Abstract
Background
Little is known of the impact of social factors on mortality after coronary artery bypass grafting (CABG). We explored sex‐ and age‐specific associations between mortality risk after CABG and marital status, income, and education.
Methods and Results
This population‐based register study included 110 742 CABG patients (21.3% women) from the SWEDEHEART registry (Swedish Web‐system for Enhancement and Development of Evidence‐based Care in Heart Disease Evaluated According to Recommended Therapies) operated 1992 to 2015. Cox regression models were used to study the relation between social factors and all‐cause mortality. Never having been married compared with being married/cohabiting was associated with a higher risk in women than in men (hazard ratio 1.32, 95% CI 1.20–1.44) versus 1.17 (1.13–1.22), P=0.030 between sex. The lowest income quintile, compared with the highest, was associated with higher risk in men than in women (hazard ratio 1.44 [1.38–1.51] versus 1.25 [1.14–1.38], P=0.0036). Lowest education level was associated with higher risk without sex difference (hazard ratio 1.15 [1.11–1.19] versus 1.25 [1.16–1.35], P=0.75). For unmarried women aged 60 years at surgery with low income and low education, mortality 10 years after surgery was 18%, compared with 11% in married women with high income and higher education level. The median life expectancy was 4.8 years shorter. Corresponding figures for 60‐year‐old men were 21% versus 12% mortality risk at 10 years and 5.0 years shorter life expectancy.
Conclusions
There are strong associations between social factors and mortality risk after CABG in both men and women. These results emphasize the importance of developing and implementing secondary prevention strategies for CABG patients with disadvantages in social factors.
Keywords: coronary artery bypass grafting, mortality, social inequalities, socioeconomic factors
Subject Categories: Cardiovascular Surgery, Epidemiology, Mortality/Survival
Clinical Perspective
What Is New?
Social factors such as low education, not being married, and low household income are associated with increased mortality risk after coronary artery bypass grafting in both men and women.
Disadvantages in social risk factors shorten median life expectancy after coronary artery bypass grafting by 4 to 5 years for both men and women.
What Are the Clinical Implications?
The results emphasize the importance of developing better educational approaches for secondary prevention strategies, particularly in coronary artery bypass grafting patients with severe social disadvantages.
Healthcare professionals need to support and increase patients’ knowledge about secondary prevention strategies after coronary artery bypass grafting.
Introduction
A link between social factors and coronary artery disease has long been established,1 with education, the most frequently used indicator, associated with increased risk of ischemic heart disease mortality2 and hospitalization for acute myocardial infarction.3 Indicators of low socioeconomic status are also negatively associated with survival after acute myocardial infarction.4, 5 Low household income is associated with mortality in patients undergoing cardiac surgery,6 and low education level and not born in Sweden are associated with an increased mortality risk in patients undergoing coronary artery bypass grafting (CABG).7 Marital status has also been found to affect the incidence of coronary heart disease as well as coronary heart disease mortality, survival after acute myocardial infarction,8, 9 and outcomes after cardiac surgery, where patients who were divorced, separated, or widowed had an ≈40% greater odds of dying or developing a new functional disability compared with married patients.10 Another vulnerable group is women, particularly younger women undergoing a first CABG, for whom long‐term survival was markedly poorer than in the general population of women of the same age.11
Accordingly, several social factors have been shown to be important in long‐term survival after cardiac procedures. Still, few studies have been adequately powered to examine the impact of several factors such as education, marital status, household income, and mortality simultaneously. In addition, to our knowledge, no previous study has explored the impact of socioeconomic disadvantages in men and women of different ages. Therefore, our aim with this study was to explore sex‐ and age‐specific associations between social factors and mortality risk among CABG patients.
The Swedish Heart Surgery Registry is a part of the SWEDEHEART registry, with 99% coverage of all open‐heart surgery in Sweden12; in combination with other national registries, it offers the possibility of investigating simultaneously the influences of several dimensions of socioeconomic disadvantage and long‐term survival in patients who have undergone CABG.
Methods
Data Collection
The SWEDEHEART registry (Swedish Web‐system for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) was used to identify 112 355 men and women over 18 years of age who underwent an isolated CABG in Sweden during the period 1992 to 2015. Codes for surgical procedures were defined by The Swedish National Board of Health and Welfare (Classification of surgery, sixth edition, 1987, and Nordic Classification of surgical procedures, 1997). To obtain first isolated CABG procedures, the following codes were used: 3066, 3105, 3127, 3158, 3092, 3080, FNA, FNB, FNC, FND, FNE, FNF. The authors declare that all supporting data are available within the article, and its online supplementary files.
To obtain data on social variables, we used the Longitudinal Integration Database for Health Insurance and Labor Market Studies register (LISA). The LISA database started in 1990 with a coverage of 80% among citizens aged over 16 years, and it is updated annually. Marital status was divided into 4 levels: (1) Married/cohabitating; (2) Never been married; (3) Divorced; and (4) Widowed. Length of education was stratified into 3 levels: (1) Under 10 years (compulsory school only); (2) 10 to 12 years (upper school); and (3) Over 12 years (college/university level). Income was measured as annual household disposable income at year of surgery, stratified into 5 quintiles from Q1 (lowest) to Q5 (highest). The consumer price index according to Swedish statistics (SCB) was used to adjust for inflation rates over time. If data were missing for the year of surgery, the latest information about education, marital status, and income was imputed from records for the most recent years before the surgery. In total, 1613 (1.4%) individuals were excluded because of missing data regarding education, marital status, or income. After exclusion, a total of 110 742 men and women remained for analysis.
