Abstract
Background
Long‐term clinical studies of peripartum cardiomyopathy (PPCM) are few. We aimed to measure the long‐term effect of PPCM on cardiac function in comparison with the long‐term effects of severe preeclampsia and uncomplicated pregnancy.
Methods and Results
A nationwide Danish cohort of women diagnosed with PPCM from 2005 to 2014 (PPCMgroup) were invited to participate in a clinical follow‐up study including maximal cardiopulmonary exercise testing and cardiac magnetic resonance imaging. Matched women with previous severe preeclampsia (preeclampsia group) and previous uncomplicated pregnancies (uncomplicated pregnancies group) served as comparison groups. A total of 84 women with 28 in each group participated. Median time to follow‐up after PPCM was 91 months. Most women (85%) in the PPCM group reported no symptoms of heart failure. Mean left ventricular ejection fraction in the PPCM group was normal at 62%, but significantly lower than in the preeclampsia group and the uncomplicated pregnancies group where mean left ventricular ejection fraction was 69% and 67%, respectively (P<0.0001). Women in the PPCM group also had impaired diastolic function with reduced left ventricular peak filling rate, left atrial passive emptying volume, and left atrial passive emptying fraction. Maximal exercise capacity (peak VO 2) was also reduced in the PPCM group compared with the preeclampsia group and the uncomplicated pregnancies group, and PPCM, high body mass index, and low left ventricular ejection fraction independently predicted reduced peak VO 2. Only 1 woman with PPCM had late gadolinium enhancement.
Conclusions
Women generally recovered left ventricular ejection fraction and were asymptomatic 7 years after PPCM, but had subtle diastolic dysfunction on cardiac magnetic resonance imaging and reduced peak VO 2. Focal myocardial fibrosis assessed with late gadolinium enhancement was, however, uncommon.
Keywords: diastolic function, heart failure, peripartum cardiomyopathy, preeclampsia, pregnancy
Subject Categories: Cardiomyopathy, Heart Failure, Preeclampsia
Clinical Perspective
What Is New?
In this first nationwide, long‐term clinical follow‐up study of women with previous peripartum cardiomyopathy, women generally recovered left ventricular ejection fraction and were clinically asymptomatic 7 years after diagnosis.
However, they had diastolic dysfunction, reduced maximal exercise capacity, and higher body mass index compared with 2 matched groups of women with either previous preeclampsia or uncomplicated pregnancies.
What Are the Clinical Implications?
Despite a symptomatic recovery, some degree of cardiac dysfunction might persist or relapse late after peripartum cardiomyopathy.
Whether this is useful to predict the risk of subsequent recurrence of heart failure remains unknown and should be explored in future studies of long‐term outcome.
Introduction
Peripartum cardiomyopathy (PPCM) is defined as idiopathic heart failure with left ventricular ejection fraction (LVEF) reduced below 45% in late pregnancy or in the first months after childbirth in women with no previous heart disease or other identifiable causes of heart failure.1 Incidence varies greatly worldwide, most likely reflecting differences in population ethnicity, awareness of the disease, and rigor of definition.2
PPCM is associated with hypertensive disorders of pregnancy (HDP), including chronic hypertension, gestational hypertension, and preeclampsia.3 Recent studies report an incidence of concomitant HDP in nearly half of PPCM cases.4, 5, 6, 7 This clinical association may be explained by shared pathophysiological mechanisms such as increased levels of soluble Fms‐like tyrosine kinase (sFlt‐1).8, 9 Higher sFlt‐1 levels seem to correlate with severity of both preeclampsia10 and PPCM,11 and echocardiographic studies have also demonstrated subclinical cardiac dysfunction in women with preeclampsia,12 leading to the hypothesis that preeclampsia and PPCM represent a spectrum of disease.2, 8 Some PPCM studies have reported better 6‐ to 12‐month outcome associated with concomitant HDP,5, 6, 13 but the clinical and prognostic implications of concomitant HDP are not clear: In the multicenter IPAC (Investigations in Pregnancy‐Associated Cardiomyopathy) cohort, hypertension did not predict LVEF at 12‐month follow‐up,4 whereas Lindley et al recently noted increased morbidity and mortality among PPCM women with concomitant preeclampsia compared with PPCM women without preeclampsia.7 The impact of concomitant HDP on long‐term outcome is unknown.
We hypothesized that women with previous PPCM and women with previous severe preeclampsia have graded degrees of cardiovascular dysfunction at long‐term follow‐up compared with women with uncomplicated pregnancies. We aimed to invite all women diagnosed with PPCM in Denmark from 2005 to 2014 to a clinical follow‐up study in order to measure (1) systolic and diastolic cardiac function using cardiac magnetic resonance imaging (CMR) and (2) exercise capacity defined as peak oxygen consumption (peak VO2) during maximal exercise testing, and compare the findings with 2 age‐matched groups of women with either previous severe preeclampsia or previous uncomplicated pregnancies.
Methods
Study Population
Three groups of age‐matched women were invited by letter to participate:
Women with previous PPCM (PPCM group).
Women with a history of severe preeclampsia without cardiac complications (PE group).
Women with a history of uncomplicated pregnancies (UCP group).
The PPCM group was recruited from a nationwide Danish cohort of 61 women with a validated PPCM diagnosis during 2005–2014, as previously described.6 Women in the PE group and the UCP group were identified in an obstetric database that covers deliveries in the Capital Region of Denmark and accounts for approximately one third of all deliveries in Denmark.14 These women either had an International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis of severe preeclampsia (O14.1) or no diagnoses that indicated complications during their past pregnancy.
For women in the PE group who agreed to participate, we reviewed charts to preclude heart failure or other cardiac complications and validate the diagnosis according to the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy.3 Women in the UCP group verbally confirmed the database information.
The study complies with the Declaration of Helsinki, and the protocol was approved by the Danish Data Protection Agency (RH‐2016‐174, I‐Suite 04729) and the Capital Region's Committee on Biomedical Research Ethics (H‐1‐2014‐131). All participants provided written informed consent.
The data, analytical methods, and study materials will not be made publicly available to other researchers for purposes of reproducing the results or replicating the procedure.
Experimental Design and Procedures
All measurements were performed during 1 visit 2 to 11 years after the index delivery. In sequential order, urine and nonfasting venous blood samples were collected and height, weight, and blood pressure were measured. An ECG, exercise testing, and CMR were then performed. After exercise testing, the participating women were offered a meal and at least 2 hours of rest before CMR.
Exercise Testing
Maximal cardiopulmonary exercise test, using an upright exercise bicycle (Ergoselect; Ergoline, Windhagen, Germany), was performed. After a period of unloaded cycling, the workload, starting at 50 W, was sequentially increased by 50 W every 2 minutes at speeds of 60 to 80 revolutions per minute. During the exercise test, women were monitored with 12‐lead ECG. The anaerobic threshold was defined as the time at which the respiratory exchange ratio exceeded 1 without dropping to levels below 1 during the remaining time of exercise, and respiratory exchange ratio >1 was used as an indicator of an adequately performed test. Respiratory gas analysis was performed using the breath‐by‐breath technique (CS‐200 Ergospiro, Schiller AG, Baar, Switzerland). At exhaustion, women rated the perceived exertion on the Borg scale,15 maximal workload was noted, and peak oxygen consumption (peak VO2) was calculated as mL/kg/min (Standard Pressure Temperature Dry).
Cardiac Magnetic Resonance Imaging
CMR was performed using a 1.5 Tesla magnetic resonance scanner (GE Optima MR450; GE Healthcare, Waukesha, WI). Steady‐state free precession, end‐tidal breath‐hold images were obtained in the 2‐, 3‐, and 4‐chamber views as well as a transaxial and a short‐axis cine stack covering the whole heart with no gaps (slice thickness 8 mm, echo time 1.6 ms, field of view 320 to 370 mm, resolution matrix 256 × 256 mm, and 25 phases per cardiac cycle). Ten minutes after an intravenous bolus of gadobutrol (0.15 mmol/kg body weight, Gadovist; Bayer HealthCare AG, Leverkusen, Germany), the short‐ and long‐axis views were repeated using a T1‐weighted inversion recovery gradient echo sequence to demonstrate late gadolinium enhancement (slice thickness 8 mm, echo time 2.9 ms, inversion time 300–375 ms, field of view 320–400 mm, and resolution matrix 256×256 mm). Inversion time was continuously adjusted to null the myocardial signal.
Offline image analysis was performed using semiautomated CMR software (cvi42; Circle Cardiovascular Imaging, Calgary, AB, Canada) for manual tracing of the epi‐ and endocardial borders. For segmentation of the left ventricle (LV), papillary muscles were excluded from LV mass and LV blood pool.16 LV end‐systolic volume, LV end‐diastolic volume (LVEDV), LV peak filling rate (LVPFR), LV stroke volume, and LVEF were read from the LV volume‐time curve.
Manual tracing of the right ventricle endocardial borders on the transaxial cine stack in end‐systole and end‐diastole were used for determination of right ventricular end‐systolic volume, right ventricular end‐diastolic volume, and right ventricular ejection fraction.
In the segmentation of the left atrium (LA), the left atrial appendage was included, the pulmonary veins were excluded,17 and the mitral valve annulus was defined as the inferior LA border.18 From the transaxial cine stack, the LA volume‐time curve was constructed to asses LV diastolic function. This enabled determination of LA passive emptying volume (LAPEV), LA active emptying volume, LA passive emptying fraction (LAPEF), and LA active emptying fraction19 (Figure S1).
All volumes and LV mass were indexed to body surface area according to the Mosteller method.
Focal late gadolinium enhancement (LGE) was considered as myocardial areas of high signal intensity (>5 SDs) and required confirmation in 2 orthogonal planes.20
LVEF estimated by echocardiography ≈12 months after diagnosis was available from chart reviews that were part of the parent cohort study,6 where complete recovery was defined as LVEF ≥55% 12 months after the initial diagnosis. Improvement in LVEF from 12 months after diagnosis to participation was defined as ≥10% increase in LVEF. Correspondingly, deterioration was defined as ≥10% decrease in LVEF at participation.
Statistical Analyses
Data are presented as numbers and proportions for categorical data, as means and SDs for normally distributed continuous data, and as medians and ranges for non‐normally distributed continuous data. Categorical data were compared between groups using chi‐square test or Fisher's exact test, as appropriate. Continuous characteristics and outcome were compared between the 3 study groups using the ANOVA test in the case of normal distribution and the Kruskal–Wallis test in the case of non‐normal distribution. Continuous data were compared between 2 groups (all women with PPCM included in the main cohort by participation in the follow‐up study or not, and participating women in the PPCM group by concomitant HDP or not) by t test or Mann–Whitney U test, as appropriate. P<0.05 was considered statistically significant.
Some post‐hoc analyses were made: First, we performed multiple comparisons of outcome variables that were significant in the global ANOVA or Kruskal–Wallis tests using chi‐square test, Fisher's exact test, Student t test, or Mann–Whitney U test, as appropriate. To correct for multiple comparisons, the Bonferroni method was applied and level of significance in these post‐hoc analyses was defined as 0.05 divided by the number of comparisons per outcome (3) as we compared the PPCM group with the PE group and the UCP group, respectively, as well as the PE group with the UCP group, and by the number of dependent outcome variables examined (20). Thus, level of significance was defined as 0.05/(3×20)=0.0008. Second, analysis of covariance of key outcome variables was performed in order to report differences in means between the 3 study groups, adjusted for body mass index (BMI) and age. Furthermore, a multiple linear regression analysis was performed to assess the effect of selected candidate variables of clinical importance with a likely impact on peak VO2 among all women, who completed the exercise test. The variables chosen were: time to follow‐up, BMI at follow‐up, age at follow‐up, current use of beta‐blockers, time spent on exercise weekly, LVEF at follow‐up, and, as a marker of diastolic function, LVPFR/LVEDV ratio. We included only 1 marker of diastolic function to avoid colinearity, and we chose LVPFR/LVEDV ratio, because it is more easily obtained and clinically accessible than markers such as LAPEV and LAPEF that both require recordings and analysis of LA images.
Author A.S.E. had full access to all data and takes responsibility for its integrity and the data analysis. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the authors at the Center for Pregnancy and Heart Disease, Copenhagen University Hospital Rigshospitalet (Copenhagen, Denmark) at ajoe0026@regionh.dk or nvej0001@regionh.dk.
All statistical analyses were performed using SAS Enterprise Guide software (version 7.1; SAS Institute Inc, Cary, NC).
Results
Of 61 women in the parent PPCM cohort, 2 had died, both within a year after the diagnosis, and another was lost to follow‐up because of emigration. Of the remaining 58 women, 28 agreed to participate (48%). Among eligible women with PPCM, more participating women were of white race, whereas disease severity and incidence of major adverse events did not differ significantly between participants and decliners (Table S1). A total of 94 women with previous preeclampsia were invited by letter in order to recruit 28 participants in this PE group (30%), and 129 women with a previous uncomplicated pregnancy were invited in order to recruit 28 women in this UCP group (22%). The 3 groups of participants did not differ significantly from one another in terms of age, race, smoking habits, comorbidities, self‐reported exercise routines, or time from index delivery to follow‐up (Table 1 and Table S2). Median time to follow‐up from index delivery was 91 months for the PPCM group, 95 months for the PE group, and 101 months for the UCP‐group. There were more nulliparous women, more caesarean deliveries, and earlier mean gestational age at time of delivery in the PE group, whereas fewer women in the PPCM group started breastfeeding and breastfed for a shorter period of time. Women in the PPCM group had higher BMI and reported less time spent on exercise at follow‐up compared with the other 2 groups. They also used more antihypertensive/heart failure medications: 11 women (43%) were still on daily medication at study participation. No women in the PPCM group received bromocriptine therapy as previously reported.
Table 1.
Distribution of Baseline Characteristics Among All Participants in the Index Pregnancy and at Study Participation
| Peripartum Cardiomyopathy, n=28 | Preeclampsia, n=28 | Controls, n=28 | P Valuea | |
|---|---|---|---|---|
| Index pregnancy characteristics | ||||
| Age at delivery, y | 30.7 (6.0) | 30.5 (5.0) | 31.0 (5.2) | 0.73 |
| Race, n (%) | ||||
| White | 28 (100) | 28 (100) | 27 (96) | 0.364 |
| Black | 0 | 0 | 1 (4) | |
| Body mass index, kg/m2 | 28.3 (6.4) | 22.8 (3.2) | 21.3 (1.8) | <0.0001 |
| Concomitant HDP, n (%) | ||||
| Gestational hypertension | 2 (7) | 0 | 0 | |
| Preeclampsia | 11 (39) | 28 (100) | 0 | |
| HELLP | 2 (7) | 0 | 0 | |
| Follow‐up characteristics | ||||
| Age, y | 38.0 (6.9) | 39.1 (5.3) | 38.8 (5.6) | 0.754 |
| Median time from index delivery to follow‐up (range), mo | 91 (227–137) | 95 (26–143) | 101 (25–146) | 0.603 |
| Engaged in exercise, n (%) | 20 (71) | 26 (93) | 25 (89) | 0.060 |
| Median weekly exercise (range), h | 2 (0–14) | 5.5 (0–20) | 4 (0–8) | 0.031 |
| NYHA class, n (%) | ||||
| I | 24 (86) | 28 (100) | 28 (100) | |
| II | 3 (11) | 0 | 0 | 0.078 |
| III | 1 (3) | 0 | 0 | |
| Current antihypertensive/heart failure medicationb, n (%) | 13 (46) | 3 (11) | 0 | <0.0001 |
| Body mass index, kg/m2 | 30.0 (8.4) | 23.3 (4.1) | 22.6 (3.0) | <0.001 |
Data are presented as means±SDs, unless otherwise stated. HDP indicates hypertensive disorders of pregnancy; HELLP, hemolysis elevated liver enzymes low platelets syndrome; NYHA, New York Heart Association.
Global analyses of difference between means, medians, and proportions across the 3 groups were performed by ANOVA, Kruskal–Wallis, or chi‐square test, respectively.
Daily antihypertensive/heart failure medications: angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, beta‐blockers, calcium antagonists, and diuretics.
Eight women in the PPCM group (29%) had ECG abnormalities at follow‐up (Table S3). A normal ECG did not exclude the cardiac functional abnormalities described below.
Exercise Testing
Four women in the PPCM‐group did not participate in exercise testing because of extreme obesity (BMI >60 kg/m2), physical inability caused by concomitant multiple sclerosis developed after PPCM onset, white coat hypertension with in‐hospital resting systolic blood pressure >200 mm Hg, and technical problems with the equipment. Also, 1 woman in the UCP group did not complete the exercise test because of technical problems. The 2 women who experienced technical problems were offered new appointments to redo the test, but were both unable to attend a new appointment within the study period. Overall, women in the PPCM group had significantly lower peak VO2 compared with women in the PE group and the UCP group (Table 2). Mean peak VO2 was 29.6, 43.2, and 45.4 mL/kg/min, respectively (P<0.0001). Despite an overall high perceived exertion on the Borg scale, not all women reached the anaerobic threshold. Among women who reached the anaerobic threshold, peak VO2 was still significantly lower in the PPCM group: Mean peak VO2 was 29.1, 43.5, and 46.0 mL/kg/min, respectively (P<0.0001). After adjusting for BMI and age, this difference was attenuated (P=0.071; Table 3). Additional adjustment for amount of time spent on exercise weekly did not change the result (analysis not shown).
Table 2.
Exercise Testing and Cardiac Magnetic Imaging Findings at Study Participation
| Peripartum Cardiomyopathy | Preeclampsia | Controls | P Valuea | |
|---|---|---|---|---|
| Exercise testing | n=24 | n=28 | n=27 | |
| Peak VO2, mL/kg/min | 29.6 (7.2)b, c | 43.2 (11.1) | 45.4 (10.2) | <0.0001 |
| Heart rate at rest, bpm | 72 (17) | 68 (7) | 69 (10) | 0.418 |
| Heart rate at peak, bpm | 168 (19) | 167 (19) | 176 (16) | 0.185 |
| Systolic BP at rest, mm Hg | 129 (16) | 129 (16) | 119 (11) | 0.019 |
| Diastolic BP at rest, mm Hg | 83 (14) | 82 (10) | 73 (9) | 0.007 |
| Systolic BP at peak, mm Hg | 185 (38) | 182 (30) | 179 (21) | 0.759 |
| Diastolic BP at peak, mm Hg | 92 (27) | 94 (18) | 99 (23) | 0.571 |
| Perceived exertion, Borg scale | 18 (1) | 18 (1) | 17 (1) | 0.752 |
| Respiratory exchange ratio | 1.04 (0.14) | 1.03 (0.11) | 1.00 (0.11) | 0.496 |
| Peak workload, W | 179 (30) | 208 (34) | 207 (37) | 0.004 |
| Cardiac magnetic resonance imaging | n=25 | n=27 | n=27 | |
| Left ventricular parameters | ||||
| Left ventricular ejection fraction, % | 62 (6)b, c | 69 (4) | 67 (5) | <0.0001 |
| LVEDV, mL/m2 | 84 (14) | 78 (10) | 80 (10) | 0.233 |
| Left ventricular end‐systolic volume, mL/m2 | 31 (7) | 25 (8) | 27 (6) | 0.008 |
| Median left ventricular mass (range), g/m2 | 62 (43–143) | 60 (48–86) | 57 (44–74) | 0.205 |
| LVPFR, mL/s per m2 | 229 (49) | 276 (57) | 265 (45) | 0.005 |
| Left atrial volumes | ||||
| Left atrial passive emptying volume, mL/m2 | 13 (5)b, c | 19 (4) | 20 (3) | <0.0001 |
| Left atrial active emptying volume, mL/m2 | 11 (4) | 9 (2) | 9 (2) | 0.129 |
| LVPFR/LVEDV ratio | 2.8 (0.6)b | 3.5 (0.6) | 3.2 (0.6) | <0.0001 |
| Left atrial passive emptying fraction, % | 34 (10) | 40 (8) | 42 (8) | 0.002 |
| Left atrial active emptying fraction, % | 38 (9) | 35 (9) | 35 (8) | 0.359 |
Data are presented as means±SDs, unless otherwise stated. BP indicates blood pressure; LVEDV, left ventricular end‐diastolic volume indexed to body surface area; LVPFR, left ventricular peak filling rate indexed to body surface area.
Global analyses of difference between means, medians, and proportions across the 3 groups were performed by ANOVA, Kruskal–Wallis, or chi‐square test, respectively. P<0.05 was considered statistically significant.
PPCM group significantly different compared with the preeclampsia group. Post‐hoc analyses were performed by Student t test, Mann–Whitney U test, or chi‐square test, as appropriate. P<0.05/(3×20)=0.0008 was considered statistically significant.
PPCM group significantly different compared with the uncomplicated control group. Post‐hoc analyses were performed by Student t test, Mann–Whitney U test, or chi‐square test, as appropriate. P<0.05/(3×20)=0.0008 was considered statistically significant.
Table 3.
Difference in Means (95% Confidence Interval) of Key Outcome Variables in the 3 Study Groups Adjusted for Body Mass Index and Age at Follow‐up
| UCP Group (Reference) | PE Group | PPCM Group | P Value | |
|---|---|---|---|---|
| Peak VO2, mL/kg/min | ··· | −0.34 (−4.92 to 4.23) | −6.17 (−11.87 to −0.47) | 0.071 |
| Left ventricular ejection fraction, % | ··· | 2.11 (−0.41 to 4.63) | −7.31 (−10.51 to −4.11) | <0.0001 |
| Left ventricular peak filling rate, mL/s/m2 | ··· | 23.83 (−3.52 to 51.18) | −14.33 (−49.03 to 20.36) | 0.053 |
| LVPFR/LVEDV ratio | ··· | 0.31 (−0.01 to 0.62) | −0.56 (−0.95 to −0.17) | <0.0001 |
| Left atrial passive emptying volume, mL/m2 | ··· | −1.13 (−3.43 to 1.18) | −4.92 (−7.82 to −2.02) | 0.004 |
| Left atrial passive emptying fraction, mL/m2 | ··· | −1.66 (−6.33 to 3.01) | −6.79 (−12.66 to −0.91) | 0.074 |
| Systolic blood pressure at rest, mm Hg | ··· | 9.87 (2.48–17.3) | 5.75 (−3.47 to 15.0) | 0.034 |
| Diastolic blood pressure at rest, mm Hg | ··· | 8.36 (2.47–14.24) | 7.22 (−0.12 to 14.55) | 0.017 |
LVEDV indicates left ventricular end‐diastolic volume; LVPFR, left ventricular peak filling rate; PE, preeclampsia; PPCM, peripartum cardiomyopathy; UCP, uncomplicated pregnancy.
Cardiac Magnetic Resonance Imaging
Three women in the PPCM group did not undergo CMR: 2 had implantable cardioverter defibrillator units incompatible with magnetic resonance imaging and 1 because of claustrophobia. Both in the PE group and in the UCP group 1 participant also could not complete the CMR protocol because of claustrophobia. The 25 women in the PPCM group, who underwent CMR, had lower mean LVEF compared with the other 2 study groups: Mean LVEF was 62% in the PPCM group, 69% in the PE group, and 67% in the UCP group (P<0.0001; Table 2).
Out of the 25 women in the PPCM group who underwent CMR, 9 (36%) had further improved their LVEF after 12 months, 16 women (64%) had a stable LVEF, and no one deteriorated. The 3 women in the PPCM group, who did not undergo CMR, all had an available echocardiographic LVEF assessment within 6 months from their study participation: The 2 women with implantable cardioverter defibrillator units both had a reduced LVEF of 45% and 20%, respectively, whereas the third woman had an LVEF of 55%.
Mean LVEF at baseline, after 12 months and at last follow‐up (study CMR or echocardiography within 6 months before study enrollment), was 27±9%, 52±9%, and 60±10%, respectively (Figure).
Figure 1.
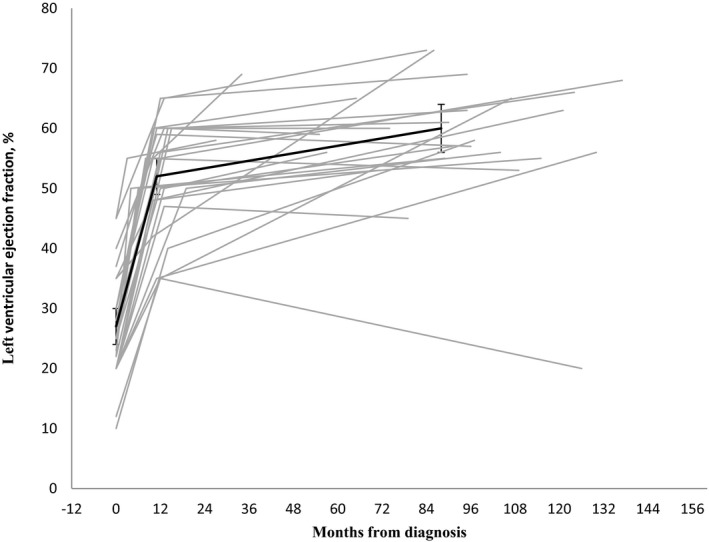
Changes in left ventricular ejection fraction. Left ventricular ejection fraction at diagnosis, at ≈12 months and at study visit among 28 women with peripartum cardiomyopathy. Mean with 95% confidence interval in bold.
Diastolic left ventricular function was affected in women in the PPCM group because both mean LVPFR and mean LAPEV were lower in this group. Mean LVPFR was 229 mL/s/m2 in the PPCM group, 276 mL/s/m2 in the PE group, and 265 mL/s/m2 in the UCP group (P=0.005). Mean LAPEV was 13, 19, and 20 mL/m2, respectively (P<0.0001). Also, LVPFR/LVEDV ratio was significantly lower in the PPCM group (Table 2). After adjusting for BMI and age, differences in mean systolic (LVEF) and some diastolic functional parameters (LVPFR/LVEDV ratio and LAPEV) remained significantly different between the 3 groups (Table 3).
In a multiple linear regression analysis of peak VO2 among all 79 women who completed the maximal exercise test, BMI, PPCM, and LVEF all independently predicted peak VO2 after adjusting for time to follow‐up, age, current use of beta‐blockers, amount of time spent on exercise weekly, and LVPFR/LVEDV ratio (Table 4).
Table 4.
Multiple Linear Regression Analysis of Predictors of Maximal Exercise Capacity (Peak VO2) Among All Women Who Completed the Maximal Exercise Test at Study Participation (N=79)
| β‐Valuea | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Time to follow‐up, mo | 0.010 | −0.056 to 0.077 | 0.754 |
| Age at follow‐up, y | −0.330 | −0.745 to 0.085 | 0.117 |
| Body mass index at follow‐up, kg/m2 | −1.228 | −1.742 to −0.7114 | <0.0001 |
| Study group | |||
| PPCM group | −11.269 | −18.257 to −4.282 | 0.008 |
| PE group | −0.999 | −5.835 to 3.838 | |
| UCP group | Reference | ||
| Current use of beta‐blockers | 4.455 | −3.817 to 12.728 | 0.286 |
| Time spent weekly on exercise, h | 0.264 | −0.283 to 0.881 | 0.339 |
| Left ventricular ejection fraction, % | −0.517 | −1.003 to −0.032 | 0.037 |
| LVPFR/LVEDV ratio | 0.990 | −2.926 to 4.905 | 0.615 |
LVEDV indicates left ventricular end‐diastolic volume; LVPFR, left ventricular peak filling rate; PE, preeclampsia; PPCM, peripartum cardiomyopathy; UCP, uncomplicated pregnancy.
The β‐value represents the slope of the linear regression or the number of units the outcome variable (peak VO2) change with a 1‐unit change in the predictor variable.
Only 1 woman with PPCM (4%) and no women in the other 2 groups had LGE. The woman who had LGE had concomitant preterm preeclampsia and a baseline LVEF of 35%, improving to 45% after 7 months and 73% after 42 months. LGE pattern was multifocal with both midwall, transmural, and epicardial foci. The left ventricle was moderately dilated (LVEDV index 119 mL/m2), and left myocardial mass was hypertrophic (mass index of 143 g/m2), but wall thickness was nowhere ≥15 mm, and the woman did not meet the criteria for hypertrophic cardiomyopathy. She had no family history of cardiomyopathy and no initial major adverse events in terms of need of inotropic therapy, mechanical circulatory support, or prolonged need for intensive care, but had a stroke 2 years after the PPCM diagnosis.
Right ventricular volumes and systolic function did not differ between the 3 groups (Table S3).
There were 15 participants in the PPCM group who had concomitant HDP (54%) and 13 who did not (46%). Of the 15 women with concomitant HDP, 12 had preeclampsia and 3 had gestational hypertension. Women who had concomitant HDP had significantly higher systolic and diastolic blood pressure at rest. They also had significantly higher LA active emptying fraction, and LAPEF was correspondingly lower, but this did not reach statistical significance (Table 5).
Table 5.
Characteristics and Clinical Findings in Women With Peripartum Cardiomyopathy by HDP in the Index Pregnancy
| HDP | No HDP | P Value | |
|---|---|---|---|
| Characteristics | n=15 | n=13 | |
| LVEF at diagnosis, % | 29 (7) | 24 (0) | 0.113 |
| Median time from index delivery to follow‐up (range), mo | 90 (26–143) | 104 (25–156) | 0.083 |
| Age at follow‐up, y | 37 (7) | 39 (7) | 0.470 |
| Median weekly exercise (range), h | 3 (1–14) | 1 (0–10) | 0.410 |
| NYHA functional class at follow‐up, n (%) | |||
| I | 13 (87) | 11 (84) | |
| II | 2 (13) | 1 (8) | 0.506 |
| III | 0 | 1 (8) | |
| Current antihypertensive/heart failure medication, n (%) | 5 (33) | 7 (54) | 0.274 |
| Body mass index at follow‐up, kg/m2 | 28.3 (1.2) | 30.1 (1.3) | 0.483 |
| Exercise testing | n=13 | n=11 | |
| Peak VO2, mL/kg/min | 29 (7) | 31 (8) | 0.456 |
| Systolic BP at rest, mm Hg | 135 (16) | 121 (14) | 0.019 |
| Diastolic BP at rest, mm Hg | 90 (13) | 74 (10) | 0.0008 |
| Cardiac magnetic resonance imaging | n=14 | n=11 | |
| LVEF, % | 63 (6) | 61 (6) | 0.460 |
| LVEDV, mL/m2 | 82 (15) | 86 (12) | 0.538 |
| Left ventricular end‐systolic volume, mL/m2 | 29 (6) | 34 (8) | 0.153 |
| Median left ventricular mass (range), g/m2 | 62 (43–143) | 62 (47–75) | 0.784 |
| LVPFR, mL/min/m2 | 224 (48) | 236 (51) | 0.535 |
| Left passive atrial emptying volume, mL/m2 | 11.8 (5) | 15.6 (5) | 0.083 |
| Left atrial active emptying volume, mL/m2 | 12 (4) | 10 (3) | 0.238 |
| LVPFR/LVEDV ratio | 2.8 (0.6) | 2.8 (0.7) | 0.909 |
| Left atrial passive emptying fraction, % | 32 (10) | 36 (8) | 0.334 |
| Left atrial active emptying fraction, % | 42 (8) | 34 (9) | 0.043 |
Data are presented as means±SDs, unless otherwise stated. BP indicates blood pressure; HDP, hypertensive disorders of pregnancy; LVEDV, left ventricular end‐diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVPFR, left ventricular peak filling rate indexed to body surface area; NYHA, New York Heart Association; RER, respiratory exchange ratio.
In a subanalysis, we compared peak VO2 and diastolic and systolic functional parameters among those women diagnosed with PPCM who reported to be free from heart failure symptoms at study participation (New York Heart Association class I, n=24) with the 2 other groups. Differences in peak VO2, LVEF, LVPFR, LAPEV, and LAPEF persisted, whereas LVEDV did not differ between the 3 groups in this analysis (not shown).
Discussion
In this Danish nationwide study of the long‐term effect of PPCM on cardiac structure and function, we observed a high rate of recovery of LV systolic function with a mean LVEF of 62% and the majority of patients (85%) reporting to be free from heart failure symptoms. Women in the PPCM group, however, had evidence of left ventricular diastolic dysfunction and much lower exercise capacity compared with those in the PE group and in the UCP group. PPCM, high BMI, and low LVEF were independent predictors of reduced peak VO2 overall. LGE was uncommon in this cohort of women with PPCM and only noted in 1 of 25.
The notion of delayed recovery beyond the early phase is in accord with other studies, showing further increased recovery rates after 12 compared with 6 months4, 13 and, in 1 study, an average time to recovery of 19 months.21 Barasa et al recently reported on 24 Swedish women with PPCM and a mean follow‐up time for echocardiography of 2.1 years, and found that the majority recovered with 54% early recovery before 100 days after diagnosis and further 21% late recovery.22
In a study of 71 Chinese PPCM women with a mean time to follow‐up of 43±33 months, 44% had persistently left ventricular systolic dysfunction at follow‐up.23 In our study, we noted a higher recovery rate with only 2 of 28 women (7%) having LVEF <50% after a median of 91 months, but similar to Li et al, we did not observe any differences in long‐term LV systolic function between women with and without concomitant HDP. In an American cohort of 39 predominantly black women with PPCM, concomitant preeclampsia was identified in 44% and was associated with higher 1‐year mortality and more hospital readmissions.7 This contrasts with the findings in our primarily white parent PPCM cohort, where all major adverse events occurred in women without HDP.6 Despite the higher early mortality and morbidity observed by Lindley et al, they found a higher mean LVEF at 1‐year follow‐up in the group with concomitant preeclampsia whereas persistent diastolic dysfunction was common in both groups, similar to our findings.
In order to explain these conflicting reports on the effect of concomitant preeclampsia on PPCM, race and genetics must be taken into account. It has previously been shown that race affects outcome and severity of both preeclampsia and PPCM, with black women experiencing more‐severe disease.4, 24, 25 Mutations in the TTN gene, encoding the cardiac sarcomere protein, titin, have been found in a subgroup of 15% of women with PPCM, predominantly in black women who did not have concomitant HDP. Black women with TTN mutations had a lower LVEF after 12 months compared with black women without TTN mutations, whereas this difference was not observed among women of white descent.25 The different impact of concomitant HDP observed in the 2 studies of primarily black and white women thus could reflect different genetic susceptibilities to, for example, sFlt‐1–mediated endothelial injury.8
In our study, subtle diastolic dysfunction was present in the setting of LVEF recovery. Residual myocardial injury correlating modestly with sFlt‐1 levels have been described previously.26 Whether persistent diastolic dysfunction as assessed by CMR is related to sFlt‐1 and other biomarkers associated with PPCM and HDP is currently unknown.
Exercise capacity was significantly reduced in women in the PPCM group, who largely reported to be free from heart failure symptoms. Also, physical activity was lower and BMI higher in the PPCM group compared with women in the PE group and in the UCP group. This may reflect exercise intolerance attributed to diastolic dysfunction, which may again precede symptomatic heart failure with preserved ejection fraction. Heart failure with preserved ejection fraction occurs more often in women and is thought to be the result of a systemic proinflammatory state with microvascular endothelial inflammation similar to that of preeclampsia.9, 27 Diastolic dysfunction expressed as reduced LAPEF has previously been noted in heart failure with preserved ejection fraction patients,28 and diastolic dysfunction further correlates with exercise capacity.29 This supports the hypothesis that the observed reduced exercise capacity in PPCM women could be related to the diastolic dysfunction observed.
BMI, a well‐known predictor of exercise capacity, also in our study proved to be an independent predictor of exercise capacity and was significantly increased in the PPCM group, both at the beginning of the index pregnancy and at study follow‐up. We were unable to match the control groups with the PPCM group on BMI, but in a recent study from Finland, previously sedentary women with a mean BMI of 26 kg/m2 underwent exercise testing after a 9‐week exercise intervention.30 In terms of BMI, this study population may be a better comparator to our PPCM group, who reported a median of 2 hours of weekly exercise. Mean peak VO2 was ≈38 mL/kg/min in the Finnish group, which is less than our UCP group but still 25% higher than the PPCM group.
Overweight has not traditionally been listed as a risk factor for PPCM,1, 2 but in a nationwide Swedish study, BMI was higher in women with PPCM compared with controls,5 and mean BMI was also above the normal range (26.4 kg/m2) in women from the worldwide EURObservational Research Programmes's PPCM registry.31
Echocardiography studies have repeatedly demonstrated diastolic, and, to some extent, systolic dysfunction in preeclamptic women and this cardiac impairment may persist or relapse several years postpartum.12, 32 This is the first study to incorporate CMR in this patient group. CMR is more accurate in terms of volumetric measurements compared with echocardiography,33 but even with CMR we were unable to detect any statistically significant differences in neither systolic nor diastolic function between the PE group and the UCP group after a mean follow‐up time of 95 months.
Similar to findings in the IPAC cohort,34 focal myocardial fibrosis assessed as LGE was rare and LVEF recovery was high in our PPCM group. This is in contrast to a German cohort of 34 PPCM women who underwent CMR at the time of diagnosis and after 5 months, where 71% had LGE.35 In a smaller study, LGE was found in 4 of 10 women and was associated with a worse prognosis.36 LGE is associated with a poorer prognosis in nonischemic cardiomyopathies,37 and its absence in our study population is reassuring in order to predict prognosis. But the observed difference in LGE prevalence after PPCM is still unexplained and should be explored in future studies that may also include measurement of diffuse myocardial fibrosis, which is associated with heart failure with preserved ejection fraction.38
Some limitations of our study must be considered. Selection bias could have been introduced given that PPCM nonparticipants could have been more affected by the disease. However, participants and nonparticipants did not differ significantly on baseline characteristics, including major adverse events. Nine of the 61 women in the parent cohort suffered a major adverse outcome, as previously reported.6 All these events occurred within 12 months after diagnosis, but upon review of charts for validation of the PPCM diagnosis and additional data collection, we screened all available chart notes up to 2016 for any major adverse events and did not find any beyond 12 months. Also, no additional deaths were noted in the Causes of Death Register beyond 12 months. The study design of a nationwide population‐based PPCM cohort reduces the risk of selection bias that is more significant in, for example, tertiary, single‐center cohorts. Our study reports on a white cohort, and the results may potentially not be extrapolated to women of black descent. Finally, complete blinding of CMR analyses was not possible in our study setting, which may be a source of bias.
Conclusion
In this nationwide long‐term follow‐up of Danish PPCM patients, the majority experienced recovery of LV systolic function. However, subtle diastolic dysfunction on CMR imaging and markedly reduced peak VO2 were common in PPCM patients and uncommon in women with previous severe preeclampsia. Improvement in LV systolic function can be expected for several years after PPCM. Focal myocardial fibrosis assessed with LGE was uncommon in this cohort.
Sources of Funding
The work was funded by The Danish Heart Foundation, Rigshospitalet's Research Foundation, Arvid Nilsson's Foundation, and Aase & Ejnar Danielsen's Foundation.
Disclosures
None.
Supporting information
Table S1. Characteristics of Women With Peripartum Cardiomyopathy by Participation in the Clinical Follow‐up Study
Table S2. Distribution of Additional Baseline Characteristics Among All Participants in the Index Pregnancy and at Study Participation
Table S3. Clinical Laboratory, ECG, Additional Exercise Testing, and Cardiac Magnetic Imaging Findings at Study Participation
Figure S1. Example of time‐volume curves constructed from the 25 left atrial (LA) volumes during 1 cardiac cycle with determination of specific volumes.
Acknowledgments
The authors thank radiographer and CMR technologist Jesper Kromann of the Department of Diagnostic Radiology, Copenhagen University Hospital, Rigshospitalet, and Marie Bayer Elming, MD, of the Department of Cardiology, Copenhagen University Hospital, Rigshospitalet, for assistance with CMR image recording and analysis. We further thank associate professor Susanne Rosthøj, Department of Biostatistics, Institute of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, for statistical consultation.
(J Am Heart Assoc. 2018;7:e008991 DOI: 10.1161/JAHA.118.008991.)
References
- 1. Sliwa K, Hilfiker‐Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. [DOI] [PubMed] [Google Scholar]
- 2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. [DOI] [PubMed] [Google Scholar]
- 3. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 4. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J III, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy‐Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barasa A, Rosengren A, Sandstrom TZ, Ladfors L, Schaufelberger M. Heart failure in late pregnancy and postpartum: incidence and long‐term mortality in Sweden 1997–2010. J Card Fail. 2017;23:370–378. [DOI] [PubMed] [Google Scholar]
- 6. Ersboll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population‐based study of incidence, management and outcome. Eur J Heart Fail. 2017;19:1712–1720. [DOI] [PubMed] [Google Scholar]
- 7. Lindley KJ, Conner SN, Cahill AG, Novak E, Mann DL. Impact of preeclampsia on clinical and functional outcomes in women with peripartum cardiomyopathy. Circ Heart Fail. 2017;10:e003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del MF, Hilfiker‐Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. [DOI] [PubMed] [Google Scholar]
- 11. Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, Felker GM, Bozkurt B, Boehmer J, Haythe J, Skopicki H, Hanley‐Yanez K, Pisarcik J, Halder I, Gorcsan J III, Rana S, Arany Z, Fett JD, McNamara DM. Relaxin‐2 and soluble Flt1 levels in peripartum cardiomyopathy: results of the multicenter IPAC study. JACC Heart Fail. 2016;4:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–714. [DOI] [PubMed] [Google Scholar]
- 13. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, Struman I, Bovy N, Sliwa K, Bauersachs J, Hilfiker‐Kleiner D. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brixval CS, Thygesen LC, Johansen NR, Rorbye C, Weber T, Due P, Koushede V. Validity of a hospital‐based obstetric register using medical records as reference. Clin Epidemiol. 2015;7:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 16. Vogel‐Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch‐Herold M, Pearson G, Sinha S, Lima JA, Bluemke DA. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr. 2006;30:426–432. [DOI] [PubMed] [Google Scholar]
- 17. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 19. Jarvinen V, Kupari M, Hekali P, Poutanen VP. Assessment of left atrial volumes and phasic function using cine magnetic resonance imaging in normal subjects. Am J Cardiol. 1994;73:1135–1138. [DOI] [PubMed] [Google Scholar]
- 20. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long‐term follow‐up and sustained therapy. Eur J Heart Fail. 2012;14:895–901. [DOI] [PubMed] [Google Scholar]
- 22. Barasa A, Goloskokova V, Ladfors L, Patel H, Schaufelberger M. Symptomatic recovery and pharmacological management in a clinical cohort with peripartum cardiomyopathy. J Matern Fetal Neonatal Med. 2018;31:1342–1349. [DOI] [PubMed] [Google Scholar]
- 23. Li W, Li H, Long Y. Clinical characteristics and long‐term predictors of persistent left ventricular systolic dysfunction in peripartum cardiomyopathy. Can J Cardiol. 2016;32:362–368. [DOI] [PubMed] [Google Scholar]
- 24. Goodwin AA, Mercer BM. Does maternal race or ethnicity affect the expression of severe preeclampsia? Am J Obstet Gynecol. 2005;193:973–978. [DOI] [PubMed] [Google Scholar]
- 25. Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker‐Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley‐Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J III, McNamara DM, Seidman CE, Seidman JG, Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goland S, Weinstein JM, Zalik A, Kuperstein R, Zilberman L, Shimoni S, Arad M, Ben GT, George J. Angiogenic imbalance and residual myocardial injury in recovered peripartum cardiomyopathy patients. Circ Heart Fail. 2016;9:e003349. [DOI] [PubMed] [Google Scholar]
- 27. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 28. von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuss G, Lucke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10:e005467. [DOI] [PubMed] [Google Scholar]
- 29. Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kyröläinen H, Hackney AC, Salminen R, Repola J, Häkkinen K, Haimi J. Effects of combined strength and endurance training on physical performance and biomarkers of healthy young women. J Strength Cond Res. 2018;32:1554–1561. [DOI] [PubMed] [Google Scholar]
- 31. Sliwa K, Mebazaa A, Hilfiker‐Kleiner D, Petrie MC, Maggioni AP, Laroche C, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, van der Meer P, Roos‐Hesselink JW, Seferovic P, van Spandonck‐Zwarts K, Mbakwem A, Bohm M, Mouquet F, Pieske B, Hall R, Ponikowski P, Bauersachs J. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 2017;19:1131–1141. [DOI] [PubMed] [Google Scholar]
- 32. Strobl I, Windbichler G, Strasak A, Weiskopf‐Schwendinger V, Schweigmann U, Ramoni A, Scheier M. Left ventricular function many years after recovery from pre‐eclampsia. BJOG. 2011;118:76–83. [DOI] [PubMed] [Google Scholar]
- 33. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- 34. Schelbert EB, Elkayam U, Cooper LT, Givertz MM, Alexis JD, Briller J, Felker GM, Chaparro S, Kealey A, Pisarcik J, Fett JD, McNamara DM. Myocardial damage detected by late gadolinium enhancement cardiac magnetic resonance is uncommon in peripartum cardiomyopathy. J Am Heart Assoc. 2017;6:e005472 DOI: 10.1161/JAHA.117.005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haghikia A, Rontgen P, Vogel‐Claussen J, Schwab J, Westenfeld R, Ehlermann P, Berliner D, Podewski E, Hilfiker‐Kleiner D, Bauersachs J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arora NP, Mohamad T, Mahajan N, Danrad R, Kottam A, Li T, Afonso LC. Cardiac magnetic resonance imaging in peripartum cardiomyopathy. Am J Med Sci. 2014;347:112–117. [DOI] [PubMed] [Google Scholar]
- 37. Patel AR, Kramer CM. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2017;10:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, Tseng WY. CMR‐verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Women With Peripartum Cardiomyopathy by Participation in the Clinical Follow‐up Study
Table S2. Distribution of Additional Baseline Characteristics Among All Participants in the Index Pregnancy and at Study Participation
Table S3. Clinical Laboratory, ECG, Additional Exercise Testing, and Cardiac Magnetic Imaging Findings at Study Participation
Figure S1. Example of time‐volume curves constructed from the 25 left atrial (LA) volumes during 1 cardiac cycle with determination of specific volumes.


