Abstract
Background
Cystatin C (Cys‐C) is a marker of renal function that has shown prognostic value for cardiovascular risk stratification across different patient populations. The incremental value of Cys‐C beyond established cardiac and renal biomarkers remains incompletely explored.
Methods and Results
SOLID‐TIMI 52 (Stabilization of Plaques Using Darapladib‐Thrombolysis in Myocardial Infarction 52; http://www.clinicaltrials.gov, NCT01000727) randomized patients ≤30 days post–acute coronary syndrome were treated with darapladib or placebo. The association between Cys‐C and long‐term risk (median follow‐up 2.5 years) was assessed in 4965 individuals with adjustments made for clinical variables and other risk markers (eg, estimated glomerular filtration rate, high‐sensitivity troponin I, brain‐type natriuretic peptide, and fibroblast growth factor‐23). The prespecified outcome of interest was cardiovascular death (CVD) or heart failure hospitalization. Cys‐C was strongly correlated with creatinine (r=0.60) and estimated glomerular filtration rate (r=−0.68), moderately correlated with fibroblast growth factor‐23 (r=0.39), and weakly correlated with brain‐type natriuretic peptide (r=0.28) and high‐sensitivity troponin I (r=0.06) (all P<0.0001). After multivariate adjustment, increasing concentration of Cys‐C (per SD of log‐transformed Cys‐C) was significantly associated with a 28% higher hazard of CVD or heart failure hospitalization (hazard ratio [HR] 1.28, 95% confidence interval [CI] 1.12‐1.46, P<0.001), including CVD (HR 1.24, 95% CI 1.04‐1.47, P=0.01) and heart failure hospitalization (HR 1.42, 95% CI 1.19‐1.69, P<0.001). Cys‐C was also associated with a higher hazard of CVD, myocardial infarction, or stroke (HR 1.15, 95% CI 1.04‐1.28, P<0.01), including myocardial infarction (HR 1.17, 95% CI 1.02‐1.33, P=0.02). The addition of Cys‐C to a fully adjusted model without estimated glomerular filtration rate improved the C‐statistic from 0.80 to 0.81 (P=0.03) for CVD or heart failure hospitalization. In contrast, the addition of estimated glomerular filtration rate to a fully adjusted model without Cys‐C failed to improve model discrimination (P=0.17).
Conclusions
Cys‐C is associated with the risk of adverse outcomes in patients after acute coronary syndrome. This relationship is independent of established and novel biomarkers of the cardiorenal axis.
Keywords: acute coronary syndrome, biomarker, cystatin C, prognosis
Subject Categories: Biomarkers, Clinical Studies, Ischemia
Clinical Perspective
What Is New?
We investigated the prognostic utility of cystatin C (Cys‐C) in 4965 patients after acute coronary syndrome in the SOLID‐TIMI 52 (Stabilization of Plaques Using Darapladib‐Thrombolysis in Myocardial Infarction 52) trial.
We found that Cys‐C was significantly associated with an increased risk of adverse cardiovascular events independent of established risk factors.
In addition, we demonstrated that Cys‐C provides incremental information for risk stratification independent of traditional and novel biomarkers of the cardiorenal axis including estimated glomerular filtration rate, high‐sensitivity troponin I, brain‐type natriuretic peptide, and fibroblast growth factor‐23.
What Are the Clinical Implications?
Cys‐C helps to identity patients at a greater risk of adverse cardiovascular events after an acute coronary syndrome.
Cys‐C may be a better cardiovascular risk marker in populations with acute coronary syndrome than traditional measures of renal function including creatinine and estimated glomerular filtration rate.
Introduction
Cystatin‐C (Cys‐C) is a cysteine protease inhibitor produced by almost all human cells. It is excreted into the bloodstream, filtered in the renal glomerulus, and metabolized by the proximal tubule.1, 2 Both creatinine and Cys‐C are serum measures of renal function, but unlike creatinine, Cys‐C is not affected by age, sex, and lean muscle mass and may be more sensitive for detecting mild to moderate changes in estimated glomerular filtration rate (eGFR).3, 4 Renal dysfunction, as measured by eGFR, has been associated with an increased risk of death among healthy elders,5 those with heart failure,6 and in stable patients with coronary artery disease,7 although the mechanisms remain incompletely defined.
Prior studies have demonstrated an association between Cys‐C and the risk of death, including cardiovascular death (CVD) and incident heart failure among ambulatory people with coronary heart disease.8 In addition, studies have shown an association between Cys‐C and the risk of adverse outcomes after acute coronary syndrome (ACS).9, 10, 11, 12 However, the incremental value of assessing Cys‐C beyond established and novel markers that reflect renal function, including eGFR and fibroblast growth factor‐23 (FGF‐23), remains disputed. Further, its independent prognostic value beyond other cardiovascular markers such as high‐sensitivity troponin (hsTn) remains unknown. Thus, we sought to investigate the independent prognostic utility of Cys‐C in patients after ACS.
Methods
The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure through the GlaxoSmithKline Data Sharing program.
Study Population and Procedures
The SOLID‐TIMI 52 (Stabilization of Plaques Using Darapladib‐Thrombolysis in Myocardial Infarction 52; http://www.clinicaltrials.gov, NCT01000727) study was a double‐blind, multicenter, phase 3 trial that enrolled 13 026 patients after ACS and randomized them to receive darapladib 160 mg daily (an inhibitor of lipoprotein‐associated phospholipase A2 activity) or placebo at 868 sites in 36 countries. Detailed descriptions of both the study design and results have been previously published.13, 14
In brief, patients were considered potentially eligible for enrollment if they had been hospitalized with an ACS in the 30 days before randomization (unstable angina, non–ST‐elevation myocardial infarction [MI], and ST‐elevation MI) and had at least 1 of the following high‐risk predictors of cardiovascular risk: age ≥60, history of documented MI before qualifying ACS event, diabetes mellitus requiring pharmacotherapy, significant renal dysfunction (defined as eGFR ≥30 and ≤59 mL/min per 1.73 m2 according to the Modification of Diet in Renal Disease Study equation), or established polyvascular disease. A random population of 5000 patients (excluding patients from China, whose samples could not be shipped) was identified before database lock and represented the planned biomarker cohort. Patients with an eGFR <30 mL/min per 1.73 m2 or receiving chronic dialysis before randomization were excluded from study participation.
