Abstract
Background
There are limited data on how the combination of diabetes mellitus (DM) and chronic kidney disease (CKD) affects cardiovascular outcomes as well as response to different P2Y12 receptor antagonists, which represented the aim of the present investigation.
Methods and Results
In this post hoc analysis of the PLATO (Platelet Inhibition and Patient Outcomes) trial, which randomized acute coronary syndrome patients to ticagrelor versus clopidogrel, patients (n=15 108) with available DM and CKD status were classified into 4 groups: DM+/CKD+ (n=1058), DM+/CKD− (n=2748), DM−/CKD+ (n=2160), and DM−/CKD− (n=9142). The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke at 12 months. The primary safety end point was PLATO major bleeding. DM+/CKD+ patients had a higher incidence of the primary end point compared with DM−/CKD− patients (23.3% versus 7.1%; adjusted hazard ratio 2.22; 95% CI 1.88–2.63; P<0.001). Patients with DM+/CKD− and DM−/CKD+ had an intermediate risk profile. The same trend was shown for the individual components of the primary end point and for major bleeding. Compared with clopidogrel, ticagrelor reduced the incidence of the primary end point consistently across subgroups (P‐interaction=0.264), but with an increased absolute risk reduction in DM+/CKD+. The effects on major bleeding were also consistent across subgroups (P‐interaction=0.288).
Conclusions
In acute coronary syndrome patients, a gradient of risk was observed according to the presence or absence of DM and CKD, with patients having both risk factors at the highest risk. Although the ischemic benefit of ticagrelor over clopidogrel was consistent in all subgroups, the absolute risk reduction was greatest in patients with both DM and CKD.
Clinical Trial Registration
URL: http://www.clinicatrials.gov. Unique identifier: NCT00391872.
Keywords: acute coronary syndrome, chronic kidney disease, clopidogrel, diabetes mellitus, ticagrelor
Subject Categories: Acute Coronary Syndromes; Thrombosis; Pharmacology; Diabetes, Type 2; Platelets
Clinical Perspective
What Is New?
Acute coronary syndrome patients with diabetes mellitus and chronic kidney disease are at markedly increased risk for long‐term atherothrombotic events compared with patients without these risk factors, as well as with those with only 1 of these.
Although the ischemic benefit of ticagrelor versus clopidogrel was consistent in all patient subgroups, the magnitude of benefit was enhanced according to the patient risk profile.
What Are the Clinical Implications?
There is a need to define the most effective treatment options for these high‐risk patients, including strategies to reduce the risk of developing chronic kidney disease in patients with diabetes mellitus.
Similarly, in patients with established chronic kidney disease, glucose control is also critical to reduce the risk of developing diabetes mellitus.
Clinicians should use more potent platelet‐inhibiting therapy in acute coronary syndrome patients with diabetes mellitus and chronic kidney disease who are often undertreated because of high perceived risk of bleeding.
Introduction
Patients with diabetes mellitus (DM) are at increased risk of atherothrombotic events.1 Importantly, DM is a key risk factor for the development of chronic kidney disease (CKD), a well‐known cardiovascular risk factor.2, 3 These observations underscore the importance of antiplatelet therapy for secondary prevention of atherothrombotic recurrences in these high‐risk patients. Dual antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor is the standard of care for secondary prevention in acute coronary syndrome (ACS) patients.4 Guidelines recommend that the more potent P2Y12 receptor inhibitors (ie, prasugrel or ticagrelor) be preferred over clopidogrel for the treatment of ACS patients because of their greater benefit in reducing the risk of cardiovascular events in these patients, albeit at the expense of increased bleeding.4, 5 Nevertheless, clopidogrel remains widely used in ACS patients.6, 7 DM patients treated with clopidogrel have increased rates of recurrent atherothrombotic events, which may be in part because of reduced platelet inhibitory effects of clopidogrel consistently observed among these subjects.1, 8, 9, 10, 11 Although studies assessing the impact of CKD status on clopidogrel‐induced antiplatelet effects have yielded conflicting findings, pharmacodynamic assessments conducted among DM patients have shown a greater magnitude of impaired clopidogrel‐induced platelet inhibition among those with CKD compared with those without CKD.12, 13, 14, 15, 16, 17, 18, 19
These observations, as well as those from other small observational studies, suggest that the concomitant presence of DM and CKD status can increase ischemic event rates, underscoring the need for more effective platelet‐inhibiting therapies in these high‐risk patients.20, 21 However, to date most large‐scale studies assessing how the presence of DM and CKD affects cardiovascular outcomes and the relative impact of specific antiplatelet treatment regimens, in particular P2Y12 receptor inhibitors, have considered these risk factors separately.1, 2 Indeed, the ever‐growing prevalence of CKD in patients with DM underscores the need to better risk stratify these patient cohorts. The aim of this analysis was to assess clinical outcomes in ACS patients from the PLATO (Platelet Inhibition and Patient Outcomes) trial according to the presence or absence of DM and CKD, as well as the differential effects of P2Y12‐inhibiting therapies (ticagrelor versus clopidogrel) in these populations.
Methods
The PLATO trial (www.ClinicalTrials.gov NCT00391872) was conducted from October 2006 to February 2009 and randomly assigned 18 624 patients with ST‐segment–elevation myocardial infarction (MI), non‐ST–segment elevation MI, or unstable angina, treated with an invasive or a noninvasive approach, to receive either ticagrelor or clopidogrel as soon as possible after admission. Details of study design, patients, outcome definitions, and results have been described elsewhere.22 In brief, ticagrelor was administered as a 180‐mg loading dose followed by 90 mg twice daily. Patients assigned to clopidogrel received a maintenance dose of 75 mg daily. Those who were clopidogrel naïve were also administered a 300‐ to 600‐mg loading dose. All patients received aspirin unless intolerant. The randomized treatment continued for a minimum of 6 to a maximum of 12 months (median duration 9.1 months). The primary efficacy end point was a composite of cardiovascular death, MI, or stroke. The primary safety end point was all major bleeding according to PLATO definition. Bleeding events were also defined according to the Thrombolysis In Myocardial Infarction (TIMI) and Global Use of Strategies to Open Occluded Arteries (GUSTO) classifications.22
Patients randomized in PLATO with available DM and CKD status at the time of randomization were included in the present analysis. Accordingly, patients were classified into 4 groups: DM+/CKD+, DM+/CKD−, DM−/CKD+, and DM−/CKD−. DM status was defined by the investigators at the time of randomization. Serum glucose and hemoglobin A1c were also measured and used to further characterize the study population, with poor glycemic control defined as levels above the median of serum glucose (6.8 mmol/L) and the median of percentage hemoglobin A1c (6.0%).23 CKD was defined as a creatinine clearance (CrCl) <60 mL/min according to the Cockcroft‐Gault equation.24 There were no exclusion criteria for renal dysfunction in the PLATO trial except for the requirement of dialysis. In an exploratory analysis, CKD status was also stratified according to the Modification of Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration equations.25 In addition, in a subgroup of patients (n=13 688), kidney function was assessed based on cystatin C levels measured on stored samples using the Creatinine‐Cystatin C Chronic Kidney Disease Epidemiology Collaboration equation.26
The PLATO trial adhered to the Declaration of Helsinki and was approved by the appropriate ethical review boards. All patients provided written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Statistical Analysis
Categorical baseline variables are presented as frequencies and percentages and compared by DM/CKD group using χ2 tests. Continuous baseline variables are presented as medians and 25th to 75th percentiles and compared by DM/CKD group using Kruskal–Wallis tests. Kaplan–Meier estimated event rates from randomization to 12 months were plotted by DM/CKD groups. Cox proportional hazards models were used to assess the associations between CKD‐DM status and clinical end points. Multivariable Cox regression models included randomized treatment, age, sex, body mass index, heart rate, prior MI, hypertension, dyslipidemia, smoking status, previous percutaneous coronary intervention or coronary artery bypass graft (CABG), and type of ACS as covariates. The interaction between DM/CKD status and randomized treatment was examined by adding an interaction term to the model. Results are presented as adjusted hazard ratios (HR) with 95% CI. In the comparisons between DM/CKD groups, HRs are reported using DM−/CKD− group as reference. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC). A 2‐sided P value of <0.05 was considered statistically significant for differences between groups and treatments.
