Abstract
Background
Reduced ventricular function and decreased exercise capacity are widespread in adults with complete transposition of the great arteries after atrial switch (TGA‐Mustard/Senning) and congenitally corrected TGA (ccTGA). Advanced imaging techniques may help to better phenotype these patients and evaluate exercise cardiac response.
Methods and Results
Thirty‐three adults with a systemic right ventricle (70% TGA‐Mustard/Senning, 37±9 years of age, 24% female, 94% New York Heart Association class I‐II) underwent echocardiogram, cardiopulmonary exercise testing, and cardiovascular magnetic resonance imaging at rest and during a 4‐stage free‐breathing bicycle test. They were compared with 12 healthy controls (39±10 years of age, 25% female, all New York Heart Association class I). TGA‐Mustard/Senning patients had a higher global circumferential strain (−15.8±3.6 versus −11.2±5.2%, P=0.008) when compared with ccTGA, whereas global longitudinal strain and systemic right ventricle contractility during exercise were similar in both groups. Septal extracellular volume (ECV) in ccTGA was significantly higher than in TGA‐Mustard/Senning (30.2±2.0 versus 27.1±2.7%, P=0.005). During exercise, TGA‐Mustard/Senning had a fall in end‐diastolic volume and stroke volume (11% and 8%, respectively; both P≤0.002), whereas ccTGA could increase their stroke volume in the same way as healthy controls. Because of a greater heart rate reserve in TGA‐Mustard/Senning (P for interaction=0.010), cardiac index and peak oxygen uptake were similar between both patient groups.
Conclusions
Caution should be exercised when evaluating pooled analyses of systemic right ventricle patients, given the differences in myocardial contraction pattern, septal extracellular volume, and the exercise response of TGA‐Mustard/Senning versus ccTGA patients. Longitudinal follow‐up will determine whether abnormal exercise cardiac response is a marker of earlier failure.
Keywords: adult congenital heart disease, exercise testing, magnetic resonance imaging, transposition of great vessels
Subject Categories: Exercise Testing, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
This is the first demonstration under physiological near‐normal exercise conditions of a divergent cardiac response in transposition of the great arteries after atrial switch (TGA)‐Mustard/Senning patients as compared with congenitally corrected TGA, patients with the former having an abnormal stroke volume response.
There is a difference in myocardial contraction pattern and the degree of septal interstitial expansion between TGA‐Mustard/Senning and congenitally corrected TGA patients.
Exercise impairment in TGA‐Mustard/Senning patients is not related to myocardial contractility but to a lower heart rate response. This could be a pure chronotropic problem or a secondary phenomenon to prevent deterioration of the cardiac output by a further decrease in stroke volume.
What Are the Clinical Implications?
These findings suggest that caution should be exercised when evaluating pooled analyses of systemic right ventricle patients, given the differences in myocardial contraction pattern, septal interstitial expansion, and exercise response of TGA‐Mustard/Senning and congenitally corrected TGA patients.
Differing physiology during exercise would suggest that the effects of β‐blockers on exercise capacity and symptoms may be different between TGA‐Mustard/Senning and ccTGA patients.
Introduction
Transposition of the great arteries (TGA) has a worldwide reported birth prevalence of 0.31 per 1000 live births, which constitutes about 5% of all patients born with a congenital heart defect.1 Congenitally corrected TGA (ccTGA) is much rarer than complete TGA, with an incidence of 1 in 33 000 live births.2 Although patients with complete TGA after atrial switch procedure (TGA‐Mustard/Senning) and ccTGA represent a rather heterogeneous patient population, they are often pooled for analysis as “patients with a systemic RV (sRV).”3, 4, 5 Better phenotyping is needed because the course of these patients is not uneventful, especially once clinical heart failure occurs.6, 7, 8, 9, 10
Reduced exercise capacity is widespread in adults with congenital heart disease, even if they consider themselves asymptomatic.11, 12 The relation between parameters of systolic sRV function at rest and exercise performance is controversial.13, 14, 15, 16 Exercise imaging may be better suited to evaluate whether sRV contractility limits exercise performance. However, the echocardiographic assessment of cardiac function during exercise is difficult, especially when cardiac geometry is less uniform. Until recently, cardiac magnetic resonance (CMR)–based exercise imaging techniques were hampered by either the need for breath holding during an exercise break17 or the use of dobutamine stress to mimic the effects of exercise.18, 19 To overcome these limitations, we developed in our institution a free breathing, real‐time exercise cardiac magnetic resonance (exCMR) protocol and validated it against invasive standards allowing volumetric assessment of cardiac function during exercise.20, 21, 22, 23, 24, 25 With exCMR, we can evaluate whether the sRV filling impairment during dobutamine‐induced tachycardia in TGA‐Mustard/Senning patients26, 27, 28, 29, 30 persists in near‐normal physiological exercise conditions. Furthermore, CMR can be simultaneously used to assess myocardial deformation13 and interstitial expansion (which is a surrogate for diffuse myocardial fibrosis).31 Fibrosis could be an additional factor explaining filling impairment during exercise in TGA‐Mustard/Senning patients.
Therefore, we aimed in this study at phenotyping both TGA‐Mustard/Senning and ccTGA patients by evaluating systolic sRV function and ventricular filling–related parameters at rest and during exercise.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. All authors have full access to the raw and derived data sets and take responsibility for the integrity of the data analysis.
Study Population
From July 2015 until April 2017, patients with a sRV were invited to participate in this study when they visited the adult congenital heart disease outpatient clinic of the University Hospitals Leuven (Belgium) for their routine clinical follow‐up. Exclusion criteria were patients with New York Heart Association functional class IV, a functionally univentricular heart, a nonrestrictive ventricular septal defect, the presence of a pacemaker/implantable cardioverter defibrillator, or other contraindications for CMR imaging. Data from a cohort of healthy volunteers who previously performed the exCMR protocol in our institution were used to illustrate the exercise response of healthy individuals. The local Ethics Committee Research UZ/KU Leuven approved the study and written informed consent was obtained from all study participants.