The study protocol from the original SOLID‐TIMI 52 trial was approved by ethics committees at each participating institution, and written informed consent was obtained from all participants. SOLID‐TIMI 52 has an active institutional review board approval for continued research with the Partners Human Research Committee.
Laboratory Analysis
At the baseline visit (median 14 days after ACS), samples were obtained and frozen before being shipped on dry ice to be stored at a central lab at −80°C. Biomarker assays were conducted by laboratory personnel blinded to treatment allocation and clinical outcome at the TIMI (Thrombolysis in Myocardial Infarction) Clinical Trials Laboratory (Boston, MA) including Cys‐C (Randox assay, Crumlin, UK; Roche Diagnostics c6000 [c501] instrument, Basel, Switzerland); FGF‐23 (Immutopics, San Clemente, CA), high‐sensitivity troponin I (hsTnI) (Architect i2000SR, Abbott Laboratories, Abbott Park, IL), and brain‐type natriuretic peptide (BNP) (Architect i2000SR, Abbott Laboratories, Abbott Park, IL). eGFR was calculated through a central laboratory using the MDRD (Modification of Diet in Renal Disease) Study equation.15
Study End Points
The prespecified outcome of interest for the current analysis was the composite of CVD or hospitalization for heart failure. Additional outcomes of interest included all‐cause mortality, (cardiovascular death, MI, or stroke [MACE]) and its individual components. Cardiovascular end points were adjudicated by a blinded clinical events committee.13, 14
Statistical Analyses
Continuous variables were compared with ANOVA with a test for trend. Categorical variables were compared with the Cochran Armitage trend test or chi‐squared test. Spearman correlation was used to assess correlations between markers. Kaplan‐Meier event rates are reported at 3 years. Cys‐C was prospectively modeled as a log‐transformed continuous variable and reported per standard deviation and categorically by quartile. Sensitivity analyses modeled Cys‐C as a dichotomous variable (fourth versus first 3 quartiles [Q4:Q1‐3]) and eGFR at its clinical threshold of 60 mL/min per 1.73 m2. Unadjusted and adjusted Cox proportional hazards models were assessed. Cox regression models were adjusted for clinical predictors of risk including age (quartiles), sex, region (North America and Western Europe versus other regions), race (white versus nonwhite), body mass index (<18.5, 18.5 to <25, 25 to <30, ≥30 kg/m2), smoking status, history of heart failure, diabetes mellitus, hypertension, hyperlipidemia, baseline low‐density lipoprotein cholesterol (quartiles), prior MI, index diagnosis (ST‐elevation MI versus non–ST‐elevation ACS), catheterization for qualifying event, days from qualifying event (≤14 days), eGFR (<60 mL/min per 1.73 m2), BNP (<80 pg/mL), hsTnI (<26 mg/dL), FGF‐23 (<93 pg/mL), and randomized treatment arm. The cut point for FGF‐23 was 93 pg/mL based on previous analyses in this post‐ACS population.16 Additional adjusted models without FGF‐23 and with creatinine rather than eGFR were run.
For the model performance of discrimination and reclassification related to the primary end point (CVD or heart failure [HF]), C‐statistics, net reclassification index, and integrated discrimination improvement were calculated in 2 ways: first in a model that contained all variables (including eGFR) for the addition of Cys‐C, and second, in a model that included all variables, excluding eGFR and Cys‐C, for the addition of the eGFR and Cys‐C, separately. All analyses were performed by the TIMI Study Group using the statistical software package SAS version 9.4 (SAS institute, Cary, NC) employing an independent copy of the trial database. A 2‐sided P<0.05 was considered statistically significant.
Results
Cys‐C was available in 4965 patients in the SOLID‐TIMI 52 study. The baseline characteristics of the biomarker cohort versus the overall study population are shown in Table S1. Patients with higher concentrations of Cys‐C tended to be older, female in gender, and to have a higher body mass index, a history of hypertension, and baseline eGFR <60 mL/min per 1.73 m2. Patients with a lower baseline Cys‐C concentration were more likely to be smokers, to have been hospitalized with an ST‐elevation MI, and more likely to undergo catheterization and percutaneous coronary intervention for the qualifying event. The use of evidence‐based therapies including aspirin, P2Y12 inhibitors, and statins was more common in patients with lower Cys‐C concentration. Notably, Cys‐C concentration was not significantly associated with race, diabetes mellitus, prior MI, or baseline low‐density lipoprotein cholesterol (Table 1).
Table 1.
Baseline Patient Characteristics of the Study Population by Quartile of Baseline Cystatin‐C
| Characteristic | Quartile 1 (≤0.78 mg/L) (N=1284) | Quartile 2 (0.78‐0.88 mg/L) (N=1218) | Quartile 3 (0.88‐1.03 mg/L) (N=1246) | Quartile 4 (>1.03 mg/L) (N=1217) | P Trend |
|---|---|---|---|---|---|
| Age (y) (median, IQR) | 61 (54, 65) | 63 (58, 69) | 65 (61, 72) | 70 (63, 76) | <0.001 |
| Age ≥60 y, n (%) | 740 (57.6%) | 879 (72.2%) | 1021 (81.9%) | 1059 (87%) | <0.001 |
| Female, n (%) | 284 (22.1%) | 312 (25.6%) | 292 (23.4%) | 396 (32.5%) | <0.001 |
| BMI (kg/m2), median (IQR) | 27.5 (24.8, 30.9) | 27.6 (25, 30.1) | 27.7 (25.1, 31.2) | 28.1 (25, 31.9) | <0.001 |
| Current Smoker, n (%) | 277 (21.6%) | 215 (17.7%) | 248 (19.9%) | 176 (14.5%) | <0.001 |
| Race | 0.59 | ||||
| White, n (%) | 1118 (87.1%) | 1079 (88.6%) | 1106 (88.8%) | 1061 (87.2%) | |
| Black, n (%) | 38 (3.0%) | 22 (1.8%) | 36 (2.9%) | 33 (2.7%) | |
| Asian, n (%) | 106 (8.3%) | 96 (7.9%) | 89 (7.1%) | 103 (8.5%) | |
| Other, n (%) | 22 (1.7%) | 21 (1.7%) | 15 (1.2%) | 20 (1.6%) | |
| Region | <0.01 | ||||
| North America, n (%) | 321 (25%) | 285 (23.4%) | 258 (20.7%) | 265 (21.8%) | |
| South America, n (%) | 82 (6.4%) | 90 (7.4%) | 120 (9.6%) | 108 (8.9%) | |
| Western Europe, n (%) | 415 (32.3%) | 350 (28.7%) | 394 (31.6%) | 332 (27.3%) | |
| Eastern Europe, n (%) | 343 (26.7%) | 385 (31.6%) | 371 (29.8%) | 394 (32.4%) | |
| Asia Pacific, n (%) | 123 (9.6%) | 108 (8.9%) | 103 (8.3%) | 118 (9.7%) | |
| Hypertension, n (%) | 879 (68.5%) | 861 (70.7%) | 909 (73%) | 1029 (84.6%) | <0.001 |
| Hyperlipidemia, n (%) | 879 (68.5%) | 802 (65.9%) | 780 (62.6%) | 794 (65.2%) | 0.03 |
| Diabetes mellitus, n (%) | 488 (38.0%) | 384 (31.5%) | 357 (28.7%) | 444 (36.5%) | 0.18 |
| Prior MI, n (%) | 423 (32.9%) | 353 (29.0%) | 387 (31.1%) | 407 (33.4%) | 0.58 |
| Index event | 0.02 | ||||
| Unstable angina, n (%) | 147 (11.4%) | 136 (11.2%) | 164 (13.2%) | 150 (12.3%) | |
| Non‐STEMI, n (%) | 537 (41.8%) | 499 (41%) | 516 (41.4%) | 565 (46.4%) | |
| STEMI, n (%) | 600 (46.7%) | 583 (47.9%) | 566 (45.4%) | 502 (41.3%) | |
| ST‐segment deviation, n (%) | 911 (71.1%) | 887 (72.8%) | 897 (72%) | 834 (68.5%) | 0.15 |
| Activities performed for qualifying event | |||||
| Catheterization, n (%) | 1169 (91.0%) | 1056 (86.7%) | 1064 (85.4%) | 966 (79.4%) | <0.001 |
| PCI, n (%) | 1075 (83.7%) | 942 (77.3%) | 932 (74.8%) | 825 (67.8%) | <0.001 |
| Fibrinolytic, n (%) | 109 (8.5%) | 115 (9.4%) | 117 (9.4%) | 108 (8.9%) | 0.75 |
| Days from qualifying event to randomization, median (IQR) | 13 (5, 22) | 15 (6, 23) | 15 (7, 23) | 14 (7, 23) | <0.001 |
| Baseline measurements | |||||
| eGFR (mL/min per 1.73 m2), median (IQR) | 96 (84, 108) | 84 (78, 96) | 78 (66, 84) | 60 (48, 72) | <0.001 |
| eGFR<60 (mL/min per 1.73 m2), n (%) | 2 (0.2%) | 16 (1.3%) | 70 (5.7%) | 505 (42.5%) | <0.001 |
| Creatinine (mg/dL), median (IQR) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1) | 1 (0.9, 1.1) | 1.2 (1, 1.4) | <0.001 |
| Concomitant medical therapy | |||||
| Aspirin, n (%) | 1256 (97.8%) | 1186 (97.4%) | 1198 (96.1%) | 1161 (95.4%) | <0.001 |
| P2Y12 Inhibitor, n (%) | 1189 (92.6%) | 1085 (89.1%) | 1083 (86.9%) | 1031 (84.7%) | <0.001 |
| Statin, n (%) | 1235 (96.2%) | 1160 (95.2%) | 1167 (93.7%) | 1144 (94%) | <0.01 |
| β‐Blocker, n (%) | 1123 (87.5%) | 1076 (88.3%) | 1095 (87.9%) | 1071 (88%) | 0.77 |
| ACE‐I or ARB, n (%) | 1045 (81.4%) | 1013 (83.2%) | 1037 (83.2%) | 1030 (84.6%) | 0.04 |
| Randomized to darapladib, n (%) | 640 (49.8%) | 606 (49.8%) | 624 (50.1%) | 614 (50.5%) | 0.74 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation MI.
Cys‐C was strongly correlated with creatinine (r=0.60, P<0.0001) and eGFR (r=−0.68, P<0.0001), moderately correlated with FGF‐23 (r=0.39, P<0.0001), and weakly correlated with BNP (r=0.28, P<0.0001) and hsTnI (r=0.06, P<0.0001).
Association With Outcomes
When modeled as a continuous variable, increasing concentration of Cys‐C (per SD of log‐transformed Cys‐C) was associated with an 89% higher hazard of CVD or HF hospitalization (unadjusted hazard ratio [HR] 1.89, 95% confidence interval [CI] 1.75‐2.04, P<0.001), including a 91% higher hazard of CVD (HR 1.91, 95% CI 1.79‐2.11) and 97% higher hazard of HF hospitalization (HR 1.97, 95% CI 1.78‐2.19). Higher Cys‐C concentration was also significantly associated with a 44% higher hazard of MACE (unadjusted HR per SD of log‐transformed Cys‐C 1.44, 95% CI 1.35‐1.54, P<0.001), including 28% higher hazard of MI (HR 1.28, 95% CI 1.18‐1.40).
