Abstract
Background
The likelihoods of valvular heart disease (VHD) and conduction abnormalities in patients with ankylosing spondylitis (ASp) are poorly defined. Knowing their lifetime risks of VHD and pacemaker use would help inform whether cardiac screening should be done.
Methods and Results
Patients with ASp and a comparison group without ASp were identified among US Medicare beneficiaries in 1999 to 2013. Frequencies of VHD and pacemaker use were compared in 4 age groups: 65 to 69 years, 70 to 74 years, 75 to 79 years, and 80 years or older, as were rates of valve surgeries, a measure of VHD severity, and new pacemaker insertions. Outcomes were compared between 42 327 patients with ASp and 19 211 703 patients without ASp. The prevalence of aortic valve disease in patients with ASp increased with age (2.6%, 6.7%, 10.9%, and 17.1%), as did the prevalence of mitral valve disease. Risks of VHD were slightly but significantly higher in patients with ASp (adjusted odds ratios 1.06–1.51). Rates of aortic valve replacement/repair were also higher in patients with ASp than in the comparison group (125 versus 93; 183 versus 149; 261 versus 208; 279 versus 191 per 100 000 patient‐years in the 4 age groups). Rates of mitral valve surgery did not differ between groups. Among patients with ASp, pacemaker use ranged from 1.0% to 7.6% across age groups, and was slightly higher than in controls (odds ratio range 1.11–1.32).
Conclusions
Lifetime risks of VHD and pacemaker use in ASp increase markedly with age, but are only slightly higher than in elderly people without ASp.
Keywords: ankylosing spondylitis, aortic valve replacement, pacemaker, valvular heart disease
Subject Categories: Valvular Heart Disease, Aging, Quality and Outcomes
Clinical Perspective
What Is New?
In this US population‐based study, the lifetime prevalence of valvular heart disease and pacemaker use in elderly patients with ankylosing spondylitis was only slightly higher than in controls.
What Are the Clinical Implications?
Absence of a highly increased risk of valvular heart disease or need for permanent pacemaker placement does not support screening for subclinical heart disease in patients with ankylosing spondylitis.
Among the cardiac conditions that occur in patients with ankylosing spondylitis (ASp), valvular heart disease (VHD) and conduction blocks are notable because they have been specifically linked to aortitis and HLA‐B27.1, 2, 3, 4 Inflammation and fibrosis of the aortic root, cardiac valves, and the subaortic and interventricular septum can result in dilation of the aortic outflow tract, valve thickening and insufficiency, and occasionally, high‐degree heart block. Up to 82% of patients with ASp may have some structural abnormality on echocardiography, although most abnormalities are subclinical.5, 6, 7 In case series, echocardiographic evidence of aortic insufficiency was present in 0% to 34% of patients, while the prevalence of mitral insufficiency ranged from 5% to 74%.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Valvular insufficiency was typically mild, and the frequency increased with age and the duration of ASp. The prevalence of clinically recognized VHD in ASp is less well established, ranging from 1% to 12%.12, 15, 16 Conduction disturbances have been reported to affect 1% to 35% of patients with ASp, depending on the degree of disturbance included, while complete heart block affects 0% to 9% of patients.5, 7, 10, 14, 16, 17, 18, 19, 20 The burden of symptomatic conduction disturbances in ASp has not been established.
The occurrence of VHD and conduction disturbances in ASp has raised the question of whether asymptomatic patients should be screened for these conditions.7, 14 Knowledge of the lifetime risk of clinically relevant VHD and conduction blocks is needed to inform this question. Because VHD and conduction disturbances in ASp are age dependent, previous studies of age‐unselected cohorts do not provide accurate estimates of the potential impact of these abnormalities, because it is not clear how many of the younger patients studied might later develop these problems. To determine the cumulative burden of these manifestations, we examined the likelihood of VHD in a population‐based sample of elderly patients with ASp. We also examined the incidence of valve replacement or valve revision surgeries as a measure of the severity of VHD, and cardiac pacemaker use as a measure of symptomatic cardiac conduction or rhythm abnormalities. High cumulative relative risks of VHD or pacemaker use would satisfy 1 important criterion for the value of screening.21
Methods
Data Source and Study Design
Analyses were based on 100% fee‐for‐service Medicare data, the federally funded medical insurance program for elderly Americans, from 1999 to 2013. The data included information on inpatient hospitalizations, including diagnosis codes (up to 25 per hospitalization) and procedure codes (up to 25 per hospitalization), outpatient visits (up to 13 possible diagnosis codes per visit), and outpatient procedures. Diagnoses and inpatient procedures were coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes, while outpatient procedures were coded using ICD‐9 codes or the Healthcare Common Procedure Coding System. The study used a cross‐sectional design to evaluate the prevalence of VHD and pacemaker use, and a longitudinal cohort design to examine the incidence of valve surgeries and new pacemaker insertions.
The study was approved by the National Institute of Arthritis and Musculoskeletal and Skin Disease institutional review board. Informed consent was not required. The US Centers for Medicare & Medicaid Services provided access to the data through a user agreement, and the author had full access to the data. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, as this is prohibited by the data use agreement with the Centers for Medicare & Medicaid Services.