Echocardiography
Echocardiographic examinations were acquired ≈2 hours after the exCMR using a Vivid E9 ultrasound system (General Electric Vingmed Ultrasound, Horten, Norway) and analyzed off‐line using dedicated software (EchoPAC; General Electric Vingmed Ultrasound, Horten, Norway). Longitudinal systolic sRV function was assessed by the tricuspid annular plane systolic excursion. The systemic atrioventricular valve (SAVV) regurgitation severity was assessed qualitatively by Doppler color flow mapping on the basis of size of the jet, and severity was graded on a 4‐point scale (none‐to‐trace, mild, moderate, or severe).32
Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing was performed within 3 months before the exCMR protocol on an upright cycle ergometer (eBike Comfort Ergometer; GE Healthcare, United States with MasterScreen™ CPX metabolic cart, CareFusion, United States) using a continuous ramp protocol until exhaustion. A 12‐lead ECG was continuously registered (Cardiosoft; CareFusion Corporation, United States). Breath‐by‐breath analysis provided measures of oxygen consumption at peak exercise (pVO2), ventilatory equivalent for carbon dioxide (VE/VCO2 ratio), and maximal power output in watts. Predicted pVO2 (%ppVO2), calculated using the Wasserman equation, was used as a measure of exercise performance. The anaerobic threshold was determined according to Binder et al.33 Having previously demonstrated that 66% of maximal power output in watts closely corresponds to the maximal sustainable exercise intensity in a supine position in the CMR bore, we defined exCMR efforts as: 0% (“rest”), 25% (“low intensity”), 50% (“moderate intensity”), and 66% (“peak intensity”) of maximal power output in watts.20, 21
CMR Equipment, Image Acquisition, and Analysis
CMR images were acquired with an Achieva 1.5‐T magnetic resonance unit (Philips Medical Systems, Best, The Netherlands). A detailed description of the CMR sequences can be found in Data S1. In brief, a stack of balanced steady‐state free precession breath‐hold cine‐images in the short‐axis and horizontal long‐axis plane were acquired with cardiac gating. Using the short‐axis image corresponding to the atrioventricular plane as guidance, the center of the SAVV annulus was identified and used to acquire 12 radial long‐axis planes passing through the middle of the SAVV annulus (each with a rotation of 15°). Afterwards, a MOLLI sequence was performed in 2 short‐axis images before and after gadolinium contrast administration. Blood was sampled right before the scan to determine the hematocrit level. A breath‐hold phase contrast sequence was acquired in a plane perpendicular to the proximal ascending aorta. Next, CMR images during supine bicycle exercise (exCMR) were acquired using a real‐time CMR method.20 Patients performed free breathing bicycle exercise at the abovementioned 4 exercise stages within the CMR bore using a cycle ergometer with adjustable electronic resistance (Lode, Groningen, The Netherlands). A stack of steady‐state free precession cine images was acquired in the short‐axis and horizontal long‐axis plane without cardiac gating but with simultaneous registration of ECG and respiratory timing. Radial artery pressure tracings were continuously obtained and saved in a PowerLab recording system (AD Instruments, Oxford, United Kingdom). Blood was sampled at rest and at peak CMR exercise to measure hematocrit, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and arterial oxygen saturation.
Global longitudinal strain (GLS) and global circumferential strain of the sRV were quantified on cine images using the strain analysis module in Segment v2.0 R5557 (Medviso, Lund, Sweden).34 This analysis consisted of manually contouring the endo‐ and epicardial borders of the sRV myocardium in 12 radial long‐axis planes (for GLS) or in 6 to 10 short‐axis planes (for global circumferential strain) during end diastole and triggering the automatic computation. The software package then estimated myocardial strain curves by computing interframe deformation fields using a tracking strategy based on nonrigid image registration.34 If necessary, the contouring was adjusted until the visually assessed tracking consistency was optimal. All other resting imaging studies were analyzed and quantified on the in‐house developed software program RightVol (KU Leuven, Leuven, Belgium). Ventricular volumes were delineated in short‐axis views with simultaneous reference to the horizontal long‐axis slices to facilitate correct identification of the atrioventricular plane. The trabeculations and papillary muscles were considered to be included within the blood pool for the purposes of volumetric measurements. Stroke volume index (SVi) was calculated as left ventricular (LV) end‐diastolic volume index (EDVi) minus LV ESVi. This formula was chosen to avoid the impact of SAVV regurgitation on the calculated flow. Myocardial mass measurements were done on the gated images in end‐systole to have the most accurate assessment of the true sRV endocardium (including the interventricular septum, trabeculations, and papillary muscles). sRV end‐systolic pressure‐volume relationship (sRVESPVR), a surrogate of sRV contractility, was calculated as (0.9×systolic blood pressure [SBP])/sRV end‐systolic volume (ESV).21, 35 Interstitial fibrosis in the septum was studied using pre‐ and postcontrast T1 mapping techniques with a region of interest within the sRV cavity (carefully excluding trabeculations) and the interventricular septum at a basal and mid–short axis slice to allow absolute quantification of the extracellular volume (ECV).36 Because of known issues of partial volume effects with the blood, especially in trabeculations, we only assessed the ECV in the compact ventricular septum.31 Residual uncorrected respiratory motion was addressed by manually adjusting the region of interest on the individual images of the T1 recovery curve instead of drawing a region of interest on the computed T1 map. The SAVV regurgitation fraction at rest was estimated by (sRV SV−aortic flow volume)/sRV SV. The exCMR images were analyzed and quantified on RightVol (KU Leuven, Leuven, Belgium). For this we retrospectively synchronized the images with ECG and respiratory timing such that contouring could be performed at the same point of the respiratory cycle for all slices.20 Ventricular volumes were delineated in short‐axis views and the trabeculations and papillary muscles were considered to be included within the blood pool. Changes in SAVV regurgitation fraction during exercise were estimated by calculating the difference in SV between the sRV and LV during the 4‐stage exercise test. The evolution of sRVESPVR during exercise was used as a surrogate of sRV contractile reserve. Heart rate reserve (HRR) was calculated as peak exercise HR minus resting HR; age‐adjusted HRR was obtained by dividing the HRR by ([220−age]−resting HR).