Results
Patients and Outcomes According to CKD and DM Status
Among patients randomized in the PLATO trial, 15 108 had DM and CKD status available and were classified as follows: DM+/CKD+ (n=1058), DM+/CKD− (n=2748), DM−/CKD+ (n=2160), and DM−/CKD− (n=9142). Baseline characteristics are reported in Table 1. After excluding patients who prematurely discontinued because of death, the number of patients who discontinued treatment during follow‐up was low (43 in the CKD+DM+ group [0.28%], 71 in the CKD−DM+ group [0.47%], 83 in the CKD+DM− group [0.55%], and 206 in the CKD−DM− group [1.36%]). Patients with DM+/CKD+ more frequently had a prior history of cardiovascular disease, including MI, stroke, and peripheral arterial disease; were more frequently diagnosed with non‐ST‐elevation ACS rather than ST‐elevation MI; and were more frequently treated with a noninvasive approach.
Table 1.
Baseline Characteristics by DM/CKD Status
| Group of Characteristics | Characteristic (at Baseline) | DM+/CKD+ (n=1058) | DM+/CKD− (n=2748) | DM−/CKD+ (n=2160) | DM−/CKD− (n=9142) | P Value |
|---|---|---|---|---|---|---|
| Demographics | Age (y), median (Q1–Q3) | 72 (66–78) | 61 (55–68) | 74 (68–79) | 59 (52–66) | <0.0001 |
| Age ≥75 y | 429 (40.5%) | 233 (8.5%) | 1060 (49.1%) | 604 (6.6%) | <0.0001 | |
| Female sex | 456 (43.1%) | 851 (31.0%) | 823 (38.1%) | 2176 (23.8%) | <0.0001 | |
| Weight (kg), median (Q1–Q3) | 75 (65–84) | 84 (74–95) | 72 (62–80) | 80 (70–90) | <0.0001 | |
| Weight <60 kg | 107 (10.1%) | 120 (4.4%) | 349 (16.2%) | 498 (5.4%) | <0.0001 | |
| Height (cm), median (Q1–Q3) | 165 (160–172) | 170 (163–175) | 167 (160–173) | 171 (165–177) | <0.0001 | |
| BMI (kg/m2), median (Q1–Q3) | 26.9 (24.6–30.2) | 29.3 (26.4–32.9) | 25.4 (23.2–28.1) | 27.4 (24.8–30.2) | <0.0001 | |
| Waist circumference (cm), median (Q1–Q3) | 99 (91–108) | 103 (94–112) | 95 (86–102) | 97 (90–105) | <0.0001 | |
| Race, n (%) | White | 922 (87.1) | 2515 (91.5) | 1928 (89.3) | 8553 (93.6) | <0.0001 |
| Black | 22 (2.1) | 46 (1.7) | 28 (1.3) | 71 (0.8) | ||
| Asian | 84 (7.9) | 160 (5.8) | 160 (7.4) | 457 (5.0) | ||
| Other | 30 (2.8) | 27 (1.0) | 44 (2.0) | 61 (0.7) | ||
| Cardiovascular risk factors, n (%) | Habitual smoker | 130 (12.3) | 800 (29.1) | 413 (19.1) | 4061 (44.4) | <0.0001 |
| Hypertension | 925 (87.4) | 2162 (78.7) | 1574 (72.9) | 5187 (56.7) | <0.0001 | |
| Dyslipidemia | 622 (58.8) | 1629 (59.3) | 916 (42.4) | 3816 (41.7) | <0.0001 | |
| History, n (%) | Angina pectoris | 651 (61.5) | 1423 (51.8) | 1137 (52.6) | 3647 (39.9) | <0.0001 |
| Myocardial infarction | 360 (34.0) | 676 (24.6) | 556 (25.7) | 1507 (16.5) | <0.0001 | |
| Congestive heart failure | 176 (16.6) | 188 (6.8) | 229 (10.6) | 255 (2.8) | <0.0001 | |
| PCI | 217 (20.5) | 462 (16.8) | 290 (13.4) | 1025 (11.2) | <0.0001 | |
| CABG | 139 (13.1) | 236 (8.6) | 155 (7.2) | 350 (3.8) | <0.0001 | |
| TIA | 48 (4.5) | 75 (2.7) | 81 (3.8) | 191 (2.1) | <0.0001 | |
| Nonhemorrhagic stroke | 96 (9.1) | 129 (4.7) | 117 (5.4) | 242 (2.6) | <0.0001 | |
| Peripheral arterial disease | 149 (14.1) | 210 (7.6) | 163 (7.5) | 422 (4.6) | <0.0001 | |
| Medications on arrival, n (%) | Aspirin | 1007 (95.2) | 2618 (95.3) | 2033 (94.1) | 8756 (95.8) | 0.01 |
| β‐Blockade | 842 (79.6) | 2257 (82.1) | 1613 (74.7) | 6739 (73.7) | <0.0001 | |
| ACE‐inhibition and/or ARB | 806 (76.2) | 2049 (74.6) | 1397 (64.7) | 5361 (58.6) | <0.0001 | |
| Statin | 823 (77.8) | 2230 (81.1) | 1651 (76.4) | 7350 (80.4) | <0.0001 | |
| Ca‐inhibitor | 276 (26.1) | 539 (19.6) | 352 (16.3) | 1054 (11.5) | <0.0001 | |
| Diuretic | 497 (47.0) | 793 (28.9) | 758 (35.1) | 1449 (15.8) | <0.0001 | |
| Insulin treatment before admission | 282 (26.7) | 572 (20.8) | 0.0001 | |||
| Medications index event to discharge, n (%) | GP 2b/3a inhibitor | 177 (16.7) | 734 (26.7) | 413 (19.1) | 2686 (29.4) | <0.0001 |
| Unfractionated heparin | 524 (49.5) | 1591 (57.9) | 1195 (55.3) | 5473 (59.9) | <0.0001 | |
| Low‐molecular‐weight heparin | 590 (55.8) | 1460 (53.1) | 1199 (55.5) | 4734 (51.8) | 0.003 | |
| Fondaparinux | 34 (3.2) | 74 (2.7) | 74 (3.4) | 249 (2.7) | 0.3 | |
| Bivalirudin | 25 (2.4) | 90 (3.3) | 34 (1.6) | 158 (1.7) | <0.0001 | |
| Intended approach | Invasive | 603 (57.0%) | 1912 (69.6%) | 1311 (60.7%) | 6915 (75.6%) | <0.0001 |