Identification of Patients With ASp and Controls
First we identified patients who had 2 or more inpatient or outpatient diagnosis claims for ASp (ICD‐9 code 720.0) at least 7 days apart on face‐to‐face encounters with a physician across all years of data (N=89 683). Two previous validation studies reported that this definition had a positive predictive value for ASp of 1.00 and 0.89, respectively.22, 23 Because of the interest in lifetime risks of heart disease, the study was limited to patients age 65 years and older (N=53 945). All patients were assumed to have prevalent ASp at entry. To further increase the specificity of the cohort, patients who had 2 or more diagnosis codes for rheumatoid arthritis (ICD‐9 code 714.0) or psoriatic arthritis (ICD‐9 code 696.0) were excluded, for a final sample of 42 327 patients with ASp.
Controls were a 10% stratified random sample of Medicare beneficiaries in 1999 to 2013 who had no claims coded for ASp. Because women predominate in Medicare and because the prevalence of VHD differs by sex, men were oversampled to make the sex composition of the control group more comparable to the ASp group (N=19 211 703).
Heart Disease Outcomes
All inpatient and outpatient claims were searched for relevant diagnosis codes for VHD. VHD was considered to be present from the time of the second recorded diagnosis (with a minimal interval between claims of 7 days) to the end of follow‐up. The definition of VHD included ICD‐9 codes for mitral valve disease (394.x, 424.0), aortic valve disease (395.x, 424.1), both mitral and aortic valve disease (396.x), tricuspid or pulmonic valve disease (397.x), other rheumatic heart disease (398.9), and history of valve replacement (V42.2, V43.3). A previous validation study reported that presence of a single inpatient diagnosis code in this group had a positive predictive value of 0.93 for VHD.24 Separately, mitral valve disease and aortic valve disease were identified using the specific codes above.
To examine serious and symptomatic conduction and rhythm abnormalities, we abstracted data on the use of permanent cardiac pacemakers, as defined by 2 or more inpatient or outpatient diagnosis codes for the presence of a pacemaker (V45.01) during the beneficiary's tenure in Medicare. To validate this definition, we identified 863 075 Medicare beneficiaries who met this definition in 2013 and 18 519 897 beneficiaries who did not, and who had been enrolled in Medicare since 2006. We then searched for procedure codes for pacemaker insertion or replacement in both groups in 2006 to 2013, which were present in 1 618 824 people. The presence of 2 diagnosis codes of V45.01 had a specificity of 0.99 and positive predictive value of 0.89 for having had a pacemaker insertion or replacement procedure sometime in the previous 8 years.
Procedure codes were used to identify the following interventions: mitral valve replacement or repair (ICD‐9 codes 35.02, 35.12, 35.23, 35.24, 35.97, and among hospitalizations with mitral valve disease diagnoses, 35.96), aortic valve replacement or repair or annuloplasty (ICD‐9 codes 35.01, 35.05, 35.06, 35.11, 35.21, 35.22, 35.33, and among hospitalizations with aortic valve disease diagnoses, 35.96), and pacemaker insertion (ICD‐9 codes 37.80, 37.81, 37.82, 27.83, 00.50, or Healthcare Common Procedure Coding System/Current Procedural Terminology codes 0387T, 33206, 33207, 33208, 33212, 33213, 33216, 33217, 33221, 33224, 33225).
Statistical Analysis
The prevalence of VHD and pacemaker use was estimated in 4 age groups: 65 to 69 years; 70 to 74 years; 75 to 79 years; and 80 years and older. Because Medicare beneficiaries contributed data over several years, 1 year was randomly selected for each patient and control as the basis on which to compute prevalences. Logistic regression was used to compute odds ratios of VHD and pacemaker use between patients with ASp and controls, adjusting for sex and race within age strata. Analyses were also repeated separately for men and women.
The incidence of aortic valve surgery, mitral valve surgery, and pacemaker insertion over the study period in both the ASp group and controls was also compared. For this analysis, subjects with prior valve surgery (ICD‐9 codes V42.2 or V43.3) or pacemaker use (V45.01) at entry to the cohort were excluded. Person‐years were based on periods of full Medicare coverage. Incidence rates for these procedures were computed by age group. ASp rates were sex‐ and race‐standardized to the distribution of the control group, as were relative risks for the ASp group compared with controls. Additionally, incidences in three 5‐year periods (1999–2003; 2004–2008; 2009–2013) were computed to determine trends in incidence over time.
SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. Because of the large sample, the effect size is a more meaningful indicator of association than P values. Based on correspondences with Cohen's d of 0.2, 0.5, and 0.8, an odds ratio of 1.52 was considered to represent a small effect, 2.74 to represent a medium effect, and 4.72 to represent a large effect, for associations when the prevalence in the control group was 5%.25 Odds ratios of 1.46, 2.49, and 4.13 were considered to represent small, medium, and large effects, respectively, for associations when the prevalence in the control group was 10%.25
Results
Likelihood of VHD and Pacemaker Use
Patients with ASp were predominantly white men, with an even distribution among the 4 age groups (Table 1). The demographic characteristics of the control group were comparable.
