Abstract
Background
Antazoline mesylate represents an antihistamine capable of rapid and safe cardioversion of atrial fibrillation, yet evidence concerning its efficacy in comparison to other medications is insufficient. The study aimed to evaluate the success rate and safety of pharmacological cardioversion of atrial fibrillation with intravenous antazoline (CANT [Cardioversion With Antazoline Mesylate] study) in the setting of the emergency department.
Methods and Results
After reviewing 1984 medical records, 450 eligible patients (22.7%) with short‐duration atrial fibrillation subject to pharmacological cardioversion were enrolled in a retrospective observational analysis. The choice of antiarrhythmic drug was left to the discretion of the attending physician. The primary end point was successful cardioversion in the emergency department. The safety end point comprised bradycardia <45 bpm, hypotension, syncope, or death. The study population (mean age, 65.5±11.9 years; 52.9% females) was characterized by a median atrial fibrillation episode duration of 10 hours. Antazoline, alone or in combination, was administered in 24.2% (n=109) and 40% (n=180), respectively; amiodarone was administered in 46.7% and propafenone in 9.3%, while ≥2 antiarrhythmic drugs were administered in 19.8% of patients. Antazoline had the highest success rate of pharmacological cardioversion among all drugs (85.3%), which was comparable with propafenone (78.6%; relative risk, 1.09, 95% confidence interval, 0.91–1.30; P=0.317) and higher than amiodarone treatment (66.7%; relative risk, 1.28, 95% confidence interval, 1.13–1.45; P<0.001; number needed to treat, 5.4). The rate of cardioversion with antazoline alone was higher than combined amiodarone and/or propafenone (68.1%; relative risk, 1.25; 95% confidence interval, 1.12–1.40, P=0.0001). No safety end points were reported in the antazoline group, while 5 incidents occurred in the non‐antazoline cohort (P=0.075).
Conclusions
Antazoline represents an efficacious and safe method of pharmacological cardioversion in a real‐life setting.
Keywords: amiodarone, antazoline, atrial fibrillation, pharmacological cardioversion, propafenone
Subject Categories: Atrial Fibrillation, Pharmacology, Quality and Outcomes
Clinical Perspective
What Is New?
Antazoline is an antihistamine with antiarrhythmic properties, which has been previously demonstrated to terminate episodes of atrial fibrillation (AF), but has not been systematically compared to other antiarrhythmic drugs in the setting of the emergency department.
The current study suggests that antazoline is at least as efficacious as propafenone and amiodarone for the pharmacological cardioversion of short‐duration AF, with a very low rate of clinically nonsignificant complications.
The success rate of pharmacological cardioversion was higher among patients with AF associated with tachyarrhythmia.
What Are the Clinical Implications?
The present results provide evidence supporting the use of antazoline for the rapid pharmacological cardioversion of AF, in terms of both efficacy and safety.
Widespread application of antazoline for pharmacological cardioversion of AF would require high‐volume randomized controlled trials comparing antazoline to other antiarrhythmic drugs.
Introduction
The clinical importance of atrial fibrillation (AF) is related not only to the increased risk of cardiovascular morbidity and mortality,1 but also to a steadily increasing number of patients with symptomatic episodes of AF requiring acute management in the emergency department (ED).2 Timely successful cardioversion provides symptomatic relief and may prevent redundant hospitalizations.3 Barring acute hemodynamic instability, the choice between pharmacological and electrical cardioversion relies on individual clinical decisions and the patient's preference.4, 5 Because of the need for fasting state and anesthesia, the majority of patients initially undergo a pharmacological approach at rhythm conversion.6 Existing antiarrhythmic drugs (AADs) either confer a risk of proarrhythmia in patients with structural heart disease (propafenone, flecainide, ibutilide, dofetilide)7 or have a delayed onset of action (amiodarone).8 Recently adopted vernakalant is characterized by a more universal clinical profile,9 yet its application is limited by its low availability in certain parts of the world10 as well as clinical contraindications, including severe heart failure, recent acute coronary syndrome, and severe aortic valve stenosis.4 Accordingly, the search for a rapid‐acting, well‐tolerated, and highly efficacious AAD continues.
Antazoline mesylate is a first‐generation antihistaminic compound that was originally intended for acute management of allergic reactions.11 Accruing evidence shed light on its antiarrhythmic effect12, 13, 14 related to an increased atrial refractory period and interatrial conduction time.15 In the clinical scenario of short‐duration AF, antazoline was proven to be clinically safe and restore sinus rhythm (SR) more effectively than both placebo16 and a propafenone‐based strategy.17 Antazoline was also found useful in AF termination in the setting of the electrophysiology laboratory.18, 19 Currently, antazoline is approved and widely applied in EDs throughout Poland in order to achieve acute rhythm control, despite not being covered in contemporary guidelines.4, 5 To date, however, none of the reports systematically explored the success rate and safety of antazoline mesylate in comparison to other forms of pharmacological cardioversion, most importantly amiodarone.
Thus, the study aimed to evaluate the success rate and safety of cardioversion with intravenous antazoline mesylate (CANT [Cardioversion With Antazoline Mesylate] study) in patients with AF in comparison to amiodarone and/or propafenone in the real‐world setting of the ED.
Methods
Study Design
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The CANT study was carried out as a retrospective, single‐center, observational research that comprised all patients with a symptomatic episode of paroxysmal or persistent AF undergoing urgent cardioversion in the ED of Upper Silesia Medical Center in Katowice, Poland, between 2015 and 2017. The study flowchart is presented in Figure 1. Initially, all 1984 patients with an I48 code of the International Classification of Diseases, Tenth Revision (ICD ‐ 10) were screened and subject to inclusion and exclusion criteria, which led to inclusion of 450 (22.7%) patients in the final analysis. The study protocol complied with Declaration of Helsinki Guidelines and was approved by the local Ethics Committee. All the participants gave their written informed consent for participation in the study.
Figure 1.
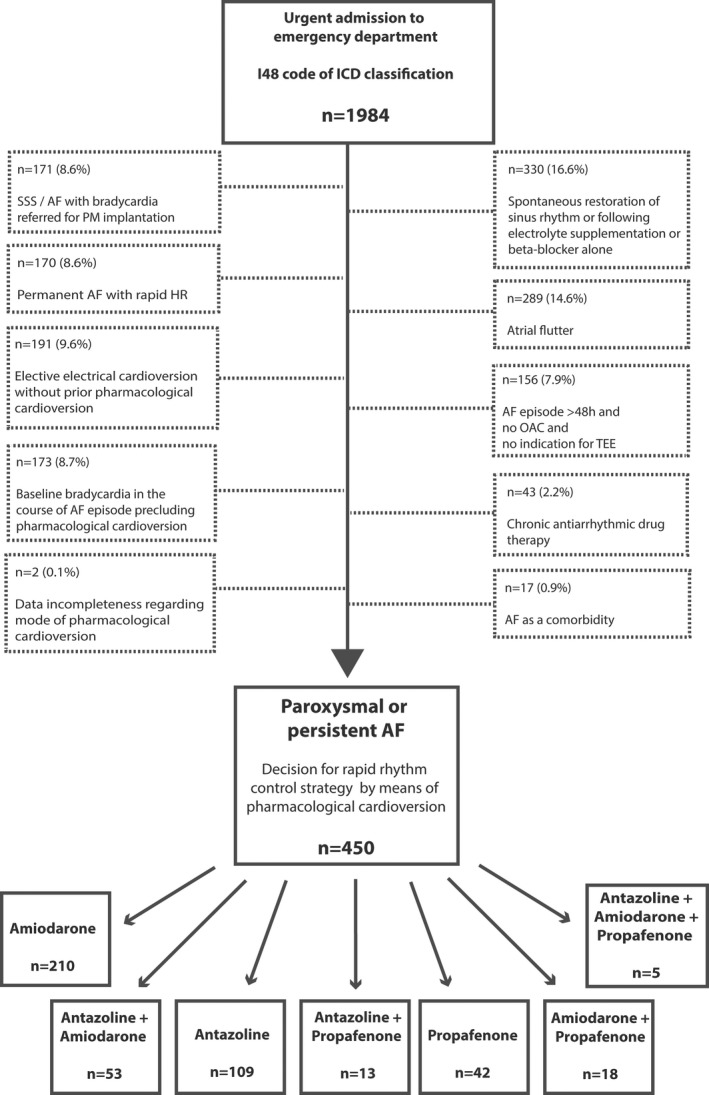
Study flowchart. AF indicates atrial fibrillation; HR, heart rate; ICD, International Classification of Diseases, Tenth Revision; OAC, oral anticoagulation therapy; PM, pacemaker; SSS, sick sinus syndrome; TEE, transesophageal echocardiography. Exclusion criteria may overlap.
The mandatory inclusion criteria comprised (1) an episode of symptomatic paroxysmal or persistent AF confirmed in 12‐lead ECG without chronic AAD therapy and (2) a decision for rhythm control by means of pharmacological cardioversion.
The exclusion criteria are listed in Figure 1 and involved (1) permanent AF; (2) AF episode duration of >48 hours without chronic oral anticoagulation or of indeterminate duration and no indication for urgent transesophageal echocardiography; (3) spontaneous rhythm conversion or secondary to infusion of intravenous potassium or β‐blockers alone; (4) AF with bradycardia referred for urgent pacemaker implantation; (5) decision for direct electrical cardioversion without a prior pharmacological approach; (6) AF as a comorbidity (eg, acute coronary syndrome and concomitant AF); (7) baseline bradycardia precluding acute pharmacological cardioversion in the ED; (8) chronic AAD therapy; (9) data incompleteness concerning mode of cardioversion; or (10) atrial flutter. Eight patients were characterized by 2 overlapping exclusion criteria.
Outcomes and Safety End Points
The primary end point was successful restoration of SR during a stay in the ED or after urgent hospital admission, which was confirmed by means of a 12‐lead ECG and persisted until discharge. In the event of lack of rhythm conversion following drug administration, patients were either observed for at least 12 hours before discharge from the ED or admitted to the hospital with the intention of further pharmacological cardioversion and/or electrical cardioversion. This concerned all the assessed drugs so as to exclude possible late arrhythmia reversal. The moment of hospital admission was not consistent with unsuccessful pharmacological cardioversion, as some patients regained SR in the cardiology department. Patients in whom SR was eventually restored by means of electrical cardioversion were regarded as patients with unsuccessful pharmacological cardioversion.
The safety end point was defined as an acute adverse event in the direct aftermath of cardioversion in the form of bradycardia <45 bpm, hypotension (decrease of systolic blood pressure of >40 mm Hg), syncope, or death.
Study Treatment
The choice of AAD was left to the discretion of attending physician, which represented a real‐life clinical approach. The attending physician took into consideration the clinical profile of each patient and current European Society of Cardiology guidelines on management of AF.4 Patients received either (1) intravenous amiodarone (Cordarone; Sanofi‐Aventis, Forest Park, GA), (2) intravenous or oral propafenone hydrochloride (Rytmonorm; Mylan Pharmaceuticals, Greensboro, NC), (3) intravenous antazoline mesylate (Phenazolinum; Polfa, Warsaw, Poland), or (4) a combination of 2 or more agents. The infusion of AAD was performed under strict electrocardiographic and blood pressure monitoring.
Amiodarone was diluted with 5% glucose and administered in an infusion pump with or without a preemptive intravenous bolus of 150 mg. Propafenone hydrochloride was administered either orally in 150‐mg pills or in a slow (3‐minute) intravenous bolus of 70 mg propafenone diluted with a 100‐mL solution of 0.9% NaCl. Antazoline was administered either as a single or repeated slow (3‐minute) undiluted intravenous bolus of 100 to 200 mg or diluted with a 100‐mL solution of 0.9% NaCl and infused over 5 to 15 minutes. The total dose of each drug, as well as adjunct use of other AADs and/or β‐blockers and/or potassium drip, were based on the individual decision of the physician.
Data Acquisition and Definitions
The data regarding demographic and clinical variables were gathered by means of meticulous review of electronic health records and discharge summaries by investigators blinded to treatment. Venous blood was withdrawn at admission to ED and comprised blood smear, serum creatinine concentration, plasma potassium and sodium concentration, high‐sensitivity troponin T, and thyroid‐stimulating hormone. Estimated glomerular filtration rate was based on Modification of Diet in Renal Disease formula. Following the evaluation of comorbidities, the CHA2DS2‐VASc score was calculated. The symptoms related to AF were graded using the European Heart Rhythm Association classification.20 Persistent AF was defined as an episode lasting for >7 days. Coronary artery disease (CAD) was defined as a history of stable angina validated by means of a noninvasive stress test or any acute coronary syndrome or any percutaneous coronary intervention in anamnesis. Peripheral artery disease was defined as presence of claudication or ankle‐brachial index <0.9, or significant stenosis of lower extremity arteries or presence of any atheromatous plaque within carotid arteries on Doppler imaging.
Transthoracic echocardiography with the assessment of left ventricular ejection fraction (LVEF) and left atrial diameter (parasternal long‐axis view) were conducted using Epiq 7G (Philips, Andover, MA) with a 2.5‐MHz probe by experienced investigators.
Statistical Analysis
Quantitative variables were expressed as mean and standard deviation or median and 1 to 3 quartile boundaries and qualitative parameters as number and percentage. Variables type of distribution was verified using the Shapiro‐Wilk test. Student t test was used in normally distributed parameters, while the 2‐tailed Mann‐Whitney U test was used in all nonnormally distributed parameters. Either ANOVA or the Kruskal‐Wallis test was used for multiple comparisons among 7 subgroups of different pharmacological cardioversions. The proportions in contingency tables were calculated using the chi‐square test with Bonferroni adjustment. Relative risk (RR) ratios with 95% confidence intervals (CIs) were calculated.
Following the univariate analysis of predictors of rhythm conversion, all the parameters with P<0.1 in univariate analysis were included in the logistic regression model. The regression model used a backward selection process. The area under the receiver operating characteristics curve for the model was calculated. A P value of <0.05 was regarded as statistically significant. Statistical analysis was performed using SPSS version 25.0 software (IBM Corporation, Armonk, NY).
Results
Demographic and Clinical Characteristics
After reviewing a total of 1984 patients, 450 (22.7%) were incorporated into the final analysis (Figure 1). The overview of demographic and clinical characteristics of the whole study population depending on successful rhythm conversion is highlighted in Table 1. The study population was characterized by a mean age of 65.5 years, slight predominance of females (52.9%), high prevalence of arterial hypertension (72.9%) and clinically proven atherosclerosis (32.4%), and chronic kidney disease (CKD; 23.3%). Impaired left ventricular systolic function reflected by LVEF <50% was found in 14.7% of patients.
Table 1.
Baseline Characteristics of Study Population Stratified by Successful and Unsuccesful Pharmacological Cardioversion
| Variable | Whole Population (N=450) | Successful Cardioversion (N=314) | Unsuccessful Cardioversion (N=136) | P Value |
|---|---|---|---|---|
| Male sex | 212 (47.1%) | 143 (45.5%) | 69 (50.7%) | 0.311a |
| Age, y | 65.5±11.9 | 65.4±11.2 | 65.9±13.2 | 0.640b |
| Weight, kg | 82.2±16.2 | 83.8±17.1 | 75.2±11.3 | 0.422b |
| Arterial hypertension | 328 (72.9%) | 226 (72.0%) | 102 (75.0%) | 0.559a |
| Diabetes mellitus | 79 (17.6%) | 57 (18.2%) | 22 (16.2%) | 0.576a |
| CAD/PAD | 144 (32.4%) | 107 (34.1%) | 37 (27.2%) | 0.154a |
| Former TIA/stroke | 20 (4.4%) | 18 (5.7%) | 2 (1.5%) | 0.043a |
| LVEF, % | 52.7±9.1 | 52.7±9.4 | 52.7±8.4 | 0.951b |
| LVEF <50% | 66 (14.7%) | 49 (15.6%) | 17 (12.5%) | 0.235a |
| LAd, mm | 42.2±5.1 | 42.0±4.8 | 42.6±5.7 | 0.320b |
| TnT, pg/mL | 10 (7; 16) | 9 (7; 16) | 10 (7; 15) | 0.795c |
| SCr, mg/dL | 0.99±0.34 | 0.98±0.36 | 1.02±0.26 | 0.293b |
| eGFR, mL/min | 71.4±17.6 | 72.5±17.4 | 68.7±17.8 | 0.046b |
| eGFR <60 mL/min per 1.73 m2 | 105 (23.3%) | 69 (22.0%) | 36 (26.5%) | 0.261a |
| K+ level, mEq/L | 4.3±0.4 | 4.2±0.4 | 4.3±0.4 | 0.023b |
| WBC, ×1000/μL | 7.8±2.9 | 7.6±3.0 | 8.1±2.5 | 0.187b |
| Hemoglobin, g/dL | 14.3±1.5 | 14.3±1.5 | 14.3±1.5 | 0.729b |
| TSH, mIU/L | 1.81 (1.10–2.72) | 1.90 (1.10–2.72) | 1.55 (1.16–2.58) | 0.749c |
CAD indicates coronary artery disease; eGFR, estimated glomerular filtration rate; LAd, left atrial diameter; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; SCr, serum creatinine concentration; SD, standard deviation; TIA, transient ischemic attack; TnT, troponin T concentration; TSH, thyroid‐stimulating hormone concentration; WBC, white blood cell count.
Chi‐squared test.
Student t test.
Mann‐Whitney U test.
The AF‐specific variables were listed in Table 2 and stratified by successful and unsuccessful pharmacological cardioversion. The vast majority of patients had paroxysmal AF (96%), while the median duration of the index AF episode was 10 hours.
Table 2.
Overview of Atrial Fibrillation Characteristics and Treatment Stratified by Successful and Unsuccessful Pharmacological Cardioversion
| Variable | Whole Population (N=450) | Successful Cardioversion (N=314) | Unsuccessful Cardioversion (N=136) | P Value |
|---|---|---|---|---|
| EHRA class | 3 (2–3) | 3 (2–3) | 2.5 (2–3) | 0.445a |
| CHA2DS2‐VASc [pts] | 3 (2–4) | 3 (2–4) | 3 (1–4) | 0.952a |
| History of PVI | 28 (6.2%) | 22 (7.0%) | 6 (4.4%) | 0.278b |
| Heart rate, bpm | 120.7±24.9 | 122.8±24.1 | 115.9±26.1 | 0.009c |
| Heart rate >130 bpm | 162 (36.0%) | 118 (37.6%) | 44 (32.3%) | 0.203b |
| Duration of AF episode, h | 10 (5–24) | 9 (4–19) | 12 (6–24) | 0.007a |
| AF episode >48 h | 54 (12.0%) | 26 (8.3%) | 28 (20.6%) | 0.001b |
| Persistent AF | 18 (4.0%) | 4 (1.3%) | 14 (10.3%) | 0.001b |
| TEE | 14 (3.1%) | 7 (2.2%) | 7 (5.1%) | 0.101b |
| Chronic oral anticoagulation | 310 (68.9%) | 208 (66.2%) | 102 (75.0%) | 0.038b |
| VKA | 98 (21.8%) | 63 (20.1%) | 35 (25.7%) | 0.178b |
| NOAC | 212 (47.1%) | 145 (46.2%) | 67 (49.3%) | 0.538b |
| Urgent hospital admission | 161 (35.8%) | 98 (31.2%) | 63 (46.3%) | 0.002b |
| Hospitalization time, d | 1 (1–2) | 1 (1–2) | 1 (1–3) | 0.010a |
| Successful cardioversion | 314 (69.8%) | ··· | ··· | ··· |
| Medications used for pharmacological cardioversion | ||||
| Amiodarone | 210 (46.7%) | 140 (44.6%) | 70 (51.5%) | 0.179b |
| Propafenone | 42 (9.3%) | 33 (10.5%) | 9 (6.6%) | 0.192b |
| Antazoline | 109 (24.2%) | 93 (29.6%) | 16 (11.8%) | 0.0001b |
| Amiodarone+antazoline | 53 (11.8%) | 29 (9.2%) | 24 (17.6%) | 0.011b |
| Propafenone+antazoline | 13 (2.9%) | 6 (1.9%) | 7 (5.1%) | 0.060b |
| Amiodarone+propafenone | 18 (4.0%) | 11 (3.5%) | 7 (5.1%) | 0.414b |
| Amiodarone+propafenone+antazoline | 5 (1.1%) | 2 (0.6%) | 3 (2.2%) | 0.145b |
| Overall antazoline use | 180 (40.0%) | 130 (41.4%) | 50 (36.8%) | 0.438b |
| Amiodarone—total dose, mg | 600 (300–750) | 600 (400–750) | 450 (300–600) | 0.008a |
| Propafenone—total dose, mg | 140 (70–210) | 140 (70–150) | 140 (70–300) | 0.047a |
| Antazoline—total dose, mg | 200 (100; 300) | 200 (100; 200) | 200 (200; 300) | 0.028a |
| Additional β‐blocker administration | 156 (34.7%) | 102 (32.5%) | 54 (39.7%) | 0.149b |
| Potassium IV | 281 (62.4%) | 198 (63.1%) | 83 (61.0%) | 0.567b |
AF indicates atrial fibrillation; bpm, beats per minute; EHRA, European Heart Rhythm Association; IV, intravenous; NOAC, non‐VKA oral anticoagulants; PVI, pulmonary vein isolation; SD, standard deviation; TEE, transesophageal echocardiography; VKA, vitamin K antagonists.
Mann‐Whitney U test.
Chi‐squared test.
Student t test.
Pharmacological Cardioversion and Success Rate
Pharmacological cardioversion was successful in 69.8% of patients. Regardless of AAD use, 34.7% of patients received an adjunct intravenous or oral β‐blocker, while 62.4% received intravenous potassium supplementation. The treatment received was delineated in Figure 1. The most common drug was amiodarone (46.7%), followed by antazoline mesylate (24.2%) and propafenone hydrochloride (9.3%) (Figure 1 and Table 2). The rest of the study population received a combined treatment of 2 or more drugs (n=89, 19.8%) (Figure 1 and Table 2). In total, antazoline was administered to 180 patients (40%). The median dose of amiodarone was 600 (300–750) mg, propafenone 140 (70–210) mg, and antazoline 200 (100–300) mg (Table 2).
No safety end points were reported in the antazoline group, whereas 3 incidents of bradycardia <45 bpm and 1 case of hypotension were reported in the amiodarone group and 1 case of bradycardia in the propafenone group. No cases of syncope or death were documented in any of the subgroups.
Antazoline Alone Versus Other Modes of Pharmacological Cardioversion
The comparison of antazoline and other regimens of cardioversion is highlighted in Table 3 and Figure 2. Patients who received antazoline alone had a higher rate of successful cardioversion than patients who were treated only with amiodarone (85.3% versus 66.7%; RR 1.28; 95% CI, 1.13–1.45; P<0.001; number needed to treat, 5.4) and comparable success rate to patients treated with propafenone alone (78.6%; RR, 1.09; 95% CI, 0.91–1.30; P=0.317).
Table 3.
Comparison of Different Modes of Pharmacological Cardioversion
| Variable | Amiodarone (N=210) | Propafenone (N=42) | Antazoline (N=109) | Antazoline+Amiodarone (N=53) | Antazoline+Propafenone (N=13) | Amiodarone+Propafenone (N=18) | Antazoline+Amiodarone+Propafenone (N=5) | Antazoline vs Amiodarone P Value | Antazoline vs Propafenone P Value | Multiple Comparisons Test P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 67.7±11.2 | 64.2±12.4 | 62.8±12.0 | 67.4±10.7 | 59.4±11.8 | 58.4±15.6 | 69.0±4.1 | <0.001a | 0.292a | 0.001b |
| Male sex | 95 (45.2%) | 9 (21.4%) | 60 (55.0%) | 29 (54.7%) | 7 (53.8%) | 10 (55.6%) | 2 (40.0%) | 0.096c | <0.001c | 0.012d |
| EHRA class | 3 (2–3) | 2 (2–3) | 3 (2–3) | 3 (2–3) | 2 (2–3) | 3 (2–3) | 3 (2.5–3) | 0.995e | 0.756e | 0.846f |
| CHA2DS2‐VASc [pts] | 2.9±1.6 | 2.6±1.7 | 2.6±1.5 | 3.0±1.5 | 2.2±1.7 | 2.1±1.5 | 2.2±0.8 | 0.082a | 0.986a | 0.072b |
| Heart rate, /min | 119.8±25.8 | 122.2±19.8 | 122.1±23.8 | 121.6±25.9 | 111.3±25.4 | 125.3±23.8 | 114.0±41.0 | 0.478a | 0.830a | 0.758b |
| AF episode duration, h | 12 (5–24) | 12 (6–15) | 7 (4–18) | 10 (4–24) | 7 (4–13) | 12 (6–15) | 9.5 (8–17) | 0.051e | 0.210e | 0.449f |
| Persistent AF | 13 (6.2%) | 2 (4.8%) | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 2 (11.1%) | 0 (0.0%) | 0.007c | 0.016c | 0.086d |
| Urgent hospital admission | 95 (45.2%) | 12 (28.6%) | 18 (16.5%) | 20 (37.7%) | 4 (30.8%) | 8 (44.4%) | 4 (80.0%) | <0.001c | 0.102c | <0.001d |
| Arterial hypertension | 156 (74.3%) | 25 (59.5%) | 77 (70.6%) | 45 (84.9%) | 10 (76.9%) | 11 (61.1%) | 4 (80.0%) | 0.435c | 0.165c | 0.087d |
| Diabetes mellitus | 40 (19.1%) | 4 (9.5%) | 17 (15.6%) | 14 (26.4%) | 2 (15.4%) | 2 (11.1%) | 0 (0.0%) | 0.422c | 0.324c | 0.299d |
| CAD/PAD | 65 (30.9%) | 9 (21.4%) | 44 (40.4%) | 17 (32.1%) | 5 (38.5%) | 4 (22.2%) | 0 (0.0%) | 0.111c | 0.029c | 0.169d |
| Former TIA/stroke | 11 (5.2%) | 3 (7.1%) | 5 (4.6%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.786c | 0.530c | 0.756d |
| TnT, pg/mL | 12 (8–21) | 9 (4–13) | 8 (7–12) | 9 (7–16) | 7 (5–10) | 8 (5–14) | 27 (7–45) | <0.001e | 0.908e | 0.001f |
| eGFR, mL/min | 70.5±18.0 | 71.9±18.8 | 72.1±16.6 | 70.6±18.0 | 71.9±15.5 | 77.1±18.6 | 70.6±12.6 | 0.576a | 0.819a | 0.694b |
| K+ level, mEq/L | 4.3±0.5 | 4.2±0.3 | 4.2±0.4 | 4.3±0.4 | 4.1±0.4 | 4.4±0.4 | 3.9±0.5 | 0.468a | 0.669a | 0.570b |
| WBC, ×1000/μL | 7.95±2.39 | 7.27±1.63 | 7.42±2.25 | 8.38±5.55 | 7.15±1.20 | 7.36±1.58 | 7.45±4.66 | 0.038a | 0.866a | 0.237b |
| Hemoglobin, g/dL | 14.1±1.6 | 14.5±1.2 | 14.5±1.5 | 14.6±1.3 | 14.6±1.4 | 14.4±1.8 | 13.5±2.3 | 0.040a | 0.583a | 0.349b |
| LVEF, % | 51.6±10.6 | 54.7±8.2 | 55.0±5.9 | 51.4±8.9 | 55.4±3.6 | 52.2±9.1 | 52.0±4.5 | 0.064a | 0.662a | 0.229b |
| LVEF <50% | 38 (18.1%) | 3 (7.1%) | 8 (7.3%) | 13 (24.5%) | 0 (0.0%) | 3 (16.7%) | 1 (20.0%) | 0.027c | 0.933c | 0.062d |
| LAd, mm | 42.6±5.1 | 39.5±5.8 | 42.3±4.5 | 42.8±4.8 | 42.5±4.7 | 41.5±6.6 | 40.4±6.7 | 0.937a | 0.009a | 0.092b |
| Electrical cardioversion | 17 (8.1%) | 4 (9.5%) | 7 (6.4%) | 5 (9.4%) | 3 (23.1%) | 3 (17.7%) | 0 (0.0%) | 0.568c | 0.485c | 0.380d |
| IV potassium use | 132 (62.9%) | 29 (69.0%) | 63 (57.8%) | 32 (60.4%) | 10 (76.9%) | 12 (66.7%) | 3 (60.0%) | 0.346c | 0.226c | 0.799d |
| β‐blocker use | 58 (27.6%) | 23 (54.8%) | 39 (35.8%) | 20 (37.7%) | 4 (30.8%) | 10 (55.5%) | 2 (40.0%) | 0.163c | 0.034c | 0.018d |
| Successful pharmacological cardioversion | 140 (66.7%) | 33 (78.6%) | 93 (85.3%) | 29 (54.7%) | 6 (46.2%) | 11 (61.1%) | 2 (40.0%) | <0.001c | 0.317c | <0.001d |
AF indicates atrial fibrillation; bpm, beats per minute; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association; IV, intravenous; LAd, left atrial diameter; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; SCr, serum creatinine concentration; SD, standard deviation; TIA, transient ischemic attack; TnT, troponin T concentration; WBC, white blood cell count.
Student t test.
ANOVA test.
Chi‐squared test.
Multiple comparisons chi‐squared test.
Mann‐Whitney U test.
Kruskal‐Wallis test.
Figure 2.
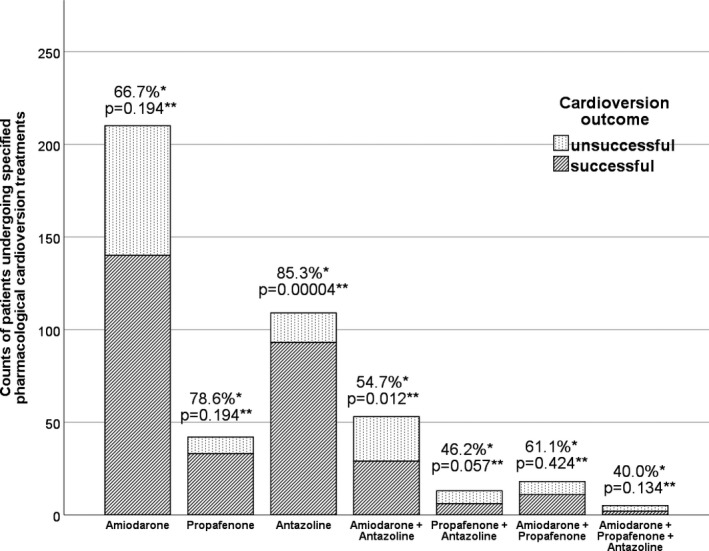
Evaluation of success rate of different forms of pharmacological cardioversion. *Rate of successful cardioversion in each treatment subgroup. **Post hoc analysis of chi‐square test; P values calculated from Z‐score–derived chi‐square; Bonferroni‐corrected P‐level of 0.0071.
The rate of rhythm conversion was also higher in antazoline group in comparison to combined antazoline and amiodarone (85.3% versus 54.7%, P<0.0001), as well as antazoline and propafenone (46.2%; P=0.002), combined amiodarone and propafenone treatment (61.1%; P=0.020), and triple therapy with antazoline, amiodarone, and propafenone (40.0%; P=0.032).
The post hoc analysis of the chi‐square test revealed that only the antazoline group differed significantly from other subgroups and had a Z‐score‐derived P value of 0.00004, which was below the Bonferroni‐corrected P value threshold of 0.0071.
Antazoline Alone or Combined Antazoline Versus Non‐Antazoline Cardioversion
Patients treated with antazoline alone (n=109) had a higher rate of SR restoration than combined treatment with amiodarone and/or propafenone (Figure 3 and Table 4; 85.3% versus 68.1%; RR, 1.25; 95% CI, 1.12–1.40; P=0.0001, number needed to treat, 5.8). Patients in the antazoline group were younger; more often were men; and had a shorter duration of hospitalization, higher rate of CAD, lower troponin T level, and less frequently had impaired LVEF (Table 4).
Figure 3.
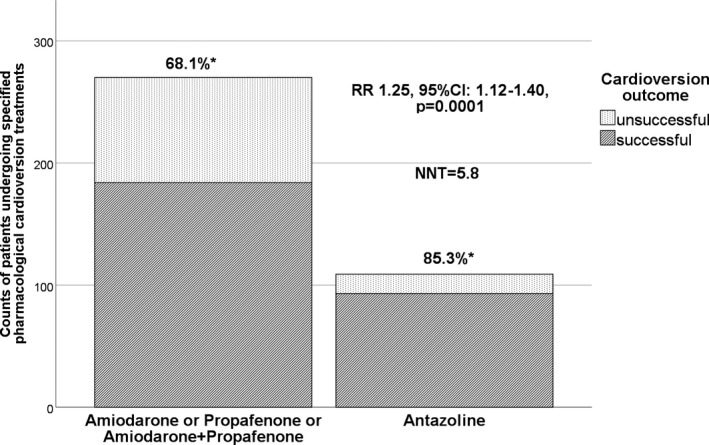
Comparison of antazoline alone versus non‐antazoline pharmacological cardioversion. *Rate of successful cardioversion in each treatment subgroup. CI indicates confidence interval; NNT, number needed to treat; RR, relative risk.
Table 4.
Comparison of Clinical Characteristics and Efficacy of Pharmacological Cardioversion in Patients Treated With Antazoline Alone (1) or Antazoline Combined With Overlapping Treatment (2) Versus Amiodarone and/or Propafenone (3)
| Variable | (1) Antazoline Alone (n=109) | (2) Antazoline Combined (n=180) | (3) Amiodarone or/and Propafenone (n=270) | P Value 1 vs 3 | P Value 2 vs 3 |
|---|---|---|---|---|---|
| Age, y | 62.8±12.0 | 64.2±11.7 | 66.5±11.9 | 0.003a | 0.023a |
| Male sex | 60 (55.0%) | 99 (55.0%) | 114 (42.2%) | 0.023b | 0.008b |
| Hospitalization time, d | 1 (1; 1) | 1 (1; 1) | 1 (1; 3) | <0.001c | 0.001c |
| EHRA class | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.976c | 0.863c |
| CHA2DS2‐VASc score [pts] | 2.6±1.5 | 2.7±1.5 | 2.8±1.6 | 0.218a | 0.414a |
| Heart rate, bpm | 122.1±23.8 | 121.0±25.0 | 120.5±24.9 | 0.708a | 0.885a |
| AF episode duration, h | 7 (4–18) | 8 (4–18) | 12 (5–24) | 0.034c | 0.029c |
| Persistent AF | 0 (0.0%) | 1 (0.6%) | 17 (6.3%) | 0.006b | 0.002b |
| Urgent hospital admission | 18 (16.5%) | 47 (26.1%) | 115 (42.6%) | <0.001b | <0.001b |
| Arterial hypertension | 77 (70.6%) | 137 (76.1%) | 192 (71.1%) | 0.905b | 0.266b |
| Diabetes mellitus | 17 (15.6%) | 33 (18.3%) | 46 (17.0%) | 0.716b | 0.750b |
| CAD/PAD | 44 (40.4%) | 66 (36.7%) | 78 (28.9%) | 0.036b | 0.087b |
| Former TIA/stroke | 5 (4.6%) | 6 (3.3%) | 14 (5.2%) | 0.798b | 0.350b |
| TnT, pg/mL | 8 (7–12) | 8 (6–14) | 11 (7–18) | 0.004c | 0.008c |
| Positive TnT >14 pg/mL | 10 (9.2%) | 26 (14.4%) | 79 (29.3%) | 0.001b | 0.003b |
| eGFR, mL/min | 72.1±16.6 | 71.5±16.7 | 71.2±18.1 | 0.868a | 0.787a |
| WBC, ×1000/μL | 7.42±2.25 | 7.69±3.64 | 7.81±2.26 | 0.077a | 0.083a |
| Hemoglobin, g/dL | 14.5±1.5 | 14.5±1.4 | 14.2±1.5 | 0.064a | 0.039a |
| LVEF <50% | 8 (7.3%) | 22 (12.2%) | 44 (16.3%) | 0.045b | 0.243b |
| LAd, mm | 42.3±4.5 | 42.4±4.7 | 42.1±5.4 | 0.587a | 0.211a |
| Electrical cardioversion | 7 (6.4%) | 16 (8.9%) | 23 (8.5%) | 0.645b | 0.963b |
| Potassium IV | 63 (57.8%) | 109 (60.6%) | 173 (64.1%) | 0.241b | 0.378b |
| β‐blocker use | 39 (35.8%) | 65 (36.1%) | 91 (33.7%) | 0.772b | 0.701b |
| Successful pharmacological cardioversion | 93 (85.3%) | 130 (72.2%) | 184 (68.1%) | 0.0001b | 0.438b |
| Safety end point | 0 (0.0%) | 0 (0.0%) | 5 (1.9%) | 0.327b | 0.075b |
AF indicates atrial fibrillation; bpm, beats per minute; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association; LAd, left atrial diameter; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; SCr, serum creatinine concentration; TIA, transient ischemic attack; TnT, troponin T concentration; WBC, white blood cell count.
Student t test 2‐tailed.
Two‐tailed chi‐squared test.
Mann‐Whitney U test.
When the antazoline group was considered as a whole with overlapping antazoline treatment (n=180), this mode of cardioversion was associated with a comparable success rate to the amiodarone and/or propafenone group (72.2% versus 68.1%; RR, 1.05; 95% CI, 0.93–1.19; P=0.438). The distribution of comorbidities was similar to antazoline alone (Table 4).
The subanalysis of patients with antazoline alone compared with the rest of the study population (amiodarone and/or propafenone and overlapping antazoline treatment) yielded a significantly higher rate of successful cardioversion in the first group (85.3% versus 64.8%; RR, 1.31; 95% CI, 1.18–1.47; P<0.0001; number needed to treat, 4.9).
Predictors of Successful Pharmacological Cardioversion
In the whole population (n=450), univariate analysis revealed that estimated glomerular filtration rate (unit odds ratio [OR], 1.012 per 1 mL/min; P=0.048), history of transient ischemic attack or stroke (OR, 4.099; P=0.061), plasma potassium level (unit OR, 0.570 per 1 mEq/L; P=0.024), baseline heart rate (unit OR, 1.011 per 1 bpm; P=0.010), AF episode duration (unit OR, 0.991 per 1 hour; P=0.060), AF episode lasting >48 hours (OR, 0.332; P=0.0002), persistent AF (OR, 0.105; P=0.0001), chronic oral anticoagulation (OR, 0.611; P=0.039), cardioversion with antazoline alone (OR, 3.156; P=0.0001), and amiodarone and antazoline (OR, 0.475; P=0.012), as well as propafenone and antazoline (OR, 0.359; P=0.070), were associated with rhythm conversion. Backward logistic regression analysis revealed that increased heart rate (unit OR, 1.012 per 1 bpm; 95% CI, 1.001–1.023; P=0.034) and the use of antazoline alone (OR, 4.028; 95% CI, 1.712–9.474; P=0.001) independently predicted restoration of SR (area under the receiver operating characteristics curve, 0.673; P=0.0001; Hosmer‐Lemeshow P=0.643).
The univariate analysis performed on the subgroup of 180 patients treated with antazoline indicated that total dose of antazoline (unit OR, 0.996 per 1 mg; P=0.030), AF episode duration (unit OR, 0.981 per 1 hour; P=0.019), estimated glomerular filtration rate (unit OR, 1.021 per 1 mL/min; P=0.045), arterial hypertension (OR, 0.433; P=0.065), baseline heart rate (unit OR, 1.0150 per 1 bpm; P=0.037), concomitant use of amiodarone (OR, 0.267; P=0.0002) and propafenone (OR, 0.262; P=0.008) were linked to AF termination. Backward logistic regression confirmed that increased heart rate (unit OR, 1.025 per 1 bpm; 95% CI, 1.001–1.049; P=0.045) and shorter duration of AF episode (unit OR, 0.975 per 1 hour; 95% CI, 0.954–0.996; P=0.020) were independently associated with successful cardioversion. In addition, the adjunct use of amiodarone was associated with a significantly lower chance of rhythm conversion (OR, 0.181; 95% CI, 0.056–0.583; P=0.004). The area under the receiver operating characteristics curve for the predictive model was 0.798 (P=0.0002; Hosmer‐Lemeshow P=0.891).
Discussion
The present study is, by far, the largest report in the literature systematically evaluating the success rate and safety of antazoline mesylate used for cardioversion in the ED and, most importantly, the first study comparing antazoline with amiodarone. Current results delivered evidence that the use of intravenous antazoline mesylate alone is associated with significantly higher rate of rhythm conversion than amiodarone (85.3% versus 66.7%; P<0.001) and combined amiodarone and/or propafenone (85.3% versus 68.1%; P=0.0001), with a comparable success rate to propafenone alone (85.3% versus 78.6%; P=0.317). When analyzed jointly (alone and overlapping treatment), antazoline was comparable to other forms of cardioversion (72.2% versus 68.1%; P=0.438) and was characterized by no adverse events in the current study (0% versus 1.9%; P=0.075).
Despite the lack of randomization, current results provide evidence for safety and a high success rate of antazoline as an AAD for termination of AF. It should be underscored that as many as 40.4% of patients treated with antazoline had CAD or peripheral artery disease diagnosis and 7.3% had impaired left ventricular systolic function and these patients did not experience any adverse events (Table 3). However, excellent results of cardioversion with antazoline might be partially attributed to a more favorable clinical profile, reflected by younger age, lower prevalence of persistent AF, lower troponin T level, and lower prevalence of LVEF impairment than patients with amiodarone (Table 3). Conversely, the antazoline group more frequently suffered from CAD and had greater left atrial diameter than the cohort treated with propafenone (Table 3).
Current findings should be interpreted in relation to a former case‐control study by Farkowski and coworkers, who found a significantly higher rate of rhythm conversion in patients treated with antazoline as compared with propafenone (71.6% versus 55.1%; RR, 1.30; 95% CI, 1.07–1.57).17 The success rate was comparable to the combined antazoline group in our research (72.2%), but the study reported several adverse events associated with antazoline infusion, such as bradycardia (n=32, 9.6%) and hypotension (n=6, 1.8%), which still were nonsignificantly less frequent than in the propafenone‐based strategy (P=0.633 and 0.244, respectively).17 This should be contrasted with no adverse events in our study, which should be interpreted with caution because of its retrospective design.
The results of the CANT study stay in line with the only randomized controlled AnPAF (Antazoline in the Rapid Cardioversion of Paroxysmal Atrial Fibrillation) study by Maciąg and coworkers, who compared antazoline mesylate with placebo.16 In this exploratory trial (n=74), antazoline mesylate was demonstrated to be superior to placebo in restoring SR in patients with short‐duration AF (72.2% versus 10.5%; RR, 6.86; 95% CI, 2.66–17.72, P<0.0001).16 Of note, antazoline facilitated rhythm conversion rapidly at a median time of 16 minutes,16 while adverse events were transient and mainly related to hot flush and drowsiness. One episode of hypotension and heart failure exacerbation and 2 episodes of bradycardia were reported.16
In the study by Balsam et al, antazoline was proven useful for termination of AF episode in 141 patients undergoing pulmonary vein isolation, as rhythm conversion rate was 83.6% in paroxysmal and 31.1% in persistent AF, while mild adverse actions were reported in 5% of patients.18
Before antiarrhythmic use, antazoline has long been utilized to treat allergic reactions with no safety concerns raised throughout decades of use. Antazoline represents a first‐generation nonspecific antihistamine with anticholinergic properties and a relatively short elimination half‐life of 2.29 hours and a high volume of distribution.21 Antazoline leads to a transient increase of heart rate by ≈8 bpm between the 2 and 4 minutes after intravenous bolus and a transient asymptomatic decrease of both stroke volume22 and blood pressure.22 In the surface ECG, antazoline was documented to prolong P‐wave duration, PQ interval, QRS complex, and corrected QT interval.22
As far as electrophysiological properties are concerned, antazoline can be classified as a quinidine‐like agent similar to Vaughan‐Williams class Ia.22 Its action is mediated by blockage of sodium and potassium channels.22 In the study performed during ablation of supraventricular arrhythmias, Bińkowski et al showed that increasing the total dose of antazoline led to the reduction of SR cycle duration and prolongation of corrected QT interval, HV time, intra‐ and interatrial conduction times and increase of right and left atrial effective refractory period.23 Antazoline did not interfere with AH time, Wenckebach point, atrioventricular node effective refractory period, and sinus node recovery period.23 Therefore, this study corroborated a unique property of antazoline mesylate: It does not cause impairment of sinus node function and atrioventricular conduction,23 which may explain no episodes of clinically significant bradycardia in the CANT study and low incidence of these complications in former trials.16, 17, 18
Last but not least, the present results of logistic regression analysis provided evidence that a higher heart rate in the course of an AF episode may paradoxically facilitate rhythm conversion both in the whole population and among patients who received antazoline. On the other hand, the additional use of other AADs was independently associated with a lower chance of SR restoration. These data may be of use in the setting of the ED, as they undermine the benefits of adjunct use of β‐blockers and lowering heart rate. The need for additional AAD use was associated with a lower chance of rhythm conversion, as it presumably identified patients resistant to pharmacological cardioversion.
Based on the results of the CANT study, antazoline emerges as a new, preferably monotherapeutic option, which could be used in patients presenting with tachyarrhythmia in the course of AF. Although antazoline was also efficacious and safe among patients with CAD and depressed left ventricular function, more safety data are required before wide application of antazoline in patients with structural heart disease.
Study Limitations
The study design was retrospective and nonrandomized; hence, it did not compensate for uneven distribution of risk factors between subgroups. Still, the study was high volume and reflected a real‐life approach to acute rhythm management in a tertiary reference center. The use of AAD did not always adhere to guidelines because of the real‐life nature of the study (eg, 3 patients in the propafenone group had depressed LVEF). The efficacy of amiodarone could be underestimated given possible delayed conversion to SR in some patients. The primary end point did not have a predefined maximal time for rhythm conversion to occur, and data concerning median time to cardioversion were unavailable. The study did not evaluate other AADs, most importantly ibutilide, flecainide, or vernakalant; however, these agents are currently unavailable in Poland. Nearly 20% of patients received 2 or more drugs, which might have distorted the properties of each evaluated agent.
Conclusion
Antazoline appears to be an efficacious and safe method of cardioversion in a real‐life setting. The use of antazoline was associated with a higher rate of successful cardioversion than amiodarone and a comparable success rate to propafenone according to the present results. Randomized controlled trials comparing antazoline to other AADs, most importantly vernakalant, amiodarone, and propafenone, are warranted to draw firmer recommendations concerning its use.
Disclosures
The study was neither funded nor supported by any external company or organization. The authors declare no conflict of interest regarding the contents of the article.
(J Am Heart Assoc. 2018;7:e010153: 10.1161/JAHA.118.010153.)
References
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogenstein C, Kelly AM, Mason S, Schneider S, Lang E, Clement CM, Stiell IG. An international view of how recent‐onset atrial fibrillation is treated in the emergency department. Acad Emerg Med. 2012;19:1255–1260. [DOI] [PubMed] [Google Scholar]
- 3. Hamilton A, Clark D, Gray A, Cragg A, Grubb N; Emergency Medicine Research Group, Edinburgh (EMERGE) . The epidemiology and management of recent‐onset atrial fibrillation and flutter presenting to the emergency department. Eur J Emerg Med. 2015;22:155–161. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 6. Gitt AK, Smolka W, Michailov G, Bernhardt A, Pittrow D, Lewalter T. Types and outcomes of cardioversion in patients admitted to hospital for atrial fibrillation: results of the German RHYTHM‐AF Study. Clin Res Cardiol. 2013;102:713–723. [DOI] [PubMed] [Google Scholar]
- 7. Kochiadakis GE, Igoumenidis NE, Hamilos ME, Marketou ME, Chlouverakis GI, Vardas PE. A comparative study of the efficacy and safety of procainamide versus propafenone versus amiodarone for the conversion of recent‐onset atrial fibrillation. Am J Cardiol. 2007;99:1721–1725. [DOI] [PubMed] [Google Scholar]
- 8. Heldal M, Atar D. Pharmacological conversion of recent‐onset atrial fibrillation: a systematic review. Scand Cardiovasc J Suppl. 2013;47:2–10. [DOI] [PubMed] [Google Scholar]
- 9. Camm AJ, Capucci A, Hohnloser SH, Torp‐Pedersen C, Van Gelder IC, Mangal B, Beatch G; AVRO Investigators . A randomized active‐controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent‐onset atrial fibrillation. J Am Coll Cardiol. 2011;57:313–321. [DOI] [PubMed] [Google Scholar]
- 10. Camm AJ. The vernakalant story: how did it come to approval in Europe and what is the delay in the U.S.A.? Curr Cardiol Rev. 2014;10:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinberg CJ. A new synthetic anti‐histaminic substance, antistine. Eye Ear Nose Throat Mon. 1947;26:639–641. [PubMed] [Google Scholar]
- 12. Kline SR, Dreifus LS, Watanabe Y, McGarry TF, Likoff W. Evaluation of the antiarrhythmic properties of antazoline: a preliminary study. Am J Cardiol. 1962;9:564–567. [DOI] [PubMed] [Google Scholar]
- 13. Kabela E, Mena MA, Martinez‐Lopez M, Mendez R. The action of the antihistaminic agents antazoline and meclizine on experimental arrhythmias. Acta Cardiol. 1967;22:113–127. [PubMed] [Google Scholar]
- 14. Gehring DA, Kehler JG. Conversion of atrial fibrillation with antazoline hydrochloride (Arithmin). Angiology. 1970;21:11–17. [DOI] [PubMed] [Google Scholar]
- 15. Frommeyer G, Sterneberg M, Dechering DG, Kaese S, Bögeholz N, Pott C, Fehr M, Bogossian H, Milberg P, Eckardt L. Effective suppression of atrial fibrillation by the antihistaminic agent antazoline: first experimental insights into a novel antiarrhythmic agent. Cardiovasc Ther. 2017;35:e12244. [DOI] [PubMed] [Google Scholar]
- 16. Maciag A, Farkowski MM, Chwyczko T, Beckowski M, Syska P, Kowalik I, Pytkowski M, Wozniak J, Dabrowski R, Szwed H. Efficacy and safety of antazoline in the rapid cardioversion of paroxysmal atrial fibrillation (the AnPAF Study). Europace. 2017;19:1637–1642. [DOI] [PubMed] [Google Scholar]
- 17. Farkowski MM, Maciąg A, Żurawska M, Pytkowski M, Kowalik I, Woźniak J, Sterliński M, Szwed H. Comparative effectiveness and safety of antazoline‐based and propafenone‐based strategies for pharmacological cardioversion of short‐duration atrial fibrillation in the emergency department. Pol Arch Med Wewn. 2016;126:381–387. [DOI] [PubMed] [Google Scholar]
- 18. Balsam P, Koźluk E, Peller M, Piątkowska A, Lodziński P, Kiliszek M, Kołtowski Ł, Grabowski M, Opolski G. Antazoline for termination of atrial fibrillation during the procedure of pulmonary veins isolation. Adv Med Sci. 2015;60:231–235. [DOI] [PubMed] [Google Scholar]
- 19. Piotrowski R, Kryński T, Baran J, Futyma P, Stec S, Kułakowski P. Antazoline for rapid termination of atrial fibrillation during ablation of accessory pathways. Cardiol J. 2014;21:299–303. [DOI] [PubMed] [Google Scholar]
- 20. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, Gupta D. The European Heart Rhythm Association symptom classification for atrial fibrillation validation and improvement through a simple modification. Europace. 2014;16:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giebułtowicz J, Piotrowski R, Baran J, Kułakowski P, Wroczyński P. Application of a novel liquid chromatography/tandem mass spectrometry method for the determination of antazoline in human plasma: result of ELEPHANT‐I [ELEctrophysiological, pharmacokinetic and hemodynamic effects of PHenazolinum (ANTazoline mesylate)] human pharmacokinetic study. J Pharm Biomed Anal. 2016;123:113–119. [DOI] [PubMed] [Google Scholar]
- 22. Piotrowski R, Giebułtowicz J, Baran J, Sikorska A, Gralak‐Łachowska D, Soszyńska M, Wroczyński P, Kułakowski P. Antazoline‐insights into drug‐induced electrocardiographic and hemodynamic effects: results of the ELEPHANT II substudy. Ann Noninvasive Electrocardiol. 2017;22:e12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bińkowski BJ, Makowski M, Kubiński P, Lubiński A. Effect of antazoline on electrophysiological properties of atrial muscle and conduction system of the heart. Cardiovasc Drugs Ther. 2018;32:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]


