Abstract
Background
Heart failure (HF) with “recovered” ejection fraction (HFrecEF) is an emerging phenotype, but no tools exist to predict ejection fraction (EF) recovery in acute HF. We hypothesized that indices of baseline cardiac structure and function predict HFrecEF in nonischemic cardiomyopathy and reduced EF.
Methods and Results
We identified a nonischemic cardiomyopathy cohort with EF<40% during the first HF hospitalization (n=166). We performed speckle‐tracking echocardiography to measure longitudinal, circumferential, and radial strain, and the average of these measures (myocardial systolic performance). HFrecEF was defined as follow‐up EF ≥40% and ≥10% improvement from baseline EF. Fifty‐nine patients (36%) achieved HFrecEF (baseline EF 26±7%; follow‐up EF 51±7%) within a median of 135 (interquartile range 58‐239) days after the first HF hospitalization. Baseline demographics, biomarker profiles, and comorbid conditions (except lower chronic kidney disease in HFrecEF) were similar between HFrecEF and persistent reduced‐EF groups. HFrecEF patients had smaller baseline left ventricular end‐systolic dimension (3.6 versus 4.8 cm; P<0.01), higher baseline myocardial systolic performance (9.2% versus 8.1%; P=0.02), and improved survival (adjusted hazard ratio 0.27, 95% confidence interval 0.11, 0.62). We found a significant interaction between baseline left ventricular end‐systolic dimension and absolute longitudinal strain. Among patients with left ventricular end‐systolic dimension >4.35 cm, higher absolute longitudinal strain (≥8%) was associated with HFrecEF (unadjusted odds ratio=3.9, 95% CI)confidence interval 1.2, 12.8). Incorporation of baseline indices of cardiac mechanics with clinical variables resulted in a predictive model for HFrecEF with c‐statistic=0.85.
Conclusions
Factors associated with achieving HFrecEF were specific to cardiac structure and indices of cardiac mechanics. Higher baseline absolute longitudinal strain is associated with HFrecEF among nonischemic cardiomyopathy patients with reduced EF and larger left ventricular dimensions.
Keywords: echocardiography, heart failure with recovered ejection fraction, longitudinal strain, nonischemic heart failure, recovery
Subject Categories: Heart Failure, Cardiomyopathy
Clinical Perspective
What Is New?
In patients with heart failure and reduced ejection fraction secondary to nonischemic cardiomyopathy, meaningful recovery of left ventricular ejection fraction is possible after a clinically pivotal event of first hospitalization for heart failure.
This study identified unique variables in cardiac structure and mechanics to create a multivariable model that was significantly associated with recovery of left ventricular ejection fraction (c‐statistic 0.85).
Relative preservation of longitudinal deformation by speckle‐tracking echocardiography in patients with a dilated left ventricle is associated with a higher likelihood of left ventricular ejection fraction recovery.
Despite recovery of left ventricular ejection fraction, over 50% of patients experienced subsequent rehospitalization for heart failure.
What Are the Clinical Implications?
In patients with a dilated left ventricle, the addition of longitudinal strain may identify a subset of patients who are more likely to recover left ventricular ejection fraction.
Our findings emphasize that recovery of left ventricular ejection fraction does not signify complete reversal of abnormal myocardial substrate as longitudinal strain remained abnormal and there was a persistent risk of rehospitalization for heart failure.
Validation of the predictive model is needed in other patient cohorts before routine implementation into clinical practice.
Heart failure (HF) with “recovered” ejection fraction (HFrecEF) has recently been recognized as an emerging entity that is clinically distinct from HF with reduced ejection fraction (HFrEF) and HF with preserved EF.1, 2, 3 Our current understanding is that HFrecEF patients previously had HFrEF and subsequently experienced reverse remodeling and improvement in left ventricular (LV) function, either spontaneously or in the setting of guideline‐directed medical and device therapy.4 Recent data among outpatients with HF suggest that this “recovered” or “improved” subgroup of patients, with an LV ejection fraction (LVEF) >40%, represent a less severe phenotype associated with improved survival.3, 5, 6 However, hospitalization for HF is often a critical event that is associated with high rates of death and rehospitalization.7 Whether or not myocardial recovery after hospitalization for HF carries a similar survival benefit to outpatient HFrecEF patients is unknown. To date, no validated clinical tools have been developed to identify those likely to recover among these high‐risk reduced‐EF patients at the time of the first HF hospitalization.
Determination of prognosis among HF patients is challenging due to high variability in the reported incidence of myocardial recovery.3, 5, 8, 9 Differences in the underlying myocardial substrate may explain some of this variability. Data suggest that the potential for myocardial recovery is highest among nonischemic cardiomyopathy (NICM) patients, which is supported by our prior work demonstrating that stable outpatients with nonischemic etiology were more likely to recover than their ischemic counterparts.8, 10
Within the NICM cohort, the potential for myocardial recovery remains heterogeneous. Reversal of the HF phenotype among patients with NICM may be related not only to the degree of adverse remodeling (eg, myocardial fibrosis),11 independent of the duration of HF, but also to the preservation of cardiac mechanics (ie, deformation). Pertinent to this discussion, speckle‐tracking echocardiography is an essential tool that allows for a noninvasive assessment of myocardial substrate and has been shown to correlate with myocardial fibrosis among patients with HF.12, 13, 14
We hypothesized that hospitalized HF patients with reduced EF who ultimately achieve HFrecEF status have unique baseline cardiac structure and function, including indices of myocardial mechanics. Therefore, we sought to (1) examine clinical and echocardiographic predictors of myocardial recovery after initial hospitalization for HF in NICM patients, specifically focusing on myocardial strain imaging, and (2) examine outcomes associated with HFrecEF status in NICM patients.