Data on comorbidities registered before the date of admission for CABG surgery were retrieved from the IPR (Swedish National Inpatient Register), where registration is mandatory for principal and contributory diagnoses. This register have complete national coverage since 1987, with an overall diagnosis validity of 85% to 95%.13 Diagnoses in IPR are based on the International Classification of Diseases system (ICD), with the 9th revision (ICD‐9) used from 1987 to 1997 and the 10th revision (ICD‐10) from 1997 to 2015. The following diagnoses were obtained: myocardial infarction (ICD‐9: 410; ICD‐10: I21), diabetes mellitus (ICD‐9: 250; ICD‐10: E10–E14), hypertension (ICD‐9: 401–405; ICD‐10: I10–I15), heart failure (ICD‐9: 427.00, 427.10; ICD‐10: I50), atrial fibrillation (ICD‐9: 427D; ICD‐10: I48), stroke (ICD‐9: 431–434, 436; ICD‐10: I61–I64, I69), chronic respiratory disease (ICD‐9: 490–496; ICD‐10: J40–J47), peripheral vascular disease; (ICD 9: 440, 441, 442, 443, 444, 447; ICD‐10: I65–I65.9, I70–174, I77), renal insufficiency (ICD‐9: 584–586; ICD‐10: N17–N19), congenital heart disease (ICD‐9: 745–747; ICD‐10: Q20–Q26), malignancy (ICD‐9: 140–208; ICD‐10: C00–C97) and PCI (ICD‐9: 3080; ICD‐10: FNG02, FNG05). The Swedish Cause of Death register includes information on all deaths since 1961.14 Data from the 4 national registers were linked together through a personal 10‐digit social security number, unique for all Swedish citizens. Mean follow‐up time for all individuals was 10.1 years (interquartile range 5.5–14.8). The primary end point for the study was all‐cause mortality. For age‐specific analysis the study population was stratified into age groups (18–54, 55–64, 65–74, and ≥75), except for the survival analysis in patients with different socioeconomic profiles, where only patients aged 60 and 70 years were included.
Statistical Analysis
All statistical analyses were performed using SAS Software version 9.4 (SAS Institute Inc., Cary, NC). Descriptive statistics for baseline characteristics are presented with frequencies and percentages for categorical variables and mean value with SD or median and interquartile range, as appropriate, for continuous variables. Fisher exact test was used for comparisons between 2 groups for dichotomous variables and Mantel–Haenszel χ2 test for ordered categorical variables. The χ2 test was used for nonordered categorical variables and the Mann–Whitney U test for continuous variables.
The present study is an observational register study and according to the purpose of the study, all analyses can be considered as exploratory. No adjustment for multiple testing was performed, but P levels should be interpreted cautiously unless very small (eg, <0.001). In order to calculate age‐ and sex‐specific hazard ratios (HR) with 95% CI, Cox proportional hazards regression analyses were conducted separately for men and women overall and by age group, 18 to 54, 55 to 64, 65 to 74, and ≥75 years. For marital status, the category married was used as reference; for education, >12 years was used, and for income, highest income quintile (Q5) was used. The first model was adjusted for age only. The final multivariable model was adjusted for age, year of surgery, baseline characteristics (previous myocardial infarction, diabetes mellitus, hypertension, heart failure, atrial fibrillation, stroke, chronic respiratory disease, peripheral vascular disease, renal insufficiency, malignancy, and congenital heart disease), and social variables other than the variable under study. The proportional hazards assumption for the main effect variables was checked by reviewing the graphs of log (−log (survival)) versus log (time) and was found to be fulfilled. The interaction between the social variables of interest and sex was included in a separate model. Additionally, we examined whether the effect of social variables statistically differed between age groups by introducing an interaction term of social variable×age group into the model.
An adjusted Cox regression as above but also including the interaction terms sex×married, sex×education, sex×income, sex×age, married×education, and education×income was conducted; this was used to construct survival probability curves for typical men and women of 60 and 70 years with the following characteristics: (1) Married/cohabiting, >12 years of education, and highest income quintile; and (2) Not married, <10 years of education, and lowest income quintile. All tests were 2‐tailed and conducted at the 0.05 significance level.
Ethical Approval
The study was approved by the regional Ethics Board of Gothenburg (approval number 139‐16). To ensure anonymity, all personal identifiers were replaced by codes in the data set before analysis.
Results
A total of 110 742 patients were included: 87 106 (78.7%) men and 23 636 (21.3%) women. The overall mean age was 65.8 years for men and 68.4 years for women (Table). Women were more likely than men to have previous diabetes mellitus, hypertension, heart failure, chronic respiratory disease, or peripheral vascular disease. Men were more likely than women to be married (68.9% versus 50.4%), whereas women were more likely to be widowed (26.1% versus 6.3%). The proportion with <10 years of education was higher among women than among men (55.7% versus 46.2%), and a higher proportion of women than men were in the lowest income quintile (36.7% versus 15.5%).
