Abstract
Background
Coronary artery calcium (CAC) predicts cardiovascular disease (CVD) events; however, less is known about how its prognostic implications vary by race/ethnicity.
Methods and Results
A total of 38 277 whites, 1621 Asians, 977 blacks, and 1349 Hispanics from the CAC Consortium (mean age 55 years, 35% women) were followed over a median of 11.7 years. Modeling CAC in continuous and categorical (CAC=0; CAC 1–99; CAC 100–399; CAC ≥400) forms, we assessed its predictive value for all‐cause and CVD mortality by race/ethnicity using Cox proportional hazards and Fine and Gray competing‐risk regression, respectively. We also assessed the impact of race/ethnicity on risk within individual CAC strata, using whites as the reference. Models were adjusted for traditional cardiovascular risk factors. Increased CAC was associated with higher total and CVD mortality risk in all race/ethnicity groups, including Asians. However, the risk gradient with increasing CAC was more pronounced in blacks and Hispanics. In Fine and Gray subdistribution hazards models adjusted for traditional cardiovascular risk factors and CAC (continuous), blacks (subdistribution hazard ratio 3.4, 95% confidence interval, 2.5–4.8) and Hispanics (subdistribution hazard ratio 2.3, 95% confidence interval, 1.6–3.2) showed greater risk of CVD mortality when compared with whites, while Asians had risk similar to whites. These race/ethnic differences persisted when CAC=0.
Conclusions
CAC predicts all‐cause and CVD mortality in all studied race/ethnicity groups, including Asians and Hispanics, who may be poorly represented by the Pooled Cohort Equations. Blacks and Hispanics may have greater mortality risk compared with whites and Asians after adjusting for atherosclerosis burden, with potential implications for US race/ethnic healthcare disparities research.
Keywords: coronary artery calcium, health disparities, race and ethnicity, risk prediction
Subject Categories: Computerized Tomography (CT), Race and Ethnicity, Cardiovascular Disease, Epidemiology
Clinical Perspective
What Is New?
This is the first study to investigate race/ethnicity differences in the prognostic implications of coronary artery calcium for all‐cause and cardiovascular disease mortality, in a referred clinical cohort.
While coronary artery calcium is strongly predictive of all‐cause and cardiovascular disease mortality in all tested race/ethnicity groups, its prognostic implications vary by race/ethnicity, with blacks and Hispanics having greater risk of all‐cause and cardiovascular disease mortality compared with whites and Asians with similar coronary artery calcium scores.
What Are the Clinical Implications?
Coronary artery calcium is a robust test for all‐cause and cardiovascular disease mortality risk stratification across tested race/ethnicity groups (including race/ethnicity groups underrepresented in the Pooled Cohort Equations).
More attention should be paid to comprehensive risk factor modification in high‐risk racial/ethnic minorities.
Introduction
Tailoring preventive therapy based on an individual's risk requires useful tools that accurately estimate atherosclerotic cardiovascular disease (ASCVD) risk, allowing reclassification of individuals with borderline risk. A criticism of nearly all risk tools, however, is their questionable applicability to ethnic minorities.1, 2, 3 To date, the possible interactions between race/ethnicity and risk prediction tools have been incompletely elucidated.4 The Pooled Cohort Equations, for example, only has equations for non‐Hispanic whites and blacks, and in the absence of good evidence, provides a general recommendation for using the non‐Hispanic white equations for all other ethnic groups.5 These assumptions fail to accurately predict risk in these populations.6, 7
Coronary artery calcium (CAC), as a direct measure of atherosclerotic disease burden, has been proposed as a tool that might overcome limitations in estimating risk in minority groups. Furthermore, CAC scoring has been shown to outperform “traditional” risk scores including the Framingham Risk Score and the Pooled Cohort Equations in the estimation of cardiovascular risk8, 9; therefore, it is widely accepted to have clinical value in risk stratification and risk reclassification among people who are estimated to have intermediate risk of ASCVD outcomes.10, 11, 12, 13
While in the MESA (Multi‐Ethnic Study of Atherosclerosis) study, CAC predicted coronary heart disease (CHD) risk similarly in 4 ethnic groups, other studies have pointed to differences in CAC distribution and interpretation across groups.14, 15, 16 The predominant limitation among these studies is small sample sizes and insufficient power to demonstrate differences in the cardiovascular predictive value of CAC between race/ethnicity groups.14, 17
We therefore aimed to address these limitations using data from the CAC Consortium, the largest racially heterogeneous cohort of CAC scoring assembled.18 Specifically, we sought to assess the utility of CAC within each race/ethnicity group, whether CAC scores portend similar levels of all‐cause and CVD mortality across select race/ethnicity groups, and whether the addition of CAC to traditional risk assessment improves risk prediction to a similar degree across these race/ethnicity groups.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Study Population
In brief, the CAC Consortium is a multicenter prospective cohort study of 66 636 patients, aimed at studying the association of clinical CAC scoring with long‐term cause‐specific mortality.18, 19 Four participating medical institutions with longstanding experience in CAC scoring from 3 states in the United States (California, Minnesota, and Ohio) contributed patient data to the consortium. Patients were at least 18 years of age and were free of clinically important CVD symptoms (ie, typical angina or angina equivalent) and overt clinical CVD at the time of a clinically indicated CAC scan. Baseline data for the CAC consortium represent the years 1991 through 2010, with follow‐up of the cohort through June 2014.
