Abstract
Background
Liver cirrhotic patients with nonvalvular atrial fibrillation have been excluded from randomized clinical studies regarding oral anticoagulants for stroke prevention. Whether non–vitamin K antagonist oral anticoagulants (NOACs) are superior to warfarin for these patients remains unclear.
Methods and Results
This nationwide retrospective cohort study, with data collected from the Taiwan National Health Insurance Research Database, enrolled 2428 liver cirrhotic patients with nonvalvular atrial fibrillation taking apixaban (n=171), dabigatran (n=535), rivaroxaban (n=732), or warfarin (n=990) from June 1, 2012, to December 31, 2016. We used propensity score–based stabilized weights to balance covariates across study groups. Patients were followed until the occurrence of an event or the end date of study. The NOAC group (n=1438) showed risk of ischemic stroke/systemic embolism and intracranial hemorrhage comparable to that of the warfarin group (n=990) after adjustment. The NOAC group showed significantly lower risk of gastrointestinal bleeding (hazard ratio: 0.51 [95% CI, 0.32–0.79]; P=0.0030) and all major bleeding (hazard ratio: 0.51 [95% CI, 0.32–0.74]; P=0.0003) compared with warfarin group. Overall, 90% (n=1290) of patients were taking a low‐dose NOAC (apixaban 2.5 mg twice daily, rivaroxaban 10–15 mg daily, or dabigatran 110 mg twice daily). The subgroup analysis indicated that both dabigatran and rivaroxaban showed lower risk of all major bleeding than warfarin. The advantage of lower gastrointestinal and all major bleeding with NOACs over warfarin is contributed by those subgroups with either nonalcoholic or nonadvanced liver cirrhosis.
Conclusions
NOACs have a risk of thromboembolism comparable to that of warfarin and a lower risk of major bleeding among liver cirrhotic Asian patients with nonvalvular atrial fibrillation. Consequently, thromboprophylaxis with low‐dose NOACs may be considered for such patients.
Keywords: atrial fibrillation, direct thrombin inhibitor, factor Xa inhibitor, hemorrhage, ischemic stroke, liver cirrhosis, mortality, warfarin
Subject Categories: Atrial Fibrillation
Short abstract
See Editorial by Gallagher et al
Clinical Perspective
What Is New?
Liver cirrhotic patients have been excluded from randomized clinical trials regarding non–vitamin K antagonist oral anticoagulants for stroke prevention among patients with nonvalvular atrial fibrillation.
Our study indicated that the group taking non–vitamin K antagonist oral anticoagulants showed risk of ischemic stroke/systemic embolism and intracranial hemorrhage comparable to that of the warfarin group and significantly lower risk of gastrointestinal bleeding and major bleeding among liver cirrhotic patients with nonvalvular atrial fibrillation.
What Are the Clinical Implications?
Thromboprophylaxis with non–vitamin K antagonist oral anticoagulants may be considered for liver cirrhotic Asian patients with nonvalvular atrial fibrillation in clinical practice.
Introduction
Liver cirrhosis is a condition associated with thrombocytopenia and decreased synthesis of several pro‐ and anticoagulant factors, which affects the hemostasis of several organs.1 The abnormalities in hemostasis related to liver cirrhosis may increase the risk of either bleeding or thrombosis.2, 3 Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a global prevalence of 2% to 3%, and significantly increases the risk of thromboembolic events, hospitalization, and mortality.4, 5 Cirrhotic patients with AF may have a higher risk of ischemic stroke or cerebral hemorrhage, and previous studies indicated that cirrhotic AF patients taking warfarin may be associated with better clinical outcomes compared with those taking an antiplatelet agent or going without treatment.6 However, because of the lack of established guidelines and the impaired synthesis of clotting factors interfacing the value of prothrombin time and international normalized ratio, prescription of warfarin remained challenging among cirrhotic AF patients. Several clinical trials have demonstrated that non–vitamin K antagonist oral anticoagulants (NOACs) have efficacy similar to or better than warfarin and are safer alternatives to warfarin.7, 8, 9, 10 However, those studies excluded cirrhotic AF patients because of poor underlying condition, and the effectiveness and safety profiles of NOACs among cirrhotic AF patients in clinical practice are limited. The objective of this study was to use data from the Taiwan National Health Insurance Research Database (NHIRD) to investigate the effectiveness and safety of NOACs, including apixaban, dabigatran, and rivaroxaban, compared with warfarin for cirrhotic AF patients in clinical practice.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
This study was approved by the institutional review board of Chang Gung Memorial Hospital. We analyzed the medical data of the Taiwan National Health Insurance system, which is a mandatory universal health insurance program and provides comprehensive medical care coverage to nearly all Taiwanese citizens. As of 2014, there were >23 million enrollees and a >99% coverage rate of the entire population.11 By using a consistent encrypting procedure, each patient's original identification number in NHIRD was encrypted and deidentified to protect patient privacy. Therefore, informed consent was waived by the institutional review board of Chang Gung Memorial Hospital.
Study Design
A dynamic cohort with 2 study groups (NOACs and warfarin) was used in the study. A flowchart of the study enrollment is shown in Figure 1. A total of 279 776 patients diagnosed with AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code 427.31 from January 1, 2010, to December 31, 2015, or ICD‐10‐CM code I48 from January 1, 2016, to December 31, 2016) were identified. Among those, 11 206 patients were recognized as having liver cirrhosis according to diagnosis using ICD codes indicating liver cirrhosis (ICD‐9‐CM codes 571.2, 571.5, and 571.6 or ICD‐10‐CM codes K72, K74, K70.2, K70.3, and K70.4).12, 13, 14 Those cirrhotic AF patients were included with their first prescription of a NOAC, including dabigatran as of the approval date of June 1, 2012; rivaroxaban as of February 1, 2013; or apixaban as of June 1, 2014, or warfarin after June 1, 2012. The index date for each group was defined as the first date of prescription for any NOAC or for warfarin after June 1, 2012. The follow‐up period was defined as the index date until the occurrence of any thromboembolic or major bleeding event or the end date of study period (December 31, 2016), whichever came first.
Figure 1.
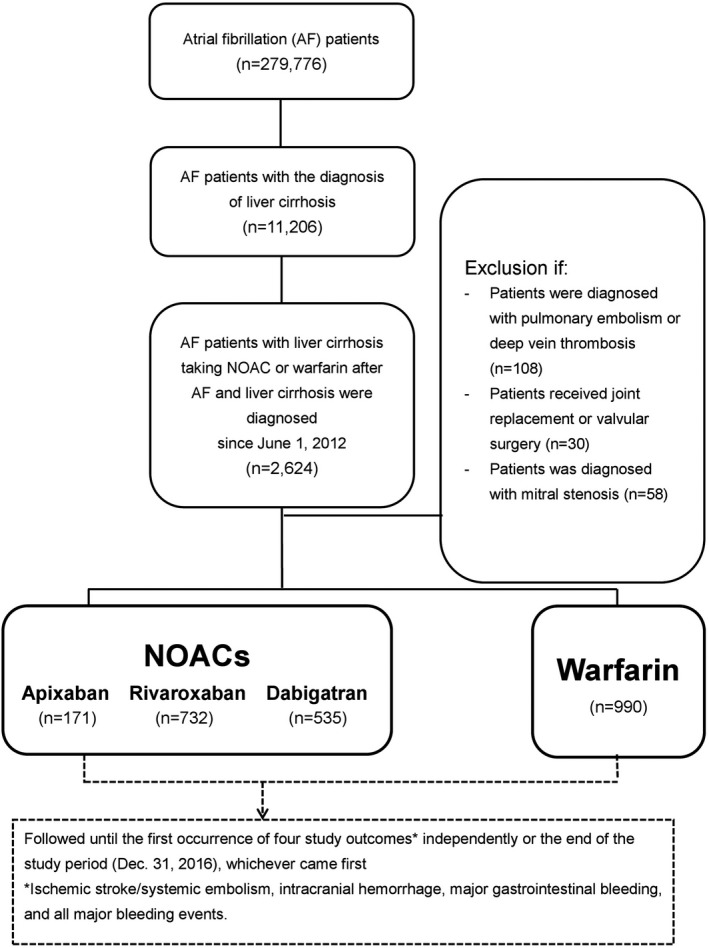
Enrollment of patients with liver cirrhosis and nonvalvular atrial fibrillation. From June 1, 2012, to December 31, 2016, a total of 171, 535, and 732 NVAF patients with liver cirrhosis taking apixaban, dabigatran, and rivaroxaban and 990 patients taking warfarin were enrolled in the study. AF indicates atrial fibrillation; NOAC, non–vitamin K antagonist oral anticoagulant.
Exclusion Criteria
Those patients with diagnoses indicating venous thromboembolism (pulmonary embolism or deep vein thrombosis), joint replacement therapy, or valvular AF (mitral stenosis or valvular surgery) within 6 months before the index date were excluded from this study to establish a cohort of NVAF patients taking an oral anticoagulant for the primary purpose of stroke prevention. Patients who took >1 kind of NOAC during their whole treatment course were also excluded from this study.
Study Outcomes
Four study outcomes were defined to determine the effectiveness and safety for cirrhotic AF patients taking NOACs and warfarin: ischemic stroke/systemic embolism (IS/SE), intracranial hemorrhage (ICH), major gastrointestinal bleeding (GIB), and all major bleeding events. All study outcomes were required to be a discharge diagnosis to avoid misclassification. ICH was defined with the use of codes for atraumatic hemorrhage. Major GIB was defined as a hospitalized primary code indicating bleeding in the gastrointestinal tract.15, 16, 17 All major bleeding events were defined as the total hospitalized events of ICH, major GIB, and other critical‐site bleeding. The diagnosis codes for NHIRD shifted from ICD‐9‐CM to ICD‐10‐CM after January 1, 2016. The ICD‐9‐CM and ICD‐10‐CM codes used to identify the study outcomes and the baseline covariates are summarized in Table S1. The same patient may have had >1 study outcome during the study duration, and all study outcomes were reported independently in the study.
Covariates
Baseline covariates referred to any claim record with the noted diagnoses or medication codes before the index date. Bleeding history was confined to events within 6 months preceding the index date. History of prescription for medicine was confined to at least once within 3 months preceding the index date. The CHA2DS2‐VASc score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, age 65–74 years, female sex) was adopted to predict the risk of ischemic stroke or thromboembolic events in AF patients, and the HAS‐BLED score (hypertension, abnormal renal or liver function, stroke, bleeding history, labile international normalized ratio, age ≥65 years, and antiplatelet drug or alcohol use) was adopted to predict the risk of bleeding in AF patients treated with oral anticoagulant.18, 19
Statistical Analysis
The method of propensity score–based stabilized weights (PSSWs), which attempts to approximate the randomized clinical trial for observational cohort data by balancing covariates across the study groups,20 was used to estimate the 4 study outcomes of NOACs and warfarin. The advantage of using PSSWs is preservation of the sample size of the original data to appropriately estimate the variance of the main effect and to maintain the designated type I error. The nonparametric generalized boosted model was used to obtain the PSSWs for optimal balance among study groups. The advantage of the generalized boosted model is automatic selection of which covariates to include and the best functional form including interactions.21 The covariates in Table 1 were included in the generalized boosted model except for CHA2DS2‐VASc and HAS‐BLED scores, which were a combination of other covariates. The balance of potential confounders at baseline (index date) between study groups was assessed using the absolute standardized mean difference rather than statistical testing because balance is a property of the sample and not of an underlying population. An absolute standardized mean difference ≤0.1 indicated an insignificant difference in potential confounders between the study groups.22 When comparing baseline characteristics among 3 NOAC groups, ANOVA, the χ2 test, and the Fisher exact test were used, as appropriate (Table 2). The incidence rates were computed using the total number of study outcomes during the follow‐up period divided by person‐years at risk. The risk of study outcomes for NOACs versus warfarin (reference) was obtained using survival analysis (Kaplan–Meier method and log‐rank test for univariate analysis and Cox proportional hazards regression for multivariate analysis). Specific subgroups were analyzed to determine whether the NOAC group continued to have a lower risk of study outcomes compared with the warfarin group. As noted, the PSSWs were reestimated for each subgroup analysis so that the NOAC and warfarin groups maintained a balance of covariates across groups. Statistical significance was defined as P<0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute).
Table 1.
Baseline Characteristics of NVAF Patients With Liver Cirrhosis Taking Oral Anticoagulants Before and After PSSWs
| Before PSSWs | After PSSWs | |||||
|---|---|---|---|---|---|---|
| NOAC (n=1438) | Warfarin (n=990) | ASMD | NOAC (n=1397) | Warfarin (n=946) | ASMD | |
| Age, y, mean±SD | 74.35±10.50 | 69.93±12.42 | 0.3848 | 72.80±11.05 | 72.41±11.21 | 0.0357 |
| Age group | ||||||
| <65 | 16.97 | 33.94 | 22.93 | 24.32 | ||
| 65–74 | 27.33 | 26.06 | 26.94 | 27.22 | ||
| 75–84 | 40.47 | 28.38 | 36.37 | 35.15 | ||
| >85 | 15.23 | 11.62 | 13.76 | 13.31 | ||
| Male sex | 62.38 | 65.56 | 0.0662 | 63.65 | 63.70 | 0.0010 |
| CHA2DS2‐VASc, mean±SD | 3.88±1.53 | 3.41±1.72 | 0.2895 | 3.72±1.57 | 3.66±1.65 | 0.0386 |
| HAS‐BLED, mean±SD | 3.76±1.04 | 3.62±1.21 | 0.1226 | 3.71±1.08 | 3.69±1.11 | 0.0162 |
| Hypertension | 86.37 | 80.91 | 0.148 | 84.29 | 83.52 | 0.0211 |
| Diabetes mellitus | 46.31 | 44.55 | 0.0355 | 46.00 | 44.89 | 0.0223 |
| Dyslipidemia | 42.14 | 38.89 | 0.0663 | 41.02 | 39.80 | 0.0249 |
| Chronic kidney disease | 34.42 | 46.46 | 0.2472 | 38.67 | 39.80 | 0.0231 |
| Chronic lung disease | 3.96 | 3.33 | 0.0336 | 3.80 | 3.69 | 0.0055 |
| Gout | 30.81 | 30.81 | 0 | 30.93 | 29.85 | 0.0236 |
| Congestive heart failure | 20.51 | 25.45 | 0.1176 | 22.75 | 22.29 | 0.0108 |
| Chronic ischemic heart disease | 12.80 | 12.02 | 0.0235 | 12.71 | 12.41 | 0.0089 |
| PAD | 0.07 | 0.10 | 0.0108 | 0.07 | 0.12 | 0.0148 |
| Stroke | 20.58 | 15.45 | 0.1338 | 18.86 | 18.24 | 0.0161 |
| TIA | 2.71 | 2.93 | 0.0131 | 2.64 | 2.64 | 0.0001 |
| Malignancy | 20.38 | 16.77 | 0.0929 | 19.50 | 18.94 | 0.0141 |
| PCI | 5.42 | 5.56 | 0.0058 | 5.54 | 5.47 | 0.0030 |
| CABG | 0.35 | 1.21 | 0.0984 | 0.51 | 0.76 | 0.0305 |
| History of bleeding | 4.66 | 5.56 | 0.0407 | 4.78 | 4.89 | 0.0050 |
| Use of NSAIDs | 26.36 | 25.86 | 0.0113 | 26.46 | 25.34 | 0.0256 |
| Use of PPI | 19.19 | 27.27 | 0.1922 | 21.89 | 22.69 | 0.0192 |
| Use of H2 blocker | 39.36 | 41.92 | 0.0521 | 39.92 | 40.62 | 0.0143 |
| Use of ACEI/ARB | 17.32 | 23.43 | 0.1523 | 19.44 | 20.28 | 0.0211 |
| Use of amiodarone | 28.44 | 34.44 | 0.1295 | 30.71 | 31.71 | 0.0215 |
| Use of dronedarone | 2.09 | 1.82 | 0.0194 | 2.00 | 2.31 | 0.0211 |
| Use of β‐blocker | 57.51 | 59.39 | 0.0382 | 58.69 | 58.12 | 0.0115 |
| Use of diltiazem/verapamil | 25.80 | 25.05 | 0.0172 | 25.76 | 24.61 | 0.0263 |
| Use of digoxin | 25.24 | 32.73 | 0.1655 | 28.23 | 29.20 | 0.0215 |
| Use of statin | 7.93 | 8.69 | 0.1480 | 8.17 | 8.55 | 0.0136 |
Data are shown as percentages except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ASMD, absolute standard mean difference; CABG, coronary artery bypass grafting; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, age 65–74 years, female sex; HAS‐BLED, hypertension, abnormal renal or liver function, stroke, bleeding history, labile international normalized ratio (could not be determined from claims and was excluded from our scoring), age ≥65 years, and antiplatelet drug or alcohol use; NOAC, non–vitamin K antagonist oral anticoagulant; NVAF, nonvalvular atrial fibrillation; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PSSW, propensity score–based stabilized weights; TIA, transient ischemic attack.
Table 2.
Baseline Characteristics of NVAF Patients With Liver Cirrhosis Taking Apixaban, Dabigatran, and Rivaroxaban
| Apixaban (n=171) | Dabigatran (n=535) | Rivaroxaban (n=732) | P Value | |
|---|---|---|---|---|
| Age, y, mean±SD | 75.36±10.26 | 73.57±10.45 | 74.68±10.59 | 0.0722a |
| Age group | ||||
| <65, y | 11.70 | 18.32 | 17.21 | |
| 65–74, y | 33.92 | 27.85 | 25.41 | |
| 75–84, y | 36.26 | 40.19 | 41.67 | |
| >85, y | 18.13 | 13.64 | 15.71 | |
| Male sex | 55.56 | 65.98 | 61.34 | 0.0353 |
| CHA2DS2‐VASc, mean±SD | 3.98±1.57 | 3.82±1.46 | 3.90±1.57 | 0.4457a |
| HAS‐BLED, mean±SD | 3.90±1.04 | 3.82±1.46 | 3.90±3.76 | 0.1306a |
| Hypertension | 87.72 | 86.54 | 85.93 | 0.8192 |
| Diabetes mellitus | 43.86 | 46.36 | 46.86 | 0.7781 |
| Dyslipidemia | 41.52 | 40.37 | 43.58 | 0.5134 |
| Chronic kidney disease | 41.52 | 29.91 | 36.07 | 0.0085 |
| Chronic lung disease | 3.51 | 3.36 | 4.51 | 0.5577 |
| Gout | 30.41 | 28.41 | 32.65 | 0.2698 |
| Congestive heart failure | 18.71 | 21.50 | 20.22 | 0.7064 |
| Chronic ischemic heart disease | 15.79 | 11.78 | 12.84 | 0.3919 |
| PAD | 0.00 | 0.00 | 0.14 | 1.0000b |
| Stroke | 20.47 | 21.50 | 19.95 | 0.7962 |
| TIA | 2.92 | 2.06 | 3.14 | 0.4930 |
| Malignancy | 26.32 | 21.31 | 18.31 | 0.0513 |
| PCI | 7.02 | 4.30 | 5.87 | 0.0513 |
| CABG | 0.58 | 0.37 | 0.27 | 0.6838 |
| History of bleeding | 4.68 | 4.49 | 4.78 | 0.9700 |
| Use of NSAIDs | 30.99 | 24.11 | 26.91 | 0.1827 |
| Use of PPI | 19.88 | 18.13 | 19.81 | 0.7332 |
| Use of H2 blocker | 38.60 | 37.94 | 40.57 | 0.6240 |
| Use of ACEI/ARB | 5.85 | 24.67 | 14.62 | 0.0001 |
| Use of amiodarone | 32.16 | 25.05 | 30.05 | 0.0769 |
| Use of dronedarone | 2.34 | 1.50 | 2.46 | 0.4805 |
| Use of β‐blocker | 60.82 | 54.77 | 58.74 | 0.2381 |
| Use of diltiazem/verapamil | 25.15 | 25.42 | 26.23 | 0.9282 |
| Use of digoxin | 17.54 | 27.29 | 25.55 | 0.0370 |
| Use of statin | 4.09 | 10.09 | 7.24 | 0.0253 |
Data are shown as percentages except as noted. P values were calculated with the χ2 test except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; CABG, coronary artery bypass grafting; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke or transient ischemic attack, vascular disease, age 65–74 years, female; HAS‐BLED, hypertension, abnormal renal or liver function, stroke, bleeding history, labile international normalized ratio (could not be determined from claims and was excluded from our scoring), age ≥65 years, and antiplatelet drug or alcohol use; NVAF, nonvalvular atrial fibrillation; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; TIA, transient ischemic attack.
ANOVA.
Fisher exact test.
Results
Among the 11 206 AF patients with liver cirrhosis, we identified 1438 and 990 patients taking NOACs and warfarin, respectively (Figure 1). The mean follow‐up periods were 1.13 and 1.30 years for the NOAC and warfarin groups, respectively. Overall, 27% (n=381) of NOAC users previously took warfarin. Among the NOAC group, 171, 535, and 732 patients were taking apixaban, dabigatran, and rivaroxaban, respectively. Of patients taking apixaban, 69.0% (n=118) were prescribed the low dose (2.5 mg twice daily) and 31.0% (n=53) were prescribed the standard dose (5 mg twice daily). For dabigatran, 88.8% (n=475) were taking a low dose (110 mg twice daily) and 11.2% (n=60) were taking the standard dose (150 mg twice daily). For rivaroxaban, 95.2% (n=697) of patients were taking the low dose (10–15 mg once daily), whereas only 4.8% (n=35) were taking the standard dose (20 mg once daily).
Before PSSWs, the NOAC group had higher prevalence of age, hypertension, and stroke history but lower prevalence of chronic kidney disease and congestive heart failure than the warfarin group. The NOAC group had higher CHA2DS2‐VASc and HAS‐BLED scores than the warfarin group before propensity score weighting (absolute standardized mean difference >0.1). After PSSWs, both study groups were well balanced in all characteristics (all absolute standardized mean difference <0.1; Table 1). For the effectiveness outcome, the NOAC group had cumulative risk of IS/SE similar to the warfarin group after PSSWs. For the safety outcome, the NOAC group showed significantly lower risk of major GIB (hazard ratio [HR]: 0.51 [95% CI, 0.32–0.79]; P=0.0030) and all major bleeding (HR: 0.51 [95% CI, 0.32–0.74]; P=0.0003) compared with the warfarin group after PSSWs. The cumulative risk showed clear separation of event curves for GIB and all major bleeding for the NOAC group versus the warfarin group either before or after PSSWs (Figure 2 and Figure S1).
Figure 2.
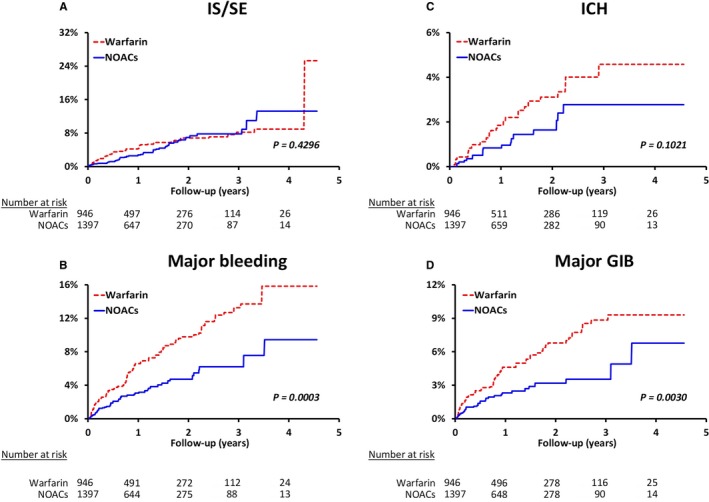
Cumulative incidence curves of IS/SE (A), all major bleeding (B), ICH (C), and major GI bleeding (D) for liver cirrhotic patients with nonvalvular atrial fibrillation according to initiated treatment after propensity score–based stabilized weighting. The NOAC group showed risk of IS/SE comparable to that of the warfarin group after adjustment. For the safety outcome, the NOAC group showed significantly lower risk of major GIB and all major bleeding than the warfarin group. GIB indicates gastrointestinal bleeding; ICH, intracranial hemorrhage; IS/SE, ischemic stroke/systemic embolism; NOAC, non–vitamin K antagonist oral anticoagulant.
After PSSWs, the annual incidence of IS/SE (3.2% versus 3.7% per year, P=0.4296) and ICH (1.0% versus 1.6% per year, P=0.1021) were comparable between the NOAC and warfarin groups. The NOAC group had significantly lower annual incidence of major GIB (1.9% versus 3.6% per year, P=0.0030) and all major bleeding (2.9% versus 5.4% per year, P=0.0003) compared with the warfarin group (Figure 2). Subgroup analysis was performed to determine whether different NOACs were superior to warfarin regarding the risk of IS/SE and bleeding among subgroups. No heterogeneity was obvious among the 3 NOAC groups (most P>0.05; Table 2). The subgroup analysis indicated that dabigatran showed a significantly lower risk of all major bleeding (2.9% versus 5.3% per year; HR: 0.54 [95% CI, 0.33–0.89]; P=0.0145) than warfarin. Rivaroxaban has a significantly lower risk of major GIB (1.6% versus 3.8% per year; HR: 0.38 [95% CI, 0.20–0.72]; P=0.0028) and all major bleeding (2.3% versus 5.7% per year; HR: 0.38 [95% CI, 0.23–0.65]; P=0.0004) than warfarin (Figure 3).
Figure 3.
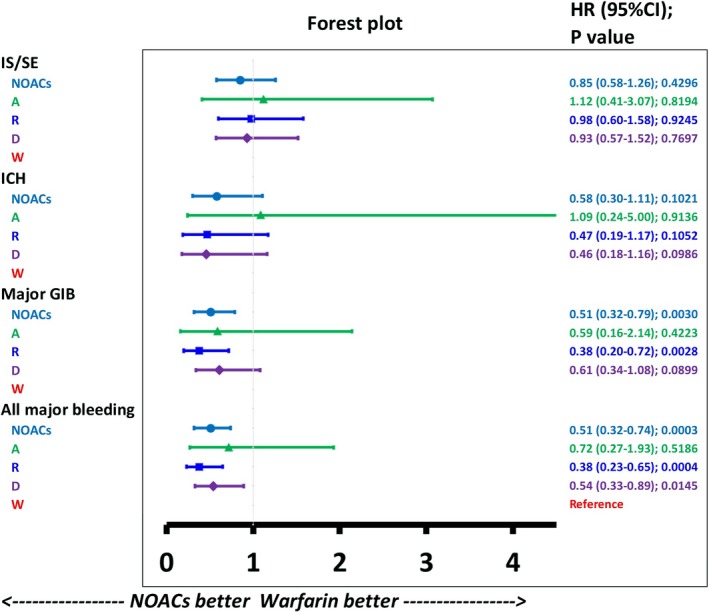
Forest plot of the hazard ratio for each NOAC vs warfarin among liver cirrhotic patients with NVAF taking oral anticoagulants. The NOAC group was associated with reduced risk of major GIB and all major bleeding compared with the warfarin group. Among NOACs, rivaroxaban was associated with lower risk of major GIB and all major bleeding than warfarin. Dabigatran was associated with lower risk of all major bleeding than warfarin. A indicates apixaban; D, dabigatran; GIB, gastrointestinal bleeding; HR, hazard ratio; ICH, intracranial hemorrhage; IS/SE, ischemic stroke/systemic embolism; NOAC, non–vitamin K antagonist oral anticoagulant; R, rivaroxaban; W, warfarin.
We also divided the cirrhotic patients taking oral anticoagulants into 2 subgroups: alcoholic versus nonalcoholic liver cirrhosis (Figure 4) and advanced versus nonadvanced liver cirrhosis (Figure 5). The definition of advanced liver cirrhosis was those cirrhotic patients who presented with any complications including ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, or esophageal varicose bleeding. In total, 222 (15%) and 143 (14%) patients with alcoholic liver cirrhosis were taking NOACs and warfarin, respectively. For those patients with alcoholic liver cirrhosis, NOACs had risks of thromboembolism and major bleeding comparable to those of warfarin. In the contrast, those patients with nonalcoholic liver cirrhosis taking NOACs had lower risks of major GIB (HR: 0.40 [95% CI, 0.24–0.68]; P=0.0006) and major bleeding (HR: 0.45 [95% CI, 0.29–0.69]; P=0.0002) than those taking warfarin (Figure 4). There were 271 (19%) and 273 (27%) patients with advanced liver cirrhosis taking NOACs and warfarin, respectively. For those patients with advanced liver cirrhosis, it is noted that the NOAC group had a lower risk of ICH (HR: 0.17 [95% CI, 0.03–0.96]; P=0.0449) than the warfarin group. For those patients with nonadvanced liver cirrhosis, the NOAC group had lower risk of major GIB (HR: 0.45 [95% CI, 0.26–0.78]; P=0.0045) and major bleeding (HR: 0.51 [95% CI, 0.33–0.79]; P=0.0027) than the warfarin group (Figure 5).
Figure 4.
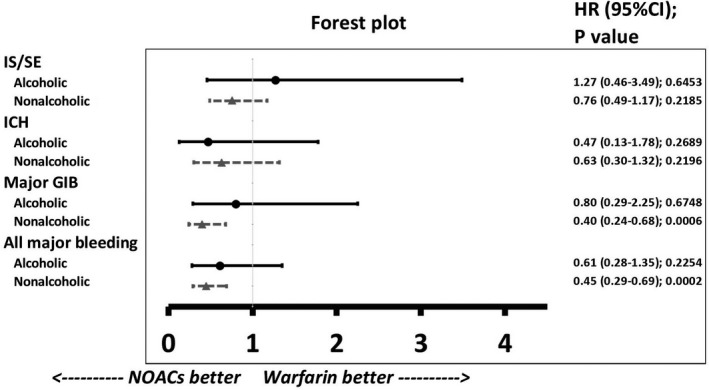
Forest plot of HRs for NOAC vs warfarin among patients with either alcoholic or nonalcoholic liver cirrhosis taking oral anticoagulants. In total, 222 (15%) and 143 (14%) patients with alcoholic liver cirrhosis were taking NOACs and warfarin, respectively. For those patients with alcoholic liver cirrhosis, the NOAC group has risks of thromboembolism and all major bleeding comparable to those of the warfarin group. For those patients with nonalcoholic liver cirrhosis, the NOAC group has lower risks of major GIB and all major bleeding than the warfarin group. GIB indicates gastrointestinal bleeding; HR, hazard ratio; ICH, intracranial hemorrhage; IS/SE, ischemic stroke/systemic embolism; NOAC, non–vitamin K antagonist oral anticoagulant.
Figure 5.
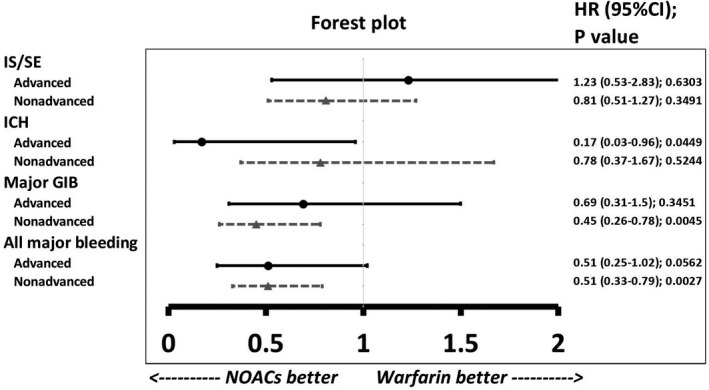
Forest plot of HRs for NOAC vs warfarin among patients with either advanced or nonadvanced liver cirrhosis taking oral anticoagulants. In total, 271 (19%) and 273 (27%) patients with advanced liver cirrhosis were taking NOACs and warfarin, respectively. For those patients with advanced liver cirrhosis, the NOAC group has lower risk of intracranial hemorrhage than the warfarin group. For those patients with nonadvanced liver cirrhosis, the NOAC group has lower risks of major GIB and all major bleeding than the warfarin group. GIB indicates gastrointestinal bleeding; HR, hazard ratio; ICH, intracranial hemorrhage; IS/SE, ischemic stroke/systemic embolism; NOAC, non–vitamin K antagonist oral anticoagulant.
Discussion
This is the first nationwide population‐based and large‐scale study to investigate the effectiveness and safety of NOACs versus warfarin in cirrhotic AF patients during a long following‐up period. Our results indicated that the NOAC group had risk of IS/SE similar to that of the warfarin group and lower risk of GIB and all major bleeding events. In subgroup analyses, dabigatran and rivaroxaban were associated with lower risk of all major bleeding events compared with warfarin. Furthermore, those patients with either nonalcoholic or nonadvanced liver cirrhosis taking NOACs were associated with lower risk of major GIB and all major bleeding than those patients taking warfarin.
The liver is a solid organ that produces most important factors involved in the coagulation and anticoagulation process, with the exception of von Willebrand factor, which is secreted by endothelial cells. Cirrhotic patients are considered to have higher risk of bleeding given decreased production of procoagulation factors II, V, VII, IX, X, and XI. Meanwhile, those patients are also prone to development of thrombosis given the decreased levels of antithrombin and anticoagulant proteins C and S and increased levels of procoagulation components factor VIII.1, 2, 3, 23 Furthermore, the coagulation laboratory tests commonly used to monitor the therapeutic effects of anticoagulants may not be useful in cirrhotic patients. Prolongation of prothrombin time and activated partial thromboplastin time are commonly found in cirrhotic patients. In contrast to prothrombin time and activated partial thromboplastin time, a lower level of antifactor Xa has been noted and correlates well with antithrombin in cirrhotic patients.24, 25
Warfarin is a vitamin K antagonist that inhibits the synthesis of vitamin K–dependent clotting factors including factor II, VII, IX, and X in the liver. In addition to several limitations, including low therapeutic index, multiple food–drug and drug–drug interactions, and a requirement for regular coagulation monitoring, warfarin use is challenging in cirrhotic patients with innately elevated international normalized ratio. It is well known that warfarin is involved in a complex pathway of drug metabolism by human cytochrome P 450 (CYP; CYP1A2, CYP2C9, and CYP3A4), which contributes a wide range of warfarin–drug interactions.26 Liver cirrhosis can affect the enzymes of warfarin metabolism.27 Instead, drug–drug interaction through CYP metabolism is generally not an important issue for NOACs except for rivaroxaban (66% CYP metabolism).28 Consequently, NOACs seem to have advantages over warfarin in cirrhotic AF patients. Apixaban and rivaroxaban directly inhibit Xa factor, and dabigatran directly inhibits thrombin (factor IIa). Those NOACs have proven to be safer and convenient alternatives to warfarin without requiring regular coagulation monitoring.7, 8, 9 However, cirrhotic patients were excluded from all large clinical trials of NOACs,7, 8, 9, 10 and little is known about the clinical outcomes of NOACs on cirrhosis AF patients. Until now, most data have been restricted to small retrospective clinical studies.29, 30, 31
Whether cirrhotic AF patients have to receive oral anticoagulants for prevention of thromboembolism remains uncertain. Several studies have indicated that cirrhotic AF patients have a higher risk of ischemic stroke than those without AF.6, 32 In addition to the risk of ischemic stroke, cirrhotic patients also have a higher risk of venous thromboembolism including pulmonary embolism, deep vein thrombosis, and splanchnic vein thrombosis.33 A previous study demonstrated that cirrhotic AF patients taking warfarin may have better clinical outcomes than those taking antiplatelet therapy or going without treatment.6 Furthermore, our present study demonstrated that in cirrhotic AF patients, NOACs have effectiveness similar to warfarin and a better safety profile. Nevertheless, further randomized and prospective studies are necessary to evaluate the effectiveness and safety of NOACs in this population with liver cirrhosis.
In subgroup analyses, patients treated with dabigatran or rivaroxaban, but not apixaban, were associated with a lower risk of major bleeding compared with those treated with warfarin. Several reasons may explain why apixaban did not have an advantage of lowering major bleeding events in cirrhotic AF patients. First, the apixaban group had a higher proportion of standard‐dose prescriptions compared with the dabigatran and rivaroxaban groups in the present study (31.0%, 11.2%, and 4.8%, respectively). Second, the apixaban group had a trend of lowering major bleeding events, and the small sample size of the apixaban group may be insufficient for statistical significance. Third, apixaban had a higher proportion of hepatobiliary and intestinal elimination (≈75–80%) and a lower proportion of renal elimination (≈20–25%) compared with dabigatran and rivaroxaban.34, 35, 36 Cirrhotic patients treated with apixaban may thus have an increased level of drug exposure, which may cause a higher risk of major bleeding. In addition, our study showed that the advantage of lower risk of major GIB or all major bleeding for NOACs was observed only in those patients with nonalcoholic or nonadvanced liver cirrhosis. A possibility is that the size of the sample with alcoholic or advanced liver cirrhosis may be insufficient for statistical significance. Nevertheless, our study indicated that NOACs may not have advantages over warfarin for those advanced cirrhotic patients with a worse condition that reduces drug‐metabolizing enzymes or impairs hepatobiliary excretion.
The present study had several limitations. First, NHIRD does not include important laboratory data for prognostic scores of liver cirrhosis, such as Child–Pugh scores.37 These nonmeasured covariates not included in PSSWs may affect our results. It remains unclear whether the grading of cirrhosis is associated with risk of thromboembolism in cirrhotic AF patients. A previous study reported that Child–Pugh score did not demonstrate a statistically significant difference between venous thromboembolism incidence in Child A versus Child B/C cirrhosis,38 in which it may be due to decreased levels of both pro‐ and anticoagulation factors to achieve rebalanced hemostasis, even in patients with advanced liver cirrhosis.1, 2, 3, 23 Second, although some studies reported that adjustment of NOAC dosage may not be necessary in patients with Child A cirrhosis, limited data are available for patients with Child B or C cirrhosis.29 Each physician's choice of treatment regarding to a specific NOAC and its dosage constitutes a major limitation of the present study. Third, the dominance of hepatitis B–related liver cirrhosis and a high prevalence of low‐dose NOAC prescription in cirrhotic Asian patients may result in a different outcome from that of cirrhotic non‐Asian patients.39 Asian patients have a higher risk of bleeding when taking warfarin compared with non‐Asian patients, and previous studies have indicated that NOACs may be more effective and safer in Asian than non‐Asian patients.40, 41, 42 Consequently, whether our results can be extrapolated to the non‐Asian population remains uncertain.
Conclusions
Our data indicated that NOACs may be an effective and safe alternative to warfarin among the Asian patients with AF complicated by liver cirrhosis, especially for those with nonalcoholic or nonadvanced liver cirrhosis. Thromboprophylaxis with low‐dose NOACs may be considered for such patients, and further prospective study is necessary to evaluate the effectiveness and safety of NOACs versus warfarin among the cirrhotic population.
Sources of Funding
This study was supported by grants 102‐2628‐B‐182‐011‐MY3, 102‐2314‐B‐182A‐053‐MY3, and 105‐2628‐B‐182A‐003‐MY3 from the Ministry of Science and Technology and CMRPG3B0991‐3, CMRPG3E1683, CMRPG3F0041, CMRPG3D1631, CMRPD1F0253, CMRPG3F0041, CMRPG3E0291, CMRPG3G1471, CMRPG3G1551‐3, and CLRPG3D0045 from the Chang Gung Memorial Hospital, Linkou, Taiwan.
Disclosures
None.
Supporting information
Table S1. International Classification of Disease, 9th and 10th Revisions, Clinical Modification (ICD 9‐CM and ICD 10‐CM) Codes Used to Define Comorbidities and Clinical Outcomes in the Study Cohort
Figure S1. Cumulative incidence curves of thromboembolism and bleeding for liver cirrhotic patients with nonvalvular atrial fibrillation according to initiated treatment before propensity score–based stabilized weighting.
Acknowledgments
The authors thank and acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0044) at Chang Gung Memorial Hospital for statistical consultation and data analysis. National Health Insurance Research Database data were provided by the Applied Health Research Data Integration Service from National Health Insurance Administration.
(J Am Heart Assoc. 2019;8:e011112 DOI: 10.1161/JAHA.118.011112.)
References
- 1. Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, de Franchis R, Colombo M, Mannucci PM. An imbalance of pro‐ vs anti‐coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–2111. [DOI] [PubMed] [Google Scholar]
- 2. Northup PG. Hypercoagulation in liver disease. Clin Liver Dis. 2009;13:109–116. [DOI] [PubMed] [Google Scholar]
- 3. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. [DOI] [PubMed] [Google Scholar]
- 4. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 5. Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuo L, Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Liao JN, Chung FP, Chen TJ, Lip GYH, Chen SA. Liver cirrhosis in patients with atrial fibrillation: would oral anticoagulation have a net clinical benefit for stroke prevention? J Am Heart Assoc. 2017;6:e005307 DOI: 10.1161/JAHA.116.005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 9. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 11. Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, Lee HF, Wang CL, Kuo CF, Kuo CT. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7:e008150 DOI: 10.1161/JAHA.117.008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagstrom H, Hoijer J, Marschall HU, Williamson C, Heneghan MA, Westbrook RH, Ludvigsson JF, Stephansson O. Outcomes of pregnancy in mothers with cirrhosis: a national population‐based cohort study of 1.3 million pregnancies. Hepatol Commun. 2018;2:1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D, Li AA, Perumpail RB, Cholankeril G, Gonzalez SA, Kim W, Ahmed A. Disparate trends in mortality of etiology‐specific chronic liver disease among Hispanic sub‐populations. Clin Gastroenterol Hepatol. 2018. Available at https://www.cghjournal.org/article/S1542-3565(18)31212-6/fulltext. Accessed February 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, Volk ML, Blow FC, Lok ASF. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872–882. [DOI] [PubMed] [Google Scholar]
- 15. Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, Tu HT, See LC. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68:1389–1401. [DOI] [PubMed] [Google Scholar]
- 16. Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, Wu LS, Tu HT, Kuo CT. Acute kidney injury in Asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68:2272–2283. [DOI] [PubMed] [Google Scholar]
- 17. Chan YH, Yen KC, See LC, Chang SH, Wu LS, Lee HF, Tu HT, Yeh YH, Kuo CT. Cardiovascular, bleeding, and mortality risks of dabigatran in Asians with nonvalvular atrial fibrillation. Stroke. 2016;47:441–449. [DOI] [PubMed] [Google Scholar]
- 18. Pamukcu B, Lip GY, Lane DA. Simplifying stroke risk stratification in atrial fibrillation patients: implications of the CHA2DS2‐VASc risk stratification scores. Age Ageing. 2010;39:533–535. [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 20. Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monroe DM, Hoffman M. The coagulation cascade in cirrhosis. Clin Liver Dis. 2009;13:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Fuentes A, Gordon‐Burroughs S, Hall JB, Putney DR, Monsour HP Jr. Comparison of anti‐Xa and activated partial thromboplastin time monitoring for heparin dosing in patients with cirrhosis. Ther Drug Monit. 2015;37:40–44. [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez‐Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;32:1465–1476. [DOI] [PubMed] [Google Scholar]
- 26. Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. [DOI] [PubMed] [Google Scholar]
- 27. Elbekai RH, Korashy HM, El‐Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5:157–167. [DOI] [PubMed] [Google Scholar]
- 28. Chiang CE, Wu TJ, Ueng KC, Chao TF, Chang KC, Wang CC, Lin YJ, Yin WH, Kuo JY, Lin WS, Tsai CT, Liu YB, Lee KT, Lin LJ, Lin LY, Wang KL, Chen YJ, Chen MC, Cheng CC, Wen MS, Chen WJ, Chen JH, Lai WT, Chiou CW, Lin JL, Yeh SJ, Chen SA. 2016 Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. [DOI] [PubMed] [Google Scholar]
- 29. Intagliata NM, Henry ZH, Maitland H, Shah NL, Argo CK, Northup PG, Caldwell SH. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61:1721–1727. [DOI] [PubMed] [Google Scholar]
- 30. Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98:393–397. [DOI] [PubMed] [Google Scholar]
- 31. Goriacko P, Veltri KT. Safety of direct oral anticoagulants versus warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100:488–493. [DOI] [PubMed] [Google Scholar]
- 32. Lai HC, Chien WC, Chung CH, Lee WL, Wu TJ, Wang KY, Liu CN, Liu TJ. Atrial fibrillation, liver disease, antithrombotics and risk of cerebrovascular events: a population‐based cohort study. Int J Cardiol. 2016;223:829–837. [DOI] [PubMed] [Google Scholar]
- 33. Dhar A, Mullish BH, Thursz MR. Anticoagulation in chronic liver disease. J Hepatol. 2017;66:1313–1326. [DOI] [PubMed] [Google Scholar]
- 34. Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81. [DOI] [PubMed] [Google Scholar]
- 35. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ganetsky M, Babu KM, Salhanick SD, Brown RS, Boyer EW. Dabigatran: review of pharmacology and management of bleeding complications of this novel oral anticoagulant. J Med Toxicol. 2011;7:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoteit MA, Ghazale AH, Bain AJ, Rosenberg ES, Easley KA, Anania FA, Rutherford RE. Model for end‐stage liver disease score versus Child score in predicting the outcome of surgical procedures in patients with cirrhosis. World J Gastroenterol. 2008;14:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012–3017. [DOI] [PubMed] [Google Scholar]
- 39. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 41. Lip GY, Wang KL, Chiang CE. Non‐vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. [DOI] [PubMed] [Google Scholar]
- 42. Wang KL, Lip GY, Lin SJ, Chiang CE. Non‐vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta‐analysis. Stroke. 2015;46:2555–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Disease, 9th and 10th Revisions, Clinical Modification (ICD 9‐CM and ICD 10‐CM) Codes Used to Define Comorbidities and Clinical Outcomes in the Study Cohort
Figure S1. Cumulative incidence curves of thromboembolism and bleeding for liver cirrhotic patients with nonvalvular atrial fibrillation according to initiated treatment before propensity score–based stabilized weighting.