Table 1.
Baseline Characteristics in 110 742 Adults Who Underwent Coronary Artery Bypass Grafting in Sweden 1992–2015
| Total | Men | Women | ||
|---|---|---|---|---|
| Number of patients | 110 742 | 87 106 (78.6%) | 23 636 (21.3%) | |
| Mean age, y (SD) | 66.4 (9.3) | 65.8 (9.2) | 68.4 (9.1) | |
| n (%) | n (%) | n (%) | P Value | |
| Myocardial infarction | 52 944 (47.8) | 41 449 (47.6) | 11 495 (48.6) | 0.0043 |
| Diabetes mellitus | 22 063 (19.9) | 16 264 (18.7) | 5799 (24.5) | <0.0001 |
| Hypertension | 37 894 (34.2) | 28 281 (32.5) | 9613 (40.7) | <0.0001 |
| Heart failure | 13 370 (12.1) | 10 080 (11.6) | 3290 (13.9) | <0.0001 |
| Atrial fibrillation | 7615 (6.9) | 6172 (7.1) | 1443 (6.1) | <0.0001 |
| Stroke | 5350 (4.8) | 4214 (4.8) | 1136 (4.8) | 0.86 |
| Chronic respiratory disease | 5172 (4.7) | 3694 (4.2) | 1478 (6.3) | <0.0001 |
| Peripheral vascular disease | 6875 (6.2) | 5104 (5.9) | 1771 (7.5) | <0.0001 |
| Renal insufficiency | 1921 (1.7) | 1471 (1.7) | 450 (1.9) | 0.028 |
| Congenital heart disease | 122 (0.1) | 97 (0.1) | 25 (0.1) | 0.92 |
| Malignancy | 7734 (7.0) | 6041 (6.9) | 1693 (7.2) | 0.23 |
| Marital status | ||||
| Married/cohabiting | 71 948 (65.0) | 60 033 (68.9) | 11 915 (50.4) | |
| Not married | 10 935 (9.9) | 9361 (10.7) | 1574 (6.7) | |
| Divorced | 16 245 (14.7) | 12 262 (14.1) | 3983 (16.9) | |
| Widowed | 11 614 (10.5) | 5450 (6.3) | 6164 (26.1) | <0.0001 |
| Education | ||||
| <10 y | 53 412 (48.2) | 40 241 (46.2) | 13 171 (55.7) | |
| 10 to 12 y | 40 733 (36.8) | 32 729 (37.6) | 8004 (33.9) | |
| >12 y | 16 597 (15.0) | 14 136 (16.2) | 2461 (10.4) | <0.0001 |
| Income | ||||
| Q1 (lowest) | 22 144 (20.0) | 13 468 (15.5) | 8676 (36.7) | |
| Q2 | 22 153 (20.0) | 17 189 (19.7) | 4964 (21.0) | |
| Q3 | 22 148 (20.0) | 17 951 (20.6) | 4197 (17.9) | |
| Q4 | 22 149 (20.0) | 18 909 (21.7) | 3240 (13.7) | |
| Q5 | 22 148 (20.0) | 19 589 (22.5) | 2559 (10.8) | <0.0001 |
In the age‐specific analysis, women had a higher proportion of diabetes mellitus, hypertension, heart failure, and chronic respiratory disease than men (Table S1). The differences in comorbidities between men and women were reduced with higher age. In all age categories, there was a larger proportion of men than women in the highest income quintile. In the oldest age category, 52.5% of the women and 19.0% of the men were in the lowest income quintile.
Marital Status
After considering differences in age, comorbidities, educational level, and income, we found that men and women who had never married, or were widowed, or divorced had higher mortality compared with married men and women, with a stronger effect in women (interaction P=0.030) and in younger patients (Figure 1). There was also a significant interaction between age and marital status in men in the overall analysis with a stronger effect in younger men (P<0.0001) (Table S2). Analyses adjusted for age only are shown in Table S3. After multivariable adjustments in the age‐specific analyses, lower estimates for mortality were observed for men in all age categories, with no significant interaction between men and women. The greatest adverse risk of not being married was observed among the youngest patients (interaction P=0.71).
Figure 1.
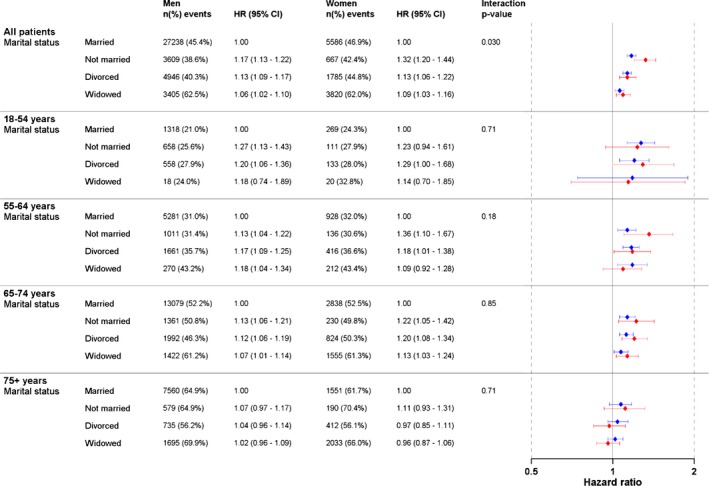
Multivariable‐adjusted hazard ratio (HR) for all‐cause mortality and marital status in 110 742 patients who underwent coronary artery bypass grafting in Sweden during the period 1992–2015. Forrest plot in blue colour = men. Forrest plot in red colour = women.
