Abstract
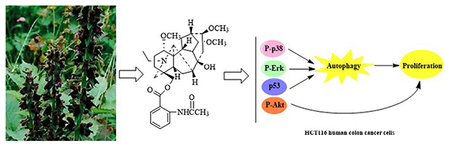
A new C19 diterpenoid alkaloid, brevicanine (1) and six known ones (2–7) were isolated from Aconitum brevicalcaratum (Finet et Gagnep.) Diels. Their structures were elucidated on the basis of extensive spectroscopic analyses. The cytotoxicity of those compounds was investigated against HCT116 human cancer cell line, which showed none of them possessing considerable anti-proliferative activities. To evaluate the autophagy effect of compounds 1–7, Western blot was used to detect the expression of autophagic marker by stimulating human cancer HCT116 cells. The results showed that compound 6 induced protective autophagy in HCT116 cells. Mechanistic insight showed that compound 6 induced protective autophagy through p53 activation, ERK1/2 and p38 MAPK signaling cascade.
Keywords: Aconitum brevicalcaratum (Finet et Gagnep.) Diels, brevicanine, autophagy, HCT116
Graphical Abstract
1. Introduction
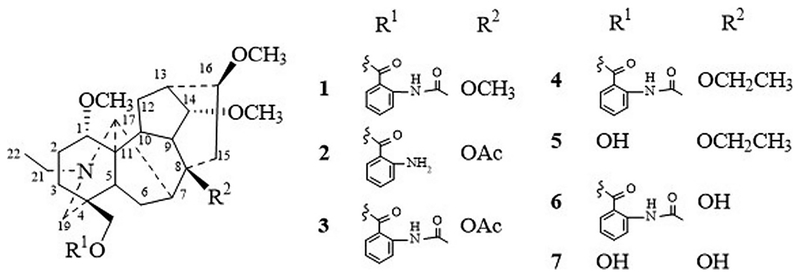
Aconitum brevicalcaratum (Finet et Gagnep.) Diels, belonging to the Ranunculaceae family, is distributed in Yunnan Province and grows at altitudes 2800–3800 m (IBCAS 1979). It has been used as a folk medicine for the treatment of coughs, colds and various types of pains for a long time (Jia & Li 2005). In previous reports, eight diterpene alkaloids from this plant have been isolated and identified (Li & Chen 1994a, 1994b). The diterpenoid alkaloids are the characteric ingredients displaying various bioactivities (Yin et al. 2014; Shyaula et al. 2015; Chen et al. 2016; Liang et al. 2017). However, the bioactivities have not been studied yet, which prompted us to undertake a systematic study of this plant. Herein, we report the isolation and structural elucidation of one new diterpene alkaloids, brevicanine (1) by 1D, 2D NMR and HR–ESI–MS experiments, along with six known compounds (Figure 1), acobretine A (2), acobretine D (3), acobretine C (4), 8-O-ethylscaconine (5), scaconitine (6) and scaconine (7).
Figure 1.
The diterpenoid alkaloids isolated from Aconitum brevicalcaratum (Finet et Gagnep.) Diels.
Accordingly, targeting autophagy may be a promising therapeutic strategy for treatment of diseases (Kamat et al. 2014). Recently, accumulating evidence has revealed many small-molecule compounds that can activate or inhibit autophagy and may therefore have remarkable therapeutic potential on diseases, such as cancers and neurodegenerative diseases (Cheng et al. 2013). In the present study, compounds 1–7 were investigated for their cytotoxicity against human colorectal cancer cell, as well as the mechanism of their autophagy-inducing activities.
2. Results and discussion
2.1. Structure elucidation and identification
Compound 1 was isolated as a white amorphous powder and gave a positive reaction to Dragendorff’s reagent. Its molecular formula was determined as C34H48N2O7 by HR–ESI–MS at m/z 597.3547 [M + H]+ (Calcd for C34H49N2O7, 597.3540). The IR spectrum indicated the presence of aromatic ring (1448, 1527, 1589 cm−1), carbonyl group (1688 cm−1). The 1H NMR spectrum of 1 (Table S1) showed characteristic signals for an N-ethyl group at (δH 1.08, 3H, t, J = 7.2 Hz; δH 2.43, 2.52, each 1H, m), and four methoxyl groups (δH 3.28, 3.13, 3.35, and 3.35, each 3H, s). In the NMR spectra (Table S1), the signals at δH 8.70 (1H, d, J = 8.4 Hz), 7.54 (1H, t, J = 8.4 Hz), 7.09 (1H, t, J = 8.4 Hz), 7.97 (1H, d, J = 7.8 Hz), together with δC 168.3, 115.0, 141.9, 120.6, 134.8, 122.5, 130.6, and an acetoxy group (δH 2.23 s; δC 169.2 s, 25.6 q) indicated the presence of an o-acetamidobenzoate moiety (-OCOC6H4-O-NHAc) (Yue et al. 1994). Besides the signal of an N-ethyl group, an acetamidobenzoate moiety and four methoxy groups in the 13C NMR spectrum, it has the surplus of 19 carbon signals including seven methylene, nine methenyl and three quaternary carbons, consistent with the molecular formula of C34H48N2O7. These data indicated that 1 was a aconitine-type C19-diterpenoid (Yin et al. 2014). The four methoxy groups were assigned to at C-1, C-8, C-14 and C-16, due to the long-range correlations between 1-OCH3 (δH 3.28) and C-1 (δC 85.5), 8-OCH3 (δH 3.13) and C-8 (δC 77.7), 14-OCH3 (δH 3.35) and C-14 (δC 83.9), 16-OCH3 (δH 3.35) and C-16 (δC 83.8) in the HMBC spectrum. The correlations in the HMBC spectrum (Figure S1) between H-18 (δH 4.98, 4.11) and C-7’ (δC 168.3) revealed that the acetamidobenzoate moiety is installed at the C-18. A correlation between H-1 and H-10 in the NOESY experiment (Figure S1), and compared with OCH3–1α compounds chasmanine (δC 86.2 d) and talatisamine (δC 86.1 d), suggested that the methoxy group at C-1 as being α-oriented (Yin et al. 2015). The NOE correlations (Figure S1) of H-14/H10 and H-16/H-17 suggested the methoxy groups at C-14 as being α-oriented, and the other one at C-16 as being β-oriented. Therefore, the structure of compound 1 was determined as shown in Figure 1, named as brevicanine.
Six known compounds, acobretine A (2) (Li & Chen 1994a), acobretine D (3) (Li & Chen 1994b), acobretine C (4) (Li & Chen 1994a), 8-O-ethylscaconine (5) (Li & Chen 1994a), scaconitine (6) (Hao et al. 1985), scaconine (7) (Hao et al. 1985) were isolated.
2.2. Cytotoxicity toward HCT116 Cells
Compounds 1–7 were evaluated for their cytotoxicity against HCT116 human colorectal cancer cell lines by MTT method (Huang et al. 2013; Guo et al. 2014). The results showed compounds 1−7 did not show obvious growth inhibitory effects at concentrations up to 200 μM (Figure S2). Interestingly, the occurrence of a hydroxy group at C-8 unit in compound 6 was much less active against the tested cell lines, even more, the results showed 6 has weak proliferative activity at low concentrations.
2.3. Scaconitine (6) Induces Autophagy in HCT116 Cells
The conversion of LC3-I to LC3-II, as detected by Western blotting, is commonly used as a marker to evaluate autophagy activity (Feng et al. 2017). Western blot analyses showed that compound 6 induced accumulation of LC3-II in HCT116 cells (Figure S3(A)), indicating that compound 6 might induce autophagy in HCT116 cells. Herein, compound 6 was chosen for further study.
To further confirm compound 6-induced autophagy, the autophagy markers of LC3-II, p62 and Beclin 1 were detected by Western blot analysis in HCT116 cells after 24 h treatment with 6 in a concentration-dependent manner. Treatment with compound 6 up-regulated the expression levels of LC3-II and Beclin 1, and down-regulated the expression of p62 in HCT116 cells, indicating that compound 6 could induce autophagy (Kim et al. 2016) (Figure S3(B)).
2.4. Effects of the P53, PI3K-Akt and MAPK Signaling Pathways on Scaconitine (6)-treated cells
To explore the signaling pathway involved in the regulation of autophagy in compound 6-treated HCT116 cells, the role of the PI3K-Akt and MAPK signaling pathways in the modulation of autophagy were investigated by Western blot analysis. As shown in Figures S4(A) and (B), treatment with compound 6 in a dose and time-dependent manner resulted in the activation of Akt (Ser473) which demonstrated that compound 6 might affect the PI3K/Akt signaling pathway to lead to cell proliferation.
To determine whether MAPK signaling pathway was closely related to compound 6-induced autophagy, we treated HCT116 with compound 6 and then detected the levels of p-ERK1/2, p-JNK and p-P38. As shown in Figures S4(A) and (B), compound 6 upregulated the phosphorylation of ERK1/2 and p38 in a dose and time-dependent manner. However, the activation of JNK was not detected in compound 6-treated HCT116 cells.
P53 is linked to autophagy via inhibition of mTOR by AMPK, TSC1, and TSC2, and plays a important role in the transcriptional regulation of autophagy-related target genes (Jing et al. 2011). The effect of compound 6 on the expression of p53 was further examined (Figure S4(B)), compound 6 enhanced the expression levels of p53 in a time-dependent manner and reached a peak at 12 h. These data indicated that compound 6 could induce autophagy via activated ERK1/2, p38 and p53, associated with activated the Akt of the PI3K/Akt signaling pathway (Figure S5).
2.5. Docking Simulations of Scaconitine (6) with ERK1/2 and p38 MAPKs
The theoretical binding mode of the compound 6 in the binding sites of the human p38 MAPK and ERK1/2 were illustrated in Figure S6. The compound 6 adopted a compact conformation to bind inside of the pocket of the p38 MAPK. The phenyl group of the compound 6 was located at the hydrophobic pocket, surrounded by the residues Leu-75, Ile-84, Leu-167 and Leu-171, while the other side of compound 6 stretched into another hydrophobic pocket that consisted of Val-30, Val-38, Leu-108, Gly-110 and Ala-111, forming a stable hydrophobic binding. Detailed analysis showed that the phenyl group in the compound 6 formed the cation-π interaction with the side chain of the residue Lys-53. In addition, the anion-π interactions were observed between the phenyl group of the compound 6 and the side chains of the residues Glu-71 and Asp-168. Importantly, two hydrogen bonds were observed between the compound 6 and residues Lys-53 and Glu-71, with bond lengths of 2.5 and 3.5 Å, respectively, which was the main interaction between the compound 6 and the p38 MAPK.
In ERK1/2, we found that the phenyl group of the compound 6 was located at the hydrophobic pocket, surrounded by the residues Ile-31, Tyr-36, Val-39, Leu-156 and Cys-166, forming a stable hydrophobic binding (Figure S7). Detailed analysis showed that the phenyl group in the compound 6 formed the π–π stacking interaction with the side chain of the residue Tyr-36. In addition, the anion-π interaction was observed between the phenyl group of the compound 6 and the side chains of the residues Asp-167. Importantly, one hydrogen bond interaction was observed between the compound 6 and residue Asp-111, with bond length of 3.1 Å, which was the main interaction between the compound 6 and the ERK1/2. The results of the docking studies should prove helpful in understanding the mechanism by which compound 6 activated p38 MAPK and ERK1/2.
3. Conclusions
Investigation on the whole plant of A. brevicalcaratum resulted in the isolation of one new diterpenoid alkaloid (1) and six known ones (2–7). Compounds 1–7 were evaluated for their cytotoxicity against HCT116 cell lines by MTT assay, all of them show no obvious growth inhibitory effects. The expression of autophagic marker of human cancer HCT116 cells by stimulating compounds 1–7 were evaluated with Western blot, which indicated that compound 6 significantly increased the LC3-II/LC3-I ratio and the expression of Beclin 1 protein, decreasing the expression of p62 protein. Our further investigations indicated that compound 6 simultaneously activated ERK1/2, p38 signaling pathways and p53 in the regulation of protective autophagy. Furthermore, binding studies revealed that the phenyl group and hydrogen bond of compound 6 play key roles in binding interactions with ERK1/2 and p38 MAPK. In addition, compound 6 could also activate AKT which demonstrated PI3K/Akt signaling pathway might induce cell proliferation and decreased apoptosis in compound 6-treated HCT116 cells.
Autophagy plays two different roles in cells, depending on cell type, it may function as a protective mechanism against cellular stress, or it may induce autophagic cell death (Zhai et al. 2014). In this investigation, compound 6 displayed weak proliferative activity to HCT116 cell lines at low concentrations, which are associated with its protective autophagy. Therefore, compound 6 may be a promising chemotherapeutic agent for some autophagic disorders.
Supplementary Material
Acknowledgement
The authors are grateful to Xiao–qing Gan, Yu–ning Hou, Xiao–nan Sun and Yu Zhou for technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81773605]; the Research Foundation for the Educational Commission of Sichuan Province [grant number 15TD0048]; the Fundamental Research Funds for the Central Universities [grant number 2682017QY04]; the United States of America National Institutes of Health [grant number R01 HL128647] to Chunying Li.
Footnotes
Supplemental data for this article can be accessed at https://doi.org/10.1080/14786419.2018.1437435
Supplementary material
Experimentals relating to this article and NMR spectra for compound 1 are available online.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen L, Shan LH, Xu WL, Zhang JF, Huang S, Zhou XL. 2016. A new C20-diterpenoid alkaloid from Aconitum soongaricum var. pubescens. Nat Prod Res. 31(5):523–528. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ren X, Hait WN, Yang JM. 2013. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 65:1162–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZL, Zhang LL, Zheng YD, Liu QY, Liu JX, Feng L, Huang L, Zhang QW, Lu JJ, Lin LG. 2017. Norditerpenoids and dinorditerpenoids from the deeds of Podocarpus nagi as cytotoxic agents and autophagy inducers. J Nat Prod. 80:2110–2117. [DOI] [PubMed] [Google Scholar]
- Guo ZJ, Xu Y, Zhang H, Li MY, Xi K. 2014. New alkaloids from Aconitum taipaicum and their cytotoxic activities. Nat Prod Res. 28(3):164–168. [DOI] [PubMed] [Google Scholar]
- Hao XJ, Chen SY, Zhou J. 1985. Three new diterpenoid alkaloids from Aconitum Scaposum. Acta Botanica Yunnanica. 7:217–224. [Google Scholar]
- Huang S, Zhou XL, Wang CJ, Wang YS, Xiao F, Shan LH, Guo ZY, Weng J. 2013. Pyrrolizidine alkaloids from Liparis nervosa with inhibitory activities against LPS–induced NO production in RAW264.7 macrophages. Phytochemistry. 93:154–161. [DOI] [PubMed] [Google Scholar]
- [IBCAS] Institute of Botany, Chinese Academy of Sciences and Institute of Materia Medica, Chinese Academy of Medical Sciences. 1979. Flora Reipublicae Populais Sinica. Beijing: Science Press; Vol. 27; p. 160. [Google Scholar]
- Jia MR, Li XW. 2005. Zhongguo Minzuzhi Yao. Beijing: China Medical Science Press; p. 8. [Google Scholar]
- Jing K, Song KS, Shin S. 2011. Abstract 2867: docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling in human cancer cells. Cancer Res. 71:2867–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Kyles P. 2014. Autophagy of mitochondria: a promising therapeutic target for neurodegenerative disease. Cell Biochem Biophys. 70:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Pyee Y, Chung HJ, Park HJ, Hong JY, Son KH, Lee SK. 2016. Antitumor activity of spicatoside a by modulation of autophagy and apoptosis in Human Colorectal Cancer cells. J Nat Prod. 79:1097. [DOI] [PubMed] [Google Scholar]
- Li YH, Chen DH. 1994a. The Alkaloidal constituents from the roots of Aconitum Brebicalcaratum Diels. I. Acta ChimF Sinica. 52:204–208. [Google Scholar]
- Li YH, Chen DH. 1994b. Two novel diterpenoid alkaloids isolated from the roots of Aconitum Brebicalcaratum. Acta Bot Sin. 36:148–152. [Google Scholar]
- Liang XX, Lan C, Lei S, Song L, Fei WB, He M, He CL, Yin ZQ. 2017. Diterpenoid alkaloids from the root of Aconitum sinchiangense W. T. Wang with their antitumor and antibacterial activities. Nat Prod Res. 31:2016–2023. [DOI] [PubMed] [Google Scholar]
- Shyaula SL, Tamang T, Ghouri N, Adhikari A, Marasini S, Bajracharya GB, Manandhar MD, Choudhary MI. 2015. Antileishmanial diterpenoid alkaloids from Aconitum spicatum (Bruhl) Stapf. Nat Prod Res. 30:2590–2593. [DOI] [PubMed] [Google Scholar]
- Yin TP, Cai L, Zhou H, Zhu XF, Chen Y, Ding ZT. 2014. A new C19-diterpenoid alkaloid from the roots of Aconitum duclouxii. Nat Prod Res. 28(19):1649–1654. [DOI] [PubMed] [Google Scholar]
- Yin TP, Cai L, Fang HX, Fang YS, Li ZJ, Ding ZT. 2015. Diterpenoid alkaloids from Aconitum vilmorinianum. Phytochemistry. 116(1):314. [DOI] [PubMed] [Google Scholar]
- Yue JM, Xu J, Chen YZ, Chen SN. 1994. Diterpenoid alkaloids from Aconitum talassicum. Phytochemistry. 37:1467–1470. [Google Scholar]
- Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, Pan S, Dong X, Tan G, Wei Z., et al. 2014. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 13:1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.