Methods
We created a single‐center, retrospective, longitudinal cohort study of patients with NICM and reduced LVEF who presented with their first hospitalization for HF between January 1, 2000 and January 1, 2013. Patients were identified using the Northwestern Medicine Enterprise Data Warehouse, which is a single, integrated database of clinical and research data from Northwestern University Medical System and affiliates of Northwestern Medicine. The Social Security Death Index is linked to the Northwestern Medicine Enterprise Data Warehouse and was used for accurate ascertainment of vital status.15, 16, 17, 18 The first hospitalization for HF was determined by International Classification of Diseases Ninth Revision (ICD‐9) codes specific to acute systolic HF (eg, 428.21, 428.2, 428.40, and 428.43 listed among the top 3 discharge diagnoses) and verified with review of the electronic medical record. Reduced LVEF was defined as LVEF ≤40% on the baseline echocardiogram within 90 days of the date of the index hospitalization (the majority n=158/166 [95%] were performed within 7 days of the index hospitalization). Patients transferred from referring centers were excluded. Additional exclusion criteria were based on a series of ICD‐9 codes for ischemic heart disease, prior coronary artery bypass grafting, and/or percutaneous intervention for coronary artery disease, valvular heart disease, rheumatic heart disease, congenital heart disease, infiltrative cardiomyopathies including cardiac sarcoidosis, cardiac amyloidosis, alcoholic cardiomyopathy, and hypertrophic cardiomyopathy. Nonischemic etiology was confirmed by cross‐referencing all patients with the cardiac catheterization laboratory data to ensure that no patients with obstructive coronary artery disease (defined as >50% stenosis in any vessel on angiogram) were included in the cohort. Clinical data for all patients were extracted from the electronic medical record. Because this was a retrospective study, no informed consent was required. The institutional review board at Northwestern University approved the study. The data that support the findings of this study are available from the corresponding author on reasonable request.
Two‐Dimensional Echocardiography and Speckle‐Tracking Protocol
All study participants had baseline echocardiographic data as per inclusion criteria. Echocardiographic images were obtained using Philips IE33 or GE Vivid 7 machines at Northwestern Memorial Hospital. All images were analyzed using Siemens (Munich, Germany) (2002‐2005) or ProSolv Cardiovascular software (Fujifilm Medical Systems, Tokyo, Japan) (2005‐2014). LVEF was calculated using the Simpson biplane method from the apical views. The LV end‐diastolic dimension (LVEDD) and LV end‐systolic dimension (LVESD) measurements were made in the parasternal long axis view. LV dimension and LVEF measurements were performed by the Northwestern Memorial Hospital echo laboratory. Digital cine loops were analyzed offline by 2 trained readers who were blinded to the clinical characteristics of the patients using 2‐dimensional echocardiography speckle‐tracking software (Epsilon Imaging, Ann Arbor, MI). All images used for speckle‐tracking echocardiographic analysis were obtained using a single software package (Epsilon Imaging) at a frame rate of 50 to 70 fps. The endocardial border was traced at end‐systole in the apical 4‐chamber and 2‐chamber views to measure longitudinal strain (LS). Speckle‐tracking analysis was performed, and endocardial border tracings were manually adjusted to optimize tracking. Strain curves were generated in each view using Epsilon Software's 6‐segment model. Circumferential strain and radial strain were obtained using the parasternal short‐axis view at the level of the papillary muscles. Speckle‐tracking analysis was not performed in patients with unacceptable image quality, defined as >1 segment dropout, missing view, or significant foreshortening of the LV in either of the apical views. For ease of reporting and interpretation, all strain values were reported as absolute values (lower absolute strain values correspond to worse cardiac mechanics). Myocardial systolic performance (MSP) was computed as the average of longitudinal, circumferential, and radial strain values.19 Interrater reliability testing was performed on all strain parameters with results showing an intraclass correlation coefficient of 0.93 with 95% confidence interval (CI) 0.85 to 0.97.
Clinical Outcomes
Myocardial recovery or HFrecEF was defined as follow‐up LVEF ≥40% and ≥10% absolute improvement in LVEF from baseline to follow‐up, assessed within 18 months of the index HF hospitalization. All‐cause mortality was ascertained using the Northwestern Medicine Enterprise Data Warehouse and confirmed using the Social Security Death Index. Longitudinal data on mortality and subsequent hospitalization for HF were ascertained from the Northwestern Medicine Enterprise Data Warehouse and used for secondary analyses.
Statistical Analysis
Baseline characteristics of the study participants were stratified by recovery status; summarized as means, medians, or proportions; and compared using chi‐squared tests, t‐tests, and Wilcoxon rank‐sum tests as appropriate. We considered any baseline demographic, clinical, laboratory, or medication characteristic that was statistically significantly (P<0.05) associated with recovery status on univariable analysis for inclusion in multivariable analyses. Baseline indices of cardiac mechanics (eg, LS) were evaluated for inclusion in multivariable models based on our a priori hypothesis for their association with myocardial recovery. We tested all other cardiac structure and function variables, age, sex, and race for first‐order interaction effects. Logistic regression models were used to evaluate association of baseline values with recovery status, and models were compared with receiver operating curves and c‐statistics. Unadjusted survival curves for all‐cause mortality were plotted by the Kaplan‐Meier method, and the log‐rank test was used to test for differences by recovery status. Cox proportional hazards regression models adjusted for age, sex, and race were used for computation of hazard ratios and 95% CIs. A P<0.05 was considered statistically significant. All analyses were performed using SAS v9.4 (Cary, NC). StatTag, version 3.2 (Northwestern Medicine, Chicago, IL) was used for the preparation of results in this article. StatTag facilitates reproducible research by embedding output from statistical programs in Microsoft Word documents.20
Results
Baseline Clinical Characteristics of the NICM Cohort
We identified 593 patients with NICM and first hospitalization for HF; 166 patients had complete follow‐up clinical and echocardiographic data available. The median (interquartile range) time to follow‐up echocardiogram was 135 (58‐239) days after hospitalization for HF. Clinical characteristics of the cohort by recovery status are presented in Table 1. The mean (SD) age was 54±16 years, and baseline LVEF was 25±8% in the total cohort. The cohort was balanced by sex, was majority black, and there was a high prevalence of comorbid conditions such as diabetes mellitus, hypertension, chronic kidney disease, and atrial fibrillation. Table S1 shows comparisons of the baseline characteristics between those with (n=166) and those without complete clinical data/follow‐up (n=427). Patients without follow‐up were older and more likely to be male; laboratory data, including brain natriuretic peptide values, were similar between groups.
Table 1.
Clinical Characteristics of 166 NICM Cohort Participants by HF Recovery Status
| Characteristics | No. of Patients | Persistent HFrEF (n=107) | HF With Recovered EF (n=59) | P Value |
|---|---|---|---|---|
| Age, mean±SD, y | 166 | 55±16 | 53±15 | 0.48 |
| Female, n (%) | 166 | 55 (51) | 32 (54) | 0.73 |
| Body mass index, mean±SD, kg/m2 | 165 | 29.3±8.1 | 29.8±8.1 | 0.72 |
| Systolic blood pressure, mean±SD, mm Hg | 166 | 136.7±31.2 | 138.2±29.8 | 0.76 |
| Diastolic blood pressure, mean±SD, mm Hg | 166 | 84.7±19.8 | 88.4±20.1 | 0.26 |
| Heart rate, median (IQR), bpm | 166 | 98 (84‐112) | 100 (87‐118) | 0.19 |
| Race | 166 | 0.46 | ||
| Black, n (%) | 63 (59) | 30 (51) | ||
| Other, n (%) | 9 (8) | 4 (7) | ||
| White, n (%) | 35 (33) | 25 (42) | ||
| Medications | ||||
| ACE/ARB, n (%) | 166 | 64 (60) | 38 (64) | 0.56 |
| Aspirin, n (%) | 166 | 37 (35) | 18 (31) | 0.59 |
| β‐Blocker, n (%) | 166 | 65 (61) | 40 (68) | 0.37 |
| Loop diuretic, n (%) | 166 | 52 (49) | 29 (49) | 0.95 |
| MRA, n (%) | 166 | 23 (21) | 9 (15) | 0.33 |
| Comorbidities | ||||
| Atrial fibrillation, n (%) | 166 | 42 (39) | 21 (36) | 0.64 |
| CKD, n (%) | 166 | 46 (43) | 16 (27) | 0.04 |
| Hypertension, n (%) | 166 | 89 (83) | 48 (81) | 0.77 |
| Diabetes mellitus, n (%) | 166 | 34 (32) | 12 (20) | 0.12 |
| COPD, n (%) | 166 | 36 (34) | 17 (29) | 0.52 |
| Laboratory findings | ||||
| Hemoglobin, mean±SD, g/dL | 163 | 12.5±2.0 | 12.5±2.9 | 0.94 |
| Sodium, mean±SD, mEq/L | 163 | 137.7±3.4 | 137.8±2.9 | 0.88 |
| Creatinine, median (IQR), mg/dL | 163 | 1.1 (0.9‐1.4) | 1.0 (0.9‐1.4) | 0.57 |
| eGFR, median (IQR), mL/[min·1.73 m2] | 162 | 68.1 (46.6‐84.9) | 69.2 (49.4‐81.4) | 0.81 |
| Troponin, median (IQR), ng/mL | 146 | 0.04 (0.02‐0.08) | 0.04 (0.02‐0.13) | 0.42 |
| BNP, median (IQR), pg/mL | 129 | 988 (485‐1568) | 605 (313‐1434) | 0.13 |
| QRS duration, median (IQR), ms | 164 | 94 (84‐120) | 88 (82‐98) | 0.05 |
| Baseline cardiac structure, function, and mechanics | ||||
| LVEF, mean±SD, % | 166 | 24.6±8.0 | 26.4±7.4 | 0.14 |
| Longitudinal strain, mean±SD | 166 | 7.4±3.3 | 8.1±2.9 | 0.16 |
| Circumferential strain, mean±SD | 166 | 11.6±4.6 | 13.0±5.0 | 0.08 |
| Radial strain, median (IQR) | 166 | 4.0 (2.0‐7.0) | 6.0 (4.0‐9.0) | 0.03 |
| MSP, mean±SD | 166 | 8.1±2.9 | 9.2±2.6 | 0.02 |
| LVEDD, mean±SD, cm | 166 | 5.7±1.0 | 4.9±0.9 | <0.01 |
| LVESD, mean±SD, cm | 166 | 4.8±1.0 | 3.6±0.9 | <0.01 |
| Follow‐up cardiac structure, function, and mechanics | ||||
| LVEF, mean±SD, % | 166 | 26.7±9.0 | 50.9±6.8 | <0.01 |
| Longitudinal strain, mean±SD | 146 | 9.0±3.8 | 13.4±4.3 | <0.01 |
| Circumferential strain, mean±SD | 141 | 15.5±5.9 | 22.5±5.9 | <0.01 |
| Radial strain, median (IQR) | 139 | 6.0 (3.0‐10.0) | 8.5 (5.0‐14.0) | 0.02 |
| MSP, mean±SD | 136 | 10.5±3.9 | 15.5±4.4 | <0.01 |
ACE/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; BNP, natriuretic brain peptide; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; MRA, mineralocorticoid receptor antagonist; MSP, myocardial systolic performance; NICM, nonischemic cardiomyopathy.
Of the 166 included patients, 59 (36%) patients experienced myocardial recovery and met our “recovered‐EF” definition. Average baseline LVEF was similar between HFrecEF patients and patients without myocardial recovery, termed “persistent HFrEF” (26.4±7.4% versus 24.6±8.0%, P=0.14). LVEF did not improve among persistent HFrEF patients (26.7±9.0%), whereas recovered‐EF patients experienced substantial improvement (50.9±6.8%). We found no significant differences between baseline demographic characteristics (including age, race, and sex), baseline laboratory data or baseline medications and recovery status between HFrecEF and persistent HFrEF. Baseline comorbid conditions (including history of hypertension and diabetes mellitus) were similar between groups, but patients with persistent HFrEF were more likely than HFrecEF patients to have chronic kidney disease (P<0.05).
Baseline Differences in Cardiac Structure and Cardiac Mechanics Between Recovered‐EF and Persistently Reduced EF Groups
There were significant differences in baseline cardiac structure, cardiac mechanics, and QRS duration between HFrecEF and persistent HFrEF groups despite similar baseline LVEF values (Table 1). Patients who achieved HFrecEF on average had 0.8 cm smaller baseline LV end‐diastolic dimension (LVEDD) and 1.2 cm smaller LV end systolic dimension (LVESD), compared with persistent HFrEF patients. Patients who achieved recovered EF had higher baseline radial strain, higher MSP, and shorter QRS duration in comparison to patients who did not recover LV function.
Table 2 shows unadjusted and adjusted associations of baseline cardiac structure, strain, and ECG variables with myocardial recovery. In age‐, sex‐, and race‐adjusted models, each 1‐SD increase in baseline LVEDD was associated with 64% (95% CI 44% to 77%) lower odds of recovery; each 1‐SD increase in baseline LVESD was associated with 78% (53% to 87%) lower odds of recovery; and each 1‐SD increase in QRS duration was associated with 40% (10% to 60%) lower odds of recovery. Individual strain variables alone were not significantly associated with recovery in adjusted models; however, each 1‐SD increase in the MSP (the average value of the 3 strain measures) was associated with 52% (8% to 113%) higher odds of recovery.
Table 2.
Unadjusted and Adjusted Association of Predictors With Myocardial Recovery on Logistic Regression Analysis Among 166 NICM Participants
| Baseline Covariate | No. of Patients | Unadjusted | Age, Race, and Sex Adjusted | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Racea | 166 | 0.46 | |||
| Black | 1.07 (0.31‐3.76) | ||||
| White | 1.61 (0.44‐5.81) | ||||
| Age, y | 166 | 0.99 (0.97‐1.01) | 0.48 | ||
| Female | 166 | 1.12 (0.59‐2.12) | 0.73 | ||
| Longitudinal strain, SD | 166 | 1.26 (0.91‐1.74) | 0.16 | 1.29 (0.93‐1.78) | 0.13 |
| Circumferential strain, SD | 166 | 1.33 (0.97‐1.84) | 0.08 | 1.39 (0.99‐1.95) | 0.05 |
| Radial strain, SD | 166 | 1.35 (0.98‐1.86) | 0.06 | 1.35 (0.98‐1.87) | 0.07 |
| MSP, SD | 166 | 1.47 (1.06‐2.04) | 0.02 | 1.52 (1.08‐2.13) | 0.02 |
| LVEDD, SD | 166 | 0.42 (0.28‐0.62) | <0.01 | 0.36 (0.23‐0.56) | <0.01 |
| LVESD, SD | 166 | 0.25 (0.15‐0.40) | <0.01 | 0.22 (0.13‐0.37) | <0.01 |
| QRS duration, ms | 164 | 0.62 (0.42‐0.91) | 0.02 | 0.60 (0.40‐0.90) | 0.01 |
CI indicates confidence interval; LVEDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end systolic dimension; MSP, myocardial systolic performance; NICM, nonischemic cardiomyopathy; OR, odds ratio.
Referent group for race is “Other.”
Outcomes Associated With Recovered‐EF Status
To further examine clinical outcomes associated with recovery status we generated Kaplan‐Meier curves as shown in Figure 1. All‐cause mortality was less likely (P<0.01) to occur among HFrecEF patients than among persistent HFrEF patients over the course of median 989 (interquartile range 386‐1859) days of follow‐up from ascertainment of recovery status. There were 7 (12%) deaths among HFrecEF patients, and 36 (34%) deaths among the persistent HFrEF patients. In an age‐, race‐, and sex‐adjusted proportional hazards model, patients who recovered had a statistically significantly reduced risk of mortality (hazars ratio [95% CI] 0.27 [0.11‐0.62]) compared with those who did not recover. Despite a reduced risk of mortality compared with persistent HFrEF patients and achieving a normal LVEF (50.9±6.8%) at follow‐up, 53% of HFrecEF patients experienced subsequent HF hospitalization occurring at a median of 113 (interquartile range 32‐517) days after ascertainment of recovery status.
Figure 1.
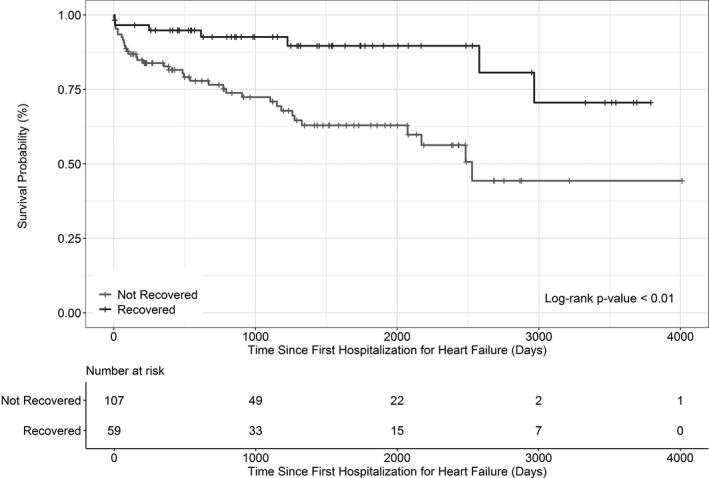
Kaplan‐Meier survival curve of 166 participants.
Strain Measures at Follow‐Up and Association With Adverse Outcomes
There were significant differences in follow‐up strain measures between HFrecEF and persistent HFrEF groups (Table 1). All measures of strain improved from the time of hospitalization (baseline) to follow‐up among both persistent HFrEF and HFrecEF patients. Circumferential strain markedly normalized at follow‐up (22.5±5.9%) among HFrecEF, whereas LS remained abnormal at follow‐up in both HFrecEF (13.4±4.3%) and persistent HFrEF (9.0±3.8) patients. After adjustment for age, sex, race, and recovery status, worse LS was the only strain measure persistently associated with death and hospitalization as a composite adverse outcome (P=0.01) (Table S2).
Predictive Model of Heart Failure With Recovered EF
In univariable and multivariable models (adjusted for age, sex, and race), LVESD, LVEDD, MSP, and QRS were significantly associated with HFrecEF by 18 months. We tested all other cardiac structure and function variables, age, sex, and race for first‐order interaction effects. Using a significance threshold α=0.025, we found significant interactions between LS and age (P<0.01), LS and LVEDD (P<0.01), and LS and LVESD (P=0.02). Our multivariable model was built using all variables that were statistically significantly associated with recovery in univariable models as well as all significant interaction terms. Nonsignificant terms were removed from the fully adjusted model. Our final model was [Age+Sex+Race+LVESD+LS+(interaction of LVESD and LS)+LVEDD], which had a c‐statistic of 0.85.
Figure 2 portrays the important interaction effects of LVESD and LS. Among patients with small LVESD (≤4.35 cm, n=83), more than 50% of patients achieved recovery of LVEF, and there is little difference in recovery status between patients with low (<8%) and those with high (≥8%) absolute LS (odds ratio=0.81; 95% CI 0.33‐1.97, P=0.64). However, among patients with larger LVESD (>4.35 cm, n=83), higher baseline absolute LS (≥8%) is associated with a nearly 4‐fold higher odds of myocardial recovery (odds ratio=3.91; 95% CI 1.20‐12.80, P=0.02) compared with patients with lower baseline absolute LS (<8%). The dichotomization point at LS we selected (8%) is supported by receiver operating characteristic analysis (shown in Figure S1) of the association between LS and recovery.
Figure 2.
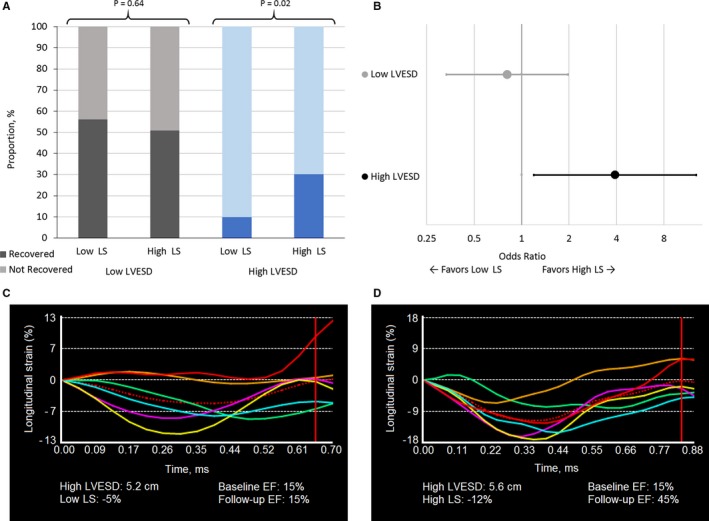
Importance of longitudinal strain and left ventricular end‐systolic dimension on recovery of left ventricular ejection fraction. A, Rate of recovery among 166 NICM participants, with low and high defined at the median and associated strain curves. B, Odds ratio for recovery among participants with high LS compared with low LS dichotomized by high and low LVESD. C, Example of LS strain curve in a patient with large LV dimension (high LVESD) and low LS who does not recover (HFrEF). D, Example of LS strain curve in a patient with large LV dimension and high LS who does recover (HFrecEF). Low LS is defined as <8, high LS as ≥8; low LVESD is defined as ≤4.35 cm, high as >4.35 cm; HFrecEF is defined as follow‐up LVEF ≥40% and ≥10% absolute improvement in LVEF from baseline to follow‐up, assessed within 18 months of the index hospitalization. Persistent HFrEF is defined as follow‐up LVEF <40% and no improvement or worsening of LVEF from baseline. EF indicates ejection fraction; LS, longitudinal strain; LVESD, left ventricular end‐systolic dimension; NICM, nonischemic cardiomyopathy.
Discussion
Myocardial Recovery After Index Hospitalization for HFrEF in NICM
Our study demonstrates that meaningful recovery of LVEF is possible among a subset of patients with NICM even after a clinically pivotal event of index hospitalization for HF. We chose to focus on this population in particular given prior work showing association of nonischemic etiology with myocardial recovery in stable outpatients with chronic HF.3, 10, 21, 22 Despite the well‐established high risk of event rates following HF hospitalization, our present study demonstrates that more than one‐third (36%) of patients experience a near doubling of baseline LVEF from 26% to 51% within a median of 135 (range 58‐239) days after first hospitalization for HF, indicative of remarkable contractile reserve, even in the setting of acute HF. Certain comorbidities may play a role in myocardial recovery. Consistent with prior studies, our study further confirms that HFrecEF patients are less likely to have chronic kidney disease.3, 5 Based on the high prevalence of hypertension, diabetes mellitus, chronic kidney disease, and atrial fibrillation, we surmise that the etiology of HF in our study population may be NICM associated with hypertensive heart disease in addition to underlying genetic predisposition to NICM, which is an area of active research.23
Outcomes in NICM Patients With Recovered EF
Despite a remarkable recovery of LVEF, HFrecEF patients exhibit a persistent risk of mortality, albeit a lower risk as compared with patients with persistent HFrEF.3, 8, 24 Although mortality was markedly reduced among HFrecEF patients in our study, with only 7 (12%) patient deaths compared with 36 (34%) with persistent HFrEF, there was still a persistent HF hospitalization risk. Fifty‐three percent of HFrecEF patients were rehospitalized for HF, with the median (range) time to rehospitalization of 113 (range 32‐517) days, suggesting that HFrecEF is not synonymous with reversal of the HF phenotype. Furthermore, our data show that with adjustment for age, sex, race, and recovery status, worse LS is associated with increased adverse outcomes (death and hospitalization).