| Noninvasive | 455 (43.0%) | 836 (30.4%) | 849 (39.3%) | 2227 (24.4%) | ||
| Final ACS diagnosis | ST‐elevation MI | 244 (23.1%) | 863 (31.4%) | 638 (29.6%) | 3980 (43.6%) | <0.0001 |
| Non‐ST‐elevation MI | 559 (52.9%) | 1259 (45.8%) | 1038 (48.2%) | 3622 (39.6%) | ||
| Unstable angina | 224 (21.2%) | 566 (20.6%) | 427 (19.8%) | 1336 (14.6%) | ||
| Other | 29 (2.7%) | 60 (2.2%) | 50 (2.3%) | 199 (2.2%) | ||
| Randomized treatment | Delay from start of pain (h), median (Q1–Q3) | 14.2 (6.8–21.2) | 12.7 (5.7–20.4) | 14.0 (5.8–21.1) | 10.2 (4.3–19.0) | <0.0001 |
| Treatment duration (d), median (Q1–Q3) | 258 (55–361) | 276 (179–365) | 265 (73–363) | 284 (184–366) | <0.0001 | |
| Biomarkers | Creatinine (μmol/L), median (Q1–Q3) | 115.0 (106.0–141.0) | 80.0 (70.7–88.0) | 106.0 (97.0–124.0) | 80.0 (71.0–88.0) | <0.0001 |
| Glucose (mmol/L), median (Q1–Q3) | 9.9 (7.2–13.5) | 9.7 (7.2–13.2) | 6.5 (5.6–7.9) | 6.4 (5.6–7.7) | <0.0001 | |
| HbA1c (mmol/mol), median (Q1–Q3) | 7.5 (6.6–8.7) | 7.6 (6.7–9.1) | 5.9 (5.6–6.2) | 5.8 (5.6–6.1) | <0.0001 | |
| Hemoglobin (mmol/mol), median (Q1–Q3) | 128.0 (116.0–140.0) | 139.0 (128.0–149.0) | 134.0 (123.0–145.0) | 142.0 (132.0–151.0) | <0.0001 | |
| NT‐proBNP (pmol/L), median (Q1–Q3) | 1734 (610.0–4071) | 395.0 (146.0–953.0) | 1002 (320.0–2544) | 277.0 (99.0–721.0) | <0.0001 | |
| Troponin I μg/L, median (Q1–Q3) | 1.10 (0.12–6.00) | 0.95 (0.11–4.30) | 1.00 (0.11–5.70) | 0.90 (0.12–4.70) | 0.01 | |
| Creatinine (mg/dL), median (Q1–Q3) | 1.3 (1.2–1.6) | 0.9 (0.8–1.0) | 1.2 (1.1–1.4) | 0.9 (0.8–1.0) | <0.0001 | |
| CrCl (mL/min), median (Q1–Q3) | 48.4 (38.9–55.1) | 86.7 (73.2–104.5) | 50.3 (42.7–55.9) | 87.7 (74.5–104.0) | <0.0001 |
ACE indicates angiotensin converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CrCl, creatinine clearance by Cockcroft‐Gault equation; DM, diabetes mellitus; GP, glycoprotein; HbA1c, hemoglobin A1c; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Patients with DM+/CKD+ had an over 3‐fold higher incidence of the primary end point at 12 months compared with DM−/CKD− patients (23.3% versus 7.1%; adjusted HR 2.22; 95% CI 1.88–2.63). Patients with DM+/CKD− (10.7%; adjusted HR 1.34; 95% CI 1.16–1.55) and DM−/CKD+ (15.8%; adjusted HR 1.60; 95% CI 1.37–1.86) had an intermediate risk profile (P for trend <0.001; Figure 1). The same trend was shown for the individual components of the primary end point, cardiovascular death, MI, and stroke, as well as for all‐cause mortality (Figure 2). Patients with DM+/CKD+ also had the highest risk of PLATO‐defined major bleeding compared with DM−/CKD− patients (14.8% versus 8.5%; adjusted HR 1.47; 95% CI 1.21–1.77) and patients with DM+/CKD− (11.7%; adjusted HR 1.34; 95%; CI: 1.17–1.54) or DM−/CKD+ (11.8%; adjusted HR 1.13; 95% CI 0.96–1.33) (Figure 3A). Non‐CABG‐related major bleeding rates were higher in patients with DM+/CKD+ and DM−/CKD+ compared with patients with DM+/CKD− and DM−/CKD− (Figure 3B). Major bleeding defined according to TIMI and GUSTO criteria showed a similar trend (Figure 4). Results were consistent when measures of poor glycemic control and alternative definitions of CKD were considered (Table 2).
Figure 1.
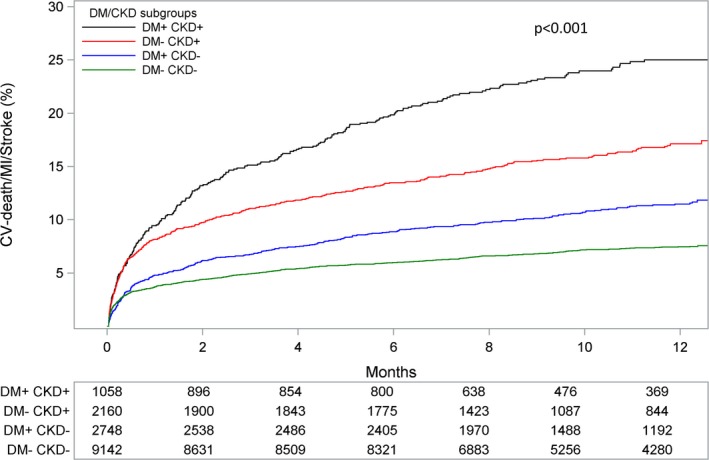
Kaplan–Meier event rate curves for the cumulative incidence of the primary composite end point of cardiovascular (CV) death, myocardial infarction (MI), and stroke stratified by DM/CKD status. P value represents the overall comparison among groups according to DM/CKD status. The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention or coronary artery bypass graft, type of acute coronary syndrome and randomized treatment. CKD indicates chronic kidney disease; DM, diabetes mellitus.
Figure 2.
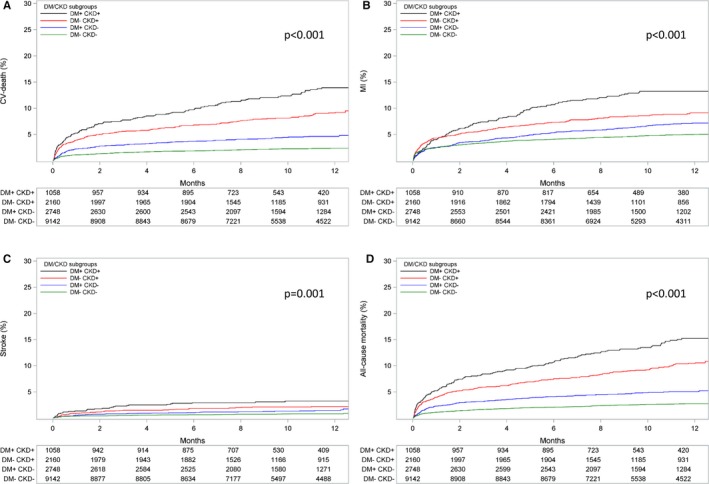
Kaplan–Meier event rate curves for the cumulative incidence of (A) cardiovascular (CV) death, (B) myocardial infarction (MI), (C) stroke, and (D) all‐cause mortality stratified by DM/CKD status. P value represents the overall comparison among groups according to DM/CKD status. The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention or coronary artery bypass graft, type of acute coronary syndrome, and randomized treatment. CKD indicates chronic kidney disease; DM, diabetes mellitus.
Figure 3.