Education
In the multivariable‐adjusted analysis, the overall mortality risk was greater for those with the lowest education (>10 years) compared with those with the highest education (high school or university) in both men (HR 1.15, 95% CI 1.11–1.19) and women (HR 1.25, 95% CI 1.16–1.35), with no significant interaction between education and sex (interaction P=0.75) (Figure 2). No significant interactions between age and education could be observed in men or women.
Figure 2.
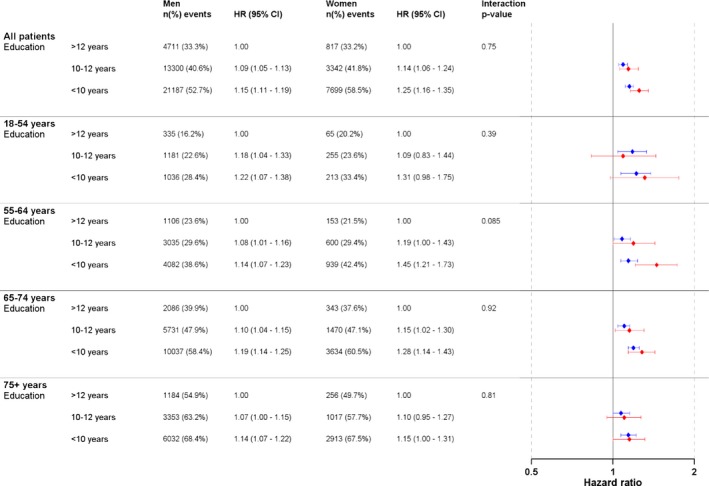
Multivariable‐adjusted hazard ratio (HR) for all‐cause mortality and education in 110 742 patients who underwent coronary artery bypass grafting in Sweden during the period 1992–2015. Forrest plot in blue colour = men. Forrest plot in red colour = women.
In the age‐specific multivariable analysis, a high education level in men was protective, irrespective of age. In women, education was also protective, although not significantly so for all age groups and not at all among the youngest women. CIs were generally wider for men than for women. The only significant interaction for education was found in men and women aged 55 to 64 years in the model with adjustments for age, sex, year of surgery, and social variables but without adjustments for comorbidities (P for interaction 0.036 [Table S4]).
Level of Income
Overall, high income was associated with lower mortality risk in both men and women in the age‐adjusted models (Table S3), and this association remained robust in the multivariable‐adjusted model (Figure 3). Although the mortality risks associated with low income were slightly reduced in the multivariable‐adjusted model, men with lower income had a more marked association with mortality risk than women. Mortality risk for men in the lowest income quintile was 44% greater than for men in the highest income quintile, with a corresponding difference of 25% in women (HR 1.44, 95% CI 1.38–1.51, versus HR 1.25, 95% CI 1.14–1.38, interaction between sex P=0.0036). The effect of income was stronger in younger, compared with older men (P for interaction <0.0001 [Table S2]). In the age‐specific analyses, HRs were generally higher for men than for women, and the only significant interaction was observed in the age group 65 to 74 years. This significant interaction for this age group was not observed in the multi‐adjusted models with adjustments for age, sex, year of surgery, and social variables but without adjustments for comorbidities (P for interaction 0.11 [Table S4]). Women aged 75 years or older with low income showed a higher mortality risk than men, but there was no significant interaction between income categories and sex in the multivariable‐adjusted model. Among patients in this age group, the HR in the lowest income quintile was 1.52 (95% CI 1.25–1.85) for women and 1.18 (95% CI 1.07–1.30) for men (interaction P=0.20).
Figure 3.
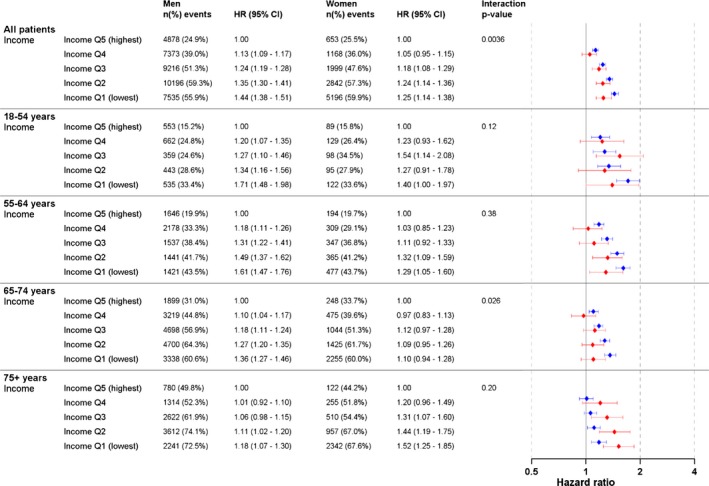
Multivariable‐adjusted hazard ratio for all‐cause mortality and income in 110 742 patients who underwent coronary artery bypass grafting in Sweden during the period 1992–2015. Forrest plot in blue colour = men. Forrest plot in red colour = women.
