Abstract
BACKGROUND:
Although surgical management of the axilla for breast cancer continues to evolve, axillary lymphadenectomy remains the standard of care for women with advanced nodal disease. We sought to evaluate national patterns of care in axillary surgery, and its association with overall survival (OS) among women with N2–N3 invasive breast cancer.
METHODS:
Women (18–90 years) with clinical N2–3 invasive breast cancer who underwent axillary surgery were identified from the National Cancer Data Base (NCDB) from 2004–2013. Axillary surgery was categorized as SLNB (1–5 nodes) or axillary lymph node dissection (ALND) (≥10 nodes). Patient and treatment characteristics, trends over time, and overall survival (OS) were compared by surgical treatment.
RESULTS:
Overall, 22,156 patients were identified. At diagnosis, 68.5% had cN2 and 31.5% had cN3 disease. Treatment included: lumpectomy (27%), mastectomy (73%), adjuvant chemotherapy (53.4%), neoadjuvant chemotherapy (NAC) (39.7%), radiation (74%), and endocrine therapy (54.4%). In total, 9.9% (n= 2,190) underwent SLNB and 90.1% (n= 19,966) underwent ALND. Receipt of SLNB was associated with private insurance, grade 3 disease, invasive ductal cancer, NAC and lumpectomy (all p<0.001). After adjustment for known covariates, including chemotherapy use, ALND was associated with improved survival (HR 0.68, p<0.001) and this effect was similar for N2 and N3 patients (axillary surgery*cN-stage interaction p=0.29).
CONCLUSIONS:
Axillary lymphadenectomy was associated with improved survival in patients presenting with clinical N2–N3 invasive breast cancer. Further studies, particularly in the neoadjuvant setting, are needed to identify breast cancer patients with advanced nodal disease that may safely avoid a lesser extent of axillary surgery.
INTRODUCTION
In the treatment of invasive breast cancer, axillary lymph node status has remained the strongest prognostic factor and guide in adjuvant decision-making. [1–3] Historically, axillary lymph node dissection (ALND) was used as part of routine breast cancer staging. In 1994, Guiliano et al validated the use of sentinel lymph node biopsy (SLNB) to reliably stage the axilla in clinically-node negative patients, [4, 5] and ALND became limited to women with known axillary nodal metastases for the purpose of local control. [6–11]
The contemporary multi-modal treatment of breast cancer has led to further reductions in axillary surgery, even among women with known axillary lymph node metastases. In 2010, results from the ACOSOG Z0011 trial supported the use of SLNB alone among women with clinically node-negative, T1–T2 invasive breast cancers undergoing breast conservation with 1–2 positive sentinel lymph nodes. Among eligible women, completion ALND did not improve overall or disease-free survival or locoregional recurrence rates. [12, 13] In 2014, the EORTC 10981–22023 AMAROS trial demonstrated that axillary-specific radiotherapy was equivalent to ALND among women with clinically node-negative, T1–T2 invasive breast cancers and positive sentinel nodes treated with either breast conservation or mastectomy. [14] Currently enrolling, the Alliance 11202 trial aims to compare ALND to axillary radiotherapy among women with residual positive sentinel lymph nodes following neoadjuvant chemotherapy, with the estimated primary completion date of January 2024. [15]
The most recent guidelines from the National Comprehensive Cancer Network (NCCN) state that SLNB alone without further axillary surgery is adequate in the setting of a clinically-negative axilla and a positive sentinel node biopsy if all of the following criteria are met: patients have T1–2 tumors with 1 to 2 positive SLNs, plan for breast conservation therapy and whole breast radiation therapy (RT), and did not receive preoperative chemotherapy. [16] Outside of the research setting, ALND continues to be considered the standard of care for women who do not meet these criteria, or those with clinically-positive, biopsy-proven axillary metastases. In light of the rapid evolution of surgical management of the axilla, and as the breast cancer community weighs the risk of distant progression against the benefits and harms of local control, uncertainties remain about the role of ALND among women with a greater burden of nodal disease. To this end, we sought to evaluate national patterns of care in axillary surgery, and its association with overall survival (OS), among women with N2–N3 invasive breast cancer.
METHODS
Adult female patients (age 18–90 years) diagnosed with T1–3, N2–3 invasive breast cancer from 2004 to 2013 were identified from the National Cancer Database (NCDB). The NCDB does not capture the specific surgical method of axillary staging for women with breast cancer. Therefore, we categorized axillary staging based on the number of lymph nodes removed as SLNB (1–5 lymph nodes examined) or ALND (10+ lymph nodes examined). Patients with non-invasive cancer, metastatic disease at diagnosis (clinical M1), missing or incomplete lymph node dissection data, or 6–9 lymph nodes examined were excluded. Patients who did not undergo surgery of the breast, or had surgery coded as local tumor destruction, surgery not otherwise specified, or unknown were also excluded. The final cohort sample size was N=22,156.
Patient characteristics are summarized as N (%) and median (IQR) for categorical and continuous variables, respectively, for all patients and by axillary surgery group (ALND or SLNB). Variables were compared between groups using the Chi-square test or Student’s t-test.
Overall survival was defined as the time from diagnosis to death. For patients that remained alive throughout the study interval, survival time was censored at the date of last follow-up. Kaplan-Meier curves and log-rank tests were used to examine unadjusted differences in survival between groups. Cox proportional hazards models were used to estimate the hazard ratio (HR) associated with type of axillary surgery after adjustment for known covariates, including age at diagnosis, race, Charlson/Deyo comorbidity score, income level, insurance type, education level, facility location and type, hormone receptor status, clinical T and N stage, grade, histology, use of endocrine therapy, RT and chemotherapy, surgery type, and number of positive lymph nodes. A subgroup analysis including only patients who received chemotherapy was conductedto estimate the effect of axillary surgery and chemotherapy sequence on survival. Additional models that included interaction terms of the axillary surgery group with select covariates were conducted to determine if the effect of axillary surgery on overall survival differed between subgroups. Because HER2-status is frequently missing in this database, a sensitivity analysis was done that excluded that variable in all models. To account for the correlation of patients treated at the same hospital, all adjusted models also included a robust sandwich covariance estimator. Only patients with available data for all covariates in a given model were included in each analysis. Sample sizes are included in the titles for each table and figure. No adjustments were made for multiple testing. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient and Treatment Characteristics
The final study cohort consisted of 22,156 patients with clinical T1–3, N2–3 invasive breast cancers treated with SLNB or ALND. Median age of the sample was 56 years and median tumor size was 3.2 cm. 68.5% had clinical N2 disease, and 31.5% had clinical N3 disease. 87% of patients had invasive ductal cancers, and 11.3% had invasive lobular cancers. On histologic review, 6% of patients had grade 1, 32.7% had grade 2 and 54.8% had grade 3 tumors. The majority had hormone receptor positive disease (53.9% ER+, 65.7% PR+). Of these women, 27% underwent lumpectomy (n=5,983) and 73% underwent mastectomy (n=16,173). 86.4% of patients received chemotherapy, of which, 39.7% received neoadjuvant chemotherapy and 53.4% received adjuvant chemotherapy. 74% of patients received radiation; 22.6% (n=5,003) after lumpectomy and 51.4% (n=11,397) after mastectomy. In total, 54.4% of all patients and 78% of hormone-receptor positive patients (ER+ and/or PR+) received endocrine therapy. Patient and treatment characteristics are summarized in Table 1.
Table 1.
Patient and treatment characteristics (N=22,156).
| All Patients (N=22156) | Axillary Surgery | P-Value | ||
|---|---|---|---|---|
| ALND (10+ LNs) (N=19966) | SLNB (1–5 LNs) (N=2190) | |||
| Age (Years) | <0.001 | |||
| ≤50 | 7814 (35.3%) | 6948 (34.8%) | 866 (39.5%) | |
| >50 | 14342 (64.7%) | 13018 (65.2%) | 1324 (60.5%) | |
| Median (IQR) | 56 (47 – 66) | 56 (47 – 66) | 54 (45 – 63) | <0.001 |
| Race | 0.002 | |||
| White | 17369 (78.4%) | 15713 (78.7%) | 1656 (75.6%) | |
| Black | 3628 (16.4%) | 3212 (16.1%) | 416 (19%) | |
| Other | 919 (4.1%) | 823 (4.1%) | 96 (4.4%) | |
| Charlson/Deyo Comorbidity Score | 0.001 | |||
| 0 | 18744 (84.6%) | 16832 (84.3%) | 1912 (87.3%) | |
| 1 | 2813 (12.7%) | 2584 (12.9%) | 229 (10.5%) | |
| ≥2 | 599 (2.7%) | 550 (2.8%) | 49 (2.2%) | |
| Facility Type | 0.003 | |||
| Academic | 6613 (29.8%) | 5969 (29.9%) | 644 (29.4%) | |
| Integrated Network | 1519 (6.9%) | 1344 (6.7%) | 175 (8%) | |
| Comprehensive | 10950 (49.4%) | 9835 (49.3%) | 1115 (50.9%) | |
| Community | 3072 (13.9%) | 2816 (14.1%) | 256 (11.7%) | |
| ER Status | <0.001 | |||
| Positive | 11942 (53.9%) | 10987 (55%) | 955 (43.6%) | |
| Negative | 9592 (43.3%) | 8412 (42.1%) | 1180 (53.9%) | |
| PR Status | <0.001 | |||
| Positive | 14552 (65.7%) | 13334 (66.8%) | 1218 (55.6%) | |
| Negative | 7065 (31.9%) | 6142 (30.8%) | 923 (42.1%) | |
| HER2 Status* | <0.001 | |||
| Positive | 2574 (11.6%) | 2210 (11.1%) | 364 (16.6%) | |
| Negative | 8132 (36.7%) | 7261 (36.4%) | 871 (39.8%) | |
| Grade | <0.001 | |||
| 1 | 1334 (6%) | 1237 (6.2%) | 97 (4.4%) | |
| 2 | 7235 (32.7%) | 6655 (33.3%) | 580 (26.5%) | |
| 3 | 12141 (54.8%) | 10805 (54.1%) | 1336 (61%) | |
| Histology | <0.001 | |||
| Invasive Ductal | 19265 (87%) | 17283 (86.6%) | 1982 (90.5%) | |
| Invasive Lobular | 2502 (11.3%) | 2343 (11.7%) | 159 (7.3%) | |
| Other Invasive | 389 (1.8%) | 340 (1.7%) | 49 (2.2%) | |
| Clinical N Stage | 0.005 | |||
| 2 | 15180 (68.5%) | 13621 (68.2%) | 1559 (71.2%) | |
| 3 | 6976 (31.5%) | 6345 (31.8%) | 631 (28.8%) | |
| Clinical T Stage | 0.05 | |||
| 1 | 4526 (20.4%) | 4114 (20.6%) | 412 (18.8%) | |
| 2 | 11886 (53.6%) | 10714 (53.7%) | 1172 (53.5%) | |
| 3 | 5744 (25.9%) | 5138 (25.7%) | 606 (27.7%) | |
| Surgery Type | <0.001 | |||
| Lumpectomy | 5983 (27%) | 5194 (26%) | 789 (36%) | |
| Mastectomy | 16173 (73%) | 14772 (74%) | 1401 (64%) | |
| Treated with Chemotherapy | 19135 (86.4%) | 17216 (86.2%) | 1919 (87.6%) | 0.21 |
| Chemotherapy Type** | <0.001 | |||
| Adjuvant | 10223 (53.4%) | 9778 (56.8%) | 445 (23.2%) | |
| Neoadjuvant | 7596 (39.7%) | 6231 (36.2%) | 1365 (71.1%) | |
| Treated with Radiation | 16400 (74%) | 14845 (74.4%) | 1555 (71%) | <0.001 |
| Surgery and Radiation Combination | ||||
| Lumpectomy + Radiation | 5003 (22.6%) | 4636 (21.9%) | 640 (29.2%) | <0.001 |
| Lumpectomy + No Radiation | 920 (4.2%) | 776 (3.9%) | 144 (6.6%) | |
| Mastectomy + Radiation | 11397 (51.4%) | 10482 (52.5%) | 915 (41.8%) | P<.001 |
| Mastectomy + No Radiation | 4574 (20.6%) | 4105 (20.6%) | 469 (21.4%) | |
| Treated with Endocrine Therapy | ||||
| Of All Patients | 12053 (54.4%) | 11053 (55.4%) | 1000 (45.7%) | <0.001 |
| Of ER+ or PR+ Patients | 11644 (78%) | 10686 (78.2%) | 958 (75.7%) | 0.02 |
| Number of LNs Examined - Median (IQR) | 16 (12 – 21) | 17 (13 – 21) | 3 (2 – 4) | <0.001 |
| Number of Positive LNs - Median (IQR) | 6 (3 – 10) | 6 (4 – 11) | 1 (0 – 3) | <0.001 |
HER2-status is frequently missing in this database due to the fact that it was only reliably collected starting in 2010.
Chemotherapy Type is out of all patients who had chemotherapy.
Predictors of Extent of Axillary Surgery
In total, 90.1% (n=19,966) of the cohort underwent ALND and 9.9% (n=2,190) underwent SLNB alone. The median number of nodes examined was 3 in the SLNB group vs. 17 in the ALND group. Receipt of axillary surgery did not differ based on clinical T-stage (p=0.05). Factors significantly associated with receipt of SLNB alone compared to ALND included: private insurance (59.9% vs. 55.1%, p<0.001), grade 3 disease (61% vs. 54.1%, p<0.001), invasive ductal cancer (90.5% vs. 86.6%, p<0.001), and receipt of lumpectomy (36% vs. 26%, p<0.001). Women who underwent ALND were more likely to have invasive lobular cancers and undergo mastectomy when compared to those treated with SLNB alone (11.7% vs. 7.3% and 74% vs. 64%, p<0.001). SLNB was associated with greater use of lumpectomy with radiation compared to those who had ALND (29.2% vs. 21.9%, p<0.001). However, ALND was associated with higher likelihood of receiving post-mastectomy radiation when compared to SLNB alone (52.5% vs. 41.8%, p<0.001).
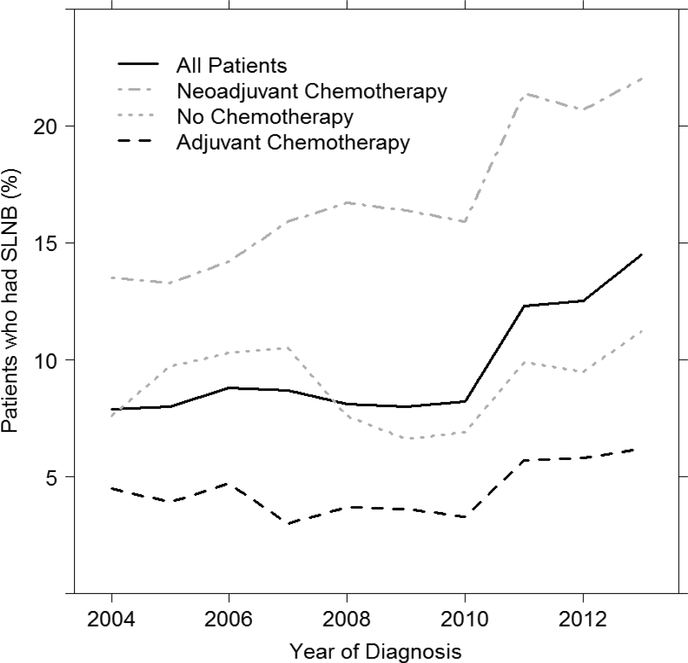
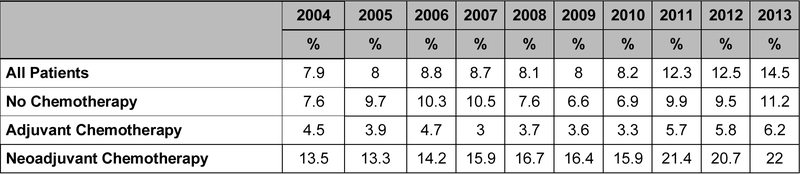
Receipt of SLNB alone was associated with neoadjuvant chemotherapy use. Among those who underwent SLNB alone, 71.1% (n=1,365) received NAC and 23.2% (n=445) received adjuvant chemotherapy, while in women who underwent ALND, 36.2% (n=6,231) received NAC and 56.8% (n=9,778) received adjuvant chemotherapy. The overall rate of SLNB alone in the NAC group was 18%. Notably, this rate increased from 13.5% in 2004 to 22% in 2013, whereas the rate of SLNB in those treated with adjuvant chemotherapy did not change appreciably over time, from 4.3% in 2004 to 6.2% in 2013 (Figure 1). Rates of SLNB over time are summarized in Figure 2.
Figure 1a. Use of sentinel lymph node biopsy (SLNB) overtime in N2–N3 breast cancer (N=19,135).
Overall Survival
After adjustment for known covariates including histology, hormone-receptor status, surgery of the primary breast tumor, receipt of chemotherapy, radiation, and endocrine therapy, ALND was associated with an improved overall survival compared to SLNB alone (HR 0.68, 95% CI 0.56–0.83, p<0.001) (Table 2). The effect of ALND vs. SLNB on survival did not differ between cN2 vs. cN3 patients (axillary Surgery*cN-stage interaction p=0.29; Table 3). There was no interaction between extent of axillary surgery (ALND versus SLNB) and chemotherapy type (axillary surgery * chemotherapy type interaction p=0.37). An adjusted overall survival model limited to women who received NAC, demonstrated a reduced risk of death with ALND when compared to SLNB alone (HR=0.75, 95% CI 0.58–0.97, p=0.03). This supports a benefit in overall survival with receipt of ALND for all patients with N2–N3 disease, including those who received NAC. Women who had a pathologic complete (pCR) response following NAC (ypN0 and ypT0), had improved overall survival compared to those who did not (HR=0.374, p<0.001). However, in patients with pCR, the survival benefit from ALND compared to SLNB remained (table 5). Additionally, receipt of lumpectomy was associated with improved overall survival compared to mastectomy (HR=0.787, p=0.002), as was use of chemotherapy (HR=0.48, p<0.001) and radiation (HR=0.676, p<0.001). (Table 2)
Table 2.
Adjusted Overall Survival Model (N=7,025).
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Axillary Surgery Group | <0.001 | ||
| SLNB (1–5 LNs) | REF | ||
| ALND (10+ LNs) | 0.678 (0.556 – 0.828) | <0.001 | |
| Age (Years) | 1.011 (1.005 – 1.017) | <0.001 | <0.001 |
| Race | 0.002 | ||
| White | REF | ||
| Black | 1.291 (1.108 – 1.505) | 0.001 | |
| Other | 0.74 (0.48 – 1.141) | 0.17 | |
| Charlson/Deyo Score | <0.001 | ||
| 0 | REF | ||
| 1 | 1.329 (1.127 – 1.567) | <.0001 | |
| ≥2 | 1.561 (1.173 – 2.077) | 0.002 | |
| Income Level | 0.03 | ||
| ≥$35,000 | REF | ||
| <$35,000 | 1.176 (1.016 – 1.362) | 0.03 | |
| Insurance Type | <0.001 | ||
| Private | REF | ||
| Government | 1.347 (1.17 – 1.551) | <0.001 | |
| Not Insured | 1.303 (0.967 – 1.755) | 0.08 | |
| Education Level | 0.49 | ||
| >80% High School Graduation Rate | REF | ||
| ≤80% High School Graduation Rate | 0.949 (0.818 – 1.1) | 0.49 | |
| ER Status | 0.25 | ||
| Positive | 0.88 (0.706 – 1.096) | 0.25 | |
| Negative | REF | ||
| PR Status | <.001 | ||
| Positive | 0.562 (0.472 – 0.669) | <0.001 | |
| Negative | REF | ||
| HER2 Status* | <0.001 | ||
| Positive | 0.585 (0.499 – 0.686) | <0.001 | |
| Negative | REF | ||
| Clinical N-Stage | 0.08 | ||
| 2 | REF | ||
| 3 | 1.137 (0.984 – 1.314) | 0.08 | |
| Clinical T-Stage | <0.001 | ||
| 1 | REF | ||
| 2 | 1.222 (1.028 – 1.453) | 0.02 | |
| 3 | 1.547 (1.276 – 1.875) | <0.001 | |
| Grade | <0.001 | ||
| 1 | REF | ||
| 2 | 1.02 (0.738 – 1.409) | 0.91 | |
| 3 | 1.613 (1.168 – 2.228) | 0.004 | |
| Histology | 0.20 | ||
| Invasive Ductal | REF | ||
| Invasive Lobular | 0.856 (0.684 – 1.072) | 0.18 | |
| Other Invasive | 0.719 (0.433 – 1.193) | 0.202 | |
| Surgery Type | 0.002 | ||
| Mastectomy | REF | ||
| Lumpectomy | 0.787 (0.675 – 0.916) | 0.002 | |
| # Positive LNs | 1.04 (1.031 −1.048) | <0.001 | <0.001 |
| Chemotherapy Use | 0.48 (0.404 – 0.571) | <0.001 | <0.001 |
| Radiation Use | 0.676 (0.586 – 0.779) | <0.001 | <0.001 |
| Endocrine Therapy Use | 0.637 (0.522 – 0.778) | <0.001 | <0.001 |
HER2 status is frequently missing in this database, hence the highly reduced sample size included in this model.
Table 3.
Adjusted Hazard Ratios by Clinical N-Stage (N=7025)
| Description | ALND vs. SLNB HR (95% CI) | |
|---|---|---|
| Clinical N-Stage | 0.29 | |
| cN3 | 0.581 (0.411 − 0.821) |
These estimates are also adjusted for all covariates included in Table 2.
Table 5.
Adjusted Hazard Ratios of ALND vs SLNB by Pathologic Response after Neoadjuvant Chemotherapy (N=2375)
| Description | ALND vs. SLNB HR (95% CI) | Interaction P-Value |
|---|---|---|
| 0.89 | ||
| No Complete Response | 0.596 (0.435–0.816) | |
| Complete Response | 0.621 (0.379–1.017) |
These estimates are also adjusted for all covariates included in Table 4.
Sensitivity Analysis
When HER2 was not included as a covariate in the adjusted survival models, results were similar to those that included HER2 (reported previously). After adjustment for other known covariates including histology, ER/PR status, breast surgery type, chemotherapy, radiation and endocrine therapy, ALND was still associated with an improved overall survival compared to SLNB (HR 0.77, 95% CI 0.68–0.86, p<0.001). The effect of ALND vs. SLNB on survival did not differ between cN2 vs. cN3 patients. (axillary Surgery*cN-stage interaction p=0.14).
DISCUSSION
Among women with invasive breast cancer, surgical management of the axilla has evolved dramatically in the past twenty years. SLNB replaced ALND for surgical staging in the setting of a clinically negative axilla, and more recently has replaced ALND in women with low-volume nodal disease. [8, 9] [12, 17] The ACOSOG Z0011, AMAROS, and Alliance 11202 trials are collectively identifying breast cancer patients with known axillary nodal metastases who can safely avoid complete lymph node dissection without compromising oncologic outcomes. [14, 15, 18]
In this study, we sought to evaluate contemporary treatment patterns for women with breast cancer and advanced axillary nodal disease, in order to understand the uptake and extrapolation of rapidly evolving evidence. Furthermore, we evaluated whether the extent of axillary surgery was associated with overall survival in a subset of breast cancer patients at higher risk of both local and distant recurrence. Our study cohort included women with clinical N2–3 invasive breast cancer, in whom axillary surgery would most likely be employed for the purpose of local control and less likely for the purpose of guiding adjuvant chemo-or-radiotherapy treatment decisions. Despite ALND being standard of care, we found that 10% of women with N2–N3 invasive breast cancer underwent surgical removal of five or fewer nodes, and that receipt of NAC or lumpectomy was associated with a greater likelihood of avoiding completion lymphadenectomy (defined as removal of ≥10 lymph nodes). 18% of women with N2–N3 disease underwent SLNB alone after neoadjuvant chemotherapy, which increased over time from 13.5% in 2004 to 22% in 2013. This suggests that reductions in the extent of axillary surgery were occurring prior to publication of the Alliance 1071 trial in 2013. [18] Kantor et al reported a similar trend among 12,063 women with cN1 invasive breast cancer, where rates of SLNB alone increased from 18.9% in 2010 to 33.3% in 2013.[19]
The ACOSOG 1071 trial evaluated the feasibility of SLNB among women with clinical N1–N2 biopsy-proven invasive breast cancer following neoadjuvant systemic therapy. [18] Among cN1 patients, use of dual tracer mapping resulted in a false negative rate (FNR) of 10.8%, which was further improved if at least three SLN were examined (FNR 9.1%). Caudle et al reported a further reduction in FNRs to less than 2% on targeted axillary dissection (TAD), defined as removal of the sentinel lymph nodes plus the biopsy-proven and clipped lymph node, among cN0 patients following NAC. [20] The ongoing Alliance A11202 study is a randomized phase III trial comparing the efficacy of ALND to axillary radiation in patients with clinical T1–T3, N1 invasive breast cancer who remain node-positive after neoadjuvant chemotherapy.[15] The sister trial NSABP B-51/RTOG 1304 is enrolling patients with biopsy-proven N1 disease who achieve a nodal pCR following NAC, randomizing women to axillary radiation or no further axillary treatment. [21, 22]
For women with advanced axillary nodal burden and a higher risk of metastatic recurrence, local axillary control could arguably have little impact on overall survival, which would instead be driven by the risk of distant metastatic disease. Our findings demonstrated that in women who presented with clinical N2–3 invasive breast cancer, axillary lymph node dissection was associated with improved survival (HR 0.68, p<0.001) in women treated with neoadjuvant chemotherapy who achieved a pCR and those who received adjuvant chemotherapy. This suggests that although SLNB alone may provide adequate control in the setting of low volume metastatic disease, axillary clearance may be associated with a survival benefit in patients with a higher burden of nodal disease where NAC use may be inadequate in down-staging advanced nodal burden. These findings differ from the results of the NSABP B-04 trial, which examined a subset of women with palpable, suspicious axillary nodes who were randomized to undergo radical mastectomy versus total mastectomy and no axillary surgery with radiation from 1971–1974 [25]. At twenty-five years of follow-up, there was no difference in disease-free or overall survival between these groups, yet a small but statistically insignificant difference in locoregional recurrence was seen in women who underwent lymphadenectomy compared to those without axillary surgery (8% vs 11%, p=0.40). As improvements in systemic therapy have lead to improved survival after breast cancer, locoregional therapy may matter more or reflect more aggressive multimodal therapy in healthier breast cancer patients with improved prognosis.
More contemporary trials evaluating less extensive axillary surgery have primarily done so in clinically node-negative patients without palpable axillary nodes. The AMAROS trial demonstrated that in women with T1–2 primary breast cancer with a clinically negative axilla but a positive sentinel lymph node, axillary radiation therapy provided equivalent local control and overall survival when compared to ALND. [14] After 5-years of follow-up, the axillary recurrence rate was 0.43% in the ALND group vs 1.2% in the radiotherapy group. Importantly, lymphedema was significantly lower in the radiotherapy group. These findings support use of axillary radiotherapy in place of ALND for patients with clinically node-negative axilla with similar oncologic outcomes and lower morbidity. Bonneau et al evaluated the use of ALND versus SLNB in 9,521 patients with 3 or 4 metastatic axillary nodes using the United States Surveillance, Epidemiology, and End Results (SEER) database. In this study, there was no difference in overall survival between patients undergoing ALND versus SLNB with 3 or more metastatic lymph nodes. [23] The authors concluded that ALND was a staging procedure and lacked therapeutic benefit in patients with T1–2 invasive breast cancer and at least 3 metastatic nodes after SLNB.
There are several limitations to our study that should be noted. As with all retrospective studies, the data is subject to bias. In this setting, women who underwent more aggressive axillary clearance with ALND may have constituted a healthier population of women with advanced breast cancer, or have received more aggressive therapy overall. Importantly, the NCDB does not capture the specific surgical method of axillary staging for women with breast cancer. Therefore, we were unable to determine if women underwent a sentinel lymph node biopsy (SLNB)) with nodal mapping or an anatomic axillary lymph node dissection (ALND) that included level 1 and 2 lymph nodes or higher. As a result, we relied on the number of lymph nodes removed as surrogates for the extent of axillary surgery based on previously published studies. [24, 25] Additionally, we were unable to determine local or disease-free recurrences, which differ by tumor phenotype and receipt of targeted therapies. [26–28] Axillary recurrence information would provide a better understanding of the actual benefit of ALND in this population at high risk of both local and distant recurrences.
In conclusion, the management of axillary disease in breast cancer has evolved significantly over the past few decades. Although randomized clinical trial data has shown that SLNB alone is adequate in women with low volume node-positive disease, our results suggest that axillary lymphadenectomy may still benefit women with clinical N2–N3 disease, regardless of the sequence of or response to systemic chemotherapy. Further studies are needed to identify select breast cancer patients with advanced nodal disease who can safely avoid surgical lymphadenectomy.
Figure 1b. Rates of SLNB alone by chemotherapy sequence in N2–N3 breast cancer.
Data presented as percentage of patients who underwent SLNB out of all patients treated in a given year.
Table 4.
Adjusted overall survival followinbg neoadjuvant chemotherapy in N2–N3 Breast Cancer (N=2375).
| HR (95% Cl) | P-Value | Overall P-Value | |
|---|---|---|---|
| Axillary Surgery Group | <.001 | ||
| SLNB (1–5 LNs) | REF | ||
| ALND (10+ LNs) | 0.604 (0.458–0.797) | <0.001 | |
| Pathologic Response | <0.001 | ||
| No Complete Response | REF | ||
| Complete Response | 0.374 (0.282–0.495) | <0.001 | |
| Age (Years) | 0.992 (0.983–1.001) | 0.06 | 0.06 |
| Race | 0.03 | ||
| White | REF | ||
| Black | 1.198 (0.959–1.497) | 0.11 | |
| Other | 0.525 (0.273–1.012) | 0.05 | |
| Charlson/Deyo Score | 0.04 | ||
| 0 | REF | ||
| 1 | 1.371 (1.020–1.843) | 0.04 | |
| ≥2 | 1.825 (0.872–3.819) | 0.11 | |
| Income Level | 0.97 | ||
| ≥$35,000 | REF | ||
| <$35,000 | 0.995 (0.787–1.258) | 0.97 | |
| Insurance Type | 0.04 | ||
| Private | REF | ||
| Government | 1.189 (0.945–1.497) | 0.14 | |
| Not Insured | 1.573 (1.070–2.313) | 0.02 | |
| Education Level | 0.15 | ||
| >80% High School Graduation Rate | REF | ||
| ≤80% High School Graduation Rate | 1.173 (0.944–1.459) | 0.15 | |
| Facility Location | 0.69 | ||
| South | REF | ||
| Midwest | 1.104 (0.877–1.389) | 0.40 | |
| Northeast | 0.938 (0.715–1.228) | 0.64 | |
| West | 1.025 (0.731–1.439) | 0.88 | |
| Facility Type | 0.06 | ||
| Academic | REF | ||
| Community | 1.521 (1.112–2.082) | 0.009 | |
| Comprehensive | 1.080 (0.871–1.339) | 0.48 | |
| Integrated Network | 1.235 (0.847–1.802) | 0.27 | |
| ER Status | 0.37 | ||
| Positive | 0.800 (0.491–1.301) | 0.37 | |
| Negative | REF | ||
| PR Status | <0.001 | ||
| Positive | 0.443 (0.312–0.628) | <0.001 | |
| Negative | REF | ||
| HER2 Status* | <0.001 | ||
| Positive | 0.559 (0.432–0.724) | <0.001 | |
| Negative | REF | ||
| Clinical N-Stage | 0.14 | ||
| 2 | REF | ||
| 3 | 1.186 (0.944–1.490) | 0.14 | |
| Clinical T-Stage | 0.82 | ||
| 1 | REF | ||
| 2 | 1.022 (0.735–1.422) | 0.90 | |
| 3 | 1.085 (0.771–1.527) | 0.64 | |
| Grade | <0.001 | ||
| 1 | REF | ||
| 2 | 1.511 (0.762–2.996) | 0.24 | |
| 3 | 2.530 (1.272–5.030) | 0.008 | |
| Histology | 0.35 | ||
| Invasive Ductal | REF | ||
| Invasive Lobular | 0.876 (0.537–1.431) | 0.60 | |
| Other Invasive | 0.587 (0.270–1.274) | 0.18 | |
| Surgery Type | 0.01 | ||
| Mastectomy | REF | ||
| Lumpectomy | 0.720 (0.555–0.933) | 0.01 | |
| # Positive LNs | 1.055 (1.041–1.070) | <0.001 | <0.001 |
| Radiation Use | 0.722 (0.550–0.949) | 0.02 | 0.02 |
| Endocrine Therapy Use | 0.727 (0.480–1.100) | 0.13 | 0.13 |
HER2 status is frequently missing in this database, hence the highly reduced sample size included in this model.
Synopsis:
Axillary lymph node dissection (removal of ten or more lymph nodes) was associated with improved overall survival in women with presenting with clinical N2–N3 invasive breast cancer, regardless of pathologic response.
Footnotes
This study was presented at the 18th Annual American Society of Breast Surgeons meeting in Las Vegas, NV in April 2017.
REFERENCES
- 1.Carter CL, Allen C, and Henson DE, Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer, 1989. 63(1): p. 181–7. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ER, Sass R, and Fisher B, Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer, 1984. 53(3 Suppl): p. 712–23. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson EN, Brown BW, and Montague ED, Tumor volume, nodal status, and metastasis in breast cancer in women. J Natl Cancer Inst, 1986. 76(2): p. 171–8. [PubMed] [Google Scholar]
- 4.Giuliano AE, et al. , Sentinel lymphadenectomy in breast cancer. J Clin Oncol, 1997. 15(6): p. 2345–50. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, et al. , Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg, 1994. 220(3): p. 391–8; discussion 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins H, et al. , Treatment of early breast cancer: a report after ten years of a clinical trial. Br Med J, 1972. 2(5811): p. 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayward J and Caleffi M, The significance of local control in the primary treatment of breast cancer. Lucy Wortham James clinical research award. Arch Surg, 1987. 122(11): p. 1244–7. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, et al. , Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol, 2000. 18(13): p. 2553–9. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, et al. , A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med, 2003. 349(6): p. 546–53. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH, et al. , American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol, 2005. 23(30): p. 7703–20. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GF, et al. , Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast April 19 to 22, 2001, Philadelphia, Pennsylvania. Hum Pathol, 2002. 33(6): p. 579–89. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano AE, et al. , Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA, 2011. 305(6): p. 569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano AE, et al. , Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg, 2010. 252(3): p. 426–32; discussion 432–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donker M, et al. , Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol, 2014. 15(12): p. 1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. Alliance 11202 Trial: p. NCT01872975 [Google Scholar]
- 16.NCCN Guidelines Invasive Breast Cancer. 2016.
- 17.Galimberti V, et al. , Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol, 2013. 14(4): p. 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boughey JC, et al. , Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA, 2013. 310(14): p. 1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantor O, et al. , Are the ACOSOG Z0011 Trial Findings Being Applied to Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy? Breast J, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Caudle AS, et al. , Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol, 2016. 34(10): p. 1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standard or Comprehensive Radiation Therapy in Treating Patients With Early-Stage Breast Cancer Previously Treated With Chemotherapy and Surgery. NSABP B-51/RTOG 1304 Trial: p. NCT01872975. [Google Scholar]
- 22. http://www.nsabp.pitt.edu/B-51.asp.
- 23.Bonneau C, et al. , Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol, 2015. 41(8): p. 998–1004. [DOI] [PubMed] [Google Scholar]
- 24.Yi M, et al. , Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol, 2010. 17 Suppl 3: p. 343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilimoria KY, et al. , Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol, 2009. 27(18): p. 2946–53. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, et al. , Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet, 2012. 379(9816): p. 633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey LA, et al. , Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J Clin Oncol, 2016. 34(6): p. 542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneeweiss A, et al. , Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol, 2013. 24(9): p. 2278–84. [DOI] [PubMed] [Google Scholar]