Abstract
A major hurdle in chemical biology is the delivery of native proteins into the cytosol of mammalian cells. Herein, we report that esterification of the carboxyl groups of an enzyme with a diazo compound enables not only its passage into the cytosol, but also the retention of its catalytic activity there. This scenario is demonstrated with human ribonuclease 1, which manifests ribonucleolytic activity that can be cytotoxic. After internalization, the nascent esters are hydrolyzed in situ by endogenous esterases, making the process traceless. This strategy provides unprecedented opportunities for the delivery of functional enzymes into human cells.
Graphical Abstract
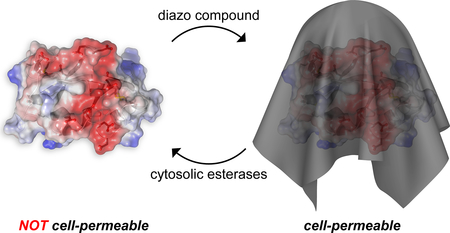
Biologics are driving the growth of the modern pharmacopeia.1 Still, nearly all of these biologics are antibodies and hormones that act on the cell surface.2,3 Virtually none are proteins that intervene beneficially in the cytosol or nucleus, despite incalculable opportunities there.4
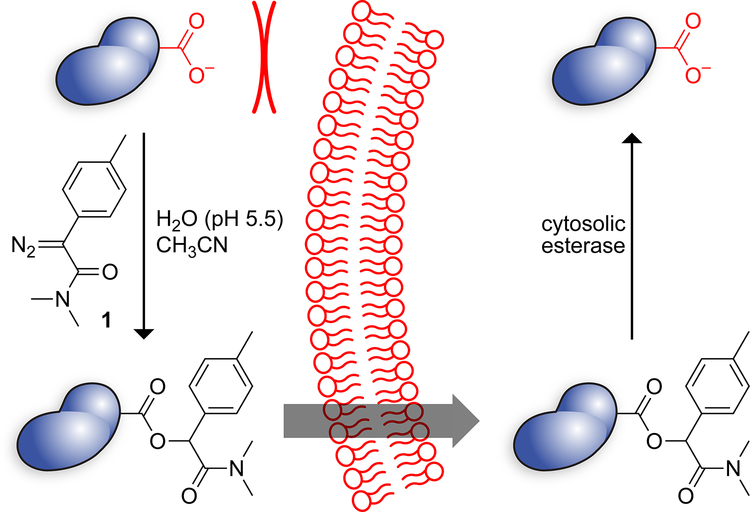
The translocation of a putative therapeutic agent across the plasma membrane is made difficult by the high anionicity of the glycocalyx and hydrophobicity of the lipid bilayer.5 Any strategy for protein delivery must overcome these two obstacles. Medicinal chemists have done so by the bioreversible masking of anionic functional groups. The ensuing “prodrugs”,6–8 which are usually esters of carboxylic acids, constitute 20% of small-molecule drugs.9 Recently, we demonstrated that the esterification of the carboxyl groups of the green fluorescent protein (GFP) with 2-diazo-2-(p-methylphenyl)-N,N-dimethylacetamide enables its passage across the plasma membrane, akin to a small-molecule prodrug (Scheme 1).10 Importantly, the nascent esters are substrates for endogenous intracellular esterases.11,12 Thus, esterification can be a “traceless” delivery method that leaves no residual atoms attached to the cargo.
Scheme 1.
Upon cytosolic entry, esterified GFP retains its fluorescence,10 consistent with the retention of its three-dimensional structure.13 The catalytic activity of an enzyme is, however, the most sensitive indicator of proper conformation.14 We sought to investigate the utility of esterification as a means to promote the delivery of a functional enzyme.
As a model enzyme, we chose human ribonuclease 1 (RNase 1; EC 3.1.27.5; UniProtKB P07998).15 RNase 1 is an efficient catalyst of RNA cleavage, and its ribonucleolytic activity can be cytotoxic.16–18 To be cytotoxic, RNase 1 must not only enter the cytosol, but also evade the ribonuclease inhibitor protein (RI) that resides there.19,20 Wild-type RNase 1 forms a complex with RI that has a Kd value of (at most21) 0.29 fM22 and is not appreciably cytotoxic because of its sequestration by RI.23
RI is a highly anionic protein, and its evasion is best achieved by installing anionic groups into RNase 1. Indeed, the most evasive known variant is R39D/N67D/N88A/G89D/R91D RNase 1, which has 1010-fold lower affinity for RI than does the wild-type enzyme.22 The DDADD variant is, however, not a potent cytotoxin because its anionicity deters cellular uptake.24 Hence, the DDADD variant along with wild-type RNase 1 were ideal enzymes for the assessment of our approach, using cytotoxicity as a readout.