Survival Probability
Patients who were unmarried, or divorced or widowed, had the lowest education, and were in the lowest income quintile had an increased mortality risk and a shorter median life expectancy compared with those who were married/cohabiting, with the highest education, and highest income quintile (Figures 4 and 5). In men with a standardized age of 60 years, and with the latter characteristics, the estimated mortality risk at 10 years after surgery was 12%, as opposed to the men with the most adverse conditions where the corresponding risk was 21% (survival probability 0.88, 95% CI 0.87–0.89) versus 0.79, 95% CI 0.78–0.79). The median life expectancy in men with the most beneficial combination of social factors was 5.0 years longer than in men with the most adverse combination (21.5 and 16.5 years, respectively, Figure 4). Corresponding results for women (standardized to 60 years) showed a better overall survival rate than in men (Figure 4). At 10 years after surgery, the mortality risk was 18% for those with the most adverse combination of social risk factors and 11% for those with the most beneficial combination (survival probability 0.82, 95% CI 0.81–0.83 versus 0.89, 95% CI 0.88–0.91), with median survival time 17.6 and 22.6 years, respectively.
Figure 4.

Expected survival probability 10 years after surgery with 95% CI from multivariable‐adjusted Cox regression model for 60‐year‐old patients who underwent coronary artery bypass grafting in Sweden during the period 1992–2015.
Figure 5.
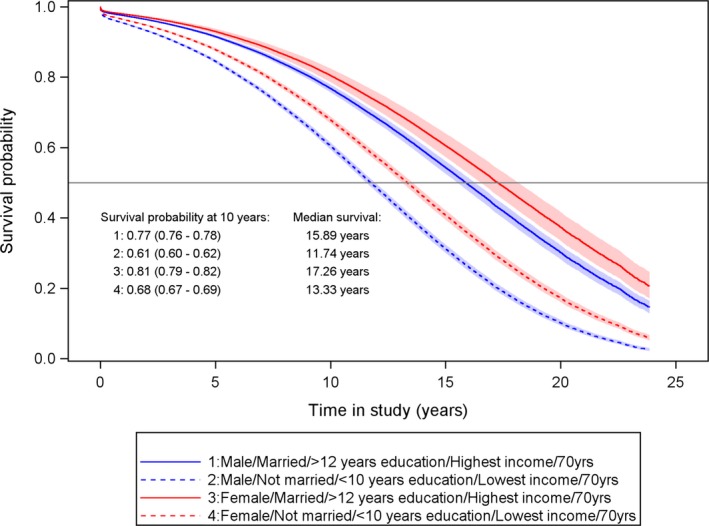
Expected survival probability 10 years after surgery with 95% CI from multivariable‐adjusted Cox regression model for 70‐year‐old patients who underwent coronary artery bypass grafting in Sweden during the period 1992–2015.
Survival probability at 10‐year follow‐up among men and women standardized to 70 years of age at the time of surgery showed a similar pattern to those standardized to 60 years of age, with lower survival probability in men compared with women. Men with the most adverse combination of social risk factors displayed the highest mortality risk of all groups, with an 39% increased risk of death at 10 years compared with 23% among men with the most beneficial combination (survival probability 0.61, 95% CI 0.60–0.62, versus 0.77, 95% CI 0.76–0.78), and they lost a median life expectancy of 4.2 years (Figure 5). A similar pattern was seen among women aged 70 years (survival probability 0.68, 95% CI 0.67–0.69 and 0.79, 95% CI 0.79–0.82, respectively), where women with the most adverse combination of social risk factors lost a median life expectancy of 3.9 years (13.3 years versus 17.3 years).
Discussion
In this large population‐based study, we explored the association between social factors, age, sex, and long‐term mortality risk in patients who underwent CABG surgery. Our results unequivocally demonstrate a strong association between disadvantages in social factors and mortality risk after CABG. The association between not being married and the increased risk of mortality was, in general, more pronounced in women than in men. In contrast, low income was associated with a higher mortality risk in men than in women. The estimated difference in survival between the lowest and the highest risk in social factors was considerable, with a median difference of 4 to 5 years when operated at age 60 years.
There is a lack of data available on the association between marital status and mortality risk in CABG populations. In this study, both men and women who were married or cohabiting showed a better survival after CABG than those who were not married, divorced, or widowed. These results are in concordance with previous findings in patients with cardiovascular disease15 and are further supported by a small study in CABG patients, which showed that men and women who live alone tended to have a reduced long‐term survival.16 However, in our study, after adjustments for comorbidities, education, and income level, we found that the association between marital status and mortality was stronger in women than in men. Several underlying factors may be responsible for the increased mortality risk in patients who live alone, including more limited family support during the extensive recovery period after surgery, substance abuse, and depression.17, 18, 19 The increased risk for unmarried women may at least partially be caused by the increased use of antidepressant medication and higher incidence of alcohol dependence in women than in men undergoing CABG.18 It is possible that the results reflect the fact that unmarried women undergoing CABG may have a lower level of social support after hospital discharge than unmarried men. Interestingly, women living alone who underwent cardiac surgery rated their health‐related quality of life lower than men.20 Women have also reported more unfavorable health‐related quality‐of‐life scores in general health, physical functioning, mental health, vitality, and bodily pain compared with men 12 months after cardiac surgery.21
Low household income was associated with an increased mortality risk in both men and women. Dalén et al reported similar associations6 in a Swedish patient cohort undergoing a wide variety of cardiac surgery procedures. In a subgroup analysis in patients who underwent isolated CABG, they reported an association between low income and increased mortality risk. In the present study, extending the analysis in CABG patients only, with sex‐ and age‐specific analyses, we were able to confirm that CABG patients with low income had increased mortality, but we also found a significant interaction between sex and income, where the inverse association between income and mortality was stronger in men than in women (P=0.0036).