Table 1.
Characteristics of Patients With ASp and Controls
| ASp (N=42 327) | Controls (N=19 211 703) | |
|---|---|---|
| Age 65–69 y | 11 477 (27.1%) | 6 052 778 (31.5%) |
| Age 70–74 y | 9097 (21.5%) | 4 285 599 (22.3%) |
| Age 75–79 y | 8631 (20.4%) | 3 424 534 (17.8%) |
| Age 80 y or older | 13 122 (31.0%) | 5 448 792 (28.4%) |
| Men | 26 796 (63.3%) | 11 438 817 (59.5%) |
| White | 38 167 (90.2%) | 16 573 134 (86.3%) |
| Black | 1863 (4.4%) | 1 538 000 (8.0%) |
| Hispanic | 542 (1.3%) | 368 637 (1.9%) |
| Asian | 776 (1.8%) | 339 034 (1.8%) |
| Other | 979 (2.3%) | 392 898 (2.0%) |
Values are number (percent). The numbers for each age group represents the number of patients and controls in these groups in the prevalence analysis. ASp indicates ankylosing spondylitis.
The prevalence of clinically diagnosed VHD among patients with ASp increased from 6.4% in those age 65 to 69 to 29.2% in those age 80 or older (Table 2). Prevalences were slightly but significantly higher in those with ASp compared with controls, with the sex‐race‐adjusted odds ratio of 1.47 in the 75‐ to 79‐year age group and the 80‐year and older age group approaching a small effect. Results were similar in men and women.
Table 2.
Prevalence of VHD and Pacemaker Use in Patients With ASp and Controls, by Age Group and Sex
| All | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ASp (%) | Controls (%) | OR (95% CI)a | ASp (%) | Controls (%) | OR (95% CI)a | ASp (%) | Controls (%) | OR (95% CI)a | |
| Valvular heart disease | |||||||||
| Age 65–69 y | 6.4 | 5.7 | 1.15 (1.06, 1.24) | 6.3 | 5.7 | 1.13 (1.03, 1.24) | 6.6 | 5.7 | 1.19 (1.04, 1.35) |
| Age 70–74 y | 15.4 | 11.6 | 1.40 (1.32, 1.48) | 14.8 | 11.5 | 1.33 (1.23, 1.43) | 16.8 | 11.6 | 1.55 (1.40, 1.71) |
| Age 75–79 y | 21.9 | 16.0 | 1.47 (1.39, 1.55) | 21.6 | 16.2 | 1.41 (1.32, 1.51) | 22.6 | 15.7 | 1.57 (1.44, 1.71) |
| Age 80 y or older | 29.2 | 21.8 | 1.47 (1.41, 1.53) | 29.2 | 22.7 | 1.39 (1.31, 1.46) | 29.4 | 20.8 | 1.58 (1.49, 1.68) |
| Aortic valve disease | |||||||||
| Age 65–69 y | 2.6 | 2.1 | 1.24 (1.10, 1.40) | 2.9 | 2.3 | 1.30 (1.14, 1.49) | 2.0 | 1.8 | 1.08 (0.86, 1.36) |
| Age 70–74 y | 6.7 | 4.7 | 1.41 (1.30, 1.54) | 7.1 | 5.1 | 1.41 (1.28, 1.56) | 5.8 | 4.2 | 1.41 (1.20, 1.66) |
| Age 75–79 y | 10.9 | 7.4 | 1.51 (1.41, 1.62) | 11.3 | 8.0 | 1.44 (1.32, 1.57) | 10.2 | 6.4 | 1.65 (1.47, 1.86) |
| Age 80 y or older | 17.1 | 11.8 | 1.51 (1.43, 1.58) | 18.0 | 13.0 | 1.45 (1.36, 1.55) | 15.8 | 10.6 | 1.58 (1.47, 1.70) |
| Mitral valve disease | |||||||||
| Age 65–69 y | 3.8 | 3.6 | 1.06 (0.96, 1.17) | 3.3 | 3.4 | 0.98 (0.86, 1.11) | 4.7 | 4.0 | 1.20 (1.03, 1.40) |
| Age 70–74 y | 9.8 | 7.7 | 1.31 (1.22, 1.41) | 8.7 | 7.3 | 1.20 (1.10, 1.32) | 12.1 | 8.3 | 1.53 (1.37, 1.72) |
| Age 75–79 y | 14.1 | 10.7 | 1.37 (1.29, 1.46) | 13.0 | 10.4 | 1.28 (1.18, 1.39) | 16.0 | 11.1 | 1.53 (1.39, 1.69) |
| Age 80 y or older | 19.2 | 14.3 | 1.41 (1.35, 1.48) | 18.2 | 14.5 | 1.30 (1.22, 1.38) | 20.5 | 14.2 | 1.56 (1.46, 1.67) |
| Pacemaker use | |||||||||
| Age 65–69 y | 1.0 | 0.9 | 1.11 (0.91, 1.33) | 1.1 | 1.1 | 1.02 (0.82, 1.28) | 0.8 | 0.6 | 1.38 (0.97, 1.96) |
| Age 70–74 y | 2.5 | 1.8 | 1.32 (1.16, 1.52) | 2.9 | 2.1 | 1.34 (1.15, 1.56) | 1.6 | 1.3 | 1.24 (0.92, 1.67) |
| Age 75–79 y | 4.0 | 3.1 | 1.29 (1.15, 1.44) | 4.8 | 3.6 | 1.32 (1.17, 1.50) | 2.6 | 2.2 | 1.18 (0.94, 1.48) |
| Age 80 y or older | 7.6 | 5.9 | 1.29 (1.21, 1.38) | 9.3 | 7.1 | 1.32 (1.21, 1.43) | 5.5 | 4.5 | 1.23 (1.10, 1.39) |
ASp indicates ankylosing spondylitis; CI, confidence interval; OR, odds ratio; VHD, valvular heart disease.