The mechanism of esterification with a diazo compound relies on a carboxyl group being in a protonated state.25,26 To encourage protonation, we performed esterification reactions at pH 5.5 in aqueous acetonitrile (Scheme 1). These conditions were well-tolerated by RNase 1 and its variants, and enabled esterification of 1/3–1/2 of enzymic carboxyl groups (Table 1). Esterification did reduce ribonucleolytic activity slightly (Table 1), but to a lesser extent than did the amidation of enzymic carboxyl groups.27
Table 1. Attributes of Untreated and Esterified RNase 1 Variants.
| RNase 1 | Za | Carboxyl groups |
1 (equiv)b | Estersc |
kcat/KM (107 M–1s–1)d |
IC50 (μM)e | |
|---|---|---|---|---|---|---|---|
| HeLa Cells | H460 Cells | ||||||
| Wild-type | +5 | 13 | 0 | — | 2.5 ± 0.1 | >100 | >100 |
| Wild-type | +5 | 13 | 100 | 4 | 1.1 ± 0.5 | 10 ± 1 | 7 ± 1 |
| Wild-type | +5 | 13 | 200 | 6 | 1.6 ± 0.1 | NDf | 5.1 ± 0.5 |
| DDADD | 0 | 17 | 0 | — | 0.022 ± 0.002 | >100 | >100 |
| DDADD | 0 | 17 | 100 | 7 | 0.023 ± 0.003 | 6 ± 1 | 8.4 ± 0.5 |
| DDADD | 0 | 17 | 200 | 11 | 0.025 ± 0.004 | ND | 1.0 ± 0.2 |
| H12A/K41A/H119A | +4 | 13 | 0 | — | <1 × 10–5 | >100 | >100 |
| H12A/K41A/H119A | +4 | 13 | 100 | 4 | <1 × 10–5 | >100 | >100 |
| FLAG-labeled | +2 | 18 | 0 | — | 0.72 ± 0.07 | ND | ND |
| FLAG-labeled | +2 | 18 | 100 | 5 | 0.35 ± 0.04 | ND | ND |
Ref. 28.
Reaction conditions: 200 μL of enzyme (0.14 μmol) in 10 mM MES–HCl buffer (pH 5.5) plus 200 μL of diazo compound 1 (14 or 28 μmol) in acetonitrile; 37 °C for 4 h.
Values are the most prevalent species apparent in the mass spectra of Figures S3–S6.
Values are the mean ± SD for the cleavage of 6-FAM–dArU(dA)2–6-TAMRA in 100 mM Tris–HCl buffer (pH 7.5), containing NaCl (100 mM). Individual values are shown in Figure S7.
ND, not determined.
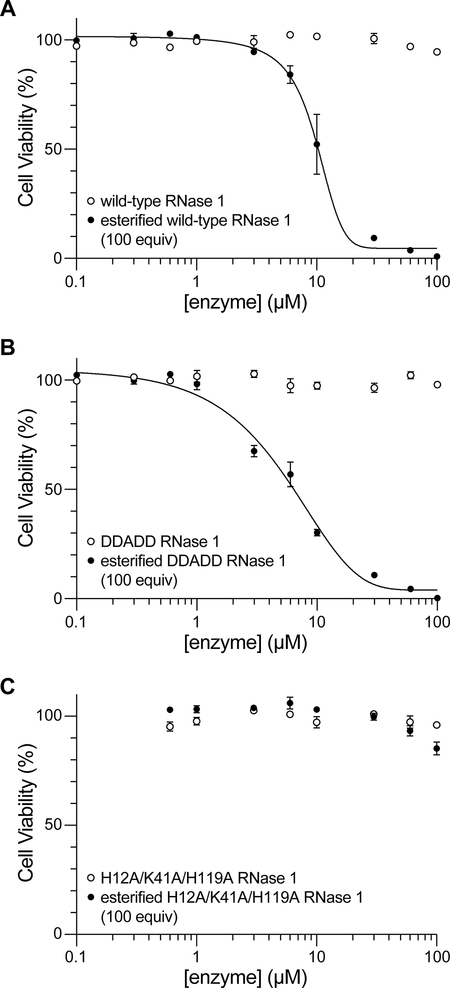
As observed previously,23 wild-type RNase 1 had no detectable effect on the viability of HeLa cells, even at a concentration of 100 μM (Figure 2A). In contrast, esterification of wild-type RNase 1 engendered toxicity towards HeLa cells with an IC50 value of (10 ± 1) μM (Figure 2A). These data are consistent with esterified RNase 1 overwhelming cytosolic RI, which is present at ~4 μM.29 Notably, only ~7% of unmodified RNase A (which is a bovine homolog of RNase 1) translocates to the cytosol from endosomes.30 Accordingly, the lack of toxicity of unmodified RNase 1 along with the near-equivalence of the IC50 value of esterified RNase 1 and the cytosolic concentration of RI suggests that esterification of RNase 1 makes its cytosolic delivery efficient.