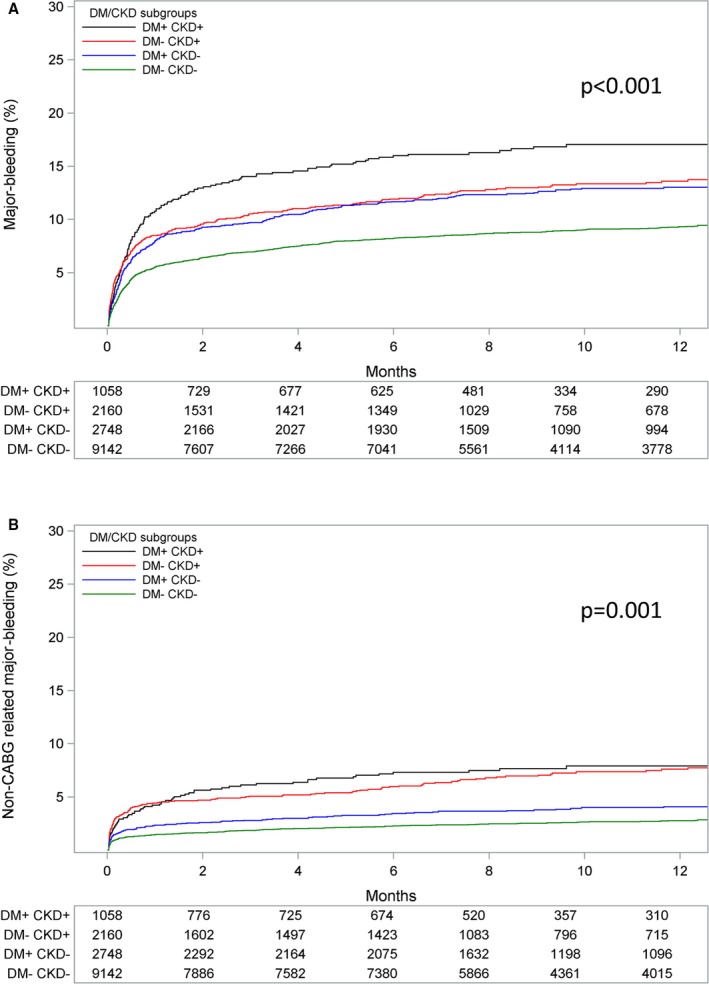
Kaplan–Meier event rate curves for the cumulative incidence of (A) major bleeding, and (B) non‐CABG‐related major bleeding stratified by DM/CKD status. P value represents the overall comparison among groups according to DM/CKD status. Bleeding is defined according to PLATO criteria. The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention, or coronary artery bypass graft, type of acute coronary syndrome, and randomized treatment. CABG indicates coronary artery bypass graft; CKD, chronic kidney disease; DM, diabetes mellitus; PLATO, Platelet Inhibition and Patient Outcomes.
Figure 4.
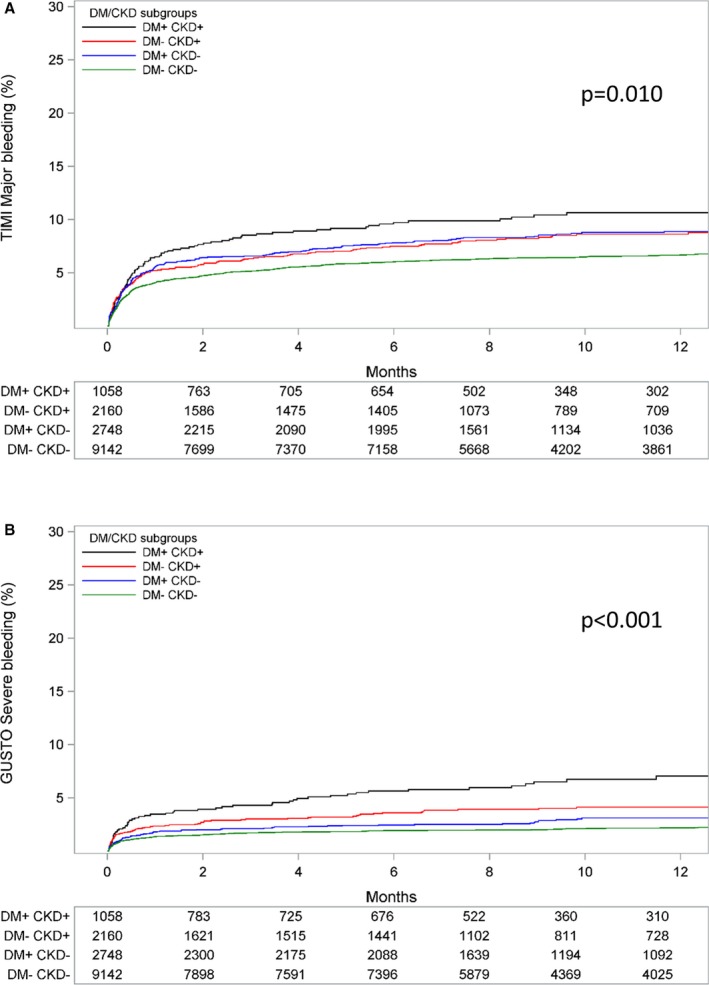
Kaplan–Meier event rate curves for the cumulative incidence of major/severe bleeding according to (A) TIMI, and (B) GUSTO criteria stratified by DM/CKD status. P value represents the overall comparison among groups according to DM/CKD status. The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention or coronary artery bypass graft, type of acute coronary syndrome, and randomized treatment. CKD indicates chronic kidney disease; DM, diabetes mellitus; GUSTO, Global Use of Strategies to Open Occluded Arteries; TIMI, thrombolysis in myocardial infarction.
Table 2.
Ischemic and Bleeding Outcomes According to DM/CKD Subgroup, With Poor Glycemic Control Defined by HbA1c and CKD Defined by the Creatinine‐Cystatin C CKD‐EPI Equation
| DM/CKD Subgroup | No. of Events | No. of Patients | Event Rate (%)a | HR (95% CI)b | P Valuec |
|---|---|---|---|---|---|
| Cardiovascular death/MI/stroke | |||||
| DM−/CKD− | 392 | 1264 | 6.9 | <0.0001 | |
| DM+/CKD− | 580 | 5726 | 10.1 | 1.33 (1.16–1.52) | |
| DM−/CKD+ | 123 | 734 | 16.8 | 1.72 (1.39–2.13) | |
| DM+/CKD+ | 263 | 1264 | 20.8 | 2.09 (1.76–2.49) | |
| Cardiovascular death | |||||
| DM−/CKD− | 121 | 5673 | 2.1 | <0.0001 | |
| DM+/CKD− | 215 | 5726 | 3.8 | 1.54 (1.23–1.94) | |
| DM−/CKD+ | 65 | 734 | 8.9 | 2.50 (1.81–3.44) | |
| DM+/CKD+ | 155 | 1264 | 12.3 | 3.44 (2.64–4.48) | |
| MI | |||||
| DM−/CKD− | 258 | 5673 | 4.5 | <0.0001 | |
| DM+/CKD− | 357 | 5726 | 6.2 | 1.24 (1.05–1.47) | |
| DM−/CKD+ | 69 | 734 | 9.4 | 1.60 (1.21–2.12) | |
| DM+/CKD+ | 130 | 1264 | 10.3 | 1.66 (1.32–2.10) | |
| All‐cause death | |||||
| DM−/CKD− | 145 | 5673 | 2.6 | <0.0001 | |
| DM+/CKD− | 238 | 5726 | 4.2 | 1.45 (1.17–1.79) | |
| DM−/CKD+ | 72 | 734 | 9.8 | 2.21 (1.63–2.99) | |
| DM+/CKD+ | 174 | 1264 | 13.8 | 3.19 (2.49–4.08) | |
| Stroke | |||||
| DM−/CKD− | 46 | 5673 | 0.8 | 0.1679 | |
| DM+/CKD− | 74 | 5726 | 1.3 | 1.43 (0.98–2.08) | |
| DM−/CKD+ | 11 | 734 | 1.5 | 1.15 (0.58–2.29) | |
| DM+/CKD+ | 27 | 1264 | 2.1 | 1.67 (0.99–2.81) | |
| Major bleeding | |||||
| DM−/CKD− | 484 | 5673 | 8.5 | 0.0039 | |
| DM+/CKD− | 629 | 5726 | 11.0 | 1.26 (1.11–1.42) | |
| DM−/CKD+ | 86 | 734 | 11.7 | 1.14 (0.90–1.45) | |
| DM+/CKD+ | 148 | 1264 | 11.7 | 1.14 (0.94–1.39) | |
| Non‐CABG‐related major bleeding | |||||
| DM−/CKD− | 161 | 5673 | 2.8 | 0.0070 | |
| DM+/CKD− | 180 | 5726 | 3.1 | 1.00 (0.81–1.25) | |
| DM−/CKD+ | 44 | 734 | 6.0 | 1.34 (0.94–1.91) | |
| DM+/CKD+ | 88 | 1264 | 7.0 | 1.55 (1.16–2.07) | |
| CABG‐related major bleeding | |||||
| DM−/CKD− | 367 | 5628 | 6.5 | 0.1678 | |
| DM+/CKD− | 366 | 5673 | 6.5 | 1.02 (0.88–1.18) | |
| DM−/CKD+ | 44 | 727 | 6.1 | 0.96 (0.69–1.32) | |
| DM+/CKD+ | 96 | 1250 | 7.7 | 1.29 (1.01–1.65) | |
The model is adjusted for age, sex, BMI, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous PCI or CABG, type of ACS define and randomized treatment. BMI indicates body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CKD‐EPI, chronic kidney disease epidemiology collaboration; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The crude event rate, (no. events/no. of subjects)×100%.