Statistical Analysis
Categorical variables are expressed as numbers and percentages. Continuous data were tested for normal distribution with the Kolmogorov‐Smirnov test and values are reported as mean±SD or as median (25 and 75% percentile [interquartile range]), as appropriate. Differences between groups for continuous variables were analyzed using unpaired t test, Kruskal–Wallis H test, Wilcoxon–Mann–Whitney test, or one‐way ANOVA, as appropriate; Fisher exact or χ2 test was used for categorical variables. The Pearson correlation test was used to quantify correlations between 2 variables. The exCMR response of TGA‐Mustard/Senning versus ccTGA patients was evaluated with repeated‐measures ANOVA (within‐subject variables: 4 exercise intensity levels, between‐subjects factor: patient group [TGA‐Mustard/Senning or ccTGA]). To compare the behavior of sRV patients with the systemic LV of healthy controls, we also performed the repeated‐measures ANOVA with 3 between‐subjects factors: TGA‐Mustard/Senning, ccTGA, and healthy controls. We then divided the TGA‐Mustard/Senning group into 2 cohorts according to their relationship with the median percentage of %ppVO2. Patients with a %ppVO2 below the median were labeled as having impaired exercise performance. Linear regression models were constructed to evaluate the variance in the %ppVO2 in this group. A repeated‐measures ANOVA was modeled to assess differences during exercise between TGA‐Mustard/Senning patients with preserved versus impaired exercise performance. All statistical tests were 2 sided, and significance was defined as P<0.05. Analyses were performed using IBM SPSS Statistics, version 24 and GraphPad Prism, version 5.
Results
Study Participants
One hundred one patients were screened, 57 patients met the inclusion criteria, and 35 patients agreed to participate in this study. Two patients were excluded from all analyses, because they did not complete the exCMR examination. The patient flow diagram is presented in Figure 1. The remaining 33 patients (37±9 years of age, 24% female sex, 94% in New York Heart Association functional class I or II) were studied. They had a median peak oxygen uptake of 72 (interquartile range 64–90) % of predicted. Comprehensive demographics and baseline characteristics of the TGA‐Mustard/Senning (n=23) and ccTGA patients (n=10) are summarized in Table. Six TGA‐Mustard patients had undergone previous successful interventions to ensure baffle patency: 2 patients had undergone a redo Mustard procedure, 4 patients balloon dilatations of which 2 had concomitant stenting of the vena cava inferior baffle. None of the TGA‐Senning patients had baffle restrictions. Three ccTGA patients had undergone previous interventions: 1 had a percutaneous atrial septal defect closure, 1 had a correction for an unroofed coronary sinus, and 1 had a surgical closure of an atrial septal defect and LV‐pulmonary artery aortic homograft.
Figure 1.
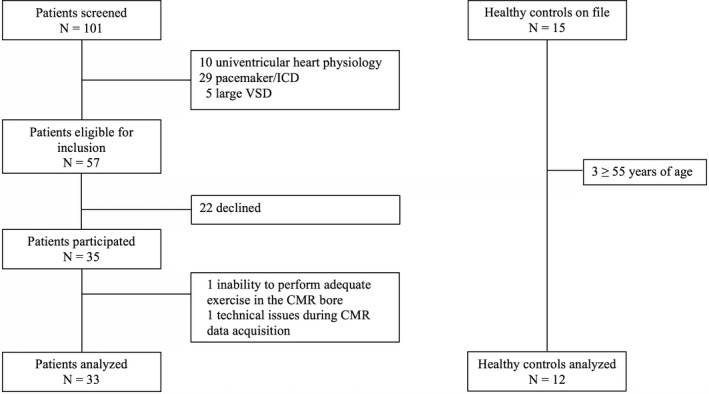
Patient and control flow diagram. CMR indicates cardiac magnetic resonance; ICD, implantable cardioverter defibrillator; VSD, ventricular septal defect.
Table 1.