We included 42 224 participants who had self‐reported data on race/ethnicity and identified as whites, blacks, Asians, or Hispanics. Our study population included 38 277 whites, 1621 Asians, 977 blacks, and 1349 Hispanics.
Consent was obtained from all study participants at individual centers at the time of CAC scanning, while Institutional Review Board approval for coordinating center activities was obtained at the Johns Hopkins Hospital.
Measurement and Definition of Risk Factors
All participants had risk factor and laboratory data collected as part of their routine clinic visit and/or at the time of the CAC scans.
Hypertension was defined based on a prior diagnosis of hypertension or treatment with antihypertensive therapy. Blood pressure taken at the time of CAC scans was not used to override a clinical diagnosis of hypertension. Diabetes mellitus was defined as a prior diagnosis of diabetes mellitus, or treatment with antidiabetic medications. Dyslipidemia was defined as a prior diagnosis of dyslipidemia (elevated triglycerides and/or low high‐density lipoprotein cholesterol), treatment with any lipid‐lowering drugs, or low‐density lipoprotein cholesterol >160 mg/dL, high‐density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women, or the presence of fasting triglycerides >150 mg/dL. Smoking status was defined based on current cigarette smoking status (Yes/No). A family history of CHD was mostly determined by the presence of a first‐degree relative with a history of CHD. A more stringent definition of premature family history (<55 years in a male or <65 years in a female relative) was applied in the Columbus, OH site. In the case of individuals with partially missing risk factor data, multiple imputation was conducted. A validation of the imputation approach has been previously reported.18
The 10‐year risk of ASCVD was calculated according to the Pooled Cohort Equations.1
Computed Tomography Data
Noncontrast cardiac‐gated computed tomography scans were performed at individual sites according to a common standard assessment protocol for each scanner technology. Most patients were scanned using electron beam tomography, accounting for ≈93% of scans, while the more recent CAC data at 2 sites were obtained using multidetector computed tomography (accounting for ≈7% of scans). There was no centralized core lab reading center; CAC scans were read locally using the Agatston method.20 Per CAC consortium study site inclusion criteria, each site had had at least 10 years of CAC scanning experience at baseline. CAC score categories were defined as CAC 0, CAC 1 to 99, CAC 100 to 399, and CAC ≥400.21
Outcome Ascertainment
Participants were followed for all‐cause and cause‐specific mortality, with mortality ascertainment accomplished through linkage to the social security death index Death Master file using a previously validated algorithm22 similar to the algorithm used by the National Death Index service. Death was considered present if there was a match on social security number and 1 additional patient identifier. Whenever social security number was not available, a complete match on all other patient identifiers was required to confirm death, thus prioritizing specificity over sensitivity.
Death certificates were obtained from the National Death Index, and the underlying cause of death was categorized into common causes of death using International Classification of Diseases, Ninth Revision (ICD‐9) and International Classification of Diseases, Tenth Revision (ICD‐10) codes as previously described.18 CVD death was defined as death from CVD causes including CHD (54% of CVD deaths), stroke (17% of CVD deaths), heart failure (5% of CVD deaths), and other circulatory diseases (24% of CVD deaths). Mean duration of follow‐up was 12±4 years with maximum follow‐up across sites ranging from 13.6 to 22.5 years.
Statistical Methods
First, we summarized baseline characteristics of the study population by race/ethnicity, reporting proportions and means±SDs as appropriate. Total and sex‐stratified prevalence of non‐zero CAC, and prevalence distributions across CAC score categories were calculated by race/ethnicity.
We then calculated overall all‐cause and CVD mortality rates by CAC category for each race/ethnicity group, expressed per 1000 person‐years, and repeated these after stratification by sex. Race/ethnicity‐specific Kaplan‐Meier survival curves were constructed for CVD death, stratified according to CAC score category and compared using log‐rank statistics.
After visually confirming the proportional hazards assumption, we used Cox proportional hazards regression models to estimate multivariable‐adjusted hazard ratios for the association between CAC and all‐cause mortality in each race/ethnicity group. Additionally, using the Fine and Gray proportional subhazards models,23 we estimated multivariable‐adjusted subdistribution hazard ratios for the association between CAC and CVD mortality, accounting for competing risks of non‐CVD mortality.
Sequential models were used as follows: unadjusted model, model 1 adjusting for age, sex, and study site, and model 2 further adjusting for hypertension, hyperlipidemia, cigarette smoking, family history of CVD, and presence of diabetes mellitus. For these analyses, CAC was modeled in categorical, dichotomous (CAC absent/present), and continuous forms [log (CAC+1)]. The addition of 1 to the absolute calcium score before logarithmic transformation allowed accounting for patients with a calcium score of 0.14
Furthermore, to assess risk inherent to individual race/ethnicity groups after accounting for atherosclerosis burden, we first estimated the relative hazards and subhazards of all‐cause and CVD mortality for each race/ethnicity group, adjusting for [log (CAC+1)], as compared with whites, using Cox proportional hazards models and competing risk models as appropriate. Additionally, we assessed the association of race/ethnicity with all‐cause and CVD mortality within individual CAC strata. Given observed differences inherent to race/ethnicity, we then conducted a separate interactive analysis where we compared all‐cause and CVD mortality risks in all other CAC‐race/ethnicity subgroups to a reference of “whites with CAC=0.”
Finally, we compared, using receiver‐operating characteristic curves, the discriminatory performance of the following risk tools versus “risk tools+CAC”: (1) risk factors; (2) Framingham risk scores.
Sensitivity analyses were conducted as follows: (1) further adjustment for baseline medication (statin and/or antihypertensive) use; (2) excluding all participants with imputed data. All analyses were performed with Stata software version 14.2 (College Station, TX).
Results
Baseline Characteristics
Overall, 65% of participants were men and the mean age of the study population was 54.7±10.6 years. Blacks had the highest prevalence of hypertension (55%) and cigarette smoking (15%), while Hispanics had the highest prevalence of diabetes mellitus (18%). Asians had the lowest prevalence of hyperlipidemia (52%). Overall, blacks had a higher mean ASCVD risk score (10.3±9.1) than other race/ethnicity groups (Table 1).
Table 1.
Baseline Demographic, Risk Factor and Risk Measure Characteristics According to Race/Ethnicity Groupa
| Characteristics | All (N=42 224) | Whites (N=38 277) | Asians (N=1621) | Blacks (N=977) | Hispanics (N=1349) |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, y | 54.7±10.6 | 54.8±10.5 | 53.8±11.2 | 55.0±11.4 | 52.9±10.8 |
| Men, % | 65.0 | 65.3 | 63.7 | 60.0 | 61.5 |
| Risk factors | |||||
| Hypertension, % | 29.9 | 28.6 | 37.1 | 54.9 | 38.0 |
| Hyperlipidemia, % | 58.4 | 58.6 | 52.1 | 57.5 | 57.8 |
| Current smoker, % | 9.6 | 9.6 | 7.5 | 14.9 | 10.0 |
| Family history of CHD, % | 49.7 | 50.2 | 45.5 | 42.4 | 45.0 |
| Diabetes mellitus, % | 6.3 | 5.4 | 11.7 | 17.3 | 18.2 |
| Total cholesterol, mg/dL | 200.2±42.4 | 200.7±42.2 | 197.4±45.1 | 191.2±42.6 | 197.3±45.1 |
| HDL‐C, mg/dL | 54.0±18.3 | 54.1±18.4 | 53.4±18.5 | 53.1±17.4 | 50±15.5 |
| Systolic blood pressure, mm Hg | 127.3±19.0 | 127.2 ±19.0 | 123.8±19.0 | 131.8±17.6 | 129.0±20.4 |
| Risk measures | |||||
| Framingham risk score | 11.3±9.1 | 11.2±9.0 | 12.1±10.7 | 12.1±10.5 | 11.7 ±10.0 |
| ASCVD risk scoreb | 7.5±8.9 | 7.4±8.8 | 8.0±10.3 | 10.3±9.1 | 7.6±9.1 |
| ASCVD risk categories, % | |||||
| <5% | 54.7 | 55.2 | 56.4 | 32.1 | 54.0 |
| 5%–7.5% | 13.4 | 13.4 | 11.9 | 17.4 | 13.1 |
| >7.5% | 32.0 | 31.5 | 31.7 | 50.5 | 32.9 |
| CAC score categories, % | |||||
| CAC 0 | 44.5 | 44.2 | 49.0 | 43.9 | 46.0 |
| CAC 1–99 | 30.7 | 30.7 | 28.6 | 32.9 | 31.9 |
| CAC 100–399 | 13.8 | 14.0 | 12.3 | 12.8 | 11.4 |
| CAC ≥400 | 11.0 | 11.0 | 10.1 | 10.4 | 10.8 |
ASCVD indicates atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CHD, coronary heart disease; HDL‐C, high‐density lipoprotein cholesterol.
Values are means±SD and percentages; percentages may not total 100 because of rounding.
ASCVD risk score is as estimated by the Pooled Cohort Equations.
Prevalence of CAC by Race/Ethnicity and Sex
Small but significant differences were noted in the overall distribution of CAC by race/ethnicity (Table 1). The prevalence of CAC was highest in blacks (56.1%), followed by whites (55.8%), Hispanics (54.0%), and Asians (51.0%). Following stratification by sex (Table S1), 40.4% of women had CAC, compared with 63.7% of men. Black women (50.6%) specifically had a higher prevalence of calcification compared with women of other race/ethnicity groups, while white (64%) and Hispanic (63.7%) men had a greater prevalence of calcification than men of other race/ethnicity groups.
All‐Cause and CVD Mortality Rates by Race/Ethnicity and CAC Group
Overall, there were 1953 deaths (608 caused by CVD, and 321 caused by CHD) over a median follow‐up duration of 11.7 years. Table 2 shows the cumulative incidence and incidence rates of all‐cause and CVD mortality by CAC group for each race/ethnicity group. For all categories of CAC including CAC 0, blacks had a disproportionately higher incidence of all‐cause and CVD mortality compared with other race/ethnicity groups, followed closely by Hispanics. Asians had the lowest all‐cause death rates across all categories of CAC. There was at least a doubling of all‐cause mortality rates with increasing CAC group in all race/ethnicity groups (Table 2). Sex‐stratified results are shown in Tables S2 and S3.
Table 2.