Subgroup DM−/CKD− is the reference category.
P value for the effect of DM/CKD subgroup.
Outcomes of Ticagrelor Versus Clopidogrel According to CKD and DM Status
Compared with clopidogrel, ticagrelor significantly reduced the incidence of the primary end point consistently across subgroups (P interaction=0.3). However, the absolute risk reduction (ARR) with ticagrelor versus clopidogrel was considerably higher in DM+/CKD+ patients (11.26%; adjusted HR 0.78; 95% CI 0.61–1.01) compared with DM−/CKD− (1.37%; adjusted HR 0.86; 95% CI 0.73–1.00) (Figures 5 and 6). Consistent findings were shown on all the components of the primary end point (Table 3). In particular, ticagrelor led to a 5.8% ARR in cardiovascular death in patients with DM+/CKD+ compared with a 0.2% reduction in DM−/CKD− patients. Accordingly, the number‐needed‐to‐treat for the primary end point was 8.9 in DM+/CKD+ and 73 in DM−/CKD−, and for cardiovascular death 17.2 in DM+/CKD+ and 500 in DM−/CKD−.
Figure 5.
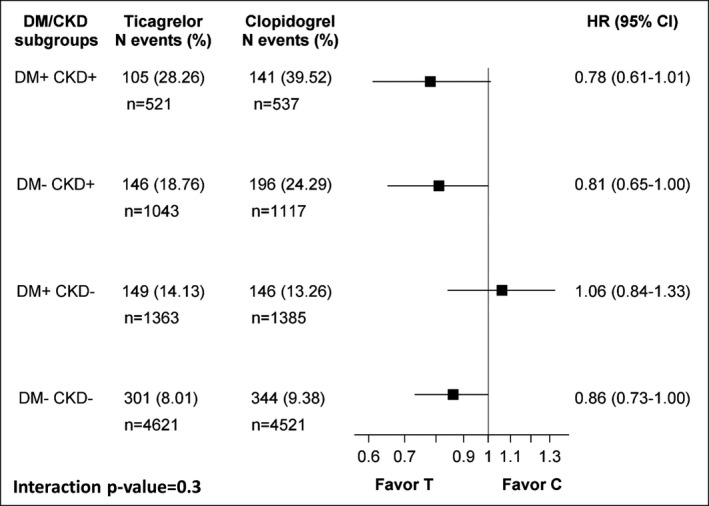
Hazard ratios (HR) with 95% CI for the primary composite end point (cardiovascular death, myocardial infarction, and stroke) of ticagrelor (T) vs clopidogrel (C) stratified by DM/CKD status. The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention or coronary artery bypass graft, type of acute coronary syndrome, and randomized treatment. CKD indicates chronic kidney disease; DM, diabetes mellitus.
Figure 6.
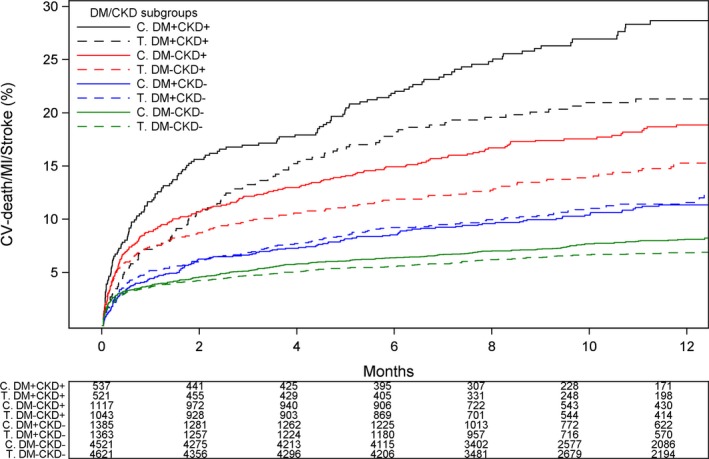
Kaplan–Meier event rate curves for the cumulative incidence of the primary composite end point of cardiovascular (CV) death, myocardial infarction, and stroke stratified by treatment group and DM/CKD status. C indicates clopidogrel; CKD, chronic kidney disease; DM, diabetes mellitus; T, ticagrelor.
Table 3.
Outcomes of Ticagrelor Versus Clopidogrel According to DM/CKD Status
| DM/CKD Subgroup | Ticagrelor Patients (N) | Clopidogrel Patients (N) | Ticagrelor Event Rate, N (%) | Clopidogrel Event Rate, N (%) | HR (95% CI) | P Value Interaction |
|---|---|---|---|---|---|---|
| Cardiovascular death | 0.3 | |||||
| DM+/CKD+ | 521 | 537 | 55 (13.60) | 77 (19.40) | 0.79 (0.55–1.11) | |
| DM−/CKD+ | 1043 | 1117 | 69 (8.33) | 111 (12.80) | 0.68 (0.51–0.92) | |
| DM+/CKD− | 1363 | 1385 | 59 (5.30) | 62 (5.38) | 1.00 (0.70–1.44) | |
| DM−/CKD− | 4621 | 4521 | 98 (2.51) | 104 (2.71) | 0.93 (0.70–1.22) | |
| MI | 0.2 | |||||
| DM+/CKD+ | 521 | 537 | 52 (13.79) | 72 (19.66) | 0.76 (0.53–1.09) | |
| DM−/CKD+ | 1043 | 1117 | 77 (9.77) | 100 (12.29) | 0.83 (0.62–1.12) | |
| DM+/CKD− | 1363 | 1385 | 93 (8.76) | 84 (7.58) | 1.13 (0.84–1.52) | |
| DM−/CKD− | 4621 | 4521 | 195 (5.16) | 233 (6.33) | 0.82 (0.67–0.99) | |
| All‐cause death | 0.5 | |||||
| DM+/CKD+ | 521 | 537 | 63 (15.58) | 82 (20.66) | 0.85 (0.61–1.18) | |
| DM−/CKD+ | 1043 | 1117 | 80 (9.66) | 125 (14.41) | 0.70 (0.53–0.93) | |
| DM+/CKD− | 1363 | 1385 | 64 (5.75) | 69 (5.98) | 0.98 (0.70–1.37) | |
| DM−/CKD− | 4621 | 4521 | 112 (2.87) | 123 (3.21) | 0.90 (0.69–1.16) | |
| Stroke | 0.6 | |||||
| DM+/CKD+ | 521 | 537 | 13 (3.26) | 18 (4.66) | 0.78 (0.38–1.59) | |
| DM−/CKD+ | 1043 | 1117 | 23 (2.81) | 20 (2.33) | 1.24 (0.68–2.26) | |
| DM+/CKD− | 1363 | 1385 | 22 (1.99) | 16 (1.40) | 1.42 (0.75–2.71) | |
| DM−/CKD− | 4621 | 4521 | 40 (1.03) | 31 (0.81) | 1.28 (0.80–2.04) | |
| Major bleeding | 0.3 | |||||
| DM+/CKD+ | 521 | 537 | 78 (27.37) | 79 (26.89) | 1.02 (0.75–1.40) | |
| DM−/CKD+ | 1043 | 1117 | 129 (21.73) | 125 (19.42) | 1.13 (0.88–1.44) | |
| DM+/CKD− | 1363 | 1385 | 150 (17.61) | 171 (18.88) | 0.91 (0.73–1.13) | |
| DM−/CKD− | 4621 | 4521 | 420 (13.23) | 355 (11.19) | 1.16 (1.01–1.34) | |
| Non‐CABG‐related major bleeding | 0.7 | |||||
| DM+/CKD+ | 521 | 537 | 39 (12.87) | 32 (10.18) | 1.32 (0.82–2.10) | |
| DM−/CKD+ | 1043 | 1117 | 75 (12.15) | 62 (9.14) | 1.34 (0.96–1.88) | |
| DM+/CKD− | 1363 | 1385 | 48 (5.30) | 50 (5.13) | 1.03 (0.69–1.52) | |
| DM−/CKD− | 4621 | 4521 | 129 (3.88) | 97 (2.93) | 1.30 (1.00–1.69) |
The model is adjusted for age, sex, body mass index, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous percutaneous coronary intervention or CABG, type of acute coronary syndrome and randomized treatment. CABG indicates coronary artery bypass graft; CKD, chronic kidney disease; DM, diabetes mellitus; HR, hazard ratio; MI, myocardial infarction.