Baseline Characteristics in sRV Patients
| Parameter | TGA‐Mustard/Senning (N=23) | ccTGA (N=10) | P Value |
|---|---|---|---|
| Age, y | 34±7 | 45±7 | <0.001 |
| Female sex | 6 (26) | 2 (20) | >0.999 |
| Mustard/Senning | 7 (30)/16 (70) | ||
| BMI, kg/m2 | 23.1±3.6 | 24.6±3.9 | 0.275 |
| NYHA class, I/II/III | 14 (61)/7 (30)/2 (9) | 7 (70)/3 (30)/0 (0) | 0.618 |
| Medical treatment | |||
| β‐Blocker | 3 (13) | 4 (40) | 0.161 |
| ACE‐I/ARB | 9 (39) | 3 (30) | 0.710 |
| Loop diuretic | 1 (4) | 0 | >0.999 |
| ECG | |||
| Heart rhythm, sinus/junctional/AF | 22 (96)/1 (4)/0 | 8 (80)/1 (10)/1 (10) | 0.240 |
| Heart rate, bpm | 79±17 | 71±18 | 0.234 |
| QRS width, ms | 103±17 | 118±18 | 0.033 |
| Hematocrit | 0.429±0.032 | 0.413±0.026 | 0.164 |
| NT‐proBNP, ng/L | 183 (89–299) | 258 (124–642) | 0.457 |
| Echocardiogram at rest | |||
| TAPSE, mm | 11 (9–12) | 15 (13–17) | 0.004 |
| SAVV regurgitation, mild/moderate/severe | 9 (39)/13 (57)/1 (4) | 1 (10)/5 (50)/4 (40) | 0.020 |
| Perimembranous VSD | 1 (4) | 1 (10) | 0.521 |
| CPET | |||
| Peak power output, W | 180±51 | 174±37 | 0.760 |
| Peak VO2, mL/kg per min | 28.6±8.3 | 25.6±7.1 | 0.333 |
| Peak VO2, % of predicted peak VO2 | 70 (64–92) | 82 (61–88) | 0.875 |
| Anaerobic threshold, % of peak VO2 | 51±14 | 51±13 | 0.913 |
| Resting CMR measures | |||
| sRVEDVi, mL/m2 | 127.1±35.5 | 126.8±40.6 | 0.983 |
| sRV/LV EDVi ratio | 1.87±0.51 | 1.84±0.34 | 0.877 |
| SVi, mL/m2 | 40.6±7.7 | 40.8±7.1 | 0.933 |
| sRV mass i, g/m2 | 81.7±17.8 | 86.7±20.6 | 0.489 |
| sRVEF, % | 40±7 | 42±10 | 0.467 |
| SAVV RF, % | 18±12 | 28±11 | 0.037 |
| GLS, % | −11.6±2.5 | −11.2±3.6 | 0.688 |
| GCS, % | −15.8±3.6 | −11.2±5.2 | 0.008 |
| sRVESPVR | 0.86±0.29 | 0.92±0.50 | 0.694 |
| Native T1 of septal myocardium, ms | 1024±40 | 1058±40 | 0.035 |
| ECV of septal myocardium, % | 27.1±2.7 | 30.2±2.0 | 0.005 |
Values are mean±SD, median (interquartile range) or number (%). ACE‐I/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; AF, atrial fibrillation; BMI, body mass index; bpm, beats per minute; cc, congenitally corrected; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise testing; ECV, extracellular volume; EDV, end‐diastolic volume; EF, ejection fraction; GCS, global circumferential strain; GLS, global longitudinal strain; i, indexed to body surface area; LV, left ventricular; NYHA, New York Heart Association; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RF, regurgitation fraction; SAVV, systemic atrioventricular valve; sRV, systemic right ventricular; sRVESPVR, sRV end‐systolic pressure/volume relationship; SV, stroke volume; sRVEDVi, systemic right ventricular end‐diastolic volume index; sRVEF, systemic right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; TGA, transposition of the great arteries; VO2, oxygen uptake; VSD, ventricular septal defect.
A cohort of 15 healthy, nonathlete controls from whom we had exCMR data was evaluated for inclusion. Three patients were excluded because of older age (≥55 years of age). The remaining 12 healthy controls (39±10 years of age, 25% female sex, all in New York Heart Association functional class I) had a mean peak oxygen uptake of 103±20% of predicted (Table S1).
TGA‐Mustard/Senning Versus ccTGA Patients
Observed differences at rest
TGA‐Mustard/Senning patients had a significantly higher global circumferential strain on CMR compared with ccTGA patients (−15.8±3.6 versus −11.2±5.2%, P=0.008). No differences in CMR‐derived GLS values were observed, although tricuspid annular plane systolic excursion was lower in TGA‐Mustard/Senning patients (11 [interquartile range 9–12] versus 15 [interquartile range 13–17] mm, P=0.004). sRV ejection fraction and sRVESPVR were comparable between both groups. There was no correlation between age and any parameter of systolic sRV function, neither in TGA‐Mustard/Senning nor in ccTGA patients.
Resting sRVEDVi and SVi were comparable in both groups. The mean septal ECV in ccTGA patients was significantly higher than that of TGA‐Mustard/Senning patients (30.2±2.0 versus 27.1±2.7%, P=0.005). Septal ECV was moderately correlated with age and NT‐proBNP levels (r=0.41, P=0.024 and r=0.41, P=0.021, respectively). There was no correlation between septal ECV and exercise performance (%ppVO2), CMR measures of sRV systolic function, or ventricular volumes.
ccTGA patients had a significantly higher degree of SAVV regurgitation with a mean estimated regurgitant fraction of 28±11% versus 18±12% for TGA‐Mustard/Senning patients (P=0.037).
Observed differences during exercise
Exercise hemodynamics of TGA‐Mustard/Senning patients, ccTGA patients, and healthy controls are demonstrated in Figure 2 and Table S2. The increase in sRV contractility (sRVESPVR) during exercise was comparable between both types of sRV (P for interaction=0.258) but markedly lower than in healthy controls (P for interaction <0.001). TGA‐Mustard/Senning patients had a linear decrease in sRVEDVi and SVi by 11% and 8%, respectively, whereas ccTGA patients showed no significant changes in sRVEDVi but increased SVi by 15%, comparable to the relative changes in LVEDVi and SVi during exercise in healthy controls. Chronotropic response differed between both patient groups (P for interaction=0.010) and the HRR was significantly higher in TGA‐Mustard/Senning patients (90±18 versus 69±28 bpm, P=0.014), although the difference in age‐adjusted HRR was not statistically significant (77±16 versus 68±29%, P=0.350). Furthermore, HRR correlated moderate‐to‐strongly with β‐blocker use (rpb=−0.595, P<0.001).
Figure 2.