OR (95% CI), adjusted for sex and race.
Among patients with ASp, the prevalence of aortic valve disease increased from 2.6% in those age 65 to 69 to 17.1% in those age 80 or older. Odds of aortic valve disease were slightly higher in patients with ASp than in controls in all age groups, with those in the 2 oldest groups representing a small effect. Mitral valve disease was also slightly more prevalent in patients with ASp than in controls in all ages, although none met criteria for a small effect. Aortic valve disease tended to be more common in men than women in both the ASp and control groups, while mitral valve disease tended to be more common among women.
Pacemakers were used by 1% of patients with ASp in those age 65 to 69 years, comparable to the prevalence in controls. The prevalence of pacemaker use increased to 7.6% in those age 80 or older, and was slightly more common in ASp than controls in the older groups. However, the association was less than a small effect in all age groups.
Incidence of Valve Procedures and Pacemaker Insertions
The incidence of aortic valve replacement or repair was 125 per 100 000 person‐years in those age 65 to 69 with ASp (Table 3). This rate increased to 279 per 100 000 person‐years in those age 80 years or older. Rates were slightly higher in patients with ASp than in controls in all age groups, with sex‐race‐standardized relative risks ranging from 1.22 to 1.46. Results were comparable in both men and women (Table 4). Transcatheter aortic valve replacement was done in 8.3% of patients with ASp and 4.4% of those in the comparison group.
Table 3.
Incidence of Aortic and Mitral Valve Replacement or Repair Surgery and Pacemaker Insertions in Patients With ASp and Controls, by Age Group
| Number of Events | Person‐Years | Incidence (per 100 000 Person‐Years)a | Relative Risk (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| ASp | Controls | ASp | Controls | ASp | Controls | ||
| Aortic valve procedures | |||||||
| Age 65–69 y | 106 | 38 816 | 79 483 | 41 540 518 | 125 | 93 | 1.34 (1.10, 1.63) |
| Age 70–74 y | 172 | 60 581 | 91 344 | 40 530 915 | 183 | 149 | 1.22 (1.05, 1.43) |
| Age 75–79 y | 241 | 72 126 | 90 032 | 34 641 013 | 261 | 208 | 1.25 (1.10, 1.43) |
| Age 80 y or older | 364 | 92 682 | 126 723 | 48 638 286 | 279 | 191 | 1.46 (1.32, 1.63) |
| Mitral valve surgery | |||||||
| Age 65–69 y | 46 | 19 262 | 84 638 | 41 574 544 | 54 | 46 | 1.17 (0.87, 1.57) |
| Age 70–74 y | 69 | 26 460 | 92 504 | 40 635 240 | 73 | 65 | 1.12 (0.88, 1.43) |
| Age 75–79 y | 67 | 27 837 | 88 731 | 34 806 678 | 72 | 80 | 0.90 (0.71, 1.15) |
| Age 80 y or older | 72 | 24 097 | 129 964 | 48 995 532 | 55 | 49 | 1.11 (0.88, 1.41) |
| Pacemaker insertion | |||||||
| Age 65–69 y | 299 | 113 382 | 81 609 | 41 024 679 | 359 | 276 | 1.30 (1.16, 1.46) |
| Age 70–74 y | 612 | 199 191 | 89 928 | 39 656 212 | 668 | 502 | 1.33 (1.22, 1.44) |
| Age 75–79 y | 886 | 271 375 | 85 736 | 33 455 987 | 1004 | 811 | 1.24 (1.15, 133) |
| Age 80 y or older | 1758 | 595 307 | 118 002 | 45 463 679 | 1460 | 1309 | 1.11 (1.06, 1.17) |
ASp indicates ankylosing spondylitis; CI, confidence interval.
Sex and race standardized to the distribution of the controls in each age group.
Table 4.