The effects of ticagrelor versus clopidogrel on PLATO‐defined major bleeding were consistent across subgroups (P interaction=0.3). In particular, there was no increased risk of major bleeding with ticagrelor compared with clopidogrel in the subgroup of patients with DM+/CKD+ (27.4% versus 26.9%; HR 1.02; 95% CI 0.75–1.40). Accordingly, the effects on non‐CABG‐related major bleeding were also consistent regardless of CKD/DM status, although the increase in bleeding risk with ticagrelor was numerically higher in patients with CKD (both DM+/CKD+ and DM−/CKD+) (Table 3). The number‐needed‐to‐harm for all major bleeding was 208 in DM+/CKD+ and 49 in DM−/CKD− and for non‐CABG‐related major bleeding was 73 in DM+/CKD+ and 105 in DM−/CKD−. Major bleeding defined according to TIMI and GUSTO criteria followed the same trend (Table 4).
Table 4.
Bleeding Outcomes of Ticagrelor Versus Clopidogrel According to DM/CKD Status According to TIMI and GUSTO Criteria
| DM/CKD Subgroup | Ticagrelor Patients (N) | Clopidogrel Patients (N) | Ticagrelor Event Rate, N (%) | Clopidogrel Event Rate, N (%) | HR (95% CI) | P Value Interaction |
|---|---|---|---|---|---|---|
| TIMI major bleeding | 0.049 | |||||
| DM+/CKD+ | 521 | 537 | 48 (16.16) | 48 (15.71) | 1.02 (0.68–1.52) | |
| DM−/CKD+ | 1043 | 1117 | 78 (12.67) | 81 (12.13) | 1.05 (0.77–1.43) | |
| DM+/CKD− | 1363 | 1385 | 93 (10.58) | 124 (13.32) | 0.77 (0.59–1.01) | |
| DM−/CKD− | 4621 | 4521 | 308 (9.56) | 252 (7.82) | 1.21 (1.02–1.42) | |
| TIMI non‐CABG‐related major bleeding | 0.219 | |||||
| DM+/CKD+ | 521 | 537 | 24 (7.84) | 15 (4.71) | 1.69 (0.89–3.23) | |
| DM−/CKD+ | 1043 | 1117 | 38 (6.01) | 36 (5.22) | 1.16 (0.74–1.83) | |
| DM+/CKD− | 1363 | 1385 | 27 (2.95) | 34 (3.47) | 0.84 (0.51–1.40) | |
| DM−/CKD− | 4621 | 4521 | 88 (2.63) | 57 (1.71) | 1.51 (1.08–2.11) | |
| GUSTO severe bleeding | 0.882 | |||||
| DM+/CKD+ | 521 | 537 | 25 (8.12) | 34 (10.88) | 0.77 (0.46–1.28) | |
| DM−/CKD+ | 1043 | 1117 | 36 (5.72) | 39 (5.70) | 0.99 (0.63–1.56) | |
| DM+/CKD− | 1363 | 1385 | 33 (3.63) | 40 (4.09) | 0.88 (0.55–1.39) | |
| DM−/CKD− | 4621 | 4521 | 89 (2.67) | 92 (2.78) | 0.95 (0.71–1.27) | |
| GUSTO non‐CABG‐related severe bleeding | 0.545 | |||||
| DM+/CKD+ | 521 | 537 | 20 (6.44) | 19 (5.98) | 1.08 (0.58–2.03) | |
| DM−/CKD+ | 1043 | 1117 | 25 (3.93) | 25 (3.61) | 1.06 (0.61–1.85) | |
| DM+/CKD− | 1363 | 1385 | 17 (1.85) | 25 (2.54) | 0.74 (0.40–1.36) | |
| DM−/CKD− | 4621 | 4521 | 54 (1.61) | 41 (1.23) | 1.28 (0.85–1.91) | |
The model is adjusted for age, sex, BMI, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous PCI or CABG, type of ACS, and randomized treatment. ACS indicates acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; DM, diabetes mellitus; GUSTO, Global Use of Strategies to Open Occluded Arteries; HR, hazard ratio; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Results were consistent when measures of poor glycemic control and alternative definitions of CKD were considered. In particular, with poor glycemic control defined by hemoglobin A1c and CKD defined by the Creatinine‐Cystatin C Chronic Kidney Disease Epidemiology Collaboration equation, the effects of ticagrelor versus clopidogrel on ischemic and bleeding events were consistent across subgroups (Table 5). In patients with DM+/CKD+, ticagrelor led to a 14% ARR in the primary end point and a 9% ARR in cardiovascular death compared with clopidogrel with no significant increase in major bleeding.
Table 5.