A stepwise increase in the incidence of CVD or HF and MACE was seen across quartiles of Cys‐C (Figures 1 and 2). Patients with Cys‐C concentration in the highest quartile had nearly a 5‐fold hazard of CVD or HF (HR Q4:Q1 4.87, 95% CI 3.48‐6.82, P<0.001) and more than a 2‐fold hazard of MACE (HR Q4:Q1 2.26, 95% CI 1.82‐2.81, P<0.001), including HF hospitalization (HR Q4:Q1 5.71, 95% CI 3.58‐9.13), CVD (HR Q4:Q1 5.54, 95% CI 3.51‐8.77), and MI (HR Q4:Q1 1.72, 95% CI 1.31‐2.27) (Table 2).
Figure 1.
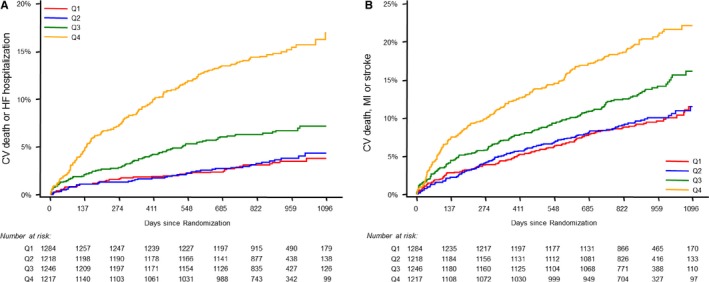
Cumulative incidence curves by cystatin‐C quartile through long‐term follow‐up for the composite end point of cardiovascular death or heart failure hospitalization (A) and MACE (B). CV indicates cardiovascular; HF, heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; Q1‐Q4, quartiles 1‐4.
Figure 2.
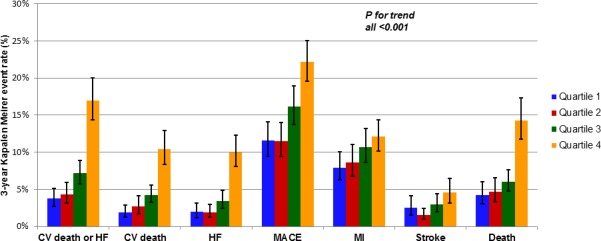
The 3‐year Kaplan‐Meier event rates of various prespecified outcomes by quartile of baseline cystatin‐C. CV indicates cardiovascular; HF, heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction.
Table 2.
Unadjusted and Adjusted Risk of Outcomes by Quartile of Baseline Cystatin‐C
| Outcome | Number of Events | Model | HR and 95% CI per 1 SD of Log Transformed cystatin‐C | P Value | Cystatin‐C Quartile | P Trend | |||
|---|---|---|---|---|---|---|---|---|---|
| Q1 Adj HR (95% CI) | Q2 Adj HR (95% CI) | Q3 Adj HR (95% CI) | Q4 Adj HR (95% CI) | ||||||
| CVD or HF | 347 | Unadjusted | 1.89 (1.75–2.04) | <0.001 | Referent | 1.10 (0.72–1.68) | 2.09 (1.44–3.03) | 4.87 (3.48–6.82) | <0.001 |
| Adjusted | 1.28 (1.12–1.46) | <0.001 | Referent | 0.84 (0.54–1.3) | 1.32 (0.89–1.96) | 1.48 (0.98–2.24) | 0.01 | ||
| CVD | 206 | Unadjusted | 1.91 (1.73–2.11) | <0.001 | Referent | 1.20 (0.68–2.12) | 2.39 (1.45–3.94) | 5.54 (3.51–8.77) | <0.001 |
| Adjusted | 1.24 (1.04–1.47) | 0.01 | Referent | 0.84 (0.47–1.52) | 1.26 (0.74–2.15) | 1.36 (0.78–2.37) | 0.14 | ||
| Hospitalization for heart ure | 186 | Unadjusted | 1.97 (1.78–2.19) | <0.001 | Referent | 1.10 (0.61–2.01) | 1.91 (1.12–3.25) | 5.71 (3.58–9.13) | <0.001 |
| Adjusted | 1.42 (1.19–1.69) | <0.001 | Referent | 0.85 (0.45–1.59) | 1.35 (0.77–2.36) | 2.08 (1.19–3.66) | <0.01 | ||
| MACE | 651 | Unadjusted | 1.44 (1.35–1.54) | <0.001 | Referent | 1.05 (0.81–1.35) | 1.48 (1.17–1.87) | 2.26 (1.82–2.81) | <0.001 |
| Adjusted | 1.15 (1.04–1.28) | <0.01 | Referent | 0.95 (0.73–1.24) | 1.21 (0.95–1.55) | 1.25 (0.94–1.65) | 0.045 | ||
| MI (fatal or nonfatal) | 406 | Unadjusted | 1.28 (1.18–1.4) | <0.001 | Referent | 1.08 (0.80–1.46) | 1.33 (1.00–1.77) | 1.72 (1.31–2.27) | <0.001 |
| Adjusted | 1.17 (1.02–1.33) | 0.02 | Referent | 1.15 (0.84–1.56) | 1.31 (0.97–1.78) | 1.34 (0.94–1.91) | 0.08 | ||
| Stroke (fatal or nonfatal) | 116 | Unadjusted | 1.36 (1.16–1.60) | <0.001 | Referent | 0.83 (0.45–1.53) | 1.38 (0.80–2.38) | 2.23 (1.35–3.68) | <0.001 |
| Adjusted | 0.94 (0.74–1.19) | 0.59 | Referent | 0.58 (0.3–1.11) | 0.95 (0.54–1.69) | 0.97 (0.52–1.81) | 0.70 | ||
| All‐cause mortality | 303 | Unadjusted | 1.76 (1.61–1.91) | <0.001 | Referent | 1.09 (0.71–1.66) | 1.74 (1.19–2.54) | 3.85 (2.74–5.41) | <0.001 |
| Adjusted | 1.15 (0.99–1.33) | 0.06 | Referent | 0.8 (0.52–1.23) | 0.96 (0.64–1.43) | 1.05 (0.69–1.6) | 0.64 | ||
Variables included in the adjusted model are age (quartiles), sex, BMI (<18.5, 18.5‐<25, 25‐<30, ≥30), history of heart failure, diabetes mellitus, hypertension, hyperlipidemia, prior MI, current smoker, region (North America and Western Europe vs other regions), race (white vs nonwhite), index diagnosis (STEMI vs non‐STE ACS), catheterization for qualifying event, baseline LDL cholesterol (quartiles), days from qualifying event (≤14 days), randomized treatment arm, baseline Cys‐C, baseline eGFR (<60 mL/min per 1.73 m2), hsTnI (<26 mg/dL), BNP (<80 pg.mL), FGF‐23 (<93 pg/mL). BMI indicates body mass index; BNP, brain‐type natriuretic peptide; CI, confidence interval; Cys‐C, cystatin C; CVD, cardiovascular death; eGFR, estimated glomerular filtration rate; FGF‐23, fibroblast growth factor‐23; HF, heart failure; HR, hazard ratio; hsTnI, high‐sensitivity troponin I; LDL, low‐density lipoprotein; MACE, major adverse cardiovascular events; MI, myocardial infarction; non‐STE ACS, non–ST‐elevation acute coronary syndrome; STEMI, ST‐elevation MI.