Incidence of Aortic and Mitral Valve Replacement or Repair Surgery and Pacemaker Insertions in Men and Women With ASp and Control Group Without ASp, by Age Group
| Number of Events | Incidence (per 100 000 Patient‐Years)a | Relative Risk (95% CI)a | |||
|---|---|---|---|---|---|
| ASp | Control | ASp | Control | ||
| Aortic valve procedures | |||||
| Men | |||||
| Age 65–69 y | 84 | 29 527 | 150 | 113 | 1.33 (1.07, 1.65) |
| Age 70–74 y | 132 | 45 511 | 212 | 182 | 1.16 (0.98, 1.38) |
| Age 75–79 y | 173 | 52 466 | 295 | 256 | 1.15 (0.99, 1.34) |
| Age 80 y or older | 224 | 64 017 | 311 | 256 | 1.21 (1.06, 1.39) |
| Women | |||||
| Age 65–69 y | 22 | 9289 | 77 | 60 | 1.27 (0.83, 1.94) |
| Age 70–74 y | 40 | 15 070 | 126 | 97 | 1.29 (0.95, 1.77) |
| Age 75–79 y | 68 | 19 660 | 202 | 139 | 1.45 (1.14, 1.84) |
| Age 80 y or older | 140 | 28 665 | 240 | 121 | 1.98 (1.67, 2.34) |
| Mitral valve surgery | |||||
| Men | |||||
| Age 65–69 y | 32 | 12 823 | 57 | 49 | 1.17 (0.82, 1.66) |
| Age 70–74 y | 49 | 17 335 | 78 | 69 | 1.13 (0.85, 1.50) |
| Age 75–79 y | 51 | 17 885 | 86 | 87 | 1.00 (0.75, 1.32) |
| Age 80 y or older | 49 | 15 206 | 67 | 60 | 1.12 (0.84, 1.48) |
| Women | |||||
| Age 65–69 y | 14 | 6439 | 49 | 42 | 1.16 (0.69, 1.98) |
| Age 70–74 y | 20 | 9125 | 63 | 59 | 1.07 (0.69, 1.67) |
| Age 75–79 y | 16 | 9952 | 47 | 70 | 0.68 (0.41, 1.11) |
| Age 80 y or older | 23 | 8891 | 39 | 37 | 1.04 (0.69, 1.58) |
| Pacemaker insertion | |||||
| Men | |||||
| Age 65–69 y | 234 | 84 312 | 426 | 327 | 1.30 (1.14, 1.48) |
| Age 70–74 y | 487 | 144 949 | 807 | 596 | 1.35 (1.23, 1.49) |
| Age 75–79 y | 662 | 190 474 | 1192 | 971 | 1.22 (1.13, 1.33) |
| Age 80 y or older | 1185 | 378 401 | 1823 | 1648 | 1.10 (1.04, 1.18) |
| Women | |||||
| Age 65–69 y | 65 | 29 070 | 229 | 190 | 1.20 (0.94, 1.54) |
| Age 70–74 y | 125 | 54 242 | 399 | 354 | 1.13 (0.94, 1.35) |
| Age 75–79 y | 224 | 80 901 | 685 | 585 | 1.17 (1.02, 1.34) |
| Age 80 y or older | 573 | 216 906 | 1035 | 964 | 1.07 (0.98, 1.17) |
ASp indicates ankylosing spondylitis; CI, confidence interval.
Race‐standardized to the composition of the control group in each age‐sex group.
Mitral valve replacement or repair was less than one‐half as common as the incidence of aortic valve surgery in all age groups (Table 3). The incidence of mitral valve surgery was not statistically different between patients with ASp and controls in any age group. Results were similar for mitral valve replacement specifically (Table S1).
Insertion of new permanent cardiac pacemakers increased dramatically with age in both the ASp and control groups, reaching incidences of 1460 and 1309 per 100 000 person‐years, respectively, in those age 80 years or older (Table 3). The most common diagnoses associated with pacemaker insertions in patients with ASp were sinoatrial node dysfunction in 41.8%, complete heart block in 11.4%, atrial fibrillation in 7.6%, and second‐degree heart block in 5.2%. The same diagnoses were identified as most common in the controls (40.0%, 11.7%, 7.9%, and 4.3%, respectively). In each age group, the sex‐race‐standardized relative risk of new pacemaker insertion was slightly higher among patients with ASp than controls. Risks were slightly higher among men than women (Table 4).
Trends in Incidence
The incidence of aortic valve replacement or repair increased substantially over time in patients with ASp, but because comparable increases occurred in the controls, the relative risks of aortic valve surgery remained constant over time (Figure 1). The incidence of mitral valve surgery was stable, while there were small decreases in the incidence of pacemaker insertions over time in patients with ASp, with slight decreases in relative risks in the younger 3 age groups (Figures 2 and 3).
Figure 1.