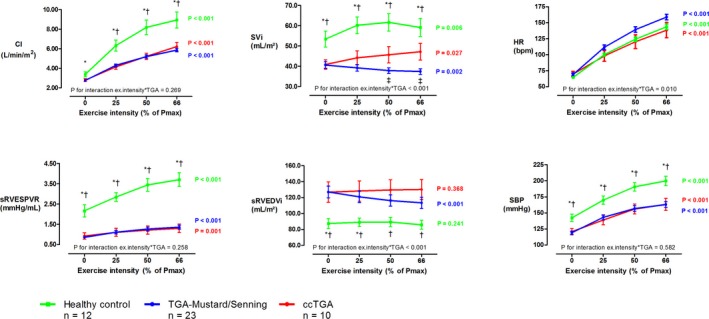
Dynamic changes during exercise in healthy controls, TGA‐Mustard/Senning patients, and ccTGA patients. To assess differences in the response of TGA‐Mustard/Senning (blue) vs ccTGA patients (red), a P for interaction was calculated. The systemic RV of patients was compared with the systemic LV of healthy controls (green). At each exercise intensity, *indicates a significant difference between healthy controls and TGA‐Mustard/Senning patients, †indicates a significant difference between healthy controls and ccTGA patients, and ‡indicates a significant difference between TGA‐Mustard/Senning and ccTGA patients. Error bars depict the standard error of the mean. bpm indicates beats per minute; CI, cardiac index; HR, heart rate; Pmax, maximal power output in watts; SBP, systolic blood pressure; sRVEDVi, systemic right ventricular end‐diastolic volume index; sRVESPVR, sRV end‐systolic pressure/volume relationship; SVi, stroke volume index; TGA, transposition of the great arteries.
Three patients (1 TGA‐Senning and 2 redo‐TGA‐Mustard) had a peak exercise arterial oxygen saturation <92%. There were no significant changes in SAVV regurgitant fraction during exercise (P=0.575).
TGA‐Mustard/Senning Patients with Impaired Versus Preserved Exercise Capacity
Demographics and baseline characteristics of TGA‐Mustard/Senning patients with a preserved (n=12) or impaired (n=11) exercise capacity are found in Table S3. These subgroups were created using the median exercise performance of TGA‐Mustard/Senning patients (70% of predicted pVO2). The HRR and the anaerobic threshold explained 77% of the variance in the %ppVO2 of TGA‐Mustard/Senning patients. There were no significant differences in the increase in sRV contractility during exercise between both groups (P for interaction=0.842). TGA‐Mustard/Senning patients with a preserved exercise capacity achieved a higher peak exercise cardiac index because of a better increase in HR (P for interaction=0.010) with a comparable decline in SVi (P for interaction=0.230) at increasing exercise intensities.
Discussion
This study indicates that (1) despite similar sRV systolic function at rest and sRV contractility during exercise, there is a distinct difference in the contraction pattern (ventricular mechanics) of TGA‐Mustard/Senning and ccTGA patients. (2) TGA‐Mustard/Senning patients have an abnormal decrease in stroke volume during exercise (even in those patients with a preserved exercise capacity), which is different from the increase observed in ccTGA patients. (3) Septal interstitial expansion seems to be more pronounced in ccTGA patients.
Systolic sRV Function
In a group of sRV patients with a mean sRV ejection fraction of 40%—which is similar to previously published series5, 13, 37, 38—we found no significant differences in overall sRV contractility between TGA‐Mustard/Senning and ccTGA patients. However, we noted interesting differences in ventricular mechanics between both groups. Previously, Pettersen et al described a shift to predominant circumferential over longitudinal free wall shortening in the sRV of TGA‐Senning patients compared with the normal RV.38 They considered it an adaptive response of the sRV to support systemic pressures. However, ventricular mechanics of the sRV of ccTGA patients were never studied. We observed the same pattern in the sRV of TGA‐Mustard/Senning patients as described by Pettersen, but we found that in ccTGA patients both GLS and global circumferential strain values of the sRV were low. Inherent differences between these 2 circulations such as the underlying anatomy, previous surgery, difference in electrical activation, and different loading conditions may contribute to differences in ventricular mechanics between both types of sRV. Note that there was a discrepancy between tricuspid annular plane systolic excursion (significantly higher in ccTGA) and GLS (no differences between the 2 groups) values. In echocardiography, tricuspid annular plane systolic excursion looks at the longitudinal displacement of the SAVV annulus towards the fixed probe near the apex and not at the real longitudinal shortening of the lateral wall of the sRV in contrast to strain measurements. Moreover, our GLS values express the global longitudinal shortening of the whole sRV myocardium and not only of the lateral wall.
sRV contractile reserve, as assessed by the evolution of sRVESPVR during exercise, was not significantly different between TGA‐Mustard/Senning patients with impaired or preserved exercise performance, which is in line with invasive measurements during dobutamine stress.26, 27
Stroke Volume
The main advantage of our supine bicycle protocol is that we test during physiological stress, maintaining preload during exercise (in contrast to dobutamine stress). Our results corroborate and strengthen previous findings of impaired stroke volume response to exercise in patients with TGA‐Mustard/Senning, especially since we observed an opposite response in ccTGA patients.26, 27, 28, 29, 30 Remarkably, even TGA‐Mustard/Senning patients with a preserved exercise performance experienced a comparable decrease in SVi during exercise. This implies that filling impairment during exercise is inherent to the circulation in TGA‐Mustard/Senning patients but not necessarily the factor that differentiates patients with a reduced versus preserved exercise performance.
Previously it has been demonstrated that TGA‐Mustard/Senning patients have higher septal ECV values compared with the general population.31 To the best of our knowledge there are currently no published ECV values in ccTGA patients. Broberg et al39 did study some ccTGA patients, but their published ECV values were pooled with TGA‐Mustard/Senning patients. In our series, ECV values were higher in the septal myocardium of ccTGA patients compared with TGA‐Mustard/Senning patients. Is this merely related to the older age of the studied ccTGA patients? One might argue that the observed differences in ECV values between TGA‐Mustard/Senning patients and ccTGA patients are much larger than one would expect when considering the slight increase in ECV values of a normal systemic LV caused by aging.40, 41 However, it could be that the age‐related increase in ECV is steeper in a sRV compared with a normal systemic LV because of inadequate perfusion and subsequent diffuse fibrosis of the sRV (related to the concordance of the coronary arteries and multiple anomalous coronary patterns in patients with an sRV).2, 42, 43 Increasing the patient numbers and subsequent longitudinal assessment of the ECV in ccTGA and TGA‐Mustard/Senning patients would be interesting to further investigate this hypothesis.
The previous point may also open the discussion about diastolic dysfunction of the sRV. Diastolic function of the sRV can deteriorate progressively over time to evolve to a restrictive filling pattern and elevated filling pressures. In this study, we did not specifically assess diastolic function, but elevated septal ECV values may indicate a certain degree of interstitial fibrosis that could be related to diastolic dysfunction. Therefore, we hypothesize that the decrease of SVi in TGA‐Mustard/Senning patients during exercise is not only related to impaired filling of the sRV, but also because of diastolic dysfunction, which may worsen during exercise. Investigation of pulmonary venous atrial function with volumetric and deformational parameters would provide additional insights.
HR Response During Exercise
The peak HR is an important determinant of the cardiac index and exercise performance. Chronotropic incompetence is frequent in patients with a sRV,44 and an abnormal HRR is an independent predictor of survival in adults with congenital heart disease.45
In this study, the HRR of ccTGA patients was lower than that of TGA‐Mustard/Senning patients. First, this could be age related. After adjusting the HRR for age, we no longer observed a significant difference between both groups. Second, it might be that ccTGA patients attain sufficient high cardiac indexes with submaximal HR increases because they have a good SVi reserve. Third, ccTGA patients have a distinctly abnormal conduction system because of the malalignment of the atrioventricular septum, making them more prone to conduction problems.2 At times, β‐blockers are an essential component in the treatment of these patients. However, in the absence of a strict indication, they may affect exercise tolerance and should be prescribed with caution.
In TGA‐Mustard/Senning patients, we observed interesting differences after dividing the patients into 2 cohorts according to their relationship with the mean pVO2. Both a lower peak HR and a worse level of physical fitness seem to be the main drivers in pVO2. The lower peak HR of the patients with impaired exercise capacity could be a primary (chronotropic) problem. Alternatively, one could speculate that the peak HR in this group of TGA‐Mustard/Senning patients could be limited because of a significant drop in venous return during exercise (“reverse Bainbridge” reflex). Thus, the lower maximal HR observed at peak exercise in these patients would be a secondary phenomenon to prevent a deterioration of the cardiac output with further increases in HR. These hypothesis‐generating findings warrant further exploration in larger series.
Limitations
Our study cohort was of modest size, excluded patients with a pacemaker or a nonrestrictive ventricular septal defect, and comprised predominately TGA‐Mustard/Senning patients. As a result, we performed no subgroup analysis on ccTGA patients. Fifty‐eight percent of eligible patients were finally studied. Although we did not find significant differences in baseline characteristics (age, sex, sRV type, or New York Heart Association class), selection bias cannot be completely excluded. Exercise response of sRV patients was compared with that of a small set of healthy volunteers, excluding the possibility for regression adjustment.
This series comprises mostly paucisymptomatic patients with a mean peak oxygen consumption on the high end of published reference values.12 It would be interesting to enroll more symptomatic patients in the future. We did not perform invasive pressure or contractility measurements. For myocardial contractile function, we relied on surrogates such as the sRVESPVR and CMR‐derived strain values. Because of technical constraints, it was not possible to perform strain analysis during exercise. The evolution of the SVi difference between the sRV and LV during the 4 exercise levels was used as a surrogate because we did not quantify the degree of SAVV regurgitation with phase‐contrast imaging during exercise. Furthermore, we have no histological myocardial samples; instead we relied on myocardial ECV measures. This is a measure of interstitial expansion that is elevated in diffuse myocardial fibrosis and a validated surrogate marker of fibrosis in the absence of confounders such as infiltration.36 ECV measurements were limited to the septum because of trabeculations in the other ventricular segments resulting in myocardial regions of interest partial voluming with the blood.31 Nonetheless, we tried in this study to shed light on a very interesting patient population by following a mechanistic approach and using state‐of‐the‐art imaging techniques.
Conclusions
These findings suggest that caution should be exercised when evaluating pooled analyses of sRV patients, given the differences in myocardial contraction pattern, septal interstitial expansion, and the exercise response of TGA‐Mustard/Senning versus ccTGA patients. Longitudinal follow‐up will determine whether this abnormal exercise cardiac response is a marker of earlier failure.
Disclosures
Dr Helsen received travel and conference grants from the Fund for Scientific Research Flanders (FWO), the European Association of Cardiovascular Imaging (EACVI), and the Society for Cardiovascular Magnetic Resonance (SCMR) to present parts of this work at the ESC Congress and the joint EuroCMR/SCMR meeting. Dr Van De Bruaene received research funding by the Frans Van de Werf Fund for Clinical Cardiovascular Research (grant no. 010116). Dr Gabriels was funded by the Agency for Innovation by Science and Technology Flanders (grant no. 131025). Dr Bogaert and Dr Budts receive project funding by KU Leuven (grant no. 3M170303).
Supporting information
Data S1. Supplemental Methods.
Table S1. Baseline Characteristics in sRV Patients and Healthy Controls
Table S2. exCMR Metrics
Table S3. Baseline Characteristics at Rest and CPET Data in TGA‐Mustard/Senning Patients With a Preserved or Impaired Exercise Capacity According to the Median %ppVO2
Acknowledgments
First and foremost, we want to recognize all patients who participated in this study. We also gratefully acknowledge Kris Byloos, Stefan Ghysels, and Guido Putzeys for their assistance in acquiring the CMR data sets. Hilde Gillijns and Maarten Vanhaverbeke are thanked for performing laboratory tests.
(J Am Heart Assoc. 2018;7:e009185 DOI: 10.1161/JAHA.118.009185.)
References
- 1. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. [DOI] [PubMed] [Google Scholar]
- 2. Filippov AA, Del Nido PJ, Vasilyev NV. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation. 2016;134:1293–1302. [DOI] [PubMed] [Google Scholar]
- 3. Winter MM, van der Bom T, de Vries LC, Balducci A, Bouma BJ, Pieper PG, van Dijk AP, van der Plas MN, Picchio FM, Mulder BJ. Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. Eur Heart J. 2012;33:1378–1385. [DOI] [PubMed] [Google Scholar]
- 4. Scherptong RW, Vliegen HW, Winter MM, Holman ER, Mulder BJ, van der Wall EE, Hazekamp MG. Tricuspid valve surgery in adults with a dysfunctional systemic right ventricle: repair or replace? Circulation. 2009;119:1467–1472. [DOI] [PubMed] [Google Scholar]
- 5. van der Bom T, Winter MM, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos‐Hesselink JW, Zwinderman AH, Mulder BJ, Bouma BJ. Right ventricular end‐diastolic volume combined with peak systolic blood pressure during exercise identifies patients at risk for complications in adults with a systemic right ventricle. J Am Coll Cardiol. 2013;62:926–936. [DOI] [PubMed] [Google Scholar]
- 6. Graham TP, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long‐term outcome in congenitally corrected transposition of the great arteries: a multi‐institutional study. J Am Coll Cardiol. 2000;36:255–261. [DOI] [PubMed] [Google Scholar]
- 7. Cuypers JA, Eindhoven JA, Slager MA, Opić P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Bogers AJ, Roos‐Hesselink JW. The natural and unnatural history of the mustard procedure: long‐term outcome up to 40 years. Eur Heart J. 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 8. Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. [DOI] [PubMed] [Google Scholar]
- 9. Van De Bruaene A, Hickey EJ, Kovacs AH, Crean AM, Wald RM, Silversides CK, Redington AN, Ross HJ, Alba AC, Billia F, Nair K, Benson L, Horlick E, Osten M, Colman J, Heggie J, Oechslin EN, Roche SL. Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. Int J Cardiol. 2018;252:80–87. [DOI] [PubMed] [Google Scholar]
- 10. Brida M, Diller GP, Gatzoulis MA. Systemic right ventricle in adults with congenital heart disease: anatomic and phenotypic spectrum and current approach to management. Circulation. 2018;137:508–518. [DOI] [PubMed] [Google Scholar]
- 11. Diller GP, Dimopoulos K, Okonko D, Li W, Babu‐Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole‐Wilson PA, Francis DP, Gatzoulis MA. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. [DOI] [PubMed] [Google Scholar]
- 12. Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life–single centre experience and review of published data. Eur Heart J. 2012;33:1386–1396. [DOI] [PubMed] [Google Scholar]
- 13. Tutarel O, Orwat S, Radke RM, Westhoff‐Bleck M, Vossler C, Schülke C, Baumgartner H, Bauersachs J, Röntgen P, Diller GP. Assessment of myocardial function using MRI‐based feature tracking in adults after atrial repair of transposition of the great arteries: reference values and clinical utility. Int J Cardiol. 2016;220:246–250. [DOI] [PubMed] [Google Scholar]
- 14. Li W, Hornung TS, Francis DP, O'Sullivan C, Duncan A, Gatzoulis M, Henein M. Relation of biventricular function quantified by stress echocardiography to cardiopulmonary exercise capacity in adults with mustard (atrial switch) procedure for transposition of the great arteries. Circulation. 2004;110:1380–1386. [DOI] [PubMed] [Google Scholar]
- 15. Ladouceur M, Redheuil A, Soulat G, Delclaux C, Azizi M, Patel M, Chatellier G, Legendre A, Iserin L, Boudjemline Y, Bonnet D, Mousseaux E, Investigators S . Longitudinal strain of systemic right ventricle correlates with exercise capacity in adult with transposition of the great arteries after atrial switch. Int J Cardiol. 2016;217:28–34. [DOI] [PubMed] [Google Scholar]
- 16. Helsen F, De Meester P, Van De Bruaene A, Gabriels C, Santens B, Claeys M, Claessen G, Goetschalckx K, Buys R, Gewillig M, Troost E, Voigt J‐U, Claus P, Bogaert J, Budts W. Right ventricular systolic dysfunction at rest is not related to decreased exercise capacity in patients with a systemic right ventricle. Int J Cardiol. 2018;260:66–71. [DOI] [PubMed] [Google Scholar]
- 17. Roest AA, Lamb HJ, van der Wall EE, Vliegen HW, van den Aardweg JG, Kunz P, de Roos A, Helbing WA. Cardiovascular response to physical exercise in adult patients after atrial correction for transposition of the great arteries assessed with magnetic resonance imaging. Heart. 2004;90:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tulevski II, Lee PL, Groenink M, van der Wall EE, Stoker J, Pieper PG, Romkes H, Hirsch A, Mulder BJ. Dobutamine‐induced increase of right ventricular contractility without increased stroke volume in adolescent patients with transposition of the great arteries: evaluation with magnetic resonance imaging. Int J Card Imaging. 2000;16:471–478. [DOI] [PubMed] [Google Scholar]
- 19. Dodge‐Khatami A, Tulevski II, Bennink GB, Hitchcock JF, de Mol BA, van der Wall EE, Mulder BJ. Comparable systemic ventricular function in healthy adults and patients with unoperated congenitally corrected transposition using MRI dobutamine stress testing. Ann Thorac Surg. 2002;73:1759–1764. [DOI] [PubMed] [Google Scholar]
- 20. La Gerche A, Claessen G, Van De Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high‐intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. [DOI] [PubMed] [Google Scholar]