After multivariable adjustment, the strength of the relationships was attenuated, but increasing concentration of Cys‐C (per SD of log‐transformed Cys‐C) remained significantly associated with a 28% higher hazard of CVD or HF (adjusted HR 1.28, 95% CI 1.12‐1.46, P<0.001), a 24% higher hazard of CVD (adjusted HR 1.24, 95% CI 1.04‐1.47, P=0.01), and a 42% higher hazard of HF hospitalization (adjusted HR 1.42, 95% CI 1.19‐1.69, P<0.001). Cys‐C was associated with a 15% higher hazard of CVD, MI, or stroke (adjusted HR 1.15, 95% CI 1.04‐1.28, P<0.01), including CVD (HR 1.24, 95% CI 1.04‐1.47) and MI (HR 1.17, 95% CI 1.02‐1.33) (Table 2). When modeling was categorical by quartile, increasing quartiles of Cys‐C were associated with a higher hazard of CVD or HF (P‐trend=0.01), HF (P‐trend≤0.01), and MACE (P‐trend=0.045). Consistent results were observed when eGFR was replaced with creatinine in the model (Table S2). Measures of association in the main model excluding FGF‐23 are presented in Table S3.
When Cys‐C and eGFR were dichotomized (Cys‐C Q4:Q1‐Q3; eGFR <60 mL/min per 1.73 m2) and entered simultaneously in a model adjusting for other covariates, higher concentrations of Cys‐C remained associated with a 34% higher hazard of CVD or HF (adjusted HR Q4:Q1‐Q3 1.34, 95% CI 1.01‐1.77, P=0.04) and an 87% higher hazard of HF (adjusted HR Q4:Q1‐Q3 1.87, 95% CI 1.28‐2.74, P<0.001). In contrast, eGFR was not significantly associated with the hazard of any cardiovascular outcomes (Figure 3).
Figure 3.
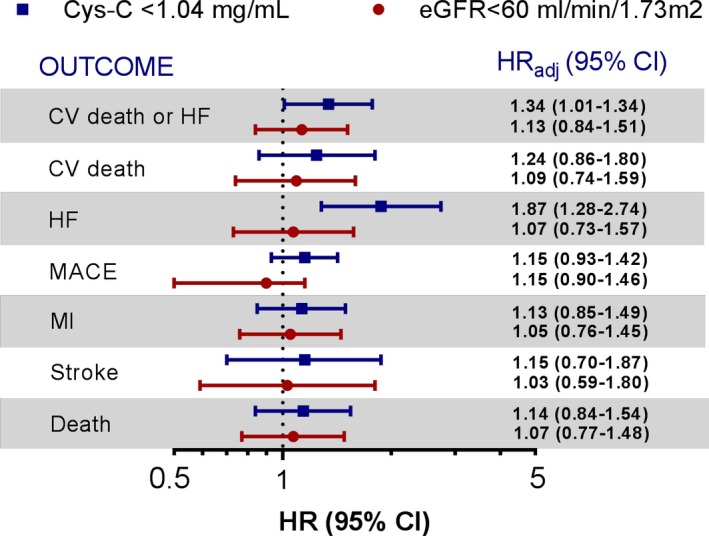
Adjusted risk of outcomes for cystatin‐C and eGFR when both are modeled as dichotomous variables and included simultaneously in a model with other risk predictors. Variables included in the model are age (quartiles), sex, BMI (<18.5, 18.5 to <25, 25 to <30, ≥30), history of HF, diabetes mellitus, hypertension, hyperlipidemia, prior MI, current smoker, region (North America and Western Europe vs other regions), race (white vs nonwhite), index diagnosis (STEMI vs non‐STE ACS), catheterization for qualifying event, baseline LDL cholesterol (quartiles), days from qualifying event (≤14 days), randomized treatment arm, hsTnI (<26 mg/dL), BNP (<80 pg/mL). BMI, body mass index; BNP, brain‐type natriuretic peptide; CI, confidence interval; CV, cardiovascular; Cys‐C, cystatin‐C; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; hsTnI, high‐sensitivity troponin I; LDL, low‐density lipoprotein; MACE, major adverse cardiovascular events; MI, myocardial infarction; non‐STE ACS, non–ST‐elevation acute coronary syndrome; STEMI, ST‐elevation MI.