The reasons for the association between low income and poor outcomes are multifactorial. For example, patients undergoing CABG who live in high deprivation areas have more clinical risk factors, such as smoking, diabetes mellitus, and cardiovascular complications than patients living in nondeprived areas.22 Associations between financial barriers and poor health have also been found in patients with myocardial infarction and in the general population.23, 24 Men and women with financial barriers rated their health status lower regarding quality of life, stress, and depression; they reported lower adherence to medication and often failed to schedule follow‐up visits, and they had higher rates of nonparticipation in cardiac rehabilitation after myocardial infarction.24 It is possible that low household income both negatively influences adherence to secondary prevention and decreases quality of life, which in turn may reduce long‐term survival after CABG.24
The association between low income and mortality was stronger in men than in women. Low household income may have a greater effect in men because of their perceived larger responsibility for the family finances. Suboptimal medical secondary prevention therapy or lack of adherence to medication could also be explanations. Pakpour et al found that women showed better adherence to medical therapy than men following CABG.25 Furthermore, men with lower income were less often prescribed secondary medication after myocardial infarction compared with men with higher income.26 A study of a general population in Japan showed that, compared with women with lower annual household income, men in this category presented lower health‐related quality‐of‐life scores in all domains of the SF 36 health questionnaire.27
When comparing men and women with the most adverse to those with the most beneficial social risk factors, a marked difference was observed in survival probability and a markedly reduced long‐term survival in the former category in both men and women. The explanation is most probably multifactorial. In addition to the factors mentioned above, social inequalities can activate and preserve a range of social patterns and health‐related behavior2 and increase the prevalence of cardiovascular risk factors such as hyperlipidemia, diabetes mellitus, and smoking.2, 5, 24 Previous studies have shown that low education is strongly associated with risk of obesity,28 smoking,29 and with lower life expectancy for both men and women.30, 31 Our study also showed that low education is associated with an increased mortality risk after CABG. Low education may be associated with difficulties in managing and understanding health advice or instructions.
In Sweden, all citizens have access to tax‐financed health care, and medications are subsidized. Despite equal access to health care and medication, our findings show that men and women with adverse social risk factors have a higher mortality risk than those with a more favorable situation. Accordingly, patients with adverse social risk factors may benefit from more attention within the healthcare service with respect to secondary prevention after CABG.
The present study shares the limitations of any population‐based registry study, including bias from unregistered variables such as smoking, obesity, diet, physical activity, stress, extent of the coronary artery disease, cardiac function, and selection bias. Although we did adjust for several factors, information about these clinical and lifestyle factors might have influenced the results. Income was measured as household income, and it is possible that this measure is not representative of each individual's economic capacity; some individuals may have a low income because of illness and inability to work but be supported by income from their spouses.
The main strength of this study is that we included a large nationwide sample, data collection from different registries and databases, and an extensive follow‐up (24 years). This large sample size allowed the analysis of smaller subgroups, such as younger men and women.
In conclusion, this large population‐based study shows that social factors are associated with increased long‐term mortality after CABG in both men and women. The association persisted after multivariable adjustments for cardiovascular and social risk factors. The association with increased mortality risk associated with being unmarried was more marked in women than in men. However, men with the most adverse social pattern had the poorest survival after surgery and the highest mortality risk associated with income level. This study demonstrates the importance of developing strategies for secondary prevention in CABG patients with social risk factors, identifying a vulnerable group. Patients who undergo CABG have advanced ischemic coronary artery disease and are often suboptimally treated.32 Limiting progress of the disease through medical interventions33 is obviously of major importance. Moreover, lifestyle changes are equally important, including support and education about the benefits of smoking cessation, diet, and physical activity.34, 35 More than half of the declining trend for coronary heart disease mortality in Sweden is explained by reductions in risk factors.36 Furthermore, a Danish study observed a similar declining trend attributable to reductions in risk factors regardless of socioeconomic position, but individuals with better socioeconomic positions had a more favorable relative reduction.37 Individuals with a stressful economic situation have fewer opportunities to follow advice about a healthier lifestyle, and educational level may also influence the chance to adopt a healthy lifestyle.38 Hence, there is room for improvement in educational approaches to increase patients’ knowledge of the importance of secondary prevention but also to make the adherence to secondary prevention strategies sustainable, particularly to those with the highest burden of social disadvantages. Customized advice in secondary prevention related to patient abilities need to be thoroughly implemented and evaluated regularly. Future research should focus on the impact of how different pedagogical approaches affect patients’ adherence to secondary prevention and whether this leads to reduced mortality, specifically in vulnerable groups undergoing CABG.
Sources of Funding
This work was supported by grants from the following funding bodies: The Swedish Heart Lung Foundation (grant number: 201604 [Nielsen] and 20150587 [Jeppsson]); Research Foundation at Skaraborg Hospital (VGSKAS‐697451); The Local Research and Development Council Skaraborg (VGFOUSKB‐703881); the Committee of the Regional Executive Board in Västra Götaland Sweden (VGFOUREG‐665591), and the Swedish Research Council (grant number 2013‐5187 [SIMSAM]).
Disclosures
None.
Supporting information
Table S1. Baseline Characteristic in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015 by Sex and Age Groups
Table S2. Interactions Between Age Categories and Social Factors in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015
Table S3. Age‐Adjusted and Multi‐Adjusted Hazard Ratios for All‐Cause Mortality in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015
Table S4. Multi‐Adjusted for All‐Cause Mortality in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015
(J Am Heart Assoc. 2019;8:e011490 DOI: 10.1161/JAHA.118.011490.)
References
- 1. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 2. Mackenbach JP, Cavelaars AE, Kunst AE, Groenhof F. Socioeconomic inequalities in cardiovascular disease mortality; an international study. Eur Heart J. 2000;21:1141–1151. [DOI] [PubMed] [Google Scholar]
- 3. Rosengren A, Subramanian SV, Islam S, Chow CK, Avezum A, Kazmi K, Sliwa K, Zubaid M, Rangarajan S, Yusuf S. Education and risk for acute myocardial infarction in 52 high, middle and low‐income countries: INTERHEART case‐control study. Heart. 2009;95:2014–2022. [DOI] [PubMed] [Google Scholar]
- 4. Klitkou ST, Wangen KR. Educational attainment and differences in relative survival after acute myocardial infarction in Norway: a registry‐based population study. BMJ Open. 2017;7:e014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergstrom G, Redfors B, Angeras O, Dworeck C, Shao Y, Haraldsson I, Petursson P, Milicic D, Wedel H, Albertsson P, Ramunddal T, Rosengren A, Omerovic E. Low socioeconomic status of a patient's residential area is associated with worse prognosis after acute myocardial infarction in Sweden. Int J Cardiol. 2015;182:141–147. [DOI] [PubMed] [Google Scholar]
- 6. Dalén M, Ivert T, Holzmann MJ, Sartipy U. Household disposable income and long‐term survival after cardiac surgery: a Swedish nationwide cohort study in 100,534 patients. J Am Coll Cardiol. 2015;66:1888–1897. [DOI] [PubMed] [Google Scholar]
- 7. Dzayee DA, Ivert T, Beiki O, Alfredsson L, Ljung R, Moradi T. Short and long term mortality after coronary artery bypass grafting (CABG) is influenced by socioeconomic position but not by migration status in Sweden, 1995–2007. PLoS One. 2013;8:e63877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA, Sperling LS. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong CW, Kwok CS, Narain A, Gulati M, Mihalidou AS, Wu P, Alasnag M, Myint PK, Mamas MA. Marital status and risk of cardiovascular diseases: a systematic review and meta‐analysis. Heart. 2018;104:1937–1948. [DOI] [PubMed] [Google Scholar]
- 10. Neuman MD, Werner RM. Marital status and postoperative functional recovery. JAMA Surg. 2016;151:194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen S, Bjorck L, Jeppsson A, Giang KW, Falk K, Maatta S, Sandstrom TZ, Rosengren A. Trends in mortality risks among 94,328 patients surviving 30 days after a first isolated coronary artery bypass graft procedure from 1987 to 2006: a population‐based study. Int J Cardiol. 2017;244:316–321. [DOI] [PubMed] [Google Scholar]
- 12. Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternstrom L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, Ragnarsson S, Friberg O. Validity of the Swedish Cardiac Surgery Registry. Interact Cardiovasc Thorac Surg. 2018;27:67–74. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manfredini R, De Giorgi A, Tiseo R, Boari B, Cappadona R, Salmi R, Gallerani M, Signani F, Manfredini F, Mikhailidis DP, Fabbian F. Marital status, cardiovascular diseases, and cardiovascular risk factors: a review of the evidence. J Womens Health (Larchmt). 2017;26:624–632. [DOI] [PubMed] [Google Scholar]
- 16. King KB, Reis HT. Marriage and long‐term survival after coronary artery bypass grafting. Health Psychol. 2012;31:55–62. [DOI] [PubMed] [Google Scholar]
- 17. Buckley JP, Furze G, Doherty P, Speck L, Connolly S, Hinton S, Jones JL. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart. 2013;99:1069–1071. [DOI] [PubMed] [Google Scholar]
- 18. Stenman M, Holzmann MJ, Sartipy U. Do socioeconomic factors modify the association between preoperative antidepressant use and survival following coronary artery bypass surgery? Int J Cardiol. 2015;198:206–212. [DOI] [PubMed] [Google Scholar]
- 19. Okkonen E, Vanhanen H. Family support, living alone, and subjective health of a patient in connection with a coronary artery bypass surgery. Heart Lung. 2006;35:234–244. [DOI] [PubMed] [Google Scholar]