Myocardial Substrate Remains Dysfunctional in NICM Patients With Recovered EF
Our work is consistent with prior studies3, 5 that have reported a continued risk of HF hospitalization among HFrecEF patients, suggesting persistent dysfunctional myocardial substrate. Mann and Burkhoff contend that complete myocardial recovery is rare, and most HFrecEF patients are in a state of myocardial “remission.”25 Our results provide further mechanistic insight. Recovered EF patients in our study experienced dramatic improvement and essentially normalization of circumferential strain values (circumferential strain, 22.5±5.9%) compared with patients with persistent HFrEF (circumferential strain, 15.5±5.9%). Recent data suggest that circumferential deformation contributes more than twice as much to LVEF than longitudinal deformation.26 However, although LVEF normalized (median LVEF 51%) along with circumferential strain values, LS remained abnormal (13.4±4.3%) in the HFrecEF patients. In fact, a recent study by Adamo et al showed worse LS to be a predictor of worsening LVEF among HFrecEF patients.24 Our results further establish the importance of LS as a prognostic marker, as worse LS is associated with an increase in composite outcome (death and hospitalization) regardless of recovery status. Our work supports the need for ongoing follow‐up for HFrecEF patients and continued treatment with medical therapy for HF because indices of myocardial deformation (specifically LS) remain abnormal despite achieving a normal or near‐normal LVEF.
Predictive Model for Recovery: Importance of Baseline Longitudinal Strain
Establishing predictors for HFrecEF status among high‐risk patients after initial hospitalization for HF provides important prognostic insights and improves risk discrimination. The ability to identify patients with a favorable prognosis of recovery, or conversely a low likelihood of recovery, may be a factor for consideration regarding decision making for referrals for advanced therapy options such as cardiac transplantation or mechanical circulatory support, and may facilitate potential clinical trial enrollment among those with low likelihood of recovery. Our study is the first, to our knowledge, to create a “signature of recovery” or predictive model for HFrecEF after incident hospitalization for HFrEF among NICM patients.
Our study findings are consistent with the published guidelines that although HFrecEF patients are clinically distinct, they are phenotypically similar to HFrEF patients.27 We found no major differences among baseline demographic characteristics (age, sex, race), biomarker profiles (eg, brain natriuretic peptide) or comorbid conditions (except chronic kidney disease) between HFrecEF and persistent HFrEF groups. Factors associated with recovery were specific to cardiac structure and indices of cardiac mechanics. In addition to baseline LS, we have identified MSP as a new marker associated with recovery of LV function.
Perhaps unsurprisingly, smaller LV dimensions were significantly associated with recovery status. Interestingly, there is recent evidence that LV geometry may confound the assessment of true LV systolic function. Specifically, less circumferential and longitudinal shortening may be required to maintain EF in smaller ventricles.26 Hence, myocardial deformation may be vastly different between hearts with similar EFs, depending on LV geometry and underlying pathophysiology. Our data support this concept. There was no difference in baseline LVEF between persistent HFrEF and HFrecEF groups. However, baseline measures of myocardial strain differed between groups, suggesting that the potential for recovery may depend more on preserved cardiac mechanics (ie, deformation).
Our findings suggest that the relative contribution of preserved cardiac mechanics (specifically LS) to eventual myocardial recovery may depend on LV geometry. Higher baseline absolute LS may signify a potential for recovery among NICM patients with larger LV dimension, who traditionally have been deemed unlikely to recover. The probability of recovery is over 50% among patients with smaller LVESD, and hence, the addition of LS may not be clinically relevant. However, patients with relatively preserved absolute LS (≥8%), despite having a larger LVESD (>4.35 cm), had a 30% chance of recovery compared with patients with worse LS (10% chance of recovery). Therefore, baseline LS dramatically changes the likelihood of recovery among those with larger LVESD.
Several limitations of our study warrant mention. Selection bias, present in all retrospective analyses due to loss to follow‐up and exposure/measurement bias, may limit the generalizability of the results. Potential associations may be the result of a factor related to the status of the individual or other unmeasured confounders such as preceding HF duration, presence of cardiac resynchronization therapy or implantable cardioverter defibrillator that was not captured in our data set. However, none of the patients included in our cohort had a QRS duration >140 milliseconds; thus, presumably cardiac resynchronization therapy would not have been indicated for a majority of patients. There is potential for survival bias in this study because only patients who lived to have repeat assessment of LV function were classified as experiencing myocardial recovery. Hospitalization rates may have been underestimated because subsequent hospitalizations outside of our own institution were not captured. Our study includes 1 follow‐up measure of strain imaging on echocardiogram, so it remains to be determined how the myocardial substrate further changes over an extended period of time. The lack of a validation cohort is also a limitation and warrants further study.
Conclusions
Among a racially diverse cohort of NICM patients (average baseline LVEF ~25%), we have shown over one‐third of patients can recover ventricular function to achieve “recovered‐EF” status after a key clinical event of first hospitalization for HF. Although HFrecEF is associated with improved survival, these patients still have a high risk for subsequent hospitalization for HF, which may be due in part to a persistent abnormal myocardial substrate. Here we present a predictive model for the emerging phenotype of HFrecEF of [Age+Race+Sex+LVESD+LS+(interaction of LVSED and LS)+LVEDD], which had a c‐statistic of 0.85 for predicting myocardial recovery. Moreover, higher baseline absolute LS may signify potential for recovery among NICM patients with larger LV dimensions, who traditionally have been deemed unlikely to recover.