Outcomes of Ticagrelor Versus Clopidogrel According to DM/CKD Status, With Poor Glycemic Control Defined by HbA1c and CKD Defined by the Creatinine‐Cystatin C CKD‐EPI Equation
| DM/CKD Subgroup | Ticagrelor Patients (N) | Clopidogrel Patients (N) | Ticagrelor Event Rate, N (%) | Clopidogrel Event Rate, N (%) | HR (95% CI) | P Value Interaction |
|---|---|---|---|---|---|---|
| Cardiovascular death/MI/stroke | 0.265 | |||||
| DM+/CKD+ | 633 | 631 | 105 (22.66) | 158 (36.57) | 0.68 (0.53–0.88) | |
| DM−/CKD+ | 344 | 390 | 49 (19.68) | 74 (27.22) | 0.77 (0.54–1.11) | |
| DM+/CKD− | 2841 | 2886 | 267 (11.79) | 313 (13.64) | 0.87 (0.74–1.03) | |
| DM−/CKD− | 2877 | 2797 | 191 (8.31) | 201 (8.99) | 0.92 (0.76–1.13) | |
| Cardiovascular death | 0.257 | |||||
| DM+/CKD+ | 633 | 631 | 57 (11.52) | 98 (20.70) | 0.63 (0.45–0.87) | |
| DM−/CKD+ | 344 | 390 | 25 (9.42) | 40 (13.63) | 0.74 (0.45–1.23) | |
| DM+/CKD− | 2841 | 2886 | 103 (4.34) | 112 (4.62) | 0.96 (0.73–1.25) | |
| DM−/CKD− | 2877 | 2797 | 57 (2.39) | 64 (2.75) | 0.87 (0.61–1.24) | |
| MI | 0.734 | |||||
| DM+/CKD+ | 633 | 631 | 53 (11.28) | 77 (17.53) | 0.71 (0.50–1.00) | |
| DM−/CKD+ | 344 | 390 | 29 (11.57) | 40 (14.61) | 0.84 (0.52–1.36) | |
| DM+/CKD− | 2841 | 2886 | 165 (7.24) | 192 (8.31) | 0.87 (0.71–1.08) | |
| DM−/CKD− | 2877 | 2797 | 124 (5.36) | 134 (5.97) | 0.89 (0.70–1.14) | |
| All‐cause death | 0.481 | |||||
| DM+/CKD+ | 633 | 631 | 68 (13.75) | 106 (22.39) | 0.70 (0.51–0.95) | |
| DM−/CKD+ | 344 | 390 | 28 (10.55) | 44 (14.99) | 0.74 (0.46–1.20) | |
| DM+/CKD− | 2841 | 2886 | 113 (4.76) | 125 (5.15) | 0.94 (0.73–1.21) | |
| DM−/CKD− | 2877 | 2797 | 64 (2.68) | 81 (3.48) | 0.77 (0.56–1.07) | |
| Stroke | 0.293 | |||||
| DM+/CKD+ | 633 | 631 | 15 (3.08) | 12 (2.57) | 1.33 (0.62–2.85) | |
| DM−/CKD+ | 344 | 390 | 5 (1.90) | 6 (2.06) | 0.95 (0.29–3.12) | |
| DM+/CKD− | 2841 | 2886 | 33 (1.40) | 41 (1.70) | 0.82 (0.52–1.30) | |
| DM−/CKD− | 2877 | 2797 | 29 (1.22) | 17 (0.73) | 1.67 (0.92–3.05) | |
| Major bleeding | 0.143 | |||||
| DM+/CKD+ | 633 | 631 | 74 (20.61) | 74 (20.51) | 1.03 (0.75–1.42) | |
| DM−/CKD+ | 344 | 390 | 43 (23.41) | 43 (19.59) | 1.12 (0.74–1.71) | |
| DM+/CKD− | 2841 | 2886 | 307 (16.44) | 322 (16.72) | 0.97 (0.83–1.14) | |
| DM−/CKD− | 2877 | 2797 | 272 (14.15) | 212 (10.97) | 1.28 (1.07–1.54) | |
| Non‐CABG‐related major bleeding | 0.782 | |||||
| DM+/CKD+ | 633 | 631 | 48 (12.89) | 40 (10.69) | 1.29 (0.84–1.96) | |
| DM−/CKD+ | 344 | 390 | 23 (12.06) | 21 (9.15) | 1.36 (0.75–2.45) | |
| DM+/CKD− | 2841 | 2886 | 93 (4.71) | 87 (4.23) | 1.12 (0.84–1.50) | |
| DM−/CKD− | 2877 | 2797 | 95 (4.71) | 66 (3.29) | 1.39 (1.02–1.91) | |
The model is adjusted for age, sex, BMI, heart rate, prior myocardial infarction, hypertension, dyslipidemia, angina pectoris, smoking status, previous PCI or CABG, type of ACS, and randomized treatment. ACS indicates acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CKD‐EPI, chronic kidney disease epidemiology collaboration; DM, diabetes mellitus; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
The data from the present post hoc analysis of the PLATO trial represent the largest exploring the impact of having DM, CKD, or both, on clinical outcomes in ACS patients. Our study showed that (1) the concomitant presence of CKD and DM is not uncommon in patients with ACS, representing 7% of the overall study population; (2) patients with CKD and DM are more likely to already have established atherosclerotic disease, more frequently present with a non‐ST‐elevation ACS and are more likely to be treated with a noninvasive approach; (3) patients with either DM or CKD are at increased risk of ischemic events compared with patients without these risk factors; and the combination of DM and CKD status is associated with an over 3‐fold increased risk of ischemic events compared with patients without these risk factors, including a 6‐fold increase in cardiovascular death; (4) the presence of DM and CKD is associated with a significant increase in major bleeding and non‐CABG‐related major bleeding, but not in CABG‐related bleeding; (5) the benefit of ticagrelor over clopidogrel on ischemic outcomes is consistent across DM and CKD status, but the magnitude of absolute benefit is enhanced in higher‐risk patients; in particular, in patients with DM and CKD ticagrelor led to a 22% relative risk reduction and an 11% ARR in the primary end point compared with clopidogrel, including a 21% relative risk reduction and an 5.8% ARR in cardiovascular death; and (6) there was no signal of increased risk of bleeding with ticagrelor in patients with CKD and DM as compared with the other subgroups.
DM and CKD have both been independently associated with an increased risk of cardiovascular events, which may be attributed to abnormalities specific to these patients favoring a prothrombotic and pro‐inflammatory status.1, 2 Among patients with DM, impaired clopidogrel‐induced antiplatelet effects leading to high levels of platelet reactivity has been largely attributed to an attenuation of clopidogrel's pharmacokinetic profile, characterized by lower active metabolite levels, and in part to dysregulation of the P2Y12 receptor signaling pathway.9, 10, 27 Subgroup analysis of major clinical trials have shown a reduced benefit of clopidogrel in CKD patients.2 Patients with CKD are characterized by upregulation of the P2Y12 signaling pathway induced by dinucleoside polyphosphates and impaired hepatic function, which can potentially impact clopidogrel metabolism.28, 29, 30, 31, 32 However, while pharmacodynamic studies have consistently shown DM to be associated with impaired clopidogrel‐induced antiplatelet effects, results have been conflicting when assessing how CKD affects clopidogrel response. These observations may be attributed to confounders within the heterogeneous study populations in which these studies have been performed.12, 13, 14, 15, 16, 17, 18, 19 Pharmacodynamic assessments specifically conducted among DM patients who also have CKD have shown these patients to have greater impairment of clopidogrel‐induced platelet inhibition compared with those without CKD.13, 15, 16 However, in the absence of DM, renal function has not always been shown to affect clopidogrel's antiplatelet effects.12, 13, 17, 18, 19 Overall, these findings suggest that there may be some level of synergism of DM and CKD on platelet reactivity in clopidogrel‐treated patients, which would be in line with the clinical observations of the present investigation.16
A post hoc analysis of the FREEDOM (Comparison of Two Treatments for Multivessel Coronary Artery Disease in Individuals With Diabetes) trial assessing revascularization strategies (surgical versus percutaneous) among DM patients (n=1843) with multivessel coronary artery disease evaluated the impact of CKD status on clinical outcomes.20 In this analysis, CKD affected clinical outcomes irrespective of the strategy used for revascularization, leading to a nearly 2‐fold risk increase in all‐cause mortality, cardiovascular death, and stroke and a 1.5‐fold risk increase in major bleeding.20 Our analysis represents the largest data set to unravel the contributing role of DM and CKD on cardiovascular outcomes. We extend the findings from the FREEDOM analysis to ACS patients receiving dual antiplatelet therapy undergoing different treatment strategies (invasive or noninvasive), showing that the presence of either DM or CKD increases long‐term cardiovascular events to a similar extent but when these risk factors are combined, this risk is further amplified. Notably, this was consistent using multiple definitions of DM and CKD, supporting the validity of our study findings. The ever‐rising prevalence of both DM and CKD underscore the relevance of these observations. In fact, both clinical disorders are pandemic public health problems. CKD has a prevalence of 13% in the United States and up to 17% in Europe.3, 33 Importantly, DM is a key risk factor for the development of CKD, and about one third of DM patients are found to have CKD.3 Therefore, with the increasing prevalence of DM, which is expected to double over the next 20 years, the prevalence of CKD is also expected to rise.34 These observations underscore the need for defining the most effective treatment options for these high‐risk patients, including strategies to reduce the risk of developing CKD in patients with DM. To this extent, sodium‐glucose cotransporter‐2 inhibitors are new antihyperglycemic therapies known to reduce long‐term decline in kidney function.35, 36 Similarly, in patients with established CKD, glucose control is also critical to reduce the risk of developing DM.