- 21. La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, Droogne W, Van Cleemput J, Claus P, Heidbuchel H. Exercise‐induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36:1998–2010. [DOI] [PubMed] [Google Scholar]
- 22. Claessen G, La Gerche A, Dymarkowski S, Claus P, Delcroix M, Heidbuchel H. Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc. 2015;4:e001602 DOI: 10.1161/JAHA.114.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Claessen G, La Gerche A, Wielandts JY, Bogaert J, Van Cleemput J, Wuyts W, Claus P, Delcroix M, Heidbuchel H. Exercise pathophysiology and sildenafil effects in chronic thromboembolic pulmonary hypertension. Heart. 2015;101:637–644. [DOI] [PubMed] [Google Scholar]
- 24. Van De Bruaene A, La Gerche A, Claessen G, De Meester P, Devroe S, Gillijns H, Bogaert J, Claus P, Heidbuchel H, Gewillig M, Budts W. Sildenafil improves exercise hemodynamics in fontan patients. Circ Cardiovasc Imaging. 2014;7:265–273. [DOI] [PubMed] [Google Scholar]
- 25. Claessen G, Schnell F, Bogaert J, Claeys M, Pattyn N, De Buck F, Dymarkowski S, Claus P, Carré F, Van Cleemput J, La Gerche A, Heidbuchel H. Exercise cardiac magnetic resonance to differentiate athlete's heart from structural heart disease. Eur Heart J Cardiovasc Imaging. 2018;19:1062–1070. [DOI] [PubMed] [Google Scholar]
- 26. Derrick GP, Narang I, White PA, Kelleher A, Bush A, Penny DJ, Redington AN. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load‐independent indexes of right ventricular performance after the mustard operation. Circulation. 2000;102:III154–III159. [DOI] [PubMed] [Google Scholar]
- 27. Vogel M, Derrick G, White PA, Cullen S, Aichner H, Deanfield J, Redington AN. Systemic ventricular function in patients with transposition of the great arteries after atrial repair: a tissue Doppler and conductance catheter study. J Am Coll Cardiol. 2004;43:100–106. [DOI] [PubMed] [Google Scholar]
- 28. Winter MM, van der Plas MN, Bouma BJ, Groenink M, Bresser P, Mulder BJ. Mechanisms for cardiac output augmentation in patients with a systemic right ventricle. Int J Cardiol. 2010;143:141–146. [DOI] [PubMed] [Google Scholar]
- 29. Fratz S, Hager A, Busch R, Kaemmerer H, Schwaiger M, Lange R, Hess J, Stern HC. Patients after atrial switch operation for transposition of the great arteries cannot increase stroke volume under dobutamine stress as opposed to patients with congenitally corrected transposition. Circ J. 2008;72:1130–1135. [DOI] [PubMed] [Google Scholar]
- 30. Dvir‐Orgad M, Anand M, De Souza AM, Zadorsky MT, Kiess MC, Potts JE, Sandor GG. Stress echocardiographic evaluation for d‐transposition of the great arteries after atrial redirection: unmasking early signs of myocardial dysfunction and baffle stenosis. J Am Soc Echocardiogr. 2017;30:80–89. [DOI] [PubMed] [Google Scholar]
- 31. Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, Moon JC. Diffuse myocardial fibrosis in the systemic right ventricle of patients late after Mustard or Senning surgery: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2013;14:963–968. [DOI] [PubMed] [Google Scholar]
- 32. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL; Echocardiography EAo . European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11:307–332. [DOI] [PubMed] [Google Scholar]
- 33. Binder RK, Wonisch M, Corra U, Cohen‐Solal A, Vanhees L, Saner H, Schmid JP. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil. 2008;15:726–734. [DOI] [PubMed] [Google Scholar]
- 34. Morais P, Marchi A, Bogaert JA, Dresselaers T, Heyde B, D'hooge J, Bogaert J. Cardiovascular magnetic resonance myocardial feature tracking using a non‐rigid, elastic image registration algorithm: assessment of variability in a real‐life clinical setting. J Cardiovasc Magn Reson. 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 36. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos‐Hesselink JW, Zwinderman AH, Mulder BJ. Effect of valsartan on systemic right ventricular function: a double‐blind, randomized, placebo‐controlled pilot trial. Circulation. 2013;127:322–330. [DOI] [PubMed] [Google Scholar]
- 38. Pettersen E, Helle‐Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, Smiseth OA, Andersen K. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol. 2007;49:2450–2456. [DOI] [PubMed] [Google Scholar]
- 39. Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch‐Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age‐related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced T1 mapping: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub‐clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta‐analysis. Circulation. 2002;106:2575–2580. [DOI] [PubMed] [Google Scholar]
- 43. Wernovsky G, Sanders SP. Coronary artery anatomy and transposition of the great arteries. Coron Artery Dis. 1993;4:148–157. [DOI] [PubMed] [Google Scholar]
- 44. Diller GP, Okonko DO, Uebing A, Dimopoulos K, Bayne S, Sutton R, Francis DP, Gatzoulis MA. Impaired heart rate response to exercise in adult patients with a systemic right ventricle or univentricular circulation: prevalence, relation to exercise, and potential therapeutic implications. Int J Cardiol. 2009;134:59–66. [DOI] [PubMed] [Google Scholar]
- 45. Diller GP, Dimopoulos K, Okonko D, Uebing A, Broberg CS, Babu‐Narayan S, Bayne S, Poole‐Wilson PA, Sutton R, Francis DP, Gatzoulis MA. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol. 2006;48:1250–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Baseline Characteristics in sRV Patients and Healthy Controls
Table S2. exCMR Metrics
Table S3. Baseline Characteristics at Rest and CPET Data in TGA‐Mustard/Senning Patients With a Preserved or Impaired Exercise Capacity According to the Median %ppVO2