Figure 2.
Effect of esterification of human RNase 1 (A) and its variants (B, C) on the viability of HeLa cells. Cell viability was measured with a tetrazolium dye-based assay for metabolic activity. Values of IC50 are listed in Table 1.
Wild-type RNase 1 has a net charge of Z = 5.28 The unmodified enzyme enters endosomes rapidly.24 In contrast, DDADD RNase 1 has a net charge of Z = 0 and enters endosomes slowly.24 As expected, DDADD RNase 1 did not have an impact on the viability of HeLa cells (Figure 2B). In contrast, esterification made the DDADD variant cytotoxic with an IC50 value of (6 ± 1) μM (Figure 2B), despite its low kcat/KM value (Table 1). This cytotoxicity is consistent with the esterified enzyme entering the cytosol and cleaving cellular RNA there.
We were aware that the observed cytotoxicity of the esterified enzymes could be due to a property other than their catalytic activity. For example, some proteins and peptides are cytotoxic because of their ability to disrupt lipid bilayers.31,32 To test this alternative mechanism, we employed H12A/K41A/H119A RNase 1, which has an eviscerated active site and no detectable ribonucleolytic activity (Table 1). We found that this variant is not cytotoxic, even upon esterification (Figure 2C). Accordingly, we conclude that the cytotoxicity of both esterified wild-type RNase 1 and esterified DDADD RNase 1 relies on the manifestation of their catalytic activity within cells. We note, too, that the toxicity of these esterified enzymes for HeLa cells exceeds that of QBI-139 (IC50 = 18 ± 2 μM),23 which is an RI-evasive variant of RNase 1 that is undergoing clinical trials as a cancer chemotherapeutic agent.33,34
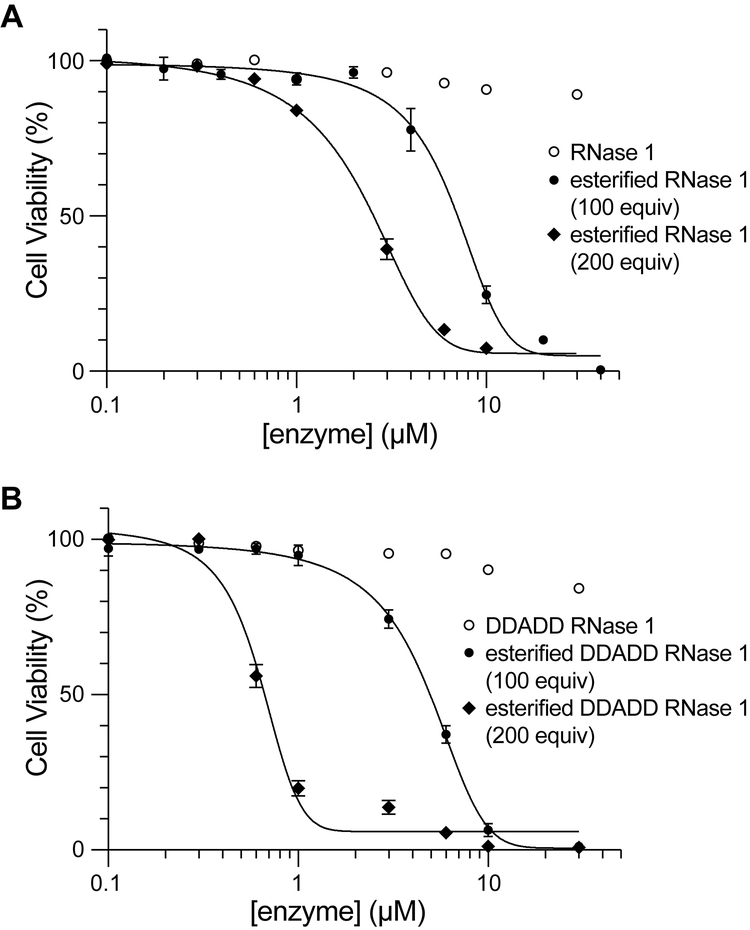
HeLa cells, which were derived from a cervical tumor, have numerous abnormalities.35 Accordingly, we sought to reproduce our results in another cell line. We chose H460 cells, which were derived from a non-small-cell lung tumor. We also used this cell line to assess the effect of esterification level on cytotoxicity. Wild-type RNase 1 and its DDADD variant were treated with either 100 or 200 equiv of diazo compound 1. We found that increasing the esters in wild-type RNase 1 from ~4 to ~6 reduced the IC50 value from (7 ± 1) μM to (5.1 ± 0.5) μM (Figure 3A). Wild-type RNase 1 has 13 carboxyl groups (Figure 1), whereas the DDADD variant has 17 carboxyl groups. The larger number of carboxyl groups amplified the effects. Specifically, we found that increasing the esters in DDADD RNase 1 from ~7 to ~11 reduced the IC50 value from (8.4 ± 0.5) μM to (1.0 ± 0.2) μM (Figure 3B).
Figure 3.
Impact of the extent of esterification of human RNase 1 (A) and its DDADD variant (B) on the viability of H460 cells. Cell viability was measured with a tetrazolium dye-based assay for metabolic activity. Values of IC50 are listed in Table 1.
Figure 1.
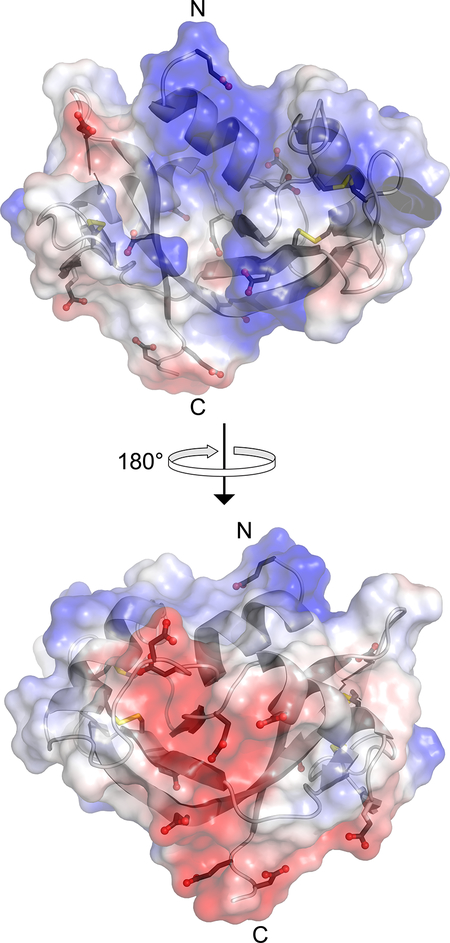
Surface electrostatic potential of human RNase 1 (blue, positive; red, negative). The side chains of the 6 aspartate, 6 glutamate, and 4 cystine residues are shown explicitly. The image was created with the program PyMOL from Schrödinger (New York, NY) and Protein Data Bank entry 1z7x, chain X.22
Finally, we investigated the reversibility of enzymic esterification in living cells. To do so, we appended an 8-residue FLAG tag to the N terminus of wild-type RNase 1 and esterified the resulting FLAG–RNase 1 by using diazo compound 1. We treated HeLa cells with untreated or esterified FLAG–RNase 1 for 24 h, washed and lysed the cells, and recovered the FLAG–RNase 1 by using anti-FLAG magnetic beads. Mass spectrometry revealed the removal of labels by intracellular esterases (Figure S6).36 These data indicate that the esters installed by diazo compound 1 are hydrolyzed by esterases in human cells.
In summary, we have used a diazo compound to esterify enzymic carboxyl groups and shown that the ensuing enzyme enters the cytosol of human cells and is functional there. Because the catalytic activity of an enzyme is fragile, its maintenance indicates that the delivery process is gentle. Notably, esterification can be performed without the need for mutagenesis,37 and the modification is traceless, being removed by cellular esterases. This facile, versatile strategy provides new opportunities for delivering native, functional proteins to intracellular targets.
Supplementary Material
Funding Sources
V.T.R. was supported by a William H. Peterson Fellowship in Biochemistry (Department of Biochemistry, University of Wisconsin–Madison). K.A.M. was supported by Molecular Biosciences Training Grant T32 GM007215 (NIH) and by the Broad Institute Chemical Biology and Therapeutics Science Shark Tank. This work was supported by Grants R01 GM044783 and R01 CA073808 (NIH).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.xxxx.
Experimental procedures, Figure S1 (DNA sequences), Figures S2 (amino acid sequences), and Figures S3–S7 (PDF).
The authors declare no competing financial interest.
REFERENCES
- (1).Mullard A Top product sales forecasts for 2018. Nat. Rev. Drug Discov 2018, 17, 86. [DOI] [PubMed] [Google Scholar]
- (2).Mitragotri S; Burke PA; Langer R Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov 2014, 13, 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fu A; Tang R; Hardie J; Farkas ME; Rotello VM Promises and pitfalls of intracellular delivery of proteins. Bioconjugate Chem. 2014, 25, 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Reineke TM; Raines RT; Rotello VM Delivery of proteins and nucleic acids: Achievements and challenges. Bioconjugate Chem. 2019, 30, 261–262. [DOI] [PubMed] [Google Scholar]
- (5).Du S; Liew SS; Li L; Yao SQ Bypassing endocytosis: Direct cytosolic delivery of proteins. J. Am. Chem. Soc 2018, 140, 15986–15996. [DOI] [PubMed] [Google Scholar]
- (6).Testa B; Mayer JM Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology. Verlag Helvetica Chimica Acta: Zürich, Switzerland, 2003. [Google Scholar]
- (7).Liederer BM; Borchardt RT Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci 2006, 95, 1177–1195. [DOI] [PubMed] [Google Scholar]
- (8).Lavis LD Ester bonds in prodrugs. ACS Chem. Biol 2008, 3, 203–206. [DOI] [PubMed] [Google Scholar]
- (9).Rautio J; Kärkkäinen J; Sloan KB Prodrugs—Recent approvals and a glimpse of the pipeline. Eur. J. Pharm. Sci 2017, 109, 146–161. [DOI] [PubMed] [Google Scholar]
- (10).Mix KA; Lomax JE; Raines RT Cytosolic delivery of proteins by bioreversible esterification. J. Am. Chem. Soc 2017, 139, 14396–14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Liederer BM; Borchardt RT Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci 2006, 95, 1177–1195. [DOI] [PubMed] [Google Scholar]
- (12).Wang D; Zou L; Jin Q; Hou J; Ge G; Yang L Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Waldo GS; Standish BM; Berendzen J; Terwilliger TC Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol 1999, 17, 691–695. [DOI] [PubMed] [Google Scholar]
- (14).Knowles JR Tinkering with enzymes: What are we learning? Science 1987, 236, 1252–1258. [DOI] [PubMed] [Google Scholar]
- (15).Sorrentino S The eight human “canonical” ribonucleases: Molecular diversity, catalytic properties, and special biological actions of the enzyme proteins. FEBS Lett. 2010, 584, 2194–2200. [DOI] [PubMed] [Google Scholar]
- (16).Kim J-S; Souček J; Matoušek J; Raines RT Catalytic activity of bovine seminal ribonuclease is essential for its immunosuppressive and other biological activities. Biochem. J 1995, 308, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Dickson KA; Dahlberg CL; Raines RT Compensating effects on the cytotoxicity of ribonuclease A variants. Arch. Biochem. Biophys 2003, 415, 172–177. [DOI] [PubMed] [Google Scholar]
- (18).Rutkoski TJ; Kurten EL; Mitchell JC; Raines RT Disruption of shape-complementarity markers to create cytotoxic variants of ribonuclease A. J. Mol. Biol 2005, 354, 41–54. [DOI] [PubMed] [Google Scholar]