Sources of Funding
Dr Wilcox has received funding from the Eleanor Wood Prince Grants Initiative and the Bluhm Cardiovascular Institute of Northwestern Memorial Hospital. Dr Shah has received funding from the National Heart, Lung, and Blood Institute. Dr Kim has received funding from the National Institutes of Health.
Disclosures
None.
Supporting information
Table S1. Clinical Characteristics of NICM Cohort Among Included and Excluded Participants
Table S2. Association of Follow‐up Echocardiogram Strain Values With Hospitalization for Heart Failure, Death, and Composite Outcome Among 166 Patients, Unadjusted and Adjusted for Age, Sex, Race, and Recovery Status
Figure S1. ROC of varied GLS cutpoints as a predictor of recovery.
(J Am Heart Assoc. 2018;7:e009841 DOI: 10.1161/JAHA.118.009841)
References
- 1. Wilcox JE, Yancy CW. Heart failure—a new phenotype emerges. JAMA Cardiol. 2016;1:507–509. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation and American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 3. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 5. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
- 6. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Cardiac Fail. 2011;17:527–532. [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the Valsartan Heart Failure Trial. Circ Heart Fail. 2016;9:e003123. [DOI] [PubMed] [Google Scholar]
- 9. Nadruz W Jr, West E, Santos M, Skali H, Groarke JD, Forman DE, Shah AM. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail. 2016;9:e002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilcox JE, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Heywood JT, Inge PJ, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Gheorghiade M. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012;163:49–56.e2. [DOI] [PubMed] [Google Scholar]
- 11. Wilcox JE, Fonarow GC, Ardehali H, Bonow RO, Butler J, Sauer AJ, Epstein SE, Khan SS, Kim RJ, Sabbah HN, Diez J, Gheorghiade M. “Targeting the Heart” in heart failure: myocardial recovery in heart failure with reduced ejection fraction. JACC Heart Fail. 2015;3:661–669. [DOI] [PubMed] [Google Scholar]
- 12. Cameli M, Mondillo S, Righini FM, Lisi M, Dokollari A, Lindqvist P, Maccherini M, Henein M. Left ventricular deformation and myocardial fibrosis in patients with advanced heart failure requiring transplantation. J Cardiac Fail. 2016;22:901–907. [DOI] [PubMed] [Google Scholar]
- 13. Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, Tsioulpas C, Bernazzali S, Tanganelli P, Maccherini M, Mondillo S, Henein MY. RV longitudinal deformation correlates with myocardial fibrosis in patients with end‐stage heart failure. JACC Cardiovasc Imaging. 2015;8:514–522. [DOI] [PubMed] [Google Scholar]
- 14. Spartera M, Damascelli A, Mozes F, De Cobelli F, La Canna G. Three‐dimensional speckle tracking longitudinal strain is related to myocardial fibrosis determined by late‐gadolinium enhancement. Int J Cardiovasc Imaging. 2017;33:1351–1360. [DOI] [PubMed] [Google Scholar]
- 15. Hauser TH, Ho KK. Accuracy of on‐line databases in determining vital status. J Clin Epidemiol. 2001;54:1267–1270. [DOI] [PubMed] [Google Scholar]
- 16. Zingmond DS, Ye Z, Ettner SL, Liu H. Linking hospital discharge and death records—accuracy and sources of bias. J Clin Epidemiol. 2004;57:21–29. [DOI] [PubMed] [Google Scholar]
- 17. Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): an important readily‐available outcomes database for researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 18. Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. [DOI] [PubMed] [Google Scholar]
- 19. Morris DA, Boldt LH, Eichstadt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2‐dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5:610–620. [DOI] [PubMed] [Google Scholar]
- 20. Welty L, Rasmussen LV, Shubat BA, Eric W. stattag . Chicago, IL: Galter Health Sciences Library. 2016. [Google Scholar]
- 21. Metra M, Nodari S, Parrinello G, Giubbini R, Manca C, Dei Cas L. Marked improvement in left ventricular ejection fraction during long‐term β‐blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292–299. [DOI] [PubMed] [Google Scholar]
- 22. de Groote P, Fertin M, Duva Pentiah A, Goeminne C, Lamblin N, Bauters C. Long‐term functional and clinical follow‐up of patients with heart failure with recovered left ventricular ejection fraction after beta‐blocker therapy. Circ Heart Fail. 2014;7:434–439. [DOI] [PubMed] [Google Scholar]
- 23. Kinnamon DD, Morales A, Bowen DJ, Burke W, Hershberger RE; Consortium DCM . Toward genetics‐driven early intervention in dilated cardiomyopathy: design and implementation of the DCM precision medicine study. Circ Cardiovasc Genet. 2017;10:e001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ Heart Fail. 2017;10:e003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60:2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. 2017;70:942–954. [DOI] [PubMed] [Google Scholar]
- 27. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: an Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Characteristics of NICM Cohort Among Included and Excluded Participants
Table S2. Association of Follow‐up Echocardiogram Strain Values With Hospitalization for Heart Failure, Death, and Composite Outcome Among 166 Patients, Unadjusted and Adjusted for Age, Sex, Race, and Recovery Status
Figure S1. ROC of varied GLS cutpoints as a predictor of recovery.