- 20. Bjornnes AK, Parry M, Falk R, Watt‐Watson J, Lie I, Leegaard M. Impact of marital status and comorbid disorders on health‐related quality of life after cardiac surgery. Qual Life Res. 2017;26:2421–2434. [DOI] [PubMed] [Google Scholar]
- 21. Gjeilo KH, Wahba A, Klepstad P, Lydersen S, Stenseth R. The role of sex in health‐related quality of life after cardiac surgery: a prospective study. Eur J Cardiovasc Prev Rehabil. 2008;15:448–452. [DOI] [PubMed] [Google Scholar]
- 22. Taylor FC, Ascione R, Rees K, Narayan P, Angelini GD. Socioeconomic deprivation is a predictor of poor postoperative cardiovascular outcomes in patients undergoing coronary artery bypass grafting. Heart. 2003;89:1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fritzell J, Nermo M, Lundberg O. The impact of income: assessing the relationship between income and health in Sweden. Scand J Public Health. 2004;32:6–16. [DOI] [PubMed] [Google Scholar]
- 24. Beckman AL, Bucholz EM, Zhang W, Xu X, Dreyer RP, Strait KM, Spertus JA, Krumholz HM, Spatz ES. Sex differences in financial barriers and the relationship to recovery after acute myocardial infarction. J Am Heart Assoc. 2016;5:e003923 DOI: 10.1161/JAHA.116.003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pakpour AH, Gellert P, Asefzadeh S, Updegraff JA, Molloy GJ, Sniehotta FF. Intention and planning predicting medication adherence following coronary artery bypass graft surgery. J Psychosom Res. 2014;77:287–295. [DOI] [PubMed] [Google Scholar]
- 26. Salomaa V, Miettinen H, Niemela M, Ketonen M, Mahonen M, Immonen‐Raiha P, Lehto S, Vuorenmaa T, Koskinen S, Palomaki P, Mustaniemi H, Kaarsalo E, Arstila M, Torppa J, Kuulasmaa K, Puska P, Pyorala K, Tuomilehto J. Relation of socioeconomic position to the case fatality, prognosis and treatment of myocardial infarction events; the FINMONICA MI Register Study. J Epidemiol Community Health. 2001;55:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamazaki S, Fukuhara S, Suzukamo Y. Household income is strongly associated with health‐related quality of life among Japanese men but not women. Public Health. 2005;119:561–567. [DOI] [PubMed] [Google Scholar]
- 28. Wardle J, Waller J, Jarvis MJ. Sex differences in the association of socioeconomic status with obesity. Am J Public Health. 2002;92:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26 year follow up of 50,000 Norwegian men and women. J Epidemiol Community Health. 2004;58:705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steingrimsdottir OA, Naess O, Moe JO, Groholt EK, Thelle DS, Strand BH, Baevre K. Trends in life expectancy by education in Norway 1961–2009. Eur J Epidemiol. 2012;27:163–171. [DOI] [PubMed] [Google Scholar]
- 31. Zajacova A, Lawrence EM. The relationship between education and health: reducing disparities through a contextual approach. Annu Rev Public Health. 2018;39:273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotseva K, Wood D, De Bacquer D, De Backer G, Ryden L, Jennings C, Gyberg V, Amouyel P, Bruthans J, Castro Conde A, Cifkova R, Deckers JW, De Sutter J, Dilic M, Dolzhenko M, Erglis A, Fras Z, Gaita D, Gotcheva N, Goudevenos J, Heuschmann P, Laucevicius A, Lehto S, Lovic D, Milicic D, Moore D, Nicolaides E, Oganov R, Pajak A, Pogosova N, Reiner Z, Stagmo M, Stork S, Tokgozoglu L, Vulic D. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23:636–648. [DOI] [PubMed] [Google Scholar]
- 33. Sousa‐Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:5–33. [DOI] [PubMed] [Google Scholar]
- 34. Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, Richardson CR, Smith DT, Swartz AM. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. [DOI] [PubMed] [Google Scholar]
- 35. Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, Lopez‐Jimenez F, McNallan SM, Patel M, Roger VL, Sellke FW, Sica DA, Zimmerman L. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation. 2015;131:927–964. [DOI] [PubMed] [Google Scholar]
- 36. Bjorck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur Heart J. 2009;30:1046–1056. [DOI] [PubMed] [Google Scholar]
- 37. Joensen AM, Joergensen T, Lundbye‐Christensen S, Johansen MB, Guzman‐Castillo M, Bandosz P, Hallas J, Prescott EIB, Capewell S, O'Flaherty M. Explaining trends in coronary heart disease mortality in different socioeconomic groups in Denmark 1991–2007 using the IMPACTSEC model. PLoS One. 2018;13:e0194793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Notara V, Panagiotakos DB, Pitsavos CE. Secondary prevention of acute coronary syndrome. Socio‐economic and lifestyle determinants: a literature review. Cent Eur J Public Health. 2014;22:175–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristic in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015 by Sex and Age Groups
Table S2. Interactions Between Age Categories and Social Factors in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015
Table S3. Age‐Adjusted and Multi‐Adjusted Hazard Ratios for All‐Cause Mortality in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015
Table S4. Multi‐Adjusted for All‐Cause Mortality in 110 742 Patients Who Underwent Coronary Artery Bypass Grafting in Sweden During 1992–2015