The addition of Cys‐C to a fully adjusted model that excluded eGFR significantly improved the C‐statistic (0.80‐0.81, P=0.03). In contrast, the addition of eGFR to a model that excluded Cys‐C failed to improve the C‐statistic (0.80‐0.80, P=0.17; Table 3). The addition of Cys‐C to a fully adjusted model that included eGFR had a borderline effect to improve the C‐statistic (0.80‐0.81, P=0.052). Integrated discrimination improvement and net reclassification index are presented in Table S4.
Table 3.
Discrimination for Cardiovascular Death or Heart Failure Hospitalization With or Without Cys‐C or eGFR
| Model | C‐Statistic (95% CI) | P Value | |
|---|---|---|---|
| Adjusted Model for Clinical Covariates and Biomarkers (Excluding eGFR and Cys‐C) | Adjusted Model Plus New Biomarker | ||
| Adjusted model*±Cys‐C | 0.80 (0.78–0.82) | 0.81 (0.78–0.83) | 0.03 |
| Adjusted model*±eGFR | 0.80 (0.78–0.83) | 0.17 | |
Variables included in the model were age (quartiles), sex, BMI (<18.5, 18.5 to <25, 25 to <30, ≥30), history of heart failure, diabetes mellitus, hypertension, hyperlipidemia, prior MI, current smoker, region (North America and Western Europe vs other regions), race (white vs nonwhite), index diagnosis (STEMI vs non‐STE ACS), catheterization for qualifying event, baseline LDL cholesterol (quartiles), days from qualifying event (≤14 days), randomized treatment arm, hsTnI (<26 mg/dL), BNP (<80 pg.mL), FGF‐23 (<93 pg/mL). BMI indicates body mass index; BNP, brain‐type natriuretic peptide; CI, confidence interval; Cys‐C, cystatin‐C; eGFR, estimated glomerular filtration rate; FGF‐23, fibroblast growth factor‐23; hsTnI, high‐sensitivity troponin I; LDL, low‐density lipoprotein; MI, myocardial infarction; non‐STE ACS, non–ST‐elevation acute coronary syndrome; STEMI, ST‐elevation MI.
Discussion
We have demonstrated in a large population of patients after ACS that Cys‐C is associated with the risk of cardiovascular outcomes independent of traditional predictors including eGFR. Further, Cys‐C provides incremental information for risk stratification that is independent of additional traditional and novel biomarkers of the cardiorenal axis including BNP, hsTnI, and FGF‐23.
Interest in Cys‐C as a marker of risk first arose when studies demonstrated that it was associated with an increased risk of all‐cause mortality, CVD, MI, and stroke among elders from the Cardiovascular Health Study.17 Later studies suggested a strong association with the risk of death, but not with MI, among patients with stable coronary heart disease8; and such results were replicated in post‐ACS populations.18 In brief, Cys‐C is a protease inhibitor produced at a constant rate by most human cells; it is filtered by the renal glomerulus, and, unlike creatinine, it is metabolized by the proximal tubule.1, 2 Thereby, it is a sensitive measure of renal function that may be less affected by age, sex, and lean muscle mass than creatinine19, 20 and offers a better estimate of GFR than creatinine and conventional creatinine‐based formulas.21, 22 A few mechanisms directly relating Cys‐C to the progression of atherosclerosis had been proposed.23, 24, 25 However, a more recent Mendelian randomization study does not support a causal role of Cys‐C in the etiology of cardiovascular disease.26
The incremental value of Cys‐C beyond simpler assessments of renal function such as eGFR has been often disputed, given the strong correlation between these methods of assessment. However, these 2 markers may indicate different biological processes. Although both creatinine and Cys‐C, are excreted by the kidney, only Cys‐C is metabolized by the kidney and thus does not reflect only clearance. In fact, a newer formula for estimating GFR combining creatinine and Cys‐C has shown better performance than equations based on 1 sole marker.27 Earlier studies have shown that Cys‐C concentration is strongly associated with long‐term mortality in a representative and multiethnic US population with normal renal function28 as well as being associated with the risk of death and cardiovascular disease among elders without chronic kidney disease. It is also predictive of all‐cause mortality and CVD among nondiabetics with stage 3 or 4 chronic kidney disease.29 Therefore, it is notable that in our data set, we did not observe a significant association between eGFR and cardiovascular risk once Cys‐C was included in the model, yet Cys‐C remained independently associated with outcomes.
In the current large‐scale study of nearly 5000 patients after ACS, we found an association between Cys‐C and the risk of CV death or HF and its individual components, independent of other cardiorenal markers. The current findings add to earlier studies in patients after ACS that showed an association between Cys‐C and the risk of death, CVD, and hospitalization for HF.9, 10, 11, 30, 31, 32 Although a few small studies have shown a weak association between Cys‐C and subsequent MI,11, 33 larger studies have failed to demonstrate a significant relationship.18, 34 Cys‐C was associated with the risk of MI in PLATO (Platelet Inhibition and Patient Outcome Study) after adjusting for clinical covariates, but this association was no longer significant when established biomarkers were considered. In the current study Cys‐C remained associated with the risk of MI after adjustment for clinical predictors and markers, although the relationship was not as strong as that observed with the risk of CVD or HF. Further, there was no apparent relationship between Cys‐C and the risk of stroke. Nonetheless, Cys‐C added incremental value for cardiovascular risk stratification independent of emerging markers including FGF‐23, a marker that is strongly correlated with renal function and the risk of cardiovascular outcomes.35, 36, 37 To the best of our knowledge, this is the first study that has had the opportunity to evaluate the incremental value of Cys‐C after ACS beyond novel prognostic markers such as FGF‐23 and hsTnI.
Limitations to our study require consideration. First, despite thorough adjustment for both clinical and biochemical variables, residual confounding cannot be excluded given the observational nature of the analysis. Second, the current observed cut points for Cys‐C would require validation in a separate data set before being considered for clinical use. Third, the temporal association and the effects of changes in biomarker concentrations over time were not assessable due to the absence of repeated measures for Cys‐C. Last, because patients were randomized within 30 days of an ACS (median 14 days), we cannot exclude that the patient's renal function was not at steady state at the time of sample collection, and this may have influenced baseline Cyc‐C concentration. Consequently, any identified cut points should be validated in additional patient populations. As well, we cannot exclude that the baseline concentration of other assessed biomarkers was influenced by the recent ACS event. The timing from the index event was included as a covariate in all multivariable models but may not have fully captured the variable nature of biomarker fluctuations.
In conclusion, in high‐risk patients after ACS, Cys‐C is a strong predictor of adverse cardiovascular outcomes, including death from cardiovascular causes and hospitalization for HF; and it provides incremental prognostic information beyond established and novel cardiorenal markers including FGF‐23, BNP, creatinine, and eGFR.
Author Contributions
Correa conducted the literature review and produced the first article draft. Goodrich and Murphy performed statistical analyses. O'Donoghue was the study supervisor and guarantor of this work. All authors interpreted the data and provided critical revision
Disclosures
Dr Morrow reports receipt of consulting fees from Abbott Laboratories, Aralez, AstraZeneca, DiaDexus, GlaxoSmithKline, Merck and Company, Peloton, Roche Diagnostics, Verseon; and research grants from Abbott, Amgen, AstraZeneca, Daichii Sankyo Ltd, GlaxoSmithKline, Merck and Company, Pfizer, Novartis Pharmaceuticals, and Roche Diagnostics. Dr Braunwald reports grant support to his institution from GlaxoSmithKline. Richard Y. Davies is a GlaxoSmithKline employee. Dr Cannon reports research grants from (all >$10 000) Amgen, Arisaph, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda; and consulting fees from Alnylam, Amgen, Arisaph, Astra Zeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Kowa, Lipimedix,* Merck, Pfizer, Regeneron,* Sanofi,* and Takeda (*>$10 000). Dr O'Donoghue reports research grants from GlaxoSmithKline, Eisai, AstraZeneca, Merck, Janssen, and The Medicines Company. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Patient Characteristics for the Biomarker Cohort Compared With the Overall Study Population in SOLID‐TIMI 52
Table S2. Adjusted Risk of Outcomes by Quartile of Cystatin‐C After Replacing eGFR With Creatinine in the Main Model
Table S3. Adjusted Risk of Outcomes by Quartile of Baseline Cystatin‐C Excluding FGF‐23
Table S4. Reclassification for Cardiovascular Death or Heart Failure Hospitalization With Cystatin C or eGFR
Acknowledgments
The SOLID‐TIMI 52 (Stabilization of Plaque Using Darapladib‐Thrombolysis in Myocardial Infarction 52; ClinicalTrials.gov NCT 01000727) was sponsored by GlaxoSmithKline.
(J Am Heart Assoc. 2018;7:e009077 DOI: 10.1161/JAHA.118.009077.)
References
- 1. Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 2. Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39:89–104. [DOI] [PubMed] [Google Scholar]
- 3. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. [DOI] [PubMed] [Google Scholar]
- 4. Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. [DOI] [PubMed] [Google Scholar]
- 5. Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5‐year mortality in older adults: the cardiovascular health study. JAMA. 1998;279:585–592. [DOI] [PubMed] [Google Scholar]
- 6. Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004;15:2195–2203. [DOI] [PubMed] [Google Scholar]
- 7. Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. [DOI] [PubMed] [Google Scholar]
- 8. Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the heart and soul study. Circulation. 2007;115:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akerblom A, Wallentin L, Siegbahn A, Becker RC, Budaj A, Buck K, Giannitsis E, Horrow J, Husted S, Katus HA, Steg PG, Storey RF, Asenblad N, James SK. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST‐elevation and non‐ST‐elevation acute coronary syndromes: results from the platelet inhibition and patient outcomes study. Clin Chem. 2012;58:190–199. [DOI] [PubMed] [Google Scholar]
- 10. Kilic T, Oner G, Ural E, Yumuk Z, Sahin T, Bildirici U, Acar E, Celikyurt U, Kozdag G, Ural D. Comparison of the long‐term prognostic value of cystatin C to other indicators of renal function, markers of inflammation and systolic dysfunction among patients with acute coronary syndrome. Atherosclerosis. 2009;207:552–558. [DOI] [PubMed] [Google Scholar]
- 11. Ristiniemi N, Lund J, Tertti R, Christensson A, Ilva T, Porela P, Pulkki K, Pettersson K. Cystatin C as a predictor of all‐cause mortality and myocardial infarction in patients with non‐ST‐elevation acute coronary syndrome. Clin Biochem. 2012;45:535–540. [DOI] [PubMed] [Google Scholar]
- 12. Taglieri N, Fernandez‐Berges DJ, Koenig W, Consuegra‐Sanchez L, Fernandez JM, Robles NR, Sanchez PL, Beiras AC, Orbe PM, Kaski JC. Plasma cystatin C for prediction of 1‐year cardiac events in Mediterranean patients with non‐ST elevation acute coronary syndrome. Atherosclerosis. 2010;209:300–305. [DOI] [PubMed] [Google Scholar]
- 13. O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, Steen DL. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID‐TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 14. O'Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, Maggioni AP, Bode C, Weaver D, Johnson JL, Cicconetti G, Lukas MA, Tarka E, Cannon CP. Study design and rationale for the stabilization of plaques using darapladib‐thrombolysis in myocardial infarction (SOLID‐TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619.e611. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 16. Bergmark BA, Udell JA, Morrow DA, Cannon CP, Steen DL, Jarolim P, Budaj A, Hamm C, Guo J, Im K, Kuder JF, Braunwald E, Sabatine MS, O'Donoghue ML. Association of fibroblast growth factor 23 with recurrent cardiovascular events in patients after an acute coronary syndrome: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2018;3:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman‐Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. [DOI] [PubMed] [Google Scholar]
- 18. Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non‐ST‐elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. [DOI] [PubMed] [Google Scholar]
- 19. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 20. Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48:712–719. [DOI] [PubMed] [Google Scholar]
- 21. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am J Kidney Dis. 2002;40:221–226. [DOI] [PubMed] [Google Scholar]
- 22. Poge U, Gerhardt T, Stoffel‐Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21:660–664. [DOI] [PubMed] [Google Scholar]
- 23. Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor‐deficient mice. J Clin Invest. 2003;111:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferraro S, Marano G, Biganzoli EM, Boracchi P, Bongo AS. Prognostic value of cystatin C in acute coronary syndromes: enhancer of atherosclerosis and promising therapeutic target. Clin Chem Lab Med. 2011;49:1397–1404. [DOI] [PubMed] [Google Scholar]
- 25. Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, Fang K, Chapman HA, Shi GP. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E‐null mice. Circ Res. 2005;96:368–375. [DOI] [PubMed] [Google Scholar]
- 26. van der Laan SW, Fall T, Soumare A, Teumer A, Sedaghat S, Baumert J, Zabaneh D, van Setten J, Isgum I, Galesloot TE, Arpegard J, Amouyel P, Trompet S, Waldenberger M, Dorr M, Magnusson PK, Giedraitis V, Larsson A, Morris AP, Felix JF, Morrison AC, Franceschini N, Bis JC, Kavousi M, O'Donnell C, Drenos F, Tragante V, Munroe PB, Malik R, Dichgans M, Worrall BB, Erdmann J, Nelson CP, Samani NJ, Schunkert H, Marchini J, Patel RS, Hingorani AD, Lind L, Pedersen NL, de Graaf J, Kiemeney LA, Baumeister SE, Franco OH, Hofman A, Uitterlinden AG, Koenig W, Meisinger C, Peters A, Thorand B, Jukema JW, Eriksen BO, Toft I, Wilsgaard T, Onland‐Moret NC, van der Schouw YT, Debette S, Kumari M, Svensson P, van der Harst P, Kivimaki M, Keating BJ, Sattar N, Dehghan A, Reiner AP, Ingelsson E, den Ruijter HM, de Bakker PI, Pasterkamp G, Arnlov J, Holmes MV, Asselbergs FW. Cystatin C and cardiovascular disease: a Mendelian randomization study. J Am Coll Cardiol. 2016;68:934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu CK, Lin JW, Caffrey JL, Chang MH, Hwang JJ, Lin YS. Cystatin C and long‐term mortality among subjects with normal creatinine‐based estimated glomerular filtration rates: NHANES III (Third National Health and Nutrition Examination Survey). J Am Coll Cardiol. 2010;56:1930–1936. [DOI] [PubMed] [Google Scholar]
- 29. Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147:19–27. [DOI] [PubMed] [Google Scholar]
- 30. Keller T, Messow CM, Lubos E, Nicaud V, Wild PS, Rupprecht HJ, Bickel C, Tzikas S, Peetz D, Lackner KJ, Tiret L, Munzel TF, Blankenberg S, Schnabel RB. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the atherogene study. Eur Heart J. 2009;30:314–320. [DOI] [PubMed] [Google Scholar]
- 31. Akgul O, Uyarel H, Ergelen M, Pusuroglu H, Gul M, Turen S, Bulut U, Baycan OF, Ozal E, Cetin M, Yildirim A, Uslu N. Predictive value of elevated cystatin C in patients undergoing primary angioplasty for ST‐elevation myocardial infarction. J Crit Care. 2013;28:882.e813–882.e820 [DOI] [PubMed] [Google Scholar]
- 32. Ichimoto E, Jo K, Kobayashi Y, Inoue T, Nakamura Y, Kuroda N, Miyazaki A, Komuro I. Prognostic significance of cystatin C in patients with ST‐elevation myocardial infarction. Circ J. 2009;73:1669–1673. [DOI] [PubMed] [Google Scholar]
- 33. Windhausen F, Hirsch A, Fischer J, van der Zee PM, Sanders GT, van Straalen JP, Cornel JH, Tijssen JG, Verheugt FW, de Winter RJ. Cystatin C for enhancement of risk stratification in non‐ST elevation acute coronary syndrome patients with an increased troponin T. Clin Chem. 2009;55:1118–1125. [DOI] [PubMed] [Google Scholar]
- 34. Kaski JC, Fernandez‐Berges DJ, Consuegra‐Sanchez L, Fernandez JM, Garcia‐Moll X, Mostaza JM, Cebada RT, Juanatey JR, Martinez GG, Marrugat J. A comparative study of biomarkers for risk prediction in acute coronary syndrome—results of the SIESTA (Systemic Inflammation Evaluation in Non‐ST‐Elevation Acute Coronary Syndrome) Study. Atherosclerosis. 2010;212:636–643. [DOI] [PubMed] [Google Scholar]
- 35. Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O'Donnell TF, Vora AN, Omland T, Solomon SD, Pfeffer MA, Braunwald E, Sabatine MS. Fibroblast growth factor‐23, cardiovascular prognosis, and benefit of angiotensin‐converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol. 2014;63:2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, Floege J, Fliser D, Heine GH. Associations of FGF‐23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2‐4. Clin J Am Soc Nephrol. 2014;9:1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin Z, Liu X, Song M, Zhou Q, Yu J, Zhou B, Wu Y, He Y, Huang L. Fibroblast growth factor 23 as a predictor of cardiovascular and all‐cause mortality in prospective studies. Atherosclerosis. 2017;261:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Patient Characteristics for the Biomarker Cohort Compared With the Overall Study Population in SOLID‐TIMI 52
Table S2. Adjusted Risk of Outcomes by Quartile of Cystatin‐C After Replacing eGFR With Creatinine in the Main Model
Table S3. Adjusted Risk of Outcomes by Quartile of Baseline Cystatin‐C Excluding FGF‐23
Table S4. Reclassification for Cardiovascular Death or Heart Failure Hospitalization With Cystatin C or eGFR