Cumulative Incidence and Incidence Rates (per 1000 Person‐Years) of All‐Cause and CVD‐Specific Mortality by CAC Group for Each Race/Ethnicity Group
| CAC 0 | CAC 1–99 | CAC 100–399 | CAC ≥400 | |
|---|---|---|---|---|
| White | ||||
| Death (n, %) | 325, 1.9% | 442, 3.8% | 365, 6.8% | 586, 13.9% |
| Per 1000 | 1.7 (1.6, 1.9) | 3.3 (3.0, 3.6) | 6.1 (5.5, 6.8) | 13.1 (12.1, 14.2) |
| CVD death (n, %) | 58, 0.34% | 114, 0.97% | 118, 2.2% | 222, 5.3% |
| Per 1000 | 0.31 (0.24, 0.40) | 0.85 (0.71, 1.03) | 2.0 (1.7, 2.4) | 5.0 (4.4, 5.7) |
| Asian | ||||
| Death (n, %) | 9, 1.1% | 15, 3.2% | 10, 5.0% | 22, 13.4% |
| Per 1000 | 0.93 (0.48, 1.8) | 2.7 (1.6, 4.5) | 4.4 (2.4. 8.2) | 12.6 (8.3, 19.2) |
| CVD death (n, %) | 0, 0% | 1, 0.22% | 4, 2.0% | 11, 6.7% |
| Per 1000 | 0 | 0.18 (0.03, 1.3) | 1.8 (0.66, 4.7) | 6.3 (3.5, 11.4) |
| Black | ||||
| Death (n, %) | 16, 3.7% | 26, 8.1% | 23, 18.4% | 32, 31.4% |
| Per 1000 | 3.7 (2.2, 6.0) | 7.9 (5.4, 11.6) | 19.9 (13.2, 29.9) | 41.4 (29.3, 58.6) |
| CVD death (n, %) | 10, 2.33% | 7, 2.2% | 11, 8.8% | 18, 17.7% |
| Per 1000 | 2.3 (1.2, 4.2) | 2.1 (1.0, 4.4) | 9.5 (5.3, 17.2) | 23.3 (14.7, 37.0) |
| Hispanic | ||||
| Death (n, %) | 13, 2.1% | 23, 5.4% | 17, 11.0% | 29, 20.0% |
| Per 1000 | 1.8 (1.1, 3.2) | 4.7 (3.1, 7.0) | 10.6 (6.6, 17.1) | 21.3 (14.8, 30.6) |
| CVD death (n, %) | 6, 0.97% | 8, 1.9% | 5, 3.3% | 15, 10.3% |
| Per 1000 | 0.85 (0.38, 1.9) | 1.6 (0.81, 3.3) | 3.1 (1.3, 7.5) | 11.0 (6.6, 18.2) |
CAC indicates coronary artery calcium; CVD, cardiovascular disease.
Predictive Value of CAC for All‐Cause and CVD Mortality by Race/Ethnicity
Significant graded CVD death‐free survival rate was observed across all race/ethnicity groups under consideration (P<0.05) (Figure).
Figure 1.
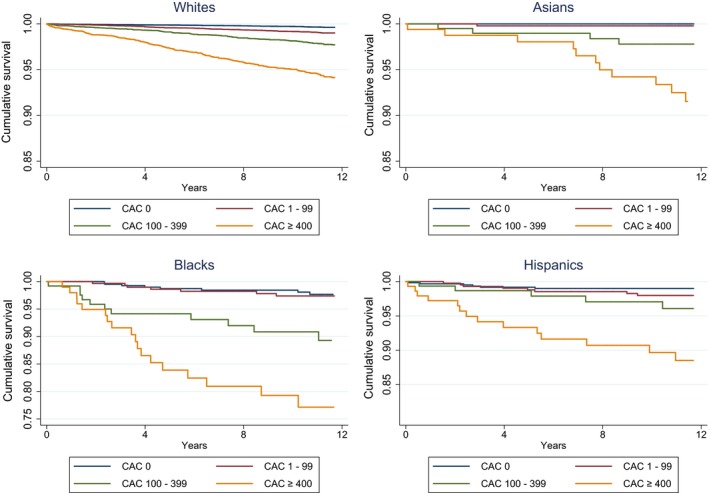
Race/ethnicity–specific Kaplan‐Meier curves for CVD mortality, by CAC group. CAC indicates coronary artery calcium; CVD, cardiovascular.
Following multivariable adjustment, we noted within each race/ethnicity group a clear trend of greater hazards of death with increasing levels of CAC when compared with a reference of CAC 0. The gradient of all‐cause and CVD mortality risk with increasing CAC was steeper in blacks and Hispanics compared with whites (Table 3). Unadjusted and minimally adjusted results for all‐cause and CVD mortality are presented in Tables S4 and S5.
Table 3.
Multivariable‐Adjusted Risk of All‐Cause and CVD Death Associated With CAC for Each Race/Ethnicity Groupa
| Adjusted HR (All‐Cause Mortality) HR (95% CI) | Adjusted SHR (CVD Mortality) SHR (95% CI) | |
|---|---|---|
| Whites | ||
| CAC 0 | 1.0 | 1.0 |
| CAC 1–99 | 1.2 (1.0–1.4) | 1.6 (1.1–2.2) |
| CAC 100–399 | 1.4 (1.2–1.7) | 2.2 (1.6–3.1) |
| CAC ≥400 | 2.2 (1.9–2.6) | 3.6 (2.5–5.1) |
| Log (CAC+1) | 1.1 (1.1–1.2) | 1.2 (1.2–1.3) |
| CAC (Yes/No) | 1.4 (1.2–1.6) | 2.1 (1.5–2.8) |
| Asians | ||
| CAC 0 | 1.0 | 1.0 |
| CAC 1–99 | 1.8 (0.7–4.2) | ···b |
| CAC 100–399 | 1.6 (0.6–4.5) | ··· |
| CAC ≥400 | 3.3 (1.2–8.5) | ··· |
| Log (CAC+1) | 1.2 (1.1–1.4) | 2.3 (1.8–2.9) |
| CAC (Yes/No) | 2.0 (0.9–4.4) | ··· |
| Blacks | ||
| CAC 0 | 1.0 | 1.0 |
| CAC 1–99 | 1.6 (0.8–3.0) | 0.7 (0.2–1.8) |
| CAC 100–399 | 3.0 (1.5–6.0) | 2.4 (0.8–7.0) |
| CAC ≥400 | 5.2 (2.7–10.0) | 4.2 (1.6–11.1) |
| Log (CAC+1) | 1.3 (1.2–1.4) | 1.3 (1.1–1.5) |
| CAC (Yes/No) | 2.4 (1.3–4.2) | 1.7 (0.8–3.8) |
| Hispanics | ||
| CAC 0 | 1.0 | 1.0 |
| CAC 1–99 | 1.8 (0.9–3.6) | 1.4 (0.5–4.1) |
| CAC 100–399 | 2.9 (1.3–6.3) | 1.9 (0.6–6.4) |
| CAC ≥400 | 4.9 (2.3–10.4) | 6.1 (2.0–18.4) |
| Log (CAC+1) | 1.3 (1.1–1.4) | 1.3 (1.1–1.5) |
| CAC (Yes/No) | 2.4 (1.3–4.6) | 2.4 (1.3–4.5) |
CAC indicates coronary artery calcium; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SHR, subdistribution hazard ratio.
Estimates are multivariable‐adjusted hazard and subdistribution hazard ratios for all‐cause and CVD mortality, respectively, adjusted for study site, age, sex, hypertension, hyperlipidemia, cigarette smoking, family history of coronary heart disease and the presence of diabetes mellitus.
No CVD deaths noted in Asians with CAC=0, analysis by CAC group and dichotomous CAC not possible.
Differences in Prognostic Implications of CAC by Race/Ethnicity
Multivariable‐adjusted effect of race/ethnicity on all‐cause and CVD mortality risk in models additionally adjusting for CAC showed blacks and Hispanics to have greater all‐cause and CVD mortality risk compared with whites, while Asians had similar mortality risk as compared with whites. Results persisted across CAC strata (Tables 4 and 5). Sensitivity analyses including (1) adjustment for statin and antihypertensive therapy, and (2) adjustment using nonimputed risk factor data yielded similar results.
Table 4.
Multivariable‐Adjusted Effect of Race/Ethnicity on All‐Cause and CVD Mortality Risk, Adjusted for CAC
| All‐Cause Mortality HR (95% CI) | CVD Mortality SHR (95% CI) | |
|---|---|---|
| Whites | 1.0 | 1.0 |
| Asians | 0.8 (0.6–1.1) | 0.7 (0.4–1.1) |
| Blacks | 2.5 (2.1–3.1) | 3.4 (2.5–4.8) |
| Hispanics | 1.9 (1.5–2.3) | 2.3 (1.6–3.2) |
CAC indicates coronary artery calcium; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SHR, subdistribution hazard ratio.
Estimates are multivariable adjusted hazard and SHRs adjusted for study site, age, sex, hypertension, hyperlipidemia, cigarette smoking, family history of coronary heart diease diabetes mellitus, and log (CAC+1).
Table 5.
Effect of Race/Ethnicity on All‐Cause and CVD‐Specific Mortality Within Individual CAC Strataa
| All‐Cause Mortality HR (95% CI) | CVD mortalityb SHR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| CAC 0 | CAC 1–99 | CAC 100–399 | CAC ≥400 | CAC 0 | CAC 1–99 | CAC 100–399 | CAC ≥400 | |
| Whites | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Asians | 0.78 (0.4–1.5) | 1.03 (0.6–1.7) | 0.68 (0.4–1.3) | 0.87 (0.6–1.3) | ··· | 0.2 (0.03–1.7) | 0.7 (0.3–2.0) | 1.1 (0.6–2.0) |
| Blacks | 1.87 (1.1–3.1) | 2.3 (1.5–3.5) | 2.5 (1.6–4.0) | 3.5 (2.4–5.0) | 5.20 (2.6–10.4) | 1.9 (0.9–4.2) | 3.0 (1.6–5.8) | 4.3 (2.5–7.4) |
| Hispanics | 1.5 (0.9–2.7) | 1.8 (1.2–2.9) | 1.9 (1.1–3.1) | 2.1 (1.4–3.0) | 3.15 (1.3–7.5) | 2.1 (1.0–4.3) | 1.3 (0.5–3.3) | 2.6 (1.5–4.5) |
CAC indicates coronary artery calcium; CI, confidence interval; CVD, cardiovascular disease; SHR, subdistribution hazard ratio.
Estimates are multivariable‐adjusted relative hazards and SHRs of all‐cause and CVD mortality, respectively, within individual CAC strata when compared with whites. Results are adjusted for study site, age, sex, hypertension, hyperlipidemia, cigarette smoking, family history of coronary heart disease and diabetes mellitus.
Estimates of CVD‐death risk to be interpreted with caution because of small numbers in corresponding cells; empty cells signify underpowered analyses.
Compared with whites with CAC 0, blacks and Hispanics had a consistent and significantly greater risk of CVD and all‐cause mortality regardless of their CAC score group. Specifically, while whites with CAC ≥400 showed about 4 times higher risk of CVD mortality compared with whites with CAC 0, Hispanics (subdistribution hazard ratio 10.7, [5.7–19.9]) and blacks (subdistribution hazard ratio 17.2, [9.3–31.8]) with CAC ≥400, when compared against the same reference showed an extremely high risk of mortality (Table 6)
Table 6.
Interplay of CAC and Race/Ethnicity on Risk of All‐Cause and CVD‐Specific Mortality
| All‐Cause Mortality HR (95% CI) | CVD Mortality SHR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| CAC 0 | CAC 1–99 | CAC 100–399 | CAC ≥400 | CAC 0 | CAC 1–99 | CAC 100 –399 | CAC ≥400 | |
| Whites | 1.0 | 1.3 (1.1–1.5) | 1.5 (1.3–1.8) | 2.4 (2.0–2.8) | 1.0 | 1.7 (1.2–2.4) | 2.5 (1.8–3.6) | 4.2 (3.0–6.0) |
| Asians | 0.7 (0.4–1.4) | 1.2 (0.7–2.1) | 1.1 (0.6–2.1) | 2.1 (1.3–3.2) | ···a | 0.37 (0.1–2.7) | 1.97 (0.7–5.6) | 4.4 (2.2–8.7) |
| Blacks | 1.9 (1.1–3.1) | 2.8 (1.9–4.2) | 4.1 (2.6–6.2) | 8.6 (5.9–12.6) | 6.0 (3.1–11.8) | 3.4 (1.5–7.5) | 7.9 (3.9–16.1) | 17.2 (9.3 –31.8) |
| Hispanics | 1.4 (0.8–2.5) | 2.2 (1.4–3.3) | 3.1 (1.9–5.1) | 5.0 (3.4–7.4) | 3.2 (1.4–7.5) | 3.4 (1.6–7.3) | 3.9 (1.5–9.9) | 10.7 (5.7–19.9) |
CAC indicates coronary artery calcium; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SHR, subdistribution hazard ratios.
Estimates are multivariable‐adjusted relative hazards and subdistribution hazards of all‐cause and CVD mortality, respectively, within individual race/ethnicity and CAC strata when compared with whites with CAC 0. Models adjusted for study site, age, sex, hypertension, hyperlipidemia, cigarette smoking, family history of coronary heart disease, and diabetes mellitus.
No CVD deaths noted in Asians with CAC=0; analysis not possible.
Discriminatory Value of CAC for All‐Cause and CVD Mortality
The addition of CAC yielded significant improvements in risk discriminatory value above risk factors alone. Importantly, the improvement in discriminatory value was most notable in nonwhite race/ethnicity groups (Table 7). Sex‐stratified assessments of improvement in risk discrimination of risk factors and Framingham risk scores on addition of CAC are shown in Tables S6 and S7.
Table 7.
Comparison of AUC Curves for Risk Factors Alone vs Risk Factors Plus CAC to Predict CVD Mortality, by Race/Ethnicity Group
| Race/Ethnicity Group | AUC for Risk Factors Alone | AUC for Risk Factors Plus CAC | P Valuea |
|---|---|---|---|
| Whites | 0.814 | 0.819 | <0.001 |
| Asians | 0.790 | 0.819 | 0.04 |
| Blacks | 0.754 | 0.790 | 0.04 |
| Hispanics | 0.745 | 0.770 | 0.05 |
AUC indicates areas under receiver‐operating‐characteristic; CAC, coronary artery calcium; CHD, coronary heart disease; CVD, cardiovascular disease.
P values test the comparison between risk factor AUCs with and without accounting for CAC score. Risk factors include age, sex, hypertension, hyperlipidemia, diabetes mellitus, cigarette smoking, and family history of CHD.
Discussion
In the largest CAC cohort yet assembled, we found that CAC has predictive value independent of other cardiovascular risk factors for both all‐cause and CVD‐specific mortality in whites, Asians, Hispanics, and blacks. These results provide a strong argument for the clinical utility of CAC as a predictive tool in all studied race/ethnicity groups.
Importantly, although CAC is predictive of all‐cause and CVD mortality within each of these race/ethnicity groups, it portends varying levels of all‐cause and CVD mortality risk across these groups. Blacks and Hispanics were at a much greater risk of all‐cause and CVD mortality than their Asian and white counterparts across all CAC strata. Interestingly, these race/ethnicity differences in risk implications were noted even in the CAC 0 category, with Hispanics and blacks with CAC 0 having significantly greater risk than whites with CAC 0.
While we recommend caution in inferring from these results that the inherent ability of CAC to predict risk differs by race/ethnicity, we think these results are important when viewed through the lens of healthcare disparities in the United States. Similar to studies that have shown that the Pooled Cohort Equation estimates higher 10‐year ASCVD risk in blacks compared with whites, given the same burden of traditional cardiovascular risk factors, our findings suggest that that blacks and Hispanics have excess CVD mortality over whites and Asians even when they have similar calcified atherosclerotic burden. Additionally, these race/ethnicity differences in prognosis of CAC may also suggest racial/ethnic differences in plaque types and pathobiological processes of vessel‐specific atherogenesis and evolution.4
Although a number of studies have investigated ethnic differences in CAC and its significance, only a few have specifically considered ethnic differences in its prognostic implications.24, 25 Doherty et al (1999)4 reported that despite similar risk factor profiles and lower prevalence of coronary calcium, blacks had significantly greater age, sex, and coronary risk‐adjusted odds of CHD death, myocardial infarction, angina, or revascularization compared with whites (odds ratio 2.16, 95% confidence interval, 1.34–3.48). Given important limitations of their study in its restriction to 2 race/ethnicity groups and its relatively small sample of blacks, our study contributes significantly to existing literature by examining these differences in a much larger and multiethnic cohort.
Nasir et al (2007)17 compared the predictive value of CAC in ethnic minorities to whites and reported the relative risk ratios of mortality in CAC ≥1000 subset to be 9.0 for Hispanics, 6.6 for Asians, and 24 for blacks. They also noted similarly higher relative risk ratios for Hispanics and blacks in the CAC ≥400 subset. This study, however, did not assess ethnic differences in prognostic significance of CAC for CVD‐specific mortality and did not assess differences in the CAC 0 group.
Detrano et al (2008)14 assessed the utility of CAC for the prediction of coronary events in a multiethnic sample of whites, blacks, Chinese, and Hispanics. Using data from 6722 participants of the MESA study who were followed for a median duration of 3.9 years, they found CAC to be a strong predictor of incident coronary heart disease in all the race/ethnicity groups tested. Our study expands upon this by considering ethnic differences in prognostic implications of CAC for all‐cause and CVD mortality in a larger sample and over a longer median follow‐up duration of 11.7 years.
Clinical Implications
These results are clinically important for several reasons. First, the improvement in risk prediction in minorities is especially important in the light of recent American College of Cardiology/American Heart Association and US Preventive Services Task Force guideline recommendations to utilize cardiovascular risk prediction tools in guiding therapy.26, 27 Since available risk calculators may not adequately capture risk in minority groups, these results provide data to support specific recommendations for considering selective use of CAC in minorities to improve ASCVD risk estimation.
Secondly, the excess cardiovascular mortality in blacks and Hispanics despite similar atherosclerotic burden may be at least partly explained by disparities in socioeconomic status and healthcare access and utilization, which according to more recent data may still be persistent.28 These findings therefore highlight the need to pay more attention to comprehensive risk factor modification in high‐risk race/ethnic minorities. Additionally, a leveling of cardiovascular mortality risk with similar CAC scores across race/ethnicity groups can be viewed as a simple marker of success in leveling disparities in cardiovascular health care.
Limitations
There are a few limitations to this study. First, all patients were referred for CAC screening and therefore may not be representative of the general population. Referral bias may impact different race/ethnicities in different ways (ie, race/ethnic minorities may be sicker). However, prior studies have shown that the CAC Consortium is largely comparable to other studies including MESA, and findings in real‐world clinical observational studies have advantages over studies like MESA that may involve healthy volunteer effects.6 Secondly, in the CAC Consortium, the CAC test results may have been used to guide treatment and follow‐up and could have improved outcomes differentially in the higher CAC groups. This, however, would bias our CAC risk estimates towards the null, although the strength of this bias may differ by race/ethnicity. Additionally, our estimates are not adjusted for control of risk factors over the duration of follow‐up, and there may exist some residual confounding as a result. However, previous studies of the predictive value of CAC in both clinical and population‐based data (including MESA) did not adjust for incident risk factors or control of these risk factors, and baseline risk factor distributions remain the standard for statistical adjustment.
Importantly, the risk estimates in our analysis were not adjusted for socioeconomic, or healthcare access and utility variables as these are not adequately captured in the CAC Consortium. We believe, however, that since study participants do not exist outside their sociodemographic and healthcare access environments and interact with these environments through their clinical courses, our results should be interpreted as more of an encompassing clinical/public health viewpoint than a purely pathobiologic description.
Finally, in considering the association of CAC with mortality, our estimates of risk are fundamentally different from those obtained in studies that have considered only ASCVD events. This is because the processes that lead to mortality outcomes are exceedingly more complex, and are influenced by several other unmeasured variables than those simply leading to sudden ASCVD events.
Conclusion
In appropriately selected asymptomatic patients referred for CAC scoring, CAC is highly predictive of all‐cause and CVD mortality in whites, Asians, blacks, and Hispanics, and its addition to risk factors substantially improves risk discrimination over risk factors alone in each of these groups. Crucially, blacks and Hispanics have a greater risk of CVD mortality than their white and Asian counterparts despite adjustment for atherosclerosis burden. These results make a strong case for strengthened guideline recommendations for CAC as a tool to improve ASCVD risk assessment and highlight a need to expand scientific knowledge by identifying biologic and social contributors to race/ethnicity differences in its prognostic implications.
Sources of Funding
Dr Blaha has received support from NIH award L30 HL110027 for this project.
Disclosures
None.
Supporting information
Table S1. Overall and Sex‐Stratified Distribution of Coronary Artery Calcium by Race/Ethnicity
Table S2. Cumulative Incidence and Incidence Rates of All‐Cause and CVD‐Specific Mortality Rates by CAC Group and Race/Ethnicity, in Women
Table S3. Cumulative Incidence and Incidence Rates of All‐Cause and CVD‐Specific Mortality Rates by CAC Group and Race/Ethnicity, in Men
Table S4. Risk of All‐Cause Death Associated With CAC for Each Race/Ethnic Group, Showing Unadjusted and Minimally Adjusted Results†
Table S5. Risk of CVD Mortality Associated With CAC by Race/Ethnic Group, Showing Unadjusted and Minimally Adjusted Results†
Table S6. C‐Statistics for the Overall and Sex‐Specific ROC Curves for All‐Cause and CVD Mortality Using the Framingham Risk Scores With and Without Accounting for CAC, by Race/Ethnicity
Table S7. C‐Statistics for the Overall and Sex‐Specific AUC Curves for All‐Cause and CVD Mortality Using Risk Factors With and Without Accounting for CAC, by Race/Ethnicity
(J Am Heart Assoc. 2018;7:e010471 DOI: 10.1161/JAHA.118.010471)
References
- 1. Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, Ballantyne CM, Go AS. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaha MJ. The critical importance of risk score calibration time for transformative approach to risk score validation? J Am Coll Cardiol. 2016;67:2131–2134. [DOI] [PubMed] [Google Scholar]
- 3. Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the Right Direction But at Risk for Miscalculation: A Critical Appraisal of the 2013 ACC/AHA Risk Assessment Guidelines. J Am Coll Cardiol. 2014;63:2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doherty TM, Tang W, Detrano RC. Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol. 1999;34:787–794. [DOI] [PubMed] [Google Scholar]
- 5. Cainzos‐Achirica M, Desai CS, Wang L, Blaha MJ, Lopez‐Jimenez F, Kopecky SL, Blumenthal RS, Martin SS. Pathways forward in cardiovascular disease prevention one and a half years after publication of the 2013 ACC/AHA cardiovascular disease prevention guidelines. Mayo Clin Proc. 2015;90:1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2886. [DOI] [PubMed] [Google Scholar]
- 8. Blaha MJ, Yeboah J, Al Rifai M, Liu K, Kronmal R, Greenland P. Providing evidence for subclinical CVD in risk assessment. Glob Heart. 2016;11:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC, Psaty BM, Greenland P, Herrington DM. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging. 2014;7:398–408; discussion 408. [DOI] [PubMed] [Google Scholar]
- 11. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elias‐Smale SE, Proença RV, Koller MT, Kavousi M, Van Rooij FJA, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JCM. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56:1407–1414. [DOI] [PubMed] [Google Scholar]
- 13. Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker‐Preuss M, Mann K, Siegrist J, Jöckel KH. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. [DOI] [PubMed] [Google Scholar]
- 14. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 15. Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. [DOI] [PubMed] [Google Scholar]
- 16. Orakzai SH, Orakzai RH, Nasir K, Santos RD, Edmundowicz D, Budoff MJ, Blumenthal RS. Subclinical coronary atherosclerosis: Racial profiling is necessary!. Am Heart J. 2006;152:819–827. [DOI] [PubMed] [Google Scholar]
- 17. Nasir K, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Flores PR, Flores FR, Raggi P, Berman DS, Blumenthal RS, Budoff MJ. Ethnic differences in the prognostic value of coronary artery calcification for all‐cause mortality. J Am Coll Cardiol. 2007;50:953–960. [DOI] [PubMed] [Google Scholar]
- 18. Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al‐Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, Miedema MD, Nasir K. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr. 2016;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al‐Mallah MH, Budoff MJ, Blaha MJ. Sex differences in calcified plaque and long‐term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018. Available at: https://academic.oup.com/eurheartj/advance-article-abstract/doi/10.1093/eurheartj/ehy534/5096672?redirectedFrom=fulltext. Accessed October 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C‐reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis heart study. J Am Coll Cardiol. 2005;46:158–165. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Mallah MH, Keteyian SJ, Brawner CA, Whelton S, Blaha MJ. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin Cardiol. 2014;37:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24. Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: The Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–1320. [DOI] [PubMed] [Google Scholar]
- 25. McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi AA, Lehmann N, Jöckel KH, Moebus S, Carr JJ, Erbel R, Burke GL. 10‐Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors Derivation in the MESA (Multi‐Ethnic Study of Atherosclerosis) with Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 27. Bibbins‐Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836. [DOI] [PubMed] [Google Scholar]
- 28. Nanna NG, Navar A, Zakroysky P, Xiang Q, Goldberg AC, Robinson J, Roger VL, Virani SS, Wilson PWF, Elassal J, Lee LV, Wang TY, Peterson ED. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the patient and provider assessment of lipid management registry. JAMA Cardiol.2018;3:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Overall and Sex‐Stratified Distribution of Coronary Artery Calcium by Race/Ethnicity
Table S2. Cumulative Incidence and Incidence Rates of All‐Cause and CVD‐Specific Mortality Rates by CAC Group and Race/Ethnicity, in Women
Table S3. Cumulative Incidence and Incidence Rates of All‐Cause and CVD‐Specific Mortality Rates by CAC Group and Race/Ethnicity, in Men
Table S4. Risk of All‐Cause Death Associated With CAC for Each Race/Ethnic Group, Showing Unadjusted and Minimally Adjusted Results†
Table S5. Risk of CVD Mortality Associated With CAC by Race/Ethnic Group, Showing Unadjusted and Minimally Adjusted Results†
Table S6. C‐Statistics for the Overall and Sex‐Specific ROC Curves for All‐Cause and CVD Mortality Using the Framingham Risk Scores With and Without Accounting for CAC, by Race/Ethnicity
Table S7. C‐Statistics for the Overall and Sex‐Specific AUC Curves for All‐Cause and CVD Mortality Using Risk Factors With and Without Accounting for CAC, by Race/Ethnicity