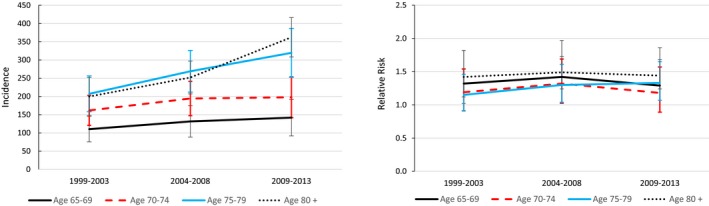
Trends in the incidence of aortic valve procedures per 100 000 patient‐years in 1999 to 2003, 2004 to 2008, and 2009 to 2013, and corresponding sex‐ and race‐standardized relative risks compared with controls.
Figure 2.
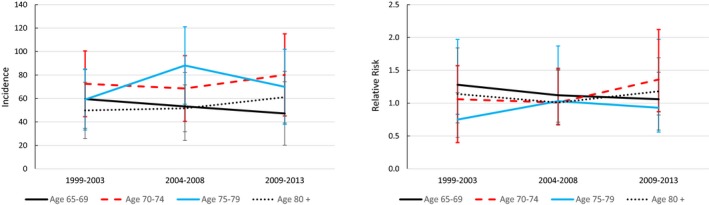
Trends in the incidence of mitral valve surgery per 100 000 patient‐years in 1999 to 2003, 2004 to 2008, and 2009 to 2013, and corresponding sex‐ and race‐standardized relative risks compared with controls.
Figure 3.
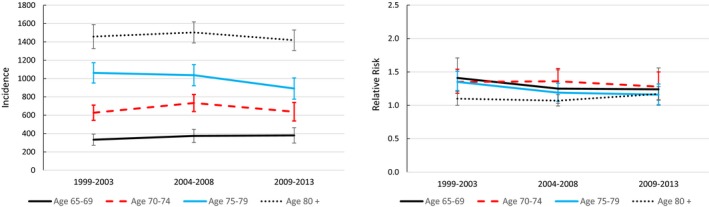
Trends in the incidence of pacemaker insertion procedures per 100 000 patient‐years in 1999 to 2003, 2004 to 2008, and 2009 to 2013, and corresponding sex‐ and race‐standardized relative risks compared with controls.
Discussion
In this population‐based sample of elderly patients with ASp, the likelihood of clinically diagnosed VHD was age dependent. Among those age 65 to 69 years, aortic valve disease and mitral valve disease were each present in fewer than 4% of patients, but approached 20% among those age 80 or older. Risks of VHD were higher among patients with ASp than controls in all age groups. Rates of aortic valve surgery, an indicator of more severe valve disease, were also slightly higher among patients with ASp, but rates of mitral valve surgery did not differ between groups. Associations between ASp and VHD were no greater than small effects.
Previous studies have not addressed the cumulative burden of VHD in ASp. Studies have largely focused on echocardiographic evidence of VHD.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Most echocardiographic abnormalities detected have been mild, and the ultimate clinical consequences of these abnormalities are uncertain. Studies also examined patients from rheumatology departments, and therefore may have included more severely affected patients.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Additionally, few studies reported age‐specific prevalences. Because VHD occurs much more commonly in older people,26 prevalences in these studies will be largely dependent on the age composition of the cohort.7 As reported in the early study of Graham, the prevalence of aortic insufficiency was 4% in ASp patients under age 30 years and 18% in those over age 50 years.15
A recent study of administrative data from Canada reported prevalences of aortic valve disease of 2.6% and 1.4% in men and women with ASp age 60 years or older, respectively, comparable to those of population controls.27 This study also reported prevalences of nonaortic valve disease of 9.1% and 5.7% in men and women with ASp age 60 or older, respectively, somewhat higher than those of controls. These prevalences were substantially lower than those found in our study, possibly because of different definitions of VHD, or that outpatient visits allowed for only a single diagnosis code. Their results were also based on a cohort defined by the presence of a single diagnosis code for ASp, which may have underestimated the prevalence of VHD by including some patients without ASp. Bengtsson et al recently reported aortic insufficiency in 1.2% and pacemaker use in 1.2% of adults in Sweden with ASp, but only 27% of their cohort was age 60 years or older, and age‐stratified results were not reported.28 New occurrences of aortic insufficiency and pacemaker insertions were about twice as likely among patients with ASp than controls. These relative risks may have been heavily influenced by results in younger patients, as these conditions are rare in the general population under age 60 years.
Among Medicare beneficiaries, although the prevalence of VHD was generally higher in patients with ASp than controls, the magnitude of the association was, at most, small. Similarly, while the higher incidence of aortic valve surgery in patients with ASp indicated that aortic valve disease tended to be more severe in ASp, the risk of aortic valve surgery was only modestly increased in those with ASp. Rates of aortic valve replacement and repair have increased recently in the general population, in part because of the introduction of percutaneous techniques.29 Similar increases occurred in patients with ASp, so that relative risks were stable over time. The transcatheter approach may be favored over a surgical approach in more patients with ASp given the likelihood of a fixed chest wall and difficulty with intubation for general anesthesia. Risks of mitral valve surgery were not higher in patients with ASp. Together, these results suggest that the cumulative burden of VHD is only slightly higher in elderly patients with ASp than those without ASp.
The prevalence of pacemaker use, an indicator of clinically important cardiac conduction or rhythm abnormalities, in ASp ranged from 1% in those age 65 to 69 years to 7.6% in those age 80 years or older. Odds of pacemaker use and the incidence of pacemaker insertions were slightly higher in patients with ASp than in controls in each age group. Pacemaker use was more common among men, as has been found in the general population.30 Although conduction blocks are specifically linked to ASp, the rhythm or conduction abnormalities reported at the time of pacemaker placement were similar in patients with ASp and controls, indicating that the disorders common in the general population also predominate in patients with ASp. Previous studies suggested that the prevalence of high‐degree atrioventricular block in ASp is 1% among all ages.28, 31 Although sinoatrial node dysfunction was the most common indication for pacemaker placement among elderly patients with ASp, conduction blocks may be a more specific finding in younger patients, who were not included here.
The strengths of this study include the population‐based sample, examination of both prevalence of disease and incident procedures, and examination of trends in procedures over time. The study focused on elderly patients to examine the lifetime risks of VHD and pacemaker use. The results may be susceptible to survival bias, as patients needed to have lived to age 65 years to be included. However, deaths before age 65 years because of VHD are likely uncommon. Relative risks may be higher in younger patients with ASp, given the rarity of VHD and pacemaker use in those under age 60 years in the general population. The estimates of VHD may be affected by ascertainment bias if evidence of VHD was sought more frequently in patients with ASp. Ascertainment bias would not be expected to affect surgery rates or pacemaker use. The prevalence of pacemaker use may be somewhat underestimated given the sensitivity of our definition of 0.53. Pacemaker insertions were not differentiated as to whether or not they were related to cardiac surgery. The study did not examine the frequency of sudden deaths because of conduction disturbances. Lastly, as in any study of administrative data, misclassification of diagnoses could be present, although steps were taken to increase the specificity of the ASp group.
A higher than expected prevalence of disease is a prerequisite for the development of targeted screening approaches for a risk group.21 That the risks of VHD and pacemaker use were only slightly higher among elderly patients with ASp in this population‐based study does not support the development of screening strategies using echocardiography or electrocardiography in asymptomatic patients. These investigations should be reserved for patients with suggestive signs or symptoms.
Sources of Funding
This work was supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (ZIA‐AR‐041153).
Disclosures
None.
Supporting information
Table S1. Incidence of Mitral Valve Replacement in Patients With Ankylosing Spondylitis (ASp) and Control Group Without ASp, by Age Group
(J Am Heart Assoc. 2018;7:e010016 DOI: 10.1161/JAHA.118.010016)
References
- 1. O'Neill TW, Bresnihan B. The heart in ankylosing spondylitis. Ann Rheum Dis. 1992;51:705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergfeldt L. HLA‐B27‐ associated cardiac disease. Ann Intern Med. 1997;127:621–629. [DOI] [PubMed] [Google Scholar]
- 3. Bergfeldt L, Insulander P, Lindblom D, Möller E, Edhag O. HLA‐B27: an important genetic risk factor for lone aortic regurgitation and severe conduction system abnormalities. Am J Med. 1988;85:12–18. [DOI] [PubMed] [Google Scholar]
- 4. Bergfeldt L, Möller E. Complete heart block—another HLA B27 associated disease manifestation. Tissue Antigens. 1983;21:385–390. [DOI] [PubMed] [Google Scholar]
- 5. Roldan CA, Chavez J, Wiest PW, Qualis CR, Crawford MH. Aortic root disease and valve disease associated with ankylosing spondylitis. J Am Coll Cardiol. 1998;32:1397–1404. [DOI] [PubMed] [Google Scholar]
- 6. Park SH, Sohn IS, Joe BH, Hwang HJ, Park CB, Jin ES, Cho JM, Kim CJ, Bae JH, Lee SH. Early cardiac valvular changes in ankylosing spondylitis: a transesophageal echocardiography study. J Cardiovasc Ultrasound. 2012;20:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klingberg E, Sveälv BG, Täng MS, Bech‐Hanssen O, Forsblad‐d'Elia H, Bergfeldt L. Aortic regurgitation is common in ankylosing spondylitis: time for routine echocardiography evaluation? Am J Med. 2015;128:1244–1250. [DOI] [PubMed] [Google Scholar]
- 8. Tucker CR, Fowles RE, Calin A, Popp RL. Aortitis in ankylosing spondylitis: early detection of aortic root abnormalities with two dimensional echocardiography. Am J Cardiol. 1982;49:680–686. [DOI] [PubMed] [Google Scholar]
- 9. Thomas D, Hill W, Geddes R, Sheppard M, Arnold J, Fritzsche J, Brooks PM. Early detection of aortic dilatation in ankylosing spondylitis using echocardiography. Aust N Z J Med. 1982;12:10–13. [DOI] [PubMed] [Google Scholar]
- 10. O'Neill TW, King G, Graham IM, Molony J, Bresnihan B. Echocardiographic abnormalities in ankylosing spondylitis. Ann Rheum Dis. 1992;51:652–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnason JA, Patel AK, Rahko PS, Sundstrom WR. Transthoracic and transesophageal echocardiographic evaluation of the aortic root and subvalvular structures in ankylosing spondylitis. J Rheumatol. 1996;23:120–123. [PubMed] [Google Scholar]
- 12. Gran JT, Skomsvoll JF. The outcome of ankylosing spondylitis: a study of 100 patients. Rheumatology. 1997;36:766–771. [DOI] [PubMed] [Google Scholar]
- 13. Alves MG, Espirito‐Santo J, Queiroz MV, Madeira H, Macieira‐Coelho E. Cardiac alterations in ankylosing spondylitis. Angiology. 1988;39:567–571. [DOI] [PubMed] [Google Scholar]
- 14. Brunner F, Kunz A, Weber U, Kissling R. Ankylosing spondylitis and heart abnormalities: do cardiac conduction disorders, valve regurgitation and diastolic dysfunction occur more often in male patients with diagnosed ankylosing spondylitis for over 15 years than in the normal population? Clin Rheumatol. 2006;25:24–29. [DOI] [PubMed] [Google Scholar]
- 15. Graham DC, Smythe HA. The carditis and aortitis of ankylosing spondylitis. Bull Rheum Dis. 1958;9:171–174. [PubMed] [Google Scholar]
- 16. Kinsella TD, Johnson LG, Sutherland RI. Cardiovascular manifestations of ankylosing spondylitis. CMAJ. 1974;111:1309–1311. [PMC free article] [PubMed] [Google Scholar]
- 17. Bergfeldt L, Edhag O, Vedin L, Vallin H. Ankylosing spondylitis: an important cause of severe disturbances of the cardiac conduction system: prevalence among 223 pacemaker‐treated men. Am J Med. 1982;73:187–191. [DOI] [PubMed] [Google Scholar]
- 18. Thomsen NH, Hørslev‐Petersen K, Beyer JM. Ambulatory 24‐hour continuous electrocardiographic monitoring in 54 patients with ankylosing spondylitis. Eur Heart J. 1986;7:240–246. [DOI] [PubMed] [Google Scholar]
- 19. Forsblad‐d'Elia H, Wallberg H, Klingberg E, Carlsten H, Bergfeldt L. Cardiac conduction system abnormalities in ankylosing spondylitis: a cross‐sectional study. BMC Musculoskeletal Disord. 2013; 14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dik VK, Peters MJ, Dijkmans PA, Van der Weijden MA, De Vries MK, Dijkmans BA, Van der Horst‐Bruinsma IE, Nurmohamed MT. The relationship between disease‐related characteristics and conduction disturbances in ankylosing spondylitis. Scand J Rheumatol. 2010;39:38–41. [DOI] [PubMed] [Google Scholar]
- 21. Obuchowski NA, Graham RJ, Baker ME, Powell KA. Ten criteria for effective screening: their application to multi‐slice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol. 2001;176:1357–1362. [DOI] [PubMed] [Google Scholar]
- 22. Singh JA, Holmgren AR, Krug H, Noorbaloochi S. Accuracy of the diagnoses of spondylarthritides in Veterans Affairs medical center databases. Arthritis Rheum. 2007;57:648–655. [DOI] [PubMed] [Google Scholar]
- 23. Dubreuil M, Peloquin C, Zhang Y, Choi HK, Inman RD, Neogi T. Validity of ankylosing spondylitis diagnoses in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2016;25:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birman‐Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 25. Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput. 2010;39:860–864. [Google Scholar]
- 26. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 27. Szabo SM, Levy AR, Rao SR, Kirbach SE, Lacaille D, Cifaldi M, Maksymowych WP. Increased risk of cardiovascular and cerebrovascular disease in individuals with ankylosing spondylitis. A population‐based study. Arthritis Rheum. 2011;63:3294–3304. [DOI] [PubMed] [Google Scholar]
- 28. Bengtsson K, Forsblad‐d'Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, Lindström U, Askling J, Jacobsson LTH. Risk of cardiac rhythm disturbances and aortic regurgitation in different spondyloarthritis subtypes in comparison with general population: a register‐based study from Sweden. Ann Rheum Dis. 2018;77:541–548. [DOI] [PubMed] [Google Scholar]
- 29. Barreto‐Filho JA, Wang Y, Dodson JA, Desai MM, Sugeng L, Geirsson A, Krumholz HM. Trends in aortic valve replacement for elderly patients in the United States, 1999‐2011. JAMA. 2013;310:2078–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown DW, Croft JB, Giles WH, Anda RF, Mensah GA. Epidemiology of pacemaker procedures among Medicare enrollees in 1990, 1995, and 2000. Am J Cardiol. 2005;95:409–411. [DOI] [PubMed] [Google Scholar]
- 31. Bremander A, Petersson IF, Bergman S, Englund M. Population‐based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res. 2011;63:550–556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Incidence of Mitral Valve Replacement in Patients With Ankylosing Spondylitis (ASp) and Control Group Without ASp, by Age Group