- (19).Leland PA; Staniszewski KE; Kim B-M; Raines RT Endowing human pancreatic ribonuclease with toxicity for cancer cells. J. Biol. Chem 2001, 276, 43095–43102. [DOI] [PubMed] [Google Scholar]
- (20).Rutkoski TJ; Raines RT Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr. Pharm. Biotechnol 2008, 9, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hoang TT; Tanrikulu IC; Vatland QA; Hoang TM; Raines RT A human ribonuclease variant and ERK-pathway inhibitors exhibit highly synergistic toxicity for cancer cells. Mol. Cancer Ther 2018, 17, 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Johnson RJ; McCoy JG; Bingman CA; Phillips GN Jr.; Raines RT Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. J. Mol. Biol 2007, 367, 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Thomas SP; Kim E; Kim J-S; Raines RT Knockout of the ribonuclease inhibitor gene leaves human cells vulnerable to secretory ribonucleases. Biochemistry 2016, 55, 6359–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Johnson RJ; Chao T-Y; Lavis LD; Raines RT Cytotoxic ribonucleases: The dichotomy of Coulombic forces. Biochemistry 2007, 46, 10308–10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Roberts JD; Watanabe W; McMahon RE The kinetics and mechanism of the reaction of diphenyldiazomethane and benzoic acid in ethanol. J. Am. Chem. Soc 1951, 73, 760–765. [Google Scholar]
- (26).Roberts JD; Watanabe W; McMahon RE The kinetics and mechanism of the reaction of diphenyldiazomethane with 2,4-dinitrophenol in ethanol. J. Am. Chem. Soc 1951, 73, 2521–2523. [Google Scholar]
- (27).Futami J; Maeda T; Kitazoe M; Nukui E; Tada H; Seno M; Kosaka M; Yamada H Preparation of potent cytotoxic ribonucleases by cationization: Enhanced cellular uptake and decreased interaction with ribonuclease inhibitor by chemical modification of carboxyl groups. Biochemistry 2001, 40, 7518–7524. [DOI] [PubMed] [Google Scholar]
- (28).The parameter Z is defined as the number of arginine + lysine – aspartate – glutamate residues.
- (29).Haigis MC; Kurten EL; Raines RT Ribonuclease inhibitor as an intracellular sentry. Nucleic Acids Res. 2003, 31, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chao T-Y; Raines RT Fluorogenic label to quantify the cytosolic delivery of macromolecules. Mol. BioSyst 2013, 9, 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Rosenberg HF Recombinant human eosinophil cationic protein. Ribonuclease activity is not essential for cytotoxicity. J. Biol. Chem 1995, 270, 7876–7881. [DOI] [PubMed] [Google Scholar]
- (32).Leuschner C; Hansel W Membrane disrupting lytic peptides for cancer treatments. Curr. Pharm. Des 2004, 10, 2299–2310. [DOI] [PubMed] [Google Scholar]
- (33).Strong LE; Kink JA; Pensinger D; Mei B; Shahan M; Raines RT Efficacy of ribonuclease QBI-139 in combination with standard of care therapies. Cancer Res. 2012, 72 (Suppl. 1), 1838. [Google Scholar]
- (34).Strong LE; Kink JA; Mei B; Shahan MN; Raines RT First in human phase I clinical trial of QBI-139, a human ribonuclease variant, in solid tumors. J. Clin. Oncol 2012, 30 (Suppl), TPS3113. [Google Scholar]
- (35).Landry JJM; Pyl PT; Rausch T; Zichner T; Tekkedil MM; Stütz AM; Jauch A; Aiyar RS; Pau G; Delhomme N; Gagneur J; Korbel JO; Huber W; Steinmetz LM The genomic and transcriptomic landscape of a HeLa cell line. G3 2013, 3, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Interestingly, we observed a ~160-Da mass increase for esterified FLAG–RNase 1 after its recovery from human cells (Figure S6). This increase likely results from the phosphorylation of FLAG–RNase 1 by cytosolic kinases, as has been observed with a homologous ribonuclease (Hoang, T. T.; Raines, R. T. Molecular basis for the autonomous promotion of cell proliferation by angiogenin. Nucleic Acids Res. 2017, 45, 818–831).
- (37).Fuchs SM; Rutkoski TJ; Kung VM; Groeschl RT; Raines RT Increasing the potency of a cytotoxin with an arginine graft. Protein Eng. Des. Sel 2007, 20, 505–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.