Ticagrelor is characterized by more potent and predictable antiplatelet effects compared with clopidogrel, which translate into better clinical outcomes in ACS patients, albeit at the expense of an increased risk of major bleeding.22, 37 Pharmacodynamic assessments have shown that the enhanced potency of ticagrelor over clopidogrel persists in patients with DM,38, 39 and in the DM subgroup of PLATO, compared with clopidogrel, ticagrelor was associated with a 2.1% ARR in the primary end point, a finding that was consistent with the overall trial results (P‐interaction: 0.49).23 In patients with CKD, ticagrelor led to a 4.7% ARR of the primary ischemic end point, which was also consistent with the overall trial results (P‐interaction: 0.13).24 However, there are limited data on the pharmacodynamic effects of ticagrelor in CKD patients.40, 41 The present study findings show that, although the benefit of ticagrelor over clopidogrel is consistent across subgroups (P‐interaction: 0.264), the enhanced benefit of ticagrelor in patients with CKD is even greater in patients who also have DM (11% ARR), including a 5.8% ARR in cardiovascular mortality. Indeed, the higher event rates that characterize these patients can contribute to the greater magnitude of the treatment effect associated with more potent platelet P2Y12 inhibition induced by ticagrelor. In addition, prior investigations supporting impaired clopidogrel‐induced platelet inhibition in DM patients, in particular those also with CKD, may contribute to these findings.9, 10, 11, 12, 14, 16, 17 However, because DM and CKD patients are characterized by enhanced vascular inflammation and endothelial dysfunction, it cannot be excluded that they could be more susceptible to the off‐target effects of ticagrelor. In fact, ticagrelor increases adenosine levels by inhibiting its reuptake by erythrocytes and adenosine may modulate inflammatory response and favor vasodilation.42
Patients with CKD and DM are overall at increased risk of bleeding. This may explain why in some studies these patients are less commonly treated with more potent platelet‐inhibiting therapies.43, 44 The increased risk for bleeding among DM and CKD patients was also confirmed in this analysis. However, there was no increased risk of major bleeding with ticagrelor versus clopidogrel in the subgroup of patients with DM+/CKD+. The increase in non‐CABG‐related major bleeding events was numerically higher in patients with DM+/CKD+, but the relative risk was similar and the effect was overall consistent across groups, also using different bleeding definitions. These findings were also consistent using multiple definitions of DM and CKD.
Study Limitations
The results of the present study should be interpreted in light of some limitations. Patients with end‐stage renal disease requiring hemodialysis were excluded from the trial; therefore, our results are not applicable to this setting. Although we used different definitions to define CKD status, we did not measure albumin–creatinine ratio and therefore may have underestimated the true prevalence of CKD. Accordingly, the number of patients with CKD+ in our study population was relatively small. CKD was defined according to baseline creatinine levels at the time of ACS presentation. Therefore, creatinine clearance may not be reflective of steady‐state kidney function. Indeed, it may be argued that the results of our study pertain to a cohort of CKD patients with mostly moderate (stage 3) degree of renal impairment and the results cannot be extrapolated to those with more advanced stages of renal disease. Moreover, the present investigation does not provide any mechanistic insights for the enhanced rates of adverse outcomes and the inconsistent response to different classes of P2Y12 inhibiting therapies among patients with concomitant DM and CKD, which is a topic of ongoing investigation (NCT02539160). It may be argued that there are large baseline differences between the DM/CKD groups that might not be possible to fully account for by covariate adjustment. Although an age/sex/comorbid matched analysis could have represented an option, this typically leads to loss of information when not all subjects can be matched, and a similar analysis would have resulted in smaller patient cohorts and ultimately not reflective of risk profile of this patient population in real‐world clinical practice. Finally, our results derive from a post hoc subgroup analysis and should as such be considered as hypothesis‐generating and requiring confirmation in prospectively designed studies.
Conclusions
In conclusion, the results of the present analysis showed that ACS patients with DM and CKD are at markedly increased risk for long‐term atherothrombotic events compared with patients without these risk factors, as well as with those with only 1 of these. Although the ischemic benefit of ticagrelor versus clopidogrel was consistent in all patient subgroups, the magnitude of benefit was enhanced according to the patient risk profile. Although patients with DM and CKD are at increased risk of bleeding, there were no signals of increased risk of major bleeding events with ticagrelor. Overall, these data underscore the need for using more potent platelet‐inhibiting therapy in ACS patients with DM and CKD who are often undertreated because of high perceived risk of bleeding.
Sources of Funding
The PLATO study was funded by AstraZeneca. Support for the analysis and interpretation of results and preparation of the manuscript was provided through funds to the Uppsala Clinical Research Center and Duke Clinical Research Institute as part of the Clinical Study Agreement.
Disclosures
James reports institutional research grant, honoraria, and consultant/advisory board fee from AstraZeneca; institutional research grant and consultant/advisory board fee from Medtronic; institutional research grants and honoraria from The Medicines Company; and consultant/advisory board fees from Janssen and Bayer. Lakic reports institutional research grants from AstraZeneca. Budaj reports consulting fees from AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, GlaxoSmithKline, Sanofi‐Aventis, Bayer, and Novartis; investigator fees from AstraZeneca, Sanofi‐Aventis, GlaxoSmithKline, Novartis, Bristol Myers Squibb/Pfizer, and Eisai; and honoraria for lectures from AstraZeneca, Bristol Myers Squibb/Pfizer, GlaxoSmithKline, Sanofi‐Aventis, and Novartis. Cornel reports consulting fees from Amgen and AstraZeneca. Katus reports personal fees from AstraZeneca, Bayer Vital, and Roche Diagnostics. Keltai has no potential conflicts to report. Kontny reports consultancy fees/honoraria for lectures, advisory board membership, and fee for research work from AstraZeneca; and advisory board membership and consultancy fees from Merck & Co. Lewis reports departmental grants for performing trials from AstraZeneca and MSD; and honoraria and speaker fees from Pfizer and Bristol‐Myers Squibb. Storey reports institutional research grants, consultancy fees, and honoraria from AstraZeneca; institutional research grants and consultancy fees from PlaqueTec; consultancy fees and honoraria from Bayer; and consultancy fees from Actelion, Avacta, Bristol‐Myers Squibb/Pfizer, Novartis, Thromboserin, and Idorsia. Himmelmann reports being an employee of AstraZeneca. Wallentin reports institutional research grants from AstraZeneca, Bristol‐Myers Squibb/Pfizer, Boehringer Ingelheim, GlaxoSmithKline, Merck & Co, and Roche Diagnostics; consultancy fees from Abbott; and holds 2 patents involving GDF‐15 licensed to Roche Diagnostics (EP2047275B1 and US8951742B2). Angiolillo reports payments as an individual for (1) consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; (2) participation in review activities from CeloNova and St. Jude Medical. He has also received institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli‐Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions; and is the recipient of funding from the Scott R. MacKenzie Foundation and the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, outside the submitted work. Franchi reports payments as an individual for consulting fee or honorarium from AstraZeneca and Sanofi.
Supporting information
Appendix S1. The members of PLATO Investigators.
Acknowledgments
This article is dedicated to the memory of the late Prof. Steen Husted.
(J Am Heart Assoc. 2019;8:e011139 DOI: 10.1161/JAHA.118.011139.)
References
- 1. Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798–813. [DOI] [PubMed] [Google Scholar]
- 2. Capodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125:2649–2661. [DOI] [PubMed] [Google Scholar]
- 3. Bonello L, Angiolillo DJ, Aradi D, Sibbing D. P2Y12‐ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes. Circulation. 2018;138:1582–1596. [DOI] [PubMed] [Google Scholar]
- 4. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. [DOI] [PubMed] [Google Scholar]
- 5. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–2931. [DOI] [PubMed] [Google Scholar]
- 6. Sherwood MW, Wiviott SD, Peng SA, Roe MT, Delemos J, Peterson ED, