certified elimination of mother-to-child HIV transmission in 2016,2 the continued spread of HIV in other high-risk groups, particularly men who have sex with men (MSM), and transgender women (TGW), remains concerning.3,4 The first cross-sectional MSM HIV prevalence survey assessment conducted in Bangkok in 2003 revealed a largely unrecognized epidemic of HIV among MSM, with a prevalence of 17.3%.5 Within a few years, HIV prevalence among MSM had risen to 28.3% using the same survey technique6; although incidence had not been assessed, it was estimated to be above 5 per 100 person-years (PY), with the prevalence remaining stable between 25 and 31% in subsequent study years.7,8 Similarly, rates of syphilis among MSM have been high and increasing in recent years, in both high-income countries in North America,9–11 Western Europe,12 and some middle-income countries13 including China,14 as well as Thailand.15,16
Although the Thailand Ministry of Public Health (MOPH) conducts HIV surveillance, the incidence of HIV infection among MSM and TGW has not been easily determined. Incidence assays can provide accurate estimates of HIV incidence in some populations, but cross-sectional studies are unable to demonstrate the dynamic nature of the HIV epidemic over time. Longitudinal data give the best estimates of incidence and can provide the means to assess the potential impact of prevention interventions in a population.17 Incidence data are most robust if quantifying HIV infections among repeatedly tested HIV-negative individuals. However, clients or participants in longitudinal assessments of HIV in a clinic or cohort study may not be representative of the general population of MSM.18–22
We assessed trends in the incidence of HIV and syphilis infections from 2005 to 2015 among MSM and TGW attending the Silom Community Clinic (SCC) located in central Bangkok. Since 2005 the clinic has provided an MSM-friendly environment and rapid voluntary counseling and testing (VCT). Services include testing and treatment for common sexually transmitted infections (STIs), counseling, and referral to care for HIV treatment.7,23 Pre-exposure prophylaxis (PrEP) against HIV was recommended in the Thai national guidelines in 2013,24 but demonstration projects in focused urban areas did not occur until 2015.25 Few if any participants were using PrEP prior to March 2016 when SCC started providing PrEP to clients. Previous analysis of participant data from 2005 to 2011 reported an HIV incidence of 6.3 per 100 PY and syphilis incidence of 3.6 per 100 PY.7,26 Updates in temporal trends of HIV and syphilis incidence provide an opportunity to evaluate the trajectory of these epidemics among MSM and TGW over the last decade in Bangkok. In addition, we evaluated factors associated with incident infections that may support public health prevention efforts.
Methods
Study participants and procedures
Between 15 September 2005 and 31 December 2015, we asked MSM and TGW clients attending SCC for VCT services’ basic demographic data at the initial visit. Thai men and TGW, aged ≥18 years, who reported oral or anal sex with another man in the past six months, were eligible for inclusion in this analysis. As VCT services were offered confidentially and anonymously, we did not ask clients to reveal their gender identity and we analyzed both MSM and TGW as one group. Clients were asked to repeat HIV and syphilis testing every six months if HIV-negative (a policy incorporated into the 2010 Thai National Guidelines).24,27 At each visit, clients were screened for HIV-1 antibody in whole blood using a rapid test and, if positive, were further tested with consecutive HIV-1 rapid tests (Determine™, Abbott, USA; DoubleCheck™, Organics Ltd, Israel or SD Bioline™, Standard Diagnostics, Inc., Korea; and Capillus™ HIV-1/2, Trinity Biotech, USA or HIV1/2 Core™, Core Diagnostic, UK), in accordance with Thai National Guidelines’ rapid HIV testing algorithm. An initial negative rapid HIV test result with a subsequent positive rapid HIV result during a follow-up visit defined incident HIV infection. All clients with incident HIV infection were referred for treatment to a Thailand MOPH HIV site according to the Thailand HIV treatment guidelines.
Clients were also screened for incident syphilis infection at each visit with a rapid plasma reagin (RPR) test, and if reactive, were tested for RPR titer (Macro-Vue™ RPR 18 mm Circle Card Test; Becton Dickinson Microbiology Systems, Sparks, Maryland, USA). If the RPR was reactive, we tested for anti-Treponema pallidum antibodies (Determine™ Syphilis TP; Inverness Medical Japan, Chiba, Japan) in serum. A positive RPR with a titer ≥1:8 and positive anti-T. pallidum antibodies were considered evidence of syphilis.13 An initial negative RPR with a subsequent positive RPR (titer ≥1:8) and confirmatory anti-T. pallidum antibodies during a follow-up visit defined incident syphilis infection. Clients with incident syphilis were treated at SCC according to Thailand treatment guidelines. We defined a cure to be a fourfold decrease in a follow-up RPR over six months.
The provision of VCT services at SCC was determined to be a public health program activity by the Office of the Associate Director of Science, CDC, and as such, this analysis was exempt from CDC Institutional Review Board review.
Statistical analysis
For incident HIV or syphilis infection during the time frame of assessment, we conducted a descriptive analysis of client demographic characteristics with comparisons using Wilcoxon nonparametric tests and Chi square tests. We calculated exact Poisson 95% confidence intervals (CIs) for HIV incidence per 100 PY. An alpha = 0.05 was used to perform two-sided significance testing, and 95% CIs were constructed for measures of effect.
Calculation of incidences per quarter assumed a uniform probability distribution throughout the serocon-version interval between the last negative and first positive HIV and syphilis test results.28 Participants were excluded from one cohort analysis or the other due to a lack of testing for one disease or lack of follow-up testing. We did not examine reinfection or multiple events. We evaluated temporal trends in HIV and syphilis incidence by quarter using a restricted cubic spline (RCS) function for time using three knots, in a Poisson regression with robust standard errors.29,30 The RCS function provides flexible fitting of curve shapes for continuous predictors in regression models, allows assessment of linearity, and graphically characterizes the association between the outcome and the predictor. The number of knots chosen was based on the recommendation that three to five knots are sufficient in practice.30 We performed all statistical analysis using Stata/SE 13.1 (Stata Press, College Station, Texas, USA).
Role of the funding source
The funder of the study had no role in study design, data gathering, analysis, or interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population
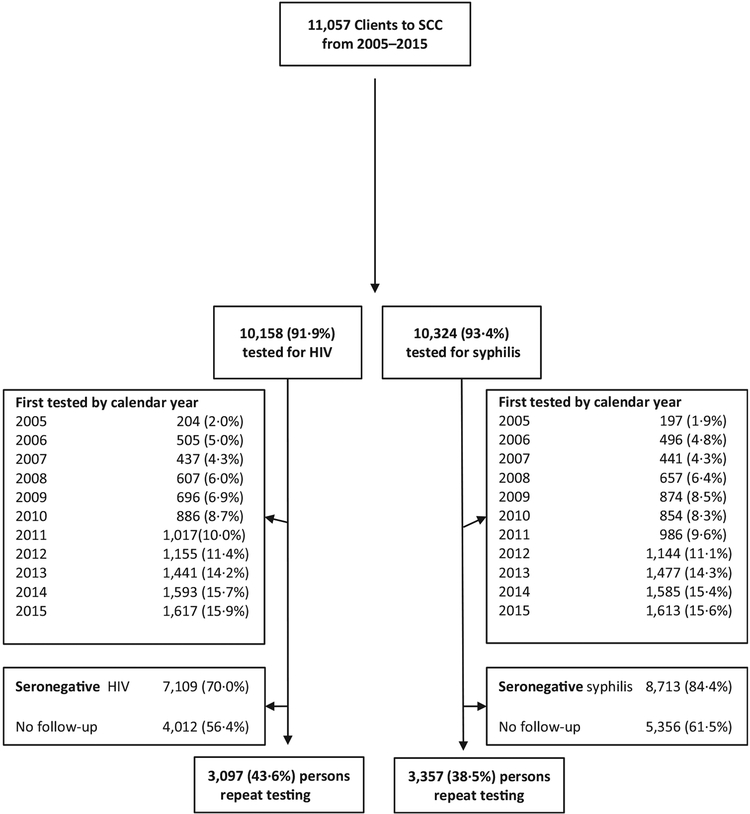
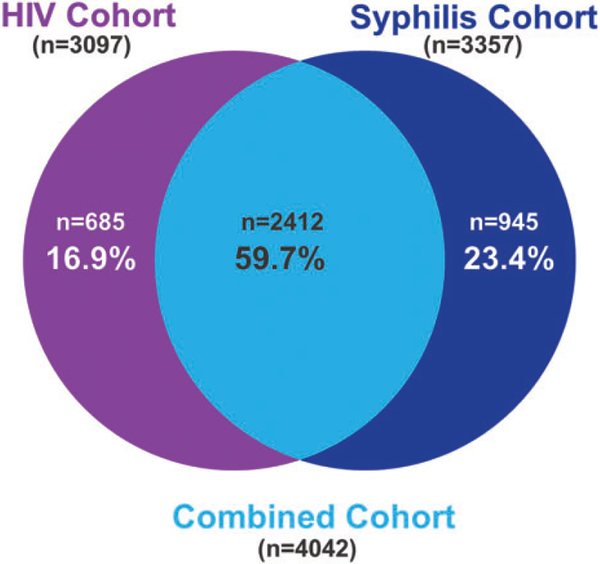
Overall, 11,057 MSM and TGW visited SCC VCT from 15 September 2005 to 31 December 2015; 10,158 were tested for HIV and 10,324 for syphilis (Figure 1). The number of first visits gradually increased during the first several years the clinic first offered services (2005–2009), to subsequently larger annual increases in the number of visits (2010–2013), and a stabilization in the number of visits through 2015. Among the 7109 clients who were seronegative for HIV, 3097 (43.6%) had a follow-up HIV test (Table 1). Among the same 3097 clients, 257 (8.3%) had syphilis infection at baseline. Among the 8713 clients who were seronegative for syphilis, 3357 (38.5%) had a follow-up syphilis test. Among the same 3357 clients, 779 (23.2%) tested positive for HIV at their first clinic visit. Overall, the overlap was 59.7%, with 16.9% only in the HIV analysis and 23.4% only in the syphilis analysis (Figure 2).
Figure 1.
HIV and syphilis screening by year among MSM, and TGW, presenting for VCT (for HIV) at the SCC in Bangkok, Thailand, 2005–2015.
SCC: Silom Community Clinic.
Table 1.
Characteristics of clients with initial negative HIV or syphilis test result,a and a follow-up test, in the VCT cohort of men who have sex with men, and transgender women, Silom Community Clinic, Bangkok, Thailand, 2005–2015.
| HIV | Syphilis | |||
|---|---|---|---|---|
| Characteristics | n | % | N | % |
| Repeat testingb | 3097 | 100.0 | 3357 | 100.0 |
| Incident infections | 347 | 11.2 | 376 | 11.2 |
| Age group (yrs) at time of incident infection | ||||
| 18–21 | 543 | 17.5 | 540 | 16.1 |
| 22–29 | 1472 | 47.5 | 1647 | 49.1 |
| ≥30 | 1082 | 34.9 | 1170 | 34.9 |
| Thai nationality, at first clinic visit | 2729 | 88.1 | 3002 | 89.4 |
| Born in Greater BMA | 1239 | 40.0 | 1322 | 39.4 |
| Living in Greater BMA, at first clinic visit | 2776 | 89.6 | 3017 | 89.9 |
| Moved to Greater BMA, at first clinic visit | 1573 | 50.8 | 1735 | 51.7 |
| History of prior HIV testing, at first clinic visit | 1809 | 58.4 | 1645 | 49.0 |
| Year of first test | ||||
| 2005 (quarter 4 only) | 88 | 2.8 | 99 | 2.9 |
| 2006 | 197 | 6.4 | 192 | 5.7 |
| 2007 | 136 | 4.4 | 156 | 4.6 |
| 2008 | 210 | 6.8 | 264 | 7.9 |
| 2009 | 268 | 8.7 | 366 | 10.9 |
| 2010 | 276 | 8.9 | 304 | 9.1 |
| 2011 | 391 | 12.6 | 412 | 12.3 |
| 2012 | 387 | 12.5 | 469 | 14.0 |
| 2013 | 437 | 14.1 | 464 | 13.8 |
| 2014 | 503 | 16.2 | 503 | 15.0 |
| 2015 | 204 | 6.6 | 128 | 3.8 |
BMA: greater Bangkok Metropolitan Area; VCT: voluntary counseling and testing.
Given the definition of how incident infection was determined, this table does not summarize characteristics by year of incident infection.
Clients who repeated HIV testing may not be the same persons as clients who repeated syphilis testing.
Figure 2:
Quarterly HIV incidence from quarter 4/2005 to quarter 4/2015, using a RCS curve, in the VCT cohort of MSM, and TGW, 18 years and older at first test, Bangkok, Thailand.
The 3097 clients who initially tested HIV-seronegative and had a follow-up visit contributed 7157 PY of follow-up (Table 2). The 3357 clients who initially tested seronegative for syphilis and had a follow-up visit contributed 8623 PY of follow-up. The median age at first registration for both groups was 27 years (interquartile range [IQR] 23–32 years) and most were Thai nationals. The median HIV testing time interval between visits (between initial and follow-up, and all follow-up visits) was 193 days (25/75 IQR 105–367 days) and the median syphilis testing time interval between visits was 278 days (25/75 IQR 162–464 days). Among the HIV-seronegative group, just over half (1809 [58.4%]) had a previous HIV testing history; only 1645 (49.0%) of those tested for syphilis reported a previous test for HIV.
Table 2.
Poisson regression results for assessing temporal trends for HIV and syphilis incidence, in the VCT cohort of men who have sex with men, and transgender women, Silom Community Clinic, Bangkok, Thailand 2005–2015.
| Variable | Incident infections | PY | Rate | (95% CI) | RR | (95% CI) | p value (adjusted) |
|---|---|---|---|---|---|---|---|
| HIV | |||||||
| Overall | 347 | 7157 | 4.84 | (4.35–5.39) | |||
| Time (trend) | |||||||
| Overall | <0.001 | ||||||
| time_RCS_linear | <0.001 | ||||||
| time_RCS_spline | <0.001 | ||||||
| Age group (yrs) | |||||||
| 30+ | 80 | 2707 | 2.96 | (2.35–3.68) | 1.0 | ||
| 22–29 | 189 | 3319 | 5.69 | (4.91–6.57) | 1.92 | (1.67–2.22) | |
| 18–21 | 78 | 1132 | 6.89 | (5.45–8.60) | 2.31 | (1.95–2.74) | <0.001 |
| Syphilis | |||||||
| Overall | 376 | 8623 | 4.36 | (3.93–4.82) | |||
| Time (trend) | |||||||
| Overall | <0.001 | ||||||
| time_RCS_linear | <0.001 | ||||||
| time_RCS_spline 1 | <0.001 | ||||||
| time_RCS_spline 2 | <0.001 | ||||||
| Age group (yrs) | |||||||
| 30+ | 113 | 3195 | 3.54 | (2.91–4.26) | 1.0 | ||
| 22–29 | 200 | 4135 | 4.84 | (4.19–5.55) | 1.36 | (1.20–1.54) | |
| 18–21 | 63 | 1293 | 4.87 | (3.74–6.23) | 1.35 | (1.15–1.59) | <0.001 |
CI: confidence Interval; PY: person-years; RCS: restricted cubic spline; RR: rate ratio; VCT: voluntary counseling and testing.
Note: (1) No estimates are given for the RCS function as they are not directly interpretable; (2) adjusted p value is from model including time represented by RCS and age; and (3) time_RCS_spline is a nonlinear component of RCS.
HIV incidence
Among the 3097 clients initially HIV-seronegative, 347 incident HIV infections occurred, with a median time between last negative and first positive HIV test result of 358 days (IQR 171–824 days). The median number of HIV tests done before seroconversion was three (IQR 2–4) tests. The overall HIV incidence was 4.8 per 100 PY (95% CI 4.4–5.4) (Table 2). Among the 347 incident HIV infections, 344 (99.1%) had a CD4 count at the time of HIV diagnosis: 95 (27.6%) had a CD4 count of <350 cells/μl, 108 (31.4%) had a CD4 count of 350–500 cells/μl, and 141 (41.0%) had a CD4 count of >500 cells/μl. For the 95 with a CD4 <350 cells/μl, the median number of tests before sero-converting was two (IQR 2–3), and the median time between last negative and first positive test result was 248 (IQR 119.5–575) days. For the 141 with a CD4 >500 cells/μl, the median number of tests before sero-converting was three (IQR 2–4), and the median time between last negative and first positive test result was 183 (IQR 91–385) days (p = 0.003 comparing median time).
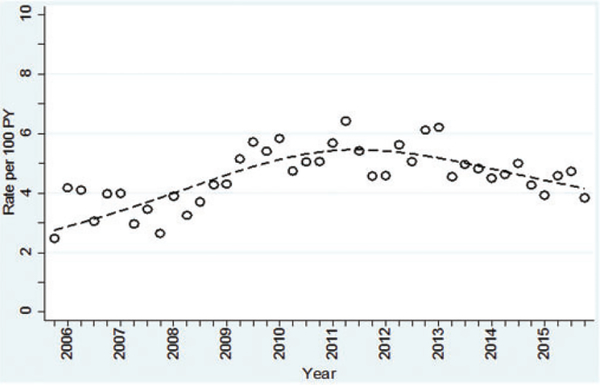
HIV incidence was significantly higher among younger persons aged 18–21 years (6.9 per 100 PY) and aged 22–29 years (5.7 per 100 PY) compared with 3.0 per 100 PY for those aged ≥30 years, p < 0.001. Over the entire period, HIV incidence fluctuated from 2.5 to 6.4 per 100 PY each quarter. Analysis noted two trends, an increased HIV incidence until quarter 2/2011, followed by stabilization in quarter 3/2011 and then a decline (inverted U-shape curve over time) through quarter 4/2015 (Figure 3) at a rate of 3.8 per 100 PY. Poisson regression of the time trend using an RCS function adjusted for age showed both a significant linear effect (p = 0.0001), and a nonlinear effect (p = 0.0001) (Table 2), with clients aged 18–21 years being at more significant risk for incident HIV infection (relative risk [RR] 2.3, 95% CI 2.0–2.7).
Figure 3.
Quarterly syphilis incidence (first episode), from quarter 4/2005 to quarter 4/2015, using a RCS curve, in the VCT cohort of MSM, and TGW, 18 years and older at first test, Bangkok, Thailand.
Syphilis incidence
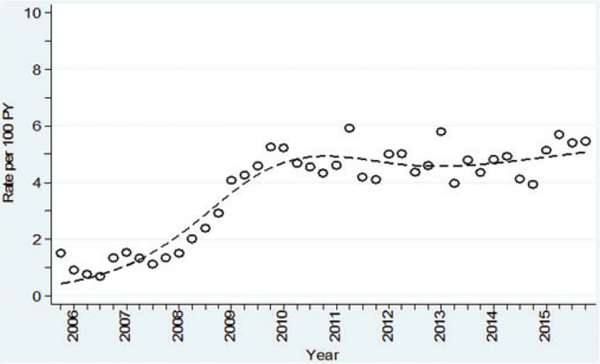
Among 3357 clients initially seronegative for syphilis, 376 (11.2%) had incident syphilis infection, with a median time between last negative and first positive serology test result of 337 days (IQR 168–784 days). The median number of syphilis tests performed before seroconverting was five (IQR 3–7). The overall incidence of syphilis infection was 4.4 per 100 PY (95% CI 3.9–4.8) (Table 2). Syphilis incidence was significantly higher in the younger age groups (4.9 per 100 PY for those aged 18–21 years, 4.8 per 100 PY for those aged 22–29 years) compared with 3.5 per 100 PY for those aged ≥30 years, p < 0.001. Syphilis incidence rose and peaked in quarter 2/2011 at 5.9 per 100 PY, then remained steady in the range of 4.2 per 100 PY in quarter 3/2011 to 5.5 per 100 PY in quarter 4/2015 (Figure 4). Poisson regression of the estimated temporal trend using an RCS function found a significant nonlinear time trend (p = 0.001). Age was also significant (p < 0.0001), with clients aged 22–29 years being at greater risk for incident syphilis infection (RR 1.4, 95% CI 1.2–1.5) (Table 2).
Figure 4.
Quarterly syphilis incidence (first episode), from quarter 4/2005 to quarter 4/2015, using a RCS curve, in the VCT cohort of MSM, and TGW, 18 years and older at first test, Bangkok, Thailand.
Incidence trends
The evaluation of the ecologic relationship between the two trend curves of HIV and syphilis incident infections using correlation (regression) analyses found a Pearson correlation of 0.75, a moderately high positive association between the two curves. However, when we divided the time into two periods, quarter 4/2005-quarter 3/2011 and quarter 4/2011-quarter 4/2015, the correlation was high during the first period (Pearson = 0.87) and low during the second period (Pearson = 0.10).
Discussion
Fifteen years have passed since the recognition of a high prevalence of HIV infection among MSM in Bangkok. Subsequent biannual surveys by the Thailand MOPH showed an increase and then stabilization of HIV prevalence in the sampled population between 25 and 31%.6–8,31 In this study of VCT clients at a large MSM and TGW clinic in Bangkok, conducted between 2005 and 2015, we found both HIV and syphilis incidence among clients to reach a peak of 5–6 per 100 PY in 2011, reflective of ongoing epidemics of HIV and syphilis infections in this population. Despite a recent drop in the number of new HIV infections in this population tested, the incidence continues to be alarmingly high. Most alarming, the youngest adults, aged 18–21 years, had the highest rate of incident infections for both HIV (more than twice the incidence than for those over 30) and syphilis, a concerning trend.16 Due to high-risk behavior, vulnerability to STIs is substantial albeit preventable for young people.
The overall HIV incidence rate found among MSM and TGW in the cohort period, in the context of the Thai epidemic, is 18 times as high as in the general population of the Thailand Eastern seaboard as seen in the placebo arm of the RV144 trial between 2003 and 2008,32 and nearly 200 times as high as the estimated incidence of 0.03% in 2011 among adults in Thailand.33 This ongoing high HIV incidence contrasts sharply with the history of the HIV epidemic among Thai heterosexuals. The rate of HIV infection in the general Thai population was brought under control by systematic interventions including the widespread use of barrier methods.1 The epidemic among MSM and TGW, however, subsequently increased and continues unabated. Two earlier reports derived from this dataset from 2005 through 2011 showed increasing incidence among MSM.7,26 Our data are similar to the high burden of infection among MSM found in an acute HIV infection study at the Bangkok Thai Red Cross anonymous clinic between 2006 and 2007, with 60% of new infections among MSM.34 Similarly, a study of VCT repeat testers in Chiang Mai, Northern Thailand during 2008–2009 found an HIV incidence of 8.2 per 100 PY.35
Our data from 2010 to 2015 show a decline in HIV incidence among the population accessing services at SCC, raising the possibility that transmission may have slowed over the past five years. This was unlikely due to increased coverage of antiretroviral therapy (ART) in the MSM population, as the UNAIDS 2016 Prevention Gap Report showed that only 10% of HIV-infected MSM were estimated to be covered by ART between 2013 and 2015.36 Prevention messaging could also have had an effect, with a resultant change in behavior.37 Social media can be used as a medium for prevention messaging, although we know of only one official Thai online intervention prior to 20 1 4.38 Anecdotal evidence suggests that during the study period MSM and TGW increased use of social networking apps and websites to find casual sexual partners, behavior found to be associated with increased risk of HIV.39
In contrast, the incidence of syphilis was initially high, reached a peak during the study period, and was sustained over time. This is consistent with the global epidemic of syphilis among MSM, a trend that is not abating.40,41 Syphilis is a recognized risk factor for transmission and acquisition of HIV infection because of both biologic and behavioral factors.42 Incident syphilis infections are a marker of ongoing high-risk sexual behaviors in the population, which may contribute to the HIV epidemic.43 Globally, syphilis infection rates are high among both HIV-infected11 and HIV-uninfected MSM,9 can be asymptomatic as well as undetected, and can lead to ‘bridging’ between the MSM population and the general community. Continued syphilis screening and treatment for MSM and TGW, including prompt partner notification and treatment, will support prevention of HIV and STIs.
If the incidence in the MSM and TGW Thai HIV epidemic is indeed declining as we found, this is welcome news, a promising sign that the high HIV incidence rates seen in the 2000s may have stabilized or even declined. The strength of this analysis is the duration of the longitudinal assessment, and a continual infusion of young MSM, although we cannot rule out a cohort effect. However, previous assessments of the incidence in VCT clients demonstrated a similar incidence to a cohort study.23 The HIV incidence trend in the VCT population described here also fit trends seen elsewhere among MSM, especially in Asia.44,45 The U.S. has noted recent declines in estimated HIV incidence (no longer directly measured by CDC)46,47 which may indicate impact of prevention strategies such as PrEP and antiretroviral treatment to reduce onward transmission.48
Although the SCC advises VCT clients to return for follow-up, many elect not to return. In this cohort, <50% of VCT clients returned for follow-up of either HIV or syphilis test results, and less for syphilis testing. Some officials in Thailand consider this better-than-expected follow-up without incentives or integrated health care delivery. In addition to being an opportunity for referral if needed, HIV testing is a decision point for conducting risk assessments and providing high-impact prevention interventions such as PrEP to reduce HIV transmission in these key populations.49 According to CDC guidelines, PrEP management also includes regular STI testing50 and is an opportunity to test and treat syphilis more frequently. We also noted a significant testing interval difference, the sero-converters with a lower CD4 count (<350 cells/μl) had a wider interval between testing than those who had a higher CD4 count (>500 cells/μl), indicating that testing messages still need to be received by those with more advanced infection.
With this expanded incidence trend line we are making a stronger argument for the need for PrEP in the national health plan, since this high incidence (even if it declined in later years) was in a country with a well-established and long-term commitment to treatment access for all. Given the high efficacy found in major PrEP trials among MSM,51–53 scale-up of PrEP is needed nationwide in Thailand for all MSM and TGW. The announcement in early 2018 that the Thailand National Health Security Office is set to approve payment for PrEP as part of a prevention package is a welcome sign that PrEP will be financially accessible to all MSM and TGW in Thailand who are at substantial risk of infection.
There are limitations to our study. For one, clients who continued to follow-up in the clinic may not be representative of the general MSM and TGW populations in Bangkok. For example, they may be motivated to follow-up because of self-recognition of ongoing risk behavior, higher knowledge, or other reasons. Our VCT services construct an open cohort, in that clients can enroll, repeat test, and follow-up (or not) as they prefer. Thus, we are not able to draw any conclusions about the causal relationship between the two incident infections. The incidence of syphilis could be underestimated. Incident syphilis cases could have been missed since our definition of incident syphilis is specific but not sensitive as it was based on seroconversion. Persons who had evidence of previous infection would be excluded with this definition, persons with a history of syphilis in a serofast state could become reinfected, and newly infected persons with RPR ≤1:4 would be missed. VCT services are offered to all who come to the clinic, but we were unable to separate the MSM and TGW into distinct groups. We agree that there is a strong need for more transgender-specific data. Testing visits varied from a median of 193 days (6.3 months) for HIV testing to 278 days (9.1 months) for syphilis testing; verification of the specific time of incident infection may be limited by the time frame between visits. In addition, the uniform probability distribution is an approximation that would not capture any recency bias toward exposure events prior to or prompting a VCT visit. Lastly, we collected minimal behavioral data and are thus unable to assess if behavioral factors in a changing client base were responsible for the shift in incidence.
In 2013, for the first time, the Thai MOPH issued national guidelines for implementing HIV prevention among MSM and TGW, which were reiterated again in 2017.24 The testing practice recommended in 2013 had been recommended at SCC VCT services since its inception in 2005, making the SCC VCT cohort a valuable and unique source of incidence data for the evaluation and monitoring of new HIV infections among the MSM population in Bangkok.
Acknowledgment
We wish to acknowledge the Silom Community Clinic, including the staff, clients, and our community partners, who work tirelessly to serve populations at risk for HIV infection in Bangkok, Thailand. The authors kindly acknowledge and thank for the support of the personnel of the Thailand Ministry of Public Health-U.S. Centers for Disease Control and Prevention Collaboration, the SCC (now SCC @TropMed), the Department of Disease Control, Thailand MOPH, the Rainbow Sky Association of Thailand, and the Service Workers in Group Foundation. We dedicate this work to the memory of Mrs Supaporn Chaikummao and to Mr Patrick J. Flaherty who were both devoted to HIV prevention at SCC. The data in this study have been presented previously at the HIV Research for Prevention conference, Chicago, IL, 17–21 October 2016, abstract 0A05.04, and at the HIV Research for Prevention conference, Cape Town, South Africa, 28–31 October 2014.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The US Centers for Disease Control and Prevention funded the research.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The authors had full access to the data and had final responsibility for the decision to submit for publication. The findings and conclusions presented in this paper are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
References
- 1.Nelson KE, Celentano DD, Eiumtrakol S, et al. Changes in sexual behavior and a decline in HIV infection among young men in Thailand. N Engl J Med 1996; 335: 297–303. [DOI] [PubMed] [Google Scholar]
- 2.Lolekha R, Boonsuk S, Plipat T, et al. Elimination of mother-to-child transmission of HIV - Thailand. Morb Mortal Wkly Rep 2016; 65: 562–566. [DOI] [PubMed] [Google Scholar]
- 3.Thanprasertsuk S, Sirivongrangson P, Ungchusak K, et al. The invisibility of the HIV epidemic among men who have sex with men in Bangkok, Thailand. AIDS 2005; 19: 1932–1933. [DOI] [PubMed] [Google Scholar]
- 4.van Griensven F, Phanuphak N and Srithanaviboonchai K. Biomedical HIV prevention research and epidemic control in Thailand: two sides of the same coin. Sex Health 2014; 11: 180–199. [DOI] [PubMed] [Google Scholar]
- 5.van Griensven F, Thanprasertsuk S, Jommaroeng R, et al. Evidence of a previously undocumented epidemic of HIV infection among men who have sex with men in Bangkok, Thailand. AIDS 2005; 19: 521–526. [DOI] [PubMed] [Google Scholar]
- 6.van Griensven F, Varangrat A, Wimonsate W, et al. HIV prevalence among populations of men who have sex with men - Thailand, 2003 and 2005. Morb Mortal Wkly Rep 2006; 55: 844–848. [PubMed] [Google Scholar]
- 7.Ananworanich J, Chitwarakorn A, Wimonsate W, et al. HIV and syphilis infection among men who have sex with men - Bangkok, Thailand, 2005–2011. Morb Mortal Wkly Rep 2013; 62: 518–520. [PMC free article] [PubMed] [Google Scholar]
- 8.van Griensven F, Varangrat A, Wimonsate W, et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr 2010; 53: 234–239. [DOI] [PubMed] [Google Scholar]
- 9.Patton ME, Su JR, Nelson R, et al. Centers for disease control and prevention. Primary and secondary syphilis-United States, 2005–2013. Morb Mortal Wkly Rep 2014; 63: 402–406. [PMC free article] [PubMed] [Google Scholar]
- 10.Peterman TA, Su J, Bernstein KT, et al. Syphilis in the United States: on the rise? Expert Rev Anti Infect Ther 2015; 13: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchell AN, Allen VG, Gardner SL, et al. High incidence of diagnosis with syphilis co-infection among men who have sex with men in an HIV cohort in Ontario, Canada. BMC Infect Dis 2015; 15: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abara WE, Hess KL, Neblett Fanfair R, et al. Syphilis trends among men who have sex with men in the United States and Western Europe: a systematic review of trend studies published between 2004 and 2015. PLoS One 2016; 11: e0159309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snowden JM, Konda KA, Leon SR, et al. Recent syphilis infection prevalence and risk factors among male low-income populations in coastal Peruvian cities. Sex Transm Dis 2010; 37: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Jia Y, Ruan Y, et al. Correlates of incident infections for HIV, syphilis, and hepatitis B virus in a cohort of men who have sex with men in Beijing. AIDS Patient Care STDS 2010; 24: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtz TH, Thienkrua W, McNicholl JM, et al. Prevalence of Treponema pallidum seropositivity and herpes simplex virus type 2 infection in a cohort of men who have sex with men, Bangkok, Thailand, 2006–2010. Int J STD AIDS 2012; 23: 424–428. [DOI] [PubMed] [Google Scholar]
- 16.Thienkrua W, Todd CS, Chonwattana W, et al. Incidence of and temporal relationships between HIV, herpes simplex II virus, and syphilis among men who have sex with men in Bangkok, Thailand: an observational cohort. BMC Infect Dis 2016; 16: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung KT, Fairley CK, Read TR, et al. HIV incidence and predictors of incident HIV among men who have sex with men attending a sexual health clinic in Melbourne, Australia. PLoS One 2016; 11: e0156160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliani M, Vescio MF, Latini A, et al. Continuous increase in HIV-1 incidence after the year 2000 among men who have sex with men in Rome: insights from a 25-year retrospective cohort study. Euro Surveill 2014; 19: 20969. [DOI] [PubMed] [Google Scholar]
- 19.Jia Z, Huang X, Wu H, et al. HIV burden in men who have sex with men: a prospective cohort study 2007–2012. Sci Rep 2015; 5: 11205–11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meireles P, Lucas R, Carvalho C, et al. Incident risk factors as predictors of HIV seroconversion in the Lisbon cohort of men who have sex with men: first results, 2011–2014. Euro Surveill 2015; 20(14): pii=21091. [DOI] [PubMed] [Google Scholar]
- 21.Meireles P, Lucas R, Martins A, et al. The Lisbon Cohort of men who have sex with men. BMJ Open 2015; 5: e007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, An M, Han X, et al. Prospective cohort study of HIV incidence and molecular characteristics of HIV among men who have sex with men (MSM) in Yunnan Province, China. BMC Infect Dis 2013; 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013; 27: 825–832. [DOI] [PubMed] [Google Scholar]
- 24.Thailand Ministry of Public Health. National guidelines for implementing HIV prevention in men who have sex with men and transgender populations. Nonthaburi: Thailand Ministry of Public Health, 2013. [Google Scholar]
- 25.Colby D, Srithanaviboonchai K, Vanichseni S, et al. HIV pre-exposure prophylaxis and health and community systems in the Global South: Thailand case study. J Int AIDS Soc 2015; 18: 19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Griensven F, Holtz TH, Thienkrua W, et al. Temporal trends in HIV-1 incidence and risk behaviours in men who have sex with men in Bangkok, Thailand, 2006–13: an observational study. Lancet HIV 2015; 2: e64–e70. [DOI] [PubMed] [Google Scholar]
- 27.Thailand Ministry of Public Health. National guidelines on HIV/AIDS diagnosis, treatment, and prevention. Nonthaburi: Thailand Ministry of Public Health, 2010. [Google Scholar]
- 28.Kitayaporn D, Uneklabh C, Weniger BG, et al. HIV-1 incidence determined retrospectively among drug users in Bangkok, Thailand. AIDS 1994; 8: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 29.Desquilbet L and Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010; 29: 1037–1057. [DOI] [PubMed] [Google Scholar]
- 30.Harrell F Jr Regression modeling strategies: with applications to linear, logistic and survival analysis. New York: Springer, 2001. [Google Scholar]
- 31.Thailand Ministry of Public Health. Results of venue-based HIV surveillance among men who have sex with men in Thailand. Nonthaburi: Thailand Ministry of Public Health, 2012. [Google Scholar]
- 32.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361: 2209–2220. [DOI] [PubMed] [Google Scholar]
- 33.Family Health International, Thailand Ministry of Public Health. The Asian epidemic model (AEM) projections for HIV/AIDS in Thailand: 2005–2025. Thailand Ministry of Public Health, https://hivhealthclearinghouse.unesco.org/library/documents/asian-epidemic-model-aem-projections-hivaids-thailand-2005-2025 (2005, accessed 13 November 2018). [Google Scholar]
- 34.Ananworanich J, Phanuphak N, de Souza M, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr 2008; 49: 151–155. [DOI] [PubMed] [Google Scholar]
- 35.Chariyalertsak S, Kosachunhanan N, Saokhieo P, et al. HIV incidence, risk factors, and motivation for biomedical intervention among gay, bisexual men, and transgender persons in Northern Thailand. PLoS One 2011; 6: e24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UNAIDS. Prevention gap report. Geneva: UNAIDS, 2016. [Google Scholar]
- 37.Holtz TH, Pattanasin S, Chonwattana W, et al. Longitudinal analysis of key HIV-risk behavior patterns and predictors in men who have sex with men, Bangkok, Thailand. Arch Sex Behav 2015; 44: 341–348. [DOI] [PubMed] [Google Scholar]
- 38.Anand T, Nitpolprasert C, Ananworanich J, et al. Innovative strategies using communications technologies to engage gay men and other men who have sex with men into early HIV testing and treatment in Thailand. J Virus Erad 2015; 1: 111–115. [PMC free article] [PubMed] [Google Scholar]
- 39.Piyaraj P, van Griensven F, Holtz TH, et al. The finding of casual sex partners on the internet, methamphetamine use for sexual pleasure, and incidence of HIV infection among men who have sex with men in Bangkok, Thailand: an observational cohort study. Lancet HIV 2018; 5: e379–e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Read P, Fairley CK and Chow EP. Increasing trends of syphilis among men who have sex with men in high income countries. Sex Health 2015; 12: 155–163. [DOI] [PubMed] [Google Scholar]
- 41.Williams LA, Klausner JD, Whittington WL, et al. Elimination and reintroduction of primary and secondary syphilis. Am J Public Health 1999; 89: 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathela P, Braunstein SL, Blank S, et al. The high risk of an HIV diagnosis following a diagnosis of syphilis: a population-level analysis of New York City men. Clin Infect Dis 2015; 61: 281–287. [DOI] [PubMed] [Google Scholar]
- 43.Solomon MM, Mayer KH, Glidden DV, et al. Syphilis predicts HIV incidence among men and transgender women who have sex with men in a preexposure prophylaxis trial. Clin Infect Dis 2014; 59: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baral SD, Grosso A, Holland C, et al. The epidemiology of HIV among men who have sex with men in countries with generalized HIV epidemics. Curr Opin HIV AIDS 2014; 9: 156–167. [DOI] [PubMed] [Google Scholar]
- 45.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA 2008; 300: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall HI, Song R, Tang T, et al. HIV trends in the United States: diagnoses and estimated incidence. JMIR Public Health Surveill 2017; 3: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson AS, Hall HI, Hu X, et al. Trends in diagnoses of HIV infection in the United States, 2002–2011. JAMA 2014; 312: 432–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Consolidated guidelines on HIV testing services. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 50.USPHS-CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States - 2017 Update: a clinical practice guideline, https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf (2018, accessed 13 November 2018).
- 51.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14: 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand preexposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4: e402–e410. [DOI] [PubMed] [Google Scholar]