Abstract
Background
It has been suggested that endocardial and epicardial ablation of ventricular tachycardia (VT) improves outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia. We investigated our sequential approach for VT ablation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia in a single center.
Methods and Results
We included 47 patients (44±16 years) with definite (81%) or borderline (19%) arrhythmogenic right ventricular cardiomyopathy/dysplasia between 1998 and 2016. Our ablation strategy was to target the endocardial substrate. Epicardial ablation was performed in case of acute ablation failure or lack of an endocardial substrate. Single and multiple procedural 1‐ and 5‐year outcome data for the first occurrence of the study end points (sustained VT/ventricular fibrillation, heart transplant, and death after the index procedure, and sustained VT/ventricular fibrillation for multiple procedures) are reported. Eighty‐one radiofrequency ablation procedures were performed (mean 1.7 per patient, range 1–4). Forty‐five (56%) ablation procedures were performed via an endocardial, 11 (13%) via an epicardial, and 25 (31%) via a combined endo‐ and epicardial approach. Complete acute success was achieved in 65 (80%) procedures, and partial success in 13 (16%). After a median follow‐up of 50.8 (interquartile range, [18.6; 99.2]) months after the index procedure, 17 (36%) patients were free from the primary end point. After multiple procedures, freedom from sustained VT/ventricular fibrillation was 63% (95% CI, 52–75) at 1 year, and 45% (95% CI, 34–61) at 5 years, with 36% of patients receiving only endocardial radiofrequency ablation. A trend (log rank P=0.058) towards an improved outcome using a combined endo‐/epicardial approach was observed after multiple procedures.
Conclusion
Endocardial ablation can be effective in a considerable number of arrhythmogenic right ventricular cardiomyopathy/dysplasia patients with VT, potentially obviating the need for an epicardial approach.
Keywords: arrhythmogenic right ventricular dysplasia/cardiomyopathy, catheter ablation, epicardial ablation, ventricular tachycardia
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Cardiomyopathy
Clinical Perspective
What Is New?
In this study we used a stepwise catheter ablation approach to treat patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and sustained ventricular arrhythmias.
Adjuvant epicardial ablation was only performed in cases of ablation failure or lack of an endocardial substrate.
What Are the Clinical Implications?
Current consensus documents propose combined endocardial and epicardial ablation during the initial procedure in these patients.
Although we observed a trend towards improved arrhythmia‐free survival with adjuvant epicardial ablation, our study shows that endocardial ablation only can be effective in a considerable number of patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia with sustained ventricular arrhythmias, obviating the need for a more risky epicardial approach that may cause pericardial adhesions potentially precluding future epicardial access.
Moreover, multiple ablation procedures are often necessary to improve long‐term arrhythmia‐free survival in this progressive and challenging disease.
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited cardiomyopathy leading to fibro‐fatty infiltration, which predominantly occurs in the right ventricular (RV) myocardium resulting in ventricular tachycardia (VT) and fibrillation (VF).1, 2 Implantable cardioverter‐defibrillators (ICD) are the only therapeutic measure that have been shown to improve prognosis in this challenging disease,3, 4 but frequent appropriate ICD interventions can lead to a reduced quality of life.5, 6 Therefore, therapies to reduce these ICD interventions are needed.
Pathologically, fibro‐fatty infiltration typically begins in the subepicardial layers and progresses towards the endocardium over time.7, 8, 9 In line with these findings, recent observational studies have suggested that additional epicardial ablation seems to be more effective than only endocardial ablation to control sustained VT.10, 11, 12, 13 This evidence is reflected in a recent task force consensus document on the treatment of ARVC/D, in which an epicardial approach is recommended in patients who fail ≥1 attempts of endocardial VT ablation, or even a combined endocardial/epicardial VT ablation approach as the initial ablation strategy.14 However, procedure‐associated complications of combined endo/epicardial ablation are thought to be higher as compared with a solely endocardial approach and occur in up to 8% of patients.15, 16, 17, 18, 19 Furthermore, epicardial fat is predominantly located in the RV free wall, especially along the tricuspid annulus. This region is frequently affected by the process of fibro‐fatty infiltration in ARVC/D, and VT often arise from this region. However, the presence of large amounts of epicardial fat in this region can reduce the efficacy of epicardial RF lesions, and potentially hamper coronary arteries that are located along the tricuspid annulus.20, 21 Therefore, in the present study, we investigated the clinical outcome of ablating sustained VT using our sequential approach in a large single‐center cohort of patients with ARVC/D.
Methods
Study Population
The study population consisted of 47 adult patients with ARVC/D who were enrolled in our center for catheter ablation of VT between 1998 and 2016. No patient with ARVC/D who received catheter ablation for VT at our center was excluded from this analysis. All patients had at least 1 symptomatic episode of sustained monomorphic VT documented by 12‐lead ECG, Holter monitoring, or interrogation of the ICD. All patients gave written informed consent to the ablation procedure. The local ethical committee approved this retrospective study (reference number PV5039). The diagnosis of ARVC/D was fulfilled according to the 2010 revised Task Force criteria22 with either definite (81%) or borderline (19%) criteria. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electrophysiologic Study
The procedure was performed in the fasting state with propofol (1–4 mg/kg per hour), midazolam, and fentanyl or sufentanyl. All antiarrhythmic drugs (AAD) (except amiodarone) were discontinued for ≥5 half‐lives before the procedure. Two 6F diagnostic catheters were positioned in the right ventricle (RV) and within the coronary sinus. The stimulation protocol consisted of programmed stimulation from the RV at 2 drive cycle lengths (CL; 510 and 440 ms) with up to 3 extra stimuli to a minimum coupling interval of 200 ms. Burst‐pacing with the shortest CL of 250 ms was also used if induction failed with programmed stimulation. If sustained VT was non‐inducible at baseline, intravenous isoproterenol infusion (2–10 μg/min) was administered to induce VT. Clinical VT was defined as the same 12‐lead surface electrocardiogram (QRS) morphology and similar CL, or similar CL to the documented VT in patients with ICDs and without a 12‐lead ECG documentation. Other induced VT were considered as non‐clinical VT.
Electroanatomical Mapping and Ablation
Our primary ablation strategy was to target the endocardial substrate. Epicardial ablation was performed only in cases of acute ablation failure or lack of an endocardial substrate (sequential approach; if required, epicardial ablation was performed within the same procedure such as endocardial ablation). For clarification, endocardial approach indicates that mapping/ablation was only performed from the endocardium, whereas epicardial approach indicates that mapping/ablation was only performed from the epicardium. Combined endo‐epicardial approach indicates that mapping/ablation was performed from both, the endocardium and epicardium within the same procedure. Mapping was performed using a 7F 3.5 mm tip (Navistar Thermocool, Biosense Webster Inc., Diamond Bar, CA) through the femoral vein under fluoroscopic guidance. Our methods were previously described in detail.16 In brief, 3D electroanatomic maps (EAM) were performed during sinus rhythm (SR) and/or during VT in case of stable and reproducible VT. Activation and voltage maps were both used to guide the ablation strategy. Low voltage amplitudes in bipolar electrograms were defined as <1.5 mV for endocardial maps, <1.0 mV for epicardial maps, and <0.5 mV for delineating dense scar. Also, endocardial unipolar voltage <5.5 mV was defined as abnormal amplitudes.23 In addition, sites with prolonged (>70 ms), fractionated (defined as >2 potentials without an isoelectric interval)24 or late potentials were tagged on 3D maps. Ablation was performed at the site of diastolic potentials during VT in case of stable VT and short post pacing intervals after entrainment of the VT or guided by pace mapping at the substrate area with fractionated or late potentials. Fragmented low voltage signals or late potentials that were judged to be crucial to maintain the induced VT circuit were targeted for ablation. If no sustained VT was inducible, all fragmented low voltage signals/late potentials in the region where the documented clinical VA were suspected were eliminated. Irrigated radiofrequency current was delivered in power‐controlled mode, power 30 to 40 W, irrigation 17 to 30 mL/min, and temperature limit 43°C.
Epicardial access was obtained through an anteriorly oriented subxiphoid puncture. In brief, after reaching the pericardial space, contrast agent was injected to verify the position of the needle in the pericardial space, and a long J‐tipped guidewire was placed via the needle in the pericardial space. After positioning a long 8.5 Fr SL‐1 sheath in the pericardial space over the guidewire, the ablation catheter was advanced into the pericardial space.
Coronary angiography was not performed on a routine basis before epicardial ablation, but only in cases where the substrate was in close proximity to the tricuspid valve and the course of the right coronary artery. Ablation energy was delivered applying a power of 30 to 40 W, flush rate 17 to 20 mL/min and maximum temperature of 43°C. Repeat aspiration of irrigation fluid was performed depending on hemodynamic status or after every fifth radiofrequency application within the epicardial space. We did not use invasive hemodynamic support like extracorporeal membrane oxygenation (ECMO) or a left ventricular assist device (LVAD system) during the procedure.
End points
The procedural end point was non‐inducibility of any sustained VT by programmed stimulation±isoproterenol at the end of the procedure (complete success). Partial success was defined as non‐inducibility of clinical VT, but remaining inducibility of non‐clinical sustained VT or fast VT (CL <240 ms). In case of lack of VT inducibility before ablation, the procedural end point was elimination of all fragmented low voltage signals/late potentials in the region where the documented clinical VT/frequent premature ventricular contractions were suspected. Acute ablation failure was defined if clinical VT remained inducible. Our combined study end point consisted of 3 components (recurrent sustained VT/VF including any appropriate ICD intervention, namely shock or anti‐tachycardia pacing [ATP], heart transplant, or death during follow‐up, whatever came first), indicating the occurrence of any of these 3 components after the first RFA procedure. Our secondary end point was recurrent sustained VT/VF after multiple procedures, indicating the occurrence of any sustained ventricular arrhythmia including ICD interventions after the last RFA procedure. Arrhythmia burden was determined in a subset of patients in whom complete ICD interrogation information was available for detailed review and analysis before and after the index ablation procedure.
Complications and Follow‐Up
Major complications were defined as transient ischemic attack (TIA), stroke, pericardial tamponade, pneumo‐ or hemothorax, or severe bleeding from the access sites or internal bleeding resulting in hemorrhagic shock. Hematoma at access sites not requiring surgical intervention or blood transfusion and pericardial effusion/pericarditis were considered minor complications.
All patients were followed routinely in our outpatient clinic or by their treating cardiologists at 3‐ to 6‐month intervals. In patients with implanted ICD, the interrogation reports were reviewed by experienced cardiologists. Recurrence of VT and appropriateness of ICD therapy were determined based on review of the stored electrograms. In patients without ICDs, VT recurrence was based on ECG or Holter documentation. Baseline and follow‐up data were obtained from hospital records, treating physicians, telephone interviews with patients and/or their family members, and a structured pre‐designed patient questionnaire at last follow‐up.
Statistical Analysis
Continuous variables were summarized as mean±SD or median (interquartile range). Categorical variables were expressed as frequencies (percentages). We examined 2 survival end points by Kaplan–Meier estimates: (1) A combined event composed of 3 components defined as the first occurrence of a sustained ventricular arrhythmia, heart transplantation, or death and (2) recurrent sustained ventricular arrhythmias (VT/VF) after multiple procedures. The log‐rank test was used to examine the end points with regard to endocardial alone versus combined endo‐/epicardial ablations. A multiple Cox proportional hazards model was used to examine effects of sex, body mass index, ARVC 2010 diagnostic score, ICD, and ablation type on the combined survival end point. Survival differences between the ablation types were expressed with P values and hazard ratios (95% CI). Sustained VT/VF burden before and after the index ablation procedure was analyzed using a linear mixed effects model to account for different time durations. Differences in procedural data between ablation types were compared with linear mixed effect models considering multiple procedures of patients. A logistic regression model was used to compare the amount of RF lesions in patients with and without pericardial adhesions. A McNemar test was used to compare AAD taken before index procedure and at last follow‐up. A Mann–Whitney‐U test was used to compare VT inducibility in patients with primary and secondary implanted ICD. All P values are 2‐sided. P<0.05 was considered statistically significant. Statistical analysis was performed using R (version 3.2.3).
Results
Patient Characteristics
Clinical characteristics are provided in Tables 1 and 2. Thirty‐eight patients (38/47, 81%) fulfilled a definite diagnosis of ARVC/D, and 9 (9/47, 19%) a borderline diagnosis according to the revised 2010 TFC. From the 6 diagnostic categories, a structural major or minor criterion was present in 64% (30/47), an ECG repolarization criterion in 66% (31/47), an ECG depolarization criterion in 51% (24/47) (Epsilon waves in 17% [8/47]), an arrhythmia criterion in 98% (46/47), and a family criterion in 19% (9/47). Myocardial biopsy to assess for tissue alterations was not performed in any patient. The majority of patients were male (38/47, 81%). An ICD was implanted in 35 (35/47, 74%) patients at the time of their first ablation procedure. Most patients were either on betablockers (n=15/44, 34%) or sotalol (n=14/44, 32%). Genetic testing was available in 11 (11/47, 23%) patients. Eight out of those 11 patients (8/11, 73%) harbored a desmosomal mutation (Table 1). There was one proband carrying a heterozygous plakophilin‐2 (PKP‐2) and desmoplakin (DSP) mutation. Genetic testing for the 3 remaining patients did not reveal any pathogenic mutation.
Table 1.
Clinical Characteristics of the Study Cohort (n=47)
| Characteristic | All Patients (n=47) |
|---|---|
| Age at index ablation, y | 44±16 |
| Age at first ARVC/D diagnosis | 40±14 |
| Male | 38/47 (81) |
| Patients alive at last follow‐up | 43/47 (91) |
| rTFC criteria | |
| Definite | 38/47 (81) |
| Borderline | 9/47 (19) |
| Presenting symptoms at baseline | n=32 (68) |
| SCD (survived) | 4/32 (13) |
| Palpitations | 19/32 (59) |
| Syncope | 6/32 (19) |
| Dyspnea | 2/32 (6) |
| Chest pain | 1/32 (3) |
| ICD at index ablation | n=35 (74) |
| Primary prophylaxis | 5/35 (14) |
| Secondary prophylaxis | 30/35 (86) |
| AAD at index ablation | 44 (94) |
| >Betablocker | 15/44 (34) |
| >Sotalol | 14/44 (32) |
| >Amiodaron | 5/44 (12) |
| >Flecainid | 1/44 (2) |
| >Betablocker+amiodaron | 7/44 (16) |
| >Betablocker+flecainid | 1/44 (2) |
| >Other combinations | 1/44 (2) |
| AAD at last follow‐up | 42 (89) |
| >Betablocker | 11/42 (26) |
| >Sotalol | 9/42 (21) |
| >Amiodaron | 4/42 (10) |
| >Flecainid | 3/42 (7) |
| >Betablocker+amiodaron | 10/42 (24) |
| >Betablocker+flecainid | 4/42 (10) |
| >Other combinations | 1/42 (2) |
| Mutation status | n=11/47 (23) |
| Single desmosomal mutation | 7/11 (64) |
| PKP‐2 | 6/7 (86) |
| DSG‐2 | 1/7 (14) |
| Digenic heterozygosity for desmosomal mutations | 1/11 (9) |
| No mutation | 3/11 (27) |
| Former endurance athlete | 22* (92) |
| Left ventricular EF <55% at baseline | 5/47 (11) |
Values are means±SD and numbers (percentages). AAD indicates antiarrhythmic drugs; ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; DSG‐2, desmoglein‐2; EF, ejection fraction; ICD, implantable cardioverter‐defibrillator; PKP‐2, plakophilin‐2; rTFC, 2010 revised ARVC/D Task Force Criteria; SCD, sudden cardiac death.
*Data available in n=24.
Table 2.
Detailed Chart Listing the Diagnostic Criteria for ARVC/D (rTFC)
| Characteristic | All Patients (n=47) |
|---|---|
| Structural rTFC | 30/47 (64) |
| Major criterion | 28/30 (60) |
| Minor criterion | 2/30 (4) |
| Tissue alterations rTFC | Not available |
| Major criterion | ··· |
| Minor criterion | ··· |
| Repolarization rTFC | 31/47 (66) |
| Major criterion | 24/31 (51) |
| Minor criterion | 7/31 (15) |
| Depolarization rTFC | 24/47 (51) |
| Major criterion | 8/24 (17) |
| Minor criterion | 16/24 (34) |
| Arrhythmia rTFC | 46/47 (98) |
| Major criterion | 34/46 (72) |
| Minor criterion | 12/46 (26) |
| Family rTFC | 9/47 (19) |
| Major criterion | 8/9 (17) |
| Minor criterion | 1/9 (2) |
Values are numbers (percentages). ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; rTFC, Task Force Criteria (revised 2010).
Five out of 47 patients (5/47, 11%) had LV involvement with a reduced LV function. Twenty‐two out of 24 patients (22/24, 92%), in whom data on sports activity were available, were former endurance athletes. In the remaining patients data on sports activity could not be retrieved.
Procedural Data and Complications
A total of 81 RFA procedures were performed during the study period (mean 1.7 per patient, range 1–4). Forty‐five (45/81, 56%) ablation procedures were performed via an endocardial approach, 11 (11/81, 13%) via an epicardial approach, and 25 (25/81, 31%) via a combined endo‐ and epicardial approach. Procedural data are provided in Table 3. The mean number of induced VT was 2.0±1.0 per procedure. VT inducibility in the subgroups of patients with a primary prophylactic ICD (n=5) and a secondary prophylactic ICD (n=30) did not differ significantly. The mean number of induced VT was 2.0±1.19 in the primary prevention group and 1.72±1.08 in the secondary prevention group.
Table 3.
Electrophysiologic Characteristics of All Procedures (n=81) in the Whole Study Cohort
| Characteristic | All Procedures (n=81) |
|---|---|
| Endocardial approach only | 45 (56) |
| Epicardial approach only | 11 (13) |
| Combined epi‐/endocardial approach | 25 (31) |
| No. of VTs induced | |
| 1 | 30 (37) |
| 2 | 16 (20) |
| ≥3 | 25 (31) |
| Only PVCs | 10 (12) |
| Cycle length of induced VTs, ms | 353±79 |
| Induction method | |
| Extra stimuli | 64 (79) |
| Isoprotenerol±burst pacing | 6 (7.5) |
| Spontaneous | 6 (7.5) |
| Multiple induction methods | 5 (6) |
| VT morphology (n=78 documented morphologies) | |
| LBBB superior axis | 25 (32) |
| LBBB inferior axis | 18 (23) |
| RBBB superior axis | 0 (0) |
| RBBB inferior axis | 0 (0) |
| Multiple morphologies | 34 (44) |
| Indeterminate axis | 1 (1) |
| Critical site for endocardial VT (n=95 documented VT sites) | |
| Subtricuspid area | 54 (57) |
| RVOT | 27 (28) |
| Inferior RV/apex | 13 (14) |
| LV | 1 (1) |
| Critical site for epicardial VT (n=48 documented VT sites) | |
| Subtricuspid area | 23 (48) |
| RVOT | 16 (33) |
| Inferior RV/apex | 7 (15) |
| LV | 2 (4) |
| Procedure time, mina | 250 (195; 345) [endo]; 300 (240; 360) [comb.] |
| Fluoroscopy time, min | 13.5 (8; 18) [endo]; 20.0 (12; 29) [combined] |
| Dose of radiation, cGy×cm2 | 1363 (770; 4042) [endo]; 2533 (1373; 5246) [c.] |
| Acute success | |
| Complete success | 65 (80) |
| Partial success | 13 (16) |
| No success | 3 (4) |
Values are means±SD and numbers (percentages). LBBB indicates left bundle branch block; LV, left ventricle; PVC, premature ventricular contraction; RBBB, right bundle branch block; RV, right ventricle; RVOT, right ventricular outflow tract; VT, ventricular tachycardia.
Defined from peripheral venous access to removal of all endovascular sheaths.
Fourteen out of 47 patients (14/47, 30%) received a single ablation procedure, whereas the majority (n=33/47, 70%) had multiple procedures. Four out of 47 patients (4/47, 9%) underwent a total of 4 ablations.
Five out of 47 patients (5/47, 11%) had LV involvement with a reduced LV function. Ablation strategy did not differ between those with a normal LVEF and those with reduced LV function.
Total procedure time tended to be shorter for endocardial procedures as compared with epicardial/combined endo‐/epicardial procedures (250 [195; 345] versus 300 [240; 360] minutes; P=0.06). Complete acute success was achieved in 65 out of 81 procedures (65/81, 80%), and partial success in 13 (13/81, 16%) procedures. Ablation failure was documented in 2 patients (3 procedures); in 1 patient this was because of the close proximity of the RV lead and the critical substrate, and in the remaining patient clinical VT was still inducible even after extensive endocardial and epicardial ablation of the substrate.
A major complication occurred in 1 patient (1/81, 1%) who developed pericardial tamponade during the procedure, which was attributed to the epicardial access and was managed without any surgical measures and without any further sequelae. Minor complications occurred in 9 patients in 81 procedures (9/81, 11%), which were associated with endocardial procedures in 5 patients. Of note, 4 patients (4/28 patients with epicardial access, 14%) developed pericardial adhesions lately after epicardial access, precluding a second epicardial access by conventional epicardial puncture during follow‐up. In these patients the total amount of RF lesions (range 0–35 per procedure) was not significantly higher as compared with those without pericardial adhesions (P=0.41).
Characteristic Pattern of the VT Substrate in ARVC/D
As indicated in Table 3, the substrate for VT in the endocardial and epicardial maps was most frequently localized in the subtricuspid area (n=54/95 documented VT sites, (57%) and n=23/48 documented VT sites, (48%), respectively) followed by the right ventricular outflow tract (RVOT) (n=27/95, (28%) and n=16/48, (33%), respectively). In those patients with both endo‐ and epicardial maps, the area of epicardial bipolar low voltage was significantly larger as compared with the endocardial low voltage area (75.9±30.8 cm2 versus 32.1±21.9 cm2; P<0.001; epicardial/endocardial low voltage ratio 2.1 for definite ARVC/D, and 1.8 for borderline ARVC/D, respectively). No significant differences in the location of low voltage areas and the total area of epicardial and endocardial low voltage were observed between both subpopulations of definite and borderline patients (74.2±35.6 cm2 versus 67.2±4.5 cm2, and 36.0±33.3 cm2 versus 38.0±20.1 cm2, respectively).
All patients had an enlarged RV measured by the endocardial 3D EAM with a volume of >100 mL/m2 (mean 247.9±91.9 mL).
Of note, 2 patients showed no low voltage/dense scar on endocardial voltage maps, but the corresponding epicardial voltage maps displayed a typical pathological substrate in the subtricuspid region. Low voltage areas often presented as a “C‐shape pattern” in the RV extending from the RVOT free wall to the inferior RV, which is more frequently visible on epicardial maps as compared with endocardial maps (61.1% versus 21.9%). This C‐shape pattern was not only present in patients with definite ARVC/D, but also in those with borderline ARVC/D. The pathological process of fibro‐fatty infiltration in ARVC/D typically begins in the subtricuspid region, then extending towards the RVOT free wall, whereas the RV apex is involved at more advanced disease stages8 (Figure 1A). Of note, the epicardial low voltage areas were confluent in all patients with definite disease having >1 RV region involved (Figure 1B). The only patient, in whom no confluent low voltage areas between the diseased RV regions were observed had borderline ARVC/D. Since bipolar endocardial voltage maps were less sensitive to display the pathologic process of fibro‐fatty infiltration as compared with the epicardial voltage maps, this typical “C‐shape pattern” of disease involvement was not present in 42.9% of the endocardial bipolar maps.
Figure 1.
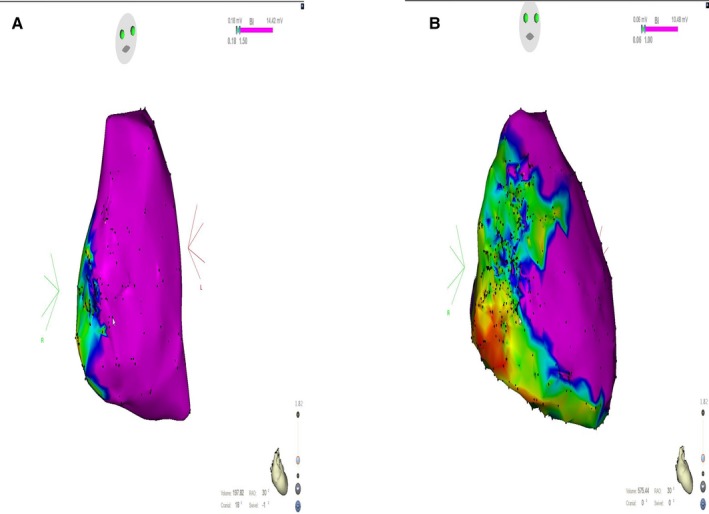
Endocardial (A) and epicardial (B) electroanatomical voltage map in right anterior oblique projection in a patient with a definite diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. The endocardial map (A) shows a low voltage area in the subtricuspid region (which is usually the first region, where the disease begins), whereas the epicardial map (B) shows more extensive scarring in a typical “C‐shape pattern” extending from the right ventricular outflow tract free wall to the inferior right ventricle and right ventricle apex.
Follow‐Up and Ventricular Arrhythmia Burden
The combined study end point was reached by 30 patients (30/47, 64%). Out of these, 11 patients (11/30, 37%) presented with an appropriate ICD intervention attributable to VT/VF, 18 patients (18/30, 60%) with sustained VT without an ICD intervention, and 1 patient (1/30, 3%) underwent heart transplantation. Four patients died during follow‐up after they had previously suffered from 1 of the other components of the primary study end point. The cause of death was decompensated heart failure in 2 patients; 1 patient developed a severe sepsis unrelated to the ablation procedure (54 days after the ablation procedure), and 1 cause of death was not clarified.
ICD interrogations before and after the index procedure were analyzed to determine the effectiveness of catheter ablation at the short term. Figure 2 illustrates the number of appropriate ICD therapies for sustained VT/VF episodes in the year before and the time (median 37.2 [2.5; 78.8] months) after the index procedure. The median number of VT/VF episodes before the index ablation procedure was 23 [7; 55] as compared with 0 [0; 1] after ablation (P<0.001).
Figure 2.
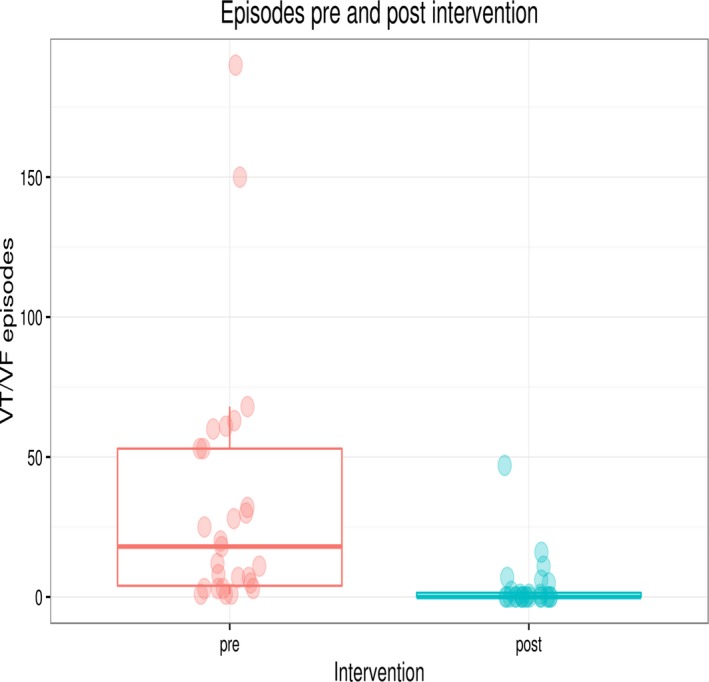
Box plot figure illustrating sustained ventricular tachycardia/ventricular fibrillation (VT/VF) episodes pre and post index procedure. N=28, since only patients with implantable cardioverter‐defibrillators (ICD) (before ablation procedure) and complete interrogation data were analyzed. Median follow‐up time in these patients was 37.2 [2.5; 78.8] months after the index procedure.
From patients with ICD shocks±ATP (n=25) before the index procedure, the majority (n=15/25, 60%) had no further ICD intervention after the index procedure. More than half of these patients (n=8/15, 53%) only received an endocardial ablation. Figure 3 shows an endocardial EAM and the targeted clinical VTs in a patient with definite ARVC/D (harboring a digenic heterozygous PKP‐2/DSP mutation), who presented with electrical storm and multiple ICD interventions before the index ablation procedure. During endocardial mapping, a pathological substrate was found in the subtricuspid area and in the RVOT. Ablation only via an endocardial approach was performed, and has been successful with no more sustained VT/VF episodes after a follow‐up of 2 years. Patients with only ATP before the index procedure (n=3/25) did not experience any further ICD intervention after their index ablation.
Figure 3.
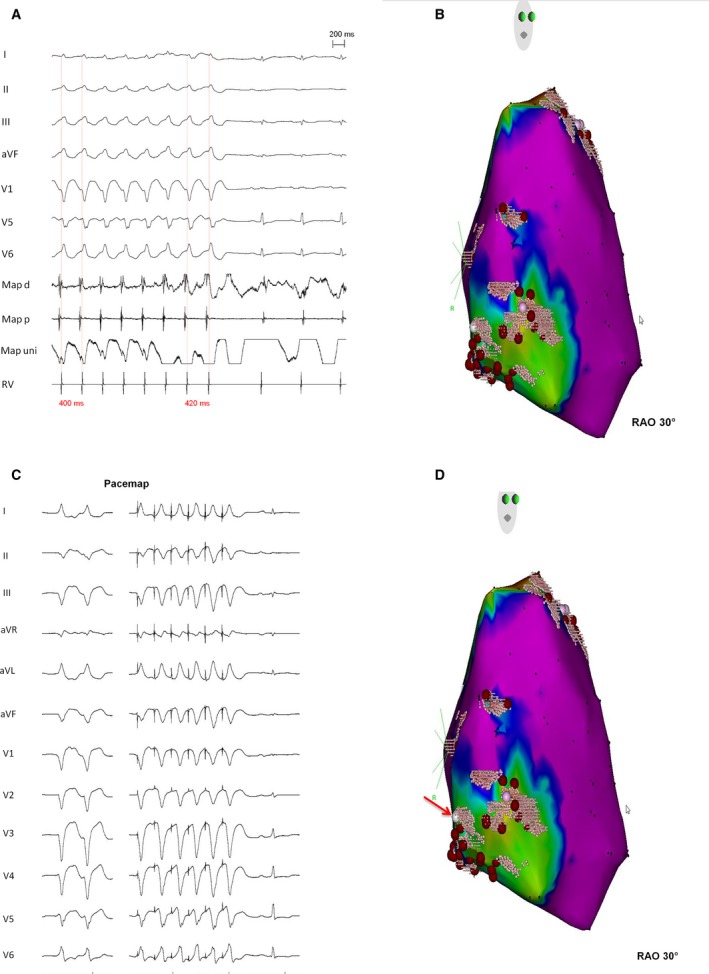
Endocardial electroanatomical voltage mapping and targeted clinical ventricular tachycardias (VT) in a patient with definite arrhythmogenic right ventricular cardiomyopathy/dysplasia (harboring a digenic heterozygous PKP‐2/DSP mutation), who presented with electrical storm and multiple implantable cardioverter‐defibrillator interventions before the index ablation procedure. During endocardial mapping, abnormal substrate was found in the subtricuspid area and in the right ventricular outflow tract. Ablation only via an endocardial approach was performed, and has been successful with no more sustained VT/ventricular fibrillation episodes after a follow‐up of 2 years. A, Intracardiac electrograms of termination of VT during catheter ablation in the RVOT region (upper pink point) and (B) shows corresponding endocardial electroanatomical map. C and D, Displays a perfect pacemap of a second VT in the subtricuspid region in the same patient (red arrow). RAO indicates right anterior oblique. I, II, III, aVR, aVL, V1‐V6 indicates surface 12‐lead ECG; Map d/p, bipolar signal on the distal (d) and proximal (p) electrodes of the ablation catheter; Map uni, unipolar signal on the ablation catheter; RV, signal from the catheter in the right ventricular apex.
There were 12 patients (12/47, 26%) in our cohort who did not have an ICD. In these patients, VT ablation was performed because of VT inducibility during the electrophysiologic study and previously observed sustained VT during Holter monitoring. All of these procedures were completely successful. During follow‐up, 6 of these patients (6/12, 50%) suffered from sustained VT which was addressed by a second ablation procedure. During follow‐up none of them suffered from sustained VT and none of them died from a cardiovascular cause. Therefore, cardiovascular mortality was not increased in this subpopulation of our patients without ICDs.
Three patients (3/35 patients with ICD, 9%), 2 of them with a definite ARVC/D diagnosis, suffered from electrical storm after the index procedure (2 with only endocardial ablation, 1 with combined endo‐/epicardial ablation).
Out of the 9 patients with a borderline ARVC/D diagnosis, 4 patients (4/9, 44%) did not have any sustained VT/VF recurrence during follow‐up. The remaining 5 patients (5/9, 56%) received ICD shocks and were admitted for a second ablation procedure after the index procedure. In comparison, out of the 38 patients with a definite ARVC/D diagnosis, a second ablation procedure because of sustained VT/VF recurrence was performed in 25 patients (66%, P=0.60).
Long‐Term Follow‐Up
After a median follow‐up of 50.8 [18.6; 99.2] months after the index procedure, 17 (17/47, 36%) patients were free from the combined study end point (sustained VT/VF, heart transplant, and death). The median time from the index procedure to this end point was 1.9 [0.2; 11.3] months. After a median follow‐up of 15.5 [0.7; 47.3] months since the last ablation procedure, 39 (39/47, 83%) patients were free from any sustained VT/VF. Eight (8/47, 17%) patients had recurrent ICD interventions after the last procedure, and 3 (3/47, 6%) of them suffered from ICD shocks.
Freedom from the combined study end point (sustained VT/VF, heart transplant, and death) for the overall population was 47% (95% CI, 34–65) at 1 year, and 31% (95% CI, 19–49) at 5 years after the index procedure (Figure 4A). After multiple procedures, freedom from sustained VT/VF recurrence for the overall population was 63% (95% CI, 52–75) at 1 year, and 45% (95% CI, 34–61) at 5 years. Of note, these rates were achieved with 36% of patients receiving only endocardial procedures. Figure 4B shows survival rates free from the combined study end point after the index procedure stratified into an endocardial only and combined endo‐/epicardial approach. Although there was a trend for improved survival rates after the index procedure in patients receiving a combined endo‐/epicardial approach as compared with an endocardial only approach, this difference was not statistically significant (hazard ratio endo‐/epicardial versus endo 0.556 [0.266, 1.162]; P=0.119, Figure 4B). Thirty‐six percent (95% CI, 22–61) of the patients in the endocardial ablation group were free from the combined end point at 1 year after the index procedure, whereas the combined endo‐/epicardial ablation group showed an event‐free survival of 63% (95% CI, 44–89) at 1 year (Figure 4B). This trend was accentuated after multiple procedures for the end point of sustained VT/VF. After multiple procedures, sustained VT/VF free survival was higher in the combined endo‐/epicardial ablation group at 1 year (75%; 95% CI, 61–93) and at 5 years (54%; 95% CI, 37–77) as compared with endocardial only ablation (53%, 95% CI, 39–71) at 1 year; and 39% (95% CI, 24–63) at 5 years, respectively; hazard ratio: endo‐/epicardial versus endocardial 0.572 (0.321, 1.020); P=0.058] (Figure 4C).
Figure 4.
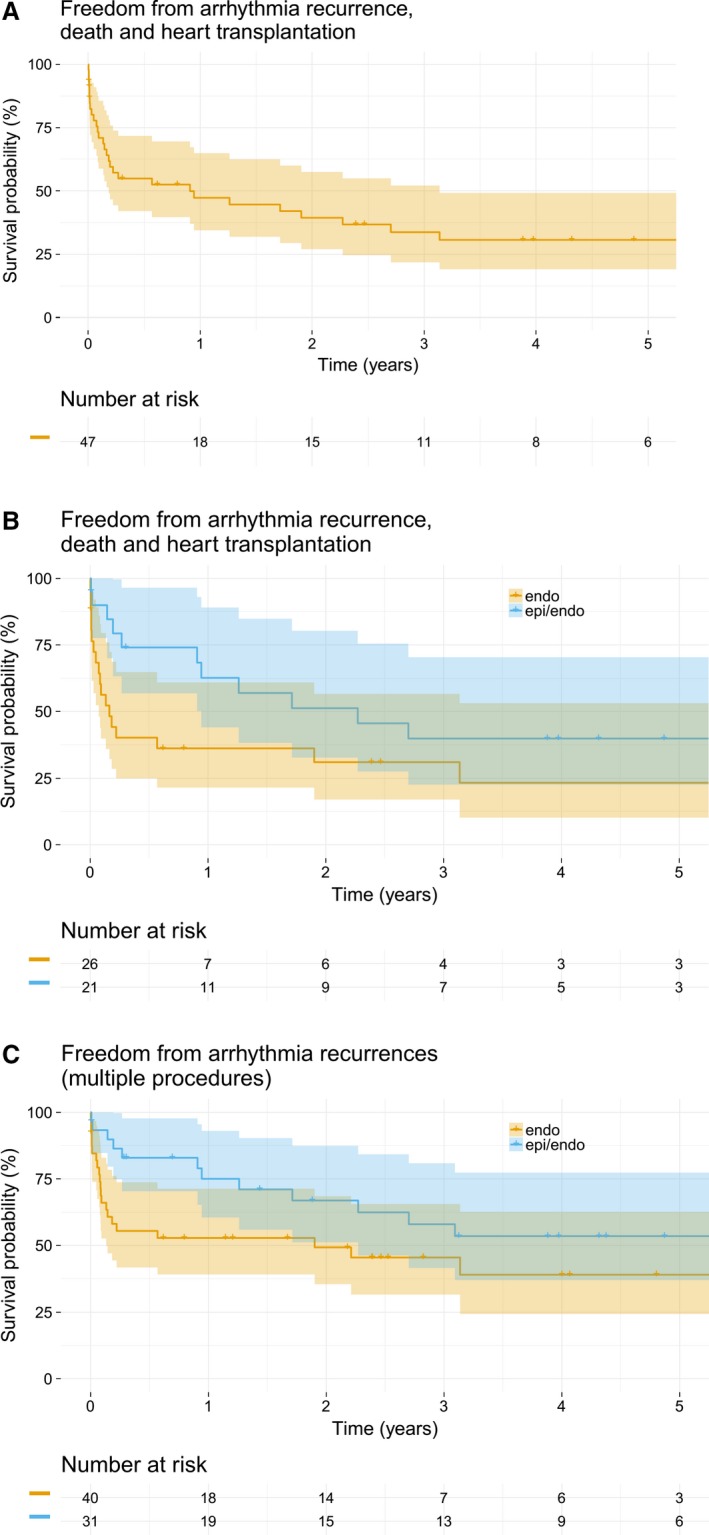
A, Kaplan–Meier plot illustrating survival rates free from the combined study end point (sustained ventricular tachycardia/ventricular fibrillation, heart transplantation, and death) after the index procedure. The graph shows data for the whole study cohort (n=47). B, Kaplan–Meier plot illustrating survival rates free from the combined study end point after the index procedure stratified into an endocardial only and combined endo‐/epicardial approach (P=0.119). C, Kaplan–Meier plot illustrating survival rates free from sustained ventricular arrhythmia recurrence after multiple procedures. It shows a statistically non‐significant trend (P=0.058) towards a better outcome for patients receiving a combined endo‐/epicardial procedure as compared with patients only receiving endocardial catheter ablation. Endo indicates endocardial; epi/endo, epi‐/endocardial.
The number of patients taking AAD at last follow‐up was not statistically different as compared with baseline (n=44/47 [94%] versus n=42/47 [89%]; P=0.72). A considerable proportion of patients (11/42, 26%) was only on beta‐blockers at last follow‐up.
Predictors of Successful Ablation
We analyzed several co‐variables to identify possible predictors of the combined study end point after catheter ablation of VT in patients with ARVC/D. Amongst the parameters age, sex, body mass index, physical activity (endurance sports), desmosomal mutation status, ARVC/D task force score, presence of an ICD, and ablation approach, we did not identify any significant variable.
Discussion
This study reports acute success and long‐term outcomes after catheter ablation of sustained VT in patients with ARVC/D at our tertiary care center using a sequential ablation strategy. This sequential strategy first targets the endocardial substrate, and epicardial ablation is performed only in cases of acute ablation failure or lack of an endocardial substrate. The main study findings are as follows: (1) High acute success rate of 96% (80% complete and 16% partial VT abolition); (2) After a median follow‐up of 50.8 (18.6; 99.2) months after the index procedure, 17 (17/47, 36%) patients were free from sustained VT/VF, heart transplant, and death; (3) After multiple procedures, freedom from sustained VT/VF for the overall population was 63% at 1 year, and 45% at 5 years; (4) A non‐significant trend towards improved outcomes using a combined endo‐/epicardial approach as compared with an endocardial only approach was observed, with a freedom from sustained VT/VF of 54% at 5 years in the endo‐/epicardial group; (5) Repeat epicardial access by needle puncture was impaired by pericardial adhesions in 14% (4/28 patients with epicardial access) of patients.
Previous Studies
Our study investigating a sequential approach for catheter ablation of sustained VT in patients with ARVC/D demonstrates a high acute success rate of 96%, but a high recurrence rate of 69% after a single procedure for the combined study end point (sustained VT/VF, heart transplant, and death), and a modest recurrence rate of 55% after multiple procedures for any sustained VT/VF during a follow‐up (F/U) of ≈5 years. An important factor is the progressive nature of the disease,25 which may explain the modest long‐term results that have been observed after catheter ablation of VT in patients with ARVC/D in the current and in previous studies.26
In line with these findings, it is well known that patients with ARVC/D may need multiple ablation procedures.27 However, previous studies on the outcome of catheter ablation for sustained VT in ARVC/D report varying results.11, 17, 27, 28 Santangeli et al28 recently demonstrated 71% freedom from VT after endocardial±adjuvant epicardial ablation at ≈4 years of F/U. Dalal et al reported a lower VT free survival of 15% at 14 months of F/U in an endocardial ablation study.27 Similar results were observed after endocardial ablation by Marchlinski and by Verma et al.29, 30 Besides adjuvant epicardial ablation, another reason for the observed differences could be the use of 3D electroanatomical mapping systems in more recent studies, since older studies have not systematically used these systems.
Our data are in line with recent studies, who reported improved outcomes using 3D mapping systems, since we know that this may facilitate characterization of the pathological substrate and ablation. An important finding of our study is that although single procedural success rates using a sequential approach are low, VT/VF free survival during long‐term F/U can be improved after multiple procedures, even in patients who never receive epicardial ablation, since more than one‐third of our patient population only received endocardial ablation. Of note, there was no significant difference in outcome between the only endocardial and the combined endo‐/epicardial approach after the index procedure (P=0.119; Figure 4B), although a trend towards improved outcomes with adjunct epicardial ablation was depicted. Similar results were recently published from another German study group. During the index procedure, patients only received adjuvant epicardial ablation if the clinical VT was still inducible after endocardial ablation. The results from Leipzig showed no difference between endocardial only (56.5%) and a combined endo‐/epicardial approach (59.1%) in freedom from sustained VT after a F/U of 31 months.18 In comparison with our study, F/U was shorter, and Müssigbrodt et al only reported recurrence‐free survival rates after the index procedure, but did not investigate VT/VF‐free survival after multiple procedures.
Pathologic Substrate in ARVC/D
In this study, the area of epicardial low voltage was ≈2 times larger as compared with the endocardial low voltage area, which seems to constitute a typical feature of ARVC/D.31 Furthermore, we observed that the pathological process in the RV of patients with ARVC/D referred for catheter ablation of VT shows a characteristic “C‐shape pattern” extending from the RVOT free wall to the inferior RV. This pattern was more frequently visible on epicardial maps. In line with previous studies, the pathological process of fibro‐fatty infiltration seems to start in the subtricuspid region, then extending towards the RVOT free wall, and the RV apex is involved at more advanced disease stages.8 Our observations on the amount and localization of the pathological substrate were similar for patients with definite and borderline ARVC/D, suggesting that they had the “same” disease.
The combination of the presence of a larger RV epicardial substrate as compared with the RV endocardium, the typical “C‐shape” low voltage area in the RV, and the disease onset in the subtricuspid region with confluent disease spreading towards the RVOT and RV inferior wall seem to be specific markers of ARVC/D. These important observations seem to contrast to phenocopies of ARVC/D such as RV myocardial infarction, myocarditis or cardiac sarcoid, with the latter 2 entities frequently showing a rather patchy scar formation.32
Endocardial or Epicardial Ablation as First Line Therapy
Recent evidence indicates that combined endo‐/epicardial ablation yields superior outcomes as compared with an endocardial only ablation strategy.10, 11, 12, 13 Yet, given the risks of an epicardial access, it is still debated whether a pure endocardial or combined endo‐/epicardial approach should be chosen as first line therapy. Garcia et al proposed an epicardial approach as first line therapy in 2009. Interestingly, with growing experience on catheter ablation of VT and improved tools, the same group proposed an endocardial approach as first line therapy and adjuvant epicardial ablation only if needed.28 Initially the authors hypothesized in their first manuscript a thicker endocardial layer in ARVC/D patients, which may only be targeted sufficiently from the epicardium. The authors performed measurements of overlapping endocardial and epicardial surfaces in 3D EAM to determine the wall thickness of the basal and free wall of the RV. However, this method lacks accuracy, and the gold standard for measuring RV wall thickness in vivo is cardiac magnetic resonance imaging (MRI), or autopsy studies ex vivo. RV thickness is usually ≤3 mm, and even thinner in diseased RV areas of patients with ARVC/D.33 Modern open‐irrigated ablation catheters using RF energy can achieve ablation lesions with a depth of up to 7 to 8 mm with standard power settings and a stable catheter position,34 which should theoretically enable a transmural lesion from the endocardial surface. Accordingly, a recent study evaluated the effect of endocardial elimination of local abnormal ventricular activities. The authors stated that local abnormal ventricular activities elimination by potent endocardial ablation could result in epicardial local abnormal ventricular activities elimination in at least 73% of patients with ARVC/D.21 In line with these considerations, our data showed no significant difference between the endocardial and epicardial approach, and a considerable number of patients receiving only endocardial ablation did not suffer from sustained VT/VF during F/U, or in case of a sustained arrhythmia, were shifted from ICD shocks to painless ATP. ATP was considered as an equivalent end point to ICD shocks, which is in line with previous studies, but ATP as compared with ICD shocks implies a significant improvement from a clinical point of view that is often neglected in ICD studies. This was shown by a recent study, where patients who only received ATP instead of high voltage shocks had a reduced mortality and hospitalization rate.35
Complications of Epicardial Ablation
Improved long‐term outcome should overweigh potential risks of complications. Sacher et al reported a complication rate of up to 8% for epicardial VT ablation,15 and studies from high‐volume centers have even reported fatalities associated with an epicardial approach.15, 16, 17, 31
In the current study, the 1 severe complication, a pericardial tamponade, was attributed to the epicardial access. Moreover, an early epicardial procedure may impair access to the pericardial space for future procedures. In the current study, this was the case in 14% of patients (4/28 patients with pericardial access) who received epicardial mapping precluding any further percutaneous epicardial ablation. In some patients this could even result in an avoidable surgical procedure with surgical adhesiolysis, which may increase the risk of severe complications. Furthermore, prolonged procedure times and increased radiation using an epicardial approach as compared with an endocardial approach, as shown in the current study, should also be considered. Given the risks of an epicardial approach, longer procedure times and higher fluoroscopy doses, and the aforementioned considerations on RV wall thickness and lesion depth, an endocardial approach as first line therapy—and only performing additional epicardial ablation if no endocardial substrate can be identified or if clinical VT is still inducible after extensive endocardial ablation—seems to be reasonable. Such a sequential approach may be considered by less experienced centers. Yet, our data underline the need of extensive endocardial ablation with good contact and stable catheter position before considering an epicardial approach. This may be particularly relevant for young patients with ARVC/D, who may need several ablation procedures, and an epicardial ablation that is performed too early may cause significant pericardial adhesions precluding further percutaneous epicardial access.
Predictors of VT/VF Recurrence
We analyzed several potential clinical predictors of VT/VF recurrence. However, no significant association between these parameters and outcome was demonstrated, which is in line with previous reports.36 In those patients in whom genetic testing was performed, no statistical difference in outcome after catheter ablation was found for those with and without desmosomal mutations. The role of physical activity and outcome in ARVC/D is debated. Endurance athletes have been shown to be associated with adverse outcome, also after catheter ablation.13 In our population, there was a high number of former endurance athletes. Yet, no patient was an active endurance or competitive athlete at last F/U, which might have influenced our results on long term F/U after VT ablation. AAD may also influence VT recurrence and outcome. We did not observe a significant reduction in the rate of patients taking betablockers and AAD at last F/U as compared with baseline, which implies that ARVC/D is a progressive disease and catheter ablation alone may not be sufficient to control arrhythmias.
Limitations
Our study has some limitations. This was not a prospective randomized trial, data on EAM and ICD interrogations were not complete in all patients, and therefore our results are rather hypothesis‐generating. It would be of great interest to analyze differences in outcome between the sequential approach and combined endo‐‐epicardial ablation as first line therapy in a randomized fashion, but in rare diseases such as ARVC/D this will need a global collaborative approach. Furthermore, patients were treated in a high‐volume center, conferring a significant risk of selection bias, and our results may not be applicable to centers with a lower ablation volume. Accordingly, the majority of patients presented with an endocardial substrate reflecting that these patients had rather advanced stages of the disease, limiting the generalizability of our data. We included some patients with borderline ARVC/D who did not fulfil the definite 2010 ARVC/D task force criteria. From a clinical perspective, these patients showed the full ARVC/D phenotype, however, since they were referred to us from other hospitals for VT ablation, we did not assign all 2010 diagnostic criteria. Furthermore, we would like to indicate that even if these patients do not fulfill definite criteria, they form an important part of our daily clinical work and it is important to assess the efficacy and safety of VT ablation also in these patients with a borderline diagnosis.
Despite being considerable for such a rare disease and complex intervention, patient numbers are still too low to account for various confounders and to analyze subgroups. Thus, our analysis on clinical predictors and VT/VF recurrence may be underpowered.
Conclusions
Catheter ablation is a generally safe and effective strategy to reduce sustained ventricular arrhythmia burden in patients with ARVC/D. Although the pathologic process in ARVC/D typically begins in the subepicardium, endocardial ablation seems to be effective in a considerable number of patients, potentially obviating the need for an epicardial approach in some of these patients.
Disclosures
Dr Kuck reports grants and personal fees from St. Jude Medical, Biosense Webster, and Medtronic, outside the submitted work. Dr Saguner reports educational grants from Biosense Webster. Dr. Mathew received travel grants and personal fees from Biosense Webster and Medtronic. Dr Rillig received travel grants from Biosense Webster, and St. Jude Medical and lecture fees from St. Jude Medical and Boehringer Ingelheim and took part at the Boston scientific electrophysiological fellowship. Dr Tilz reports grants, personal fees, and nonfinancial support from Biosense Webster, personal fees and nonfinancial support from St. Jude medical, nonfinancial support from Abbott, outside the submitted work. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e010365 DOI: 10.1161/JAHA.118.010365.)
References
- 1. Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. [DOI] [PubMed] [Google Scholar]
- 2. Saguner AM, Duru F, Brunckhorst CB. Arrhythmogenic right ventricular cardiomyopathy: a challenging disease of the intercalated disc. Circulation. 2013;128:1381–1386. [DOI] [PubMed] [Google Scholar]
- 3. Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes M, Buja G, Thiene G. Implantable cardioverter‐defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. [DOI] [PubMed] [Google Scholar]
- 4. Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes M, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 5. James CA, Tichnell C, Murray B, Daly A, Sears SF, Calkins H. General and disease‐specific psychosocial adjustment in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy with implantable cardioverter defibrillators: a large cohort study. Circ Cardiovasc Genet. 2012;5:18–24. [DOI] [PubMed] [Google Scholar]
- 6. Schinkel AFL. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and inappropriate interventions, and complications. Circ Arrhythm Electrophysiol. 2013;6:562–568. [DOI] [PubMed] [Google Scholar]
- 7. Thiene G, Basso C, Calabrese F, Angelini A, Valente M. Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz. 2000;25:210–215. [DOI] [PubMed] [Google Scholar]
- 8. Te Riele ASJM, James CA, Philips B, Rastegar N, Bhonsale A, Groeneweg JA, Murray B, Tichnell C, Judge DP, Van Der Hejden JF, Cramer MJM, Velthuis BK, Bluemke DA, Zimmerman SL, Kamel IR, Hauer RNW, Calkins H, Tandri H. Mutation‐positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol. 2013;24:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naneix AL, Perier MC, Beganton F, Jouven X, Lorin De La Grandmaison G. Sudden adult death: an autopsy series of 534 cases with gender and control comparison. J Forensic Leg Med. 2015;32:10–15. [DOI] [PubMed] [Google Scholar]
- 10. Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. [DOI] [PubMed] [Google Scholar]
- 11. Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, Saenz LC, Vacca M, Verma A, Khaykin Y, Mohanty S, Burkhardt DJ, Hongo R, Beheiry S, Dello Russo A, Casella M, Pelargonio G, Santarelli P, Sanchez J, Tondo C, Natale A. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia‐free survival after endo‐epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4:478–485. [DOI] [PubMed] [Google Scholar]
- 12. Berruezo A, Fernandez‐Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–121. [DOI] [PubMed] [Google Scholar]
- 13. Philips B, te Riele ASJM, Sawant A, Kareddy V, James CA, Murray B, Tichnell C, Kassamali B, Nazarian S, Judge DP, Calkins H, Tandri H. Outcomes and ventricular tachycardia recurrence characteristics after epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2015;12:716–725. [DOI] [PubMed] [Google Scholar]
- 14. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce1 B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes M, McKenna WJ, Thiene G, Marcus FI, Calkins H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sacher F, Roberts‐Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG. Epicardial ventricular tachycardia ablation. A multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt B, Chun KRJ, Baensch D, Baensch D, Antz M, Koektuerk B, Tilz RR, Metzner A, Ouyang F, Kuck KH. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Heart Rhythm. 2010;7:1746–1752. [DOI] [PubMed] [Google Scholar]
- 17. Philips B, Madhavan S, James C, Tichnell C, Murray B, Dalal D, Bhonsale A, Nazarian S, Judge DP, Russell SD, Abraham T, Calkins H, Tandri H. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505. [DOI] [PubMed] [Google Scholar]
- 18. Müssigbrodt A, Efimova E, Knopp H, Bertagnolli L, Dagres N, Richter S, Husser D, Bollmann A, Hindricks G, Arya A. Should all patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergo epicardial catheter ablation? J Interv Card Electrophysiol. 2017;48:193–199. [DOI] [PubMed] [Google Scholar]
- 19. Sacher F, Wright M, Derval N, Denis A, Ramoul K, Roten L, Pascale P, Bordachar P, Ritter P, Hocini M, Dos Santos P, Haissaguerre M, Jais P. Endocardial versus epicardial ventricular radiofrequency ablation: utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol. 2013;6:144–150. [DOI] [PubMed] [Google Scholar]
- 20. Desjardins B, Morady F, Bogun F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J Am Coll Cardiol. 2010;56:1320–1327. [DOI] [PubMed] [Google Scholar]
- 21. Komatsu Y, Daly M, Sacher F, Cochet H, Denis A, Derval N, Jesel L, Zellerhoff S, Lim HS, Jadidi A, Nault I, Shah A, Roten L, Pascale P, Scherr D, Aurillac‐Lavignolle V, Hocini M, Haissaguerre M, Jais P. Endocardial ablation to eliminate epicardial arrhythmia substrate in scar‐related ventricular tachycardia. J Am Coll Cardiol. 2014;63:1416–1426. [DOI] [PubMed] [Google Scholar]
- 22. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Yoerger Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polin GM, Haqqani H, Tzou W, Hutchinson MD, Garcia FC, Callans DJ, Zado ES, Marchlinski FE. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. [DOI] [PubMed] [Google Scholar]
- 24. Ino T, Fishbein MC, Mandel WJ, Chen PS, Karagueuzian HS. Cellular mechanisms of ventricular bipolar electrograms showing double and fractionated potentials. J Am Coll Cardiol. 1995;26:1080–1089. [DOI] [PubMed] [Google Scholar]
- 25. Saguner AM, Ganahl S, Kraus A, Baldinger SH, Akdis D, Saguner AR, Wolber T, Haegeli LM, Steffel J, Krasniqi N, Lüscher TF, Tanner FC, Brunckhorst C, Duru F. Electrocardiographic features of disease progression in arrhythmogenic right ventricular cardiomyopathy/dysplasia. BMC Cardiovasc Disord. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berte AB, Sacher F, Venlet J, Andreu D, Mahida S, Aldhoon B, DE Potter T, Sarkozy A, Tavernier R, Andronache M, Deneke T, Kautzner J, Berruezo A, Cochet H, Zeppenfeld K, Jaïs P. VT recurrence after ablation: incomplete ablation or disease progression? A multicentric European study. J Cardiovasc Electrophysiol. 2016;27:80–87. [DOI] [PubMed] [Google Scholar]
- 27. Dalal D, Jain R, Tandri H, Dong J, Eid SM, Prakasa K, Tichnell C, James C, Abraham T, Russell SD, Sinha S, Judge DP, Bluemke DA, Marine JE, Calkins H. Long‐term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:432–440. [DOI] [PubMed] [Google Scholar]
- 28. Santangeli P, Zado ES, Supple G, Haqqani HM, Garcia FC, Tschabrunn C, Callans DJ, Lin D, Dixit S, Hutchinsoson MD, Callans DJ, Riley M, Marchlinski FE. Long‐term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–1421. [DOI] [PubMed] [Google Scholar]
- 29. Marchlinski FE. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293–2298. [DOI] [PubMed] [Google Scholar]
- 30. Verma A, Kilicaslan F, Schweikert RA, Natale A. Short‐ and long‐term success of substrate‐based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111:3209–3216. [DOI] [PubMed] [Google Scholar]
- 31. Berruezo A, Acosta J, Fernández‐Armenta J, Pedrote A, Barrera A, Arana‐Rueda E, Bodegas AI, Anguera I, Tercedor L, Penela D, Andreu D, Perea RJ, Prat‐Gonzalez S, Mont L. Safety, long‐term outcomes and predictors of recurrence after first‐line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic substrate distribution pattern. A prospective multicentre study. Europace. 2017;19:607–616. [DOI] [PubMed] [Google Scholar]
- 32. Kumar S, Barbhaiya C, Nagashima K, Choi EK, Epstein LM, John RM, Maytin M, Albert CM, Miller AL, Koplan BA, Michaud GF, Tedrow UB, Stevenson WG. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 33. Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998;97:1571–1580. [DOI] [PubMed] [Google Scholar]
- 34. Guerra JM, Jorge E, Raga S, Galvez‐Monton C, Alonso‐Martin C, Rodriguez‐Font E, Cinca J, Vinolas X. Effects of open‐irrigated radiofrequency ablation catheter design on lesion formation and complications: in vitro comparison of 6 different devices. J Cardiovasc Electrophysiol. 2013;24:1157–1162. [DOI] [PubMed] [Google Scholar]
- 35. Sanders P, Connolly AT, Nabutovsky Y, Fischer A, Saeed M. Increased hospitalizations and overall healthcare utilization in patients receiving implantable cardioverter‐defibrillator shocks compared with antitachycardia pacing. JACC Clin Electrophysiol. 2018;4:243–253. [DOI] [PubMed] [Google Scholar]
- 36. Saguner AM, Vecchiati A, Baldinger SH, Rüeger S, Medeiros‐Domingo A, Mueller‐Burri AS, Haegeli LM, Biaggi P, Manka R, Lüscher TF, Fontaine G, Delacrétaz E, Jenni R, Held L, Brunckhorst C, Duru F, Tanner FC. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging. 2014;7:230–239. [DOI] [PubMed] [Google Scholar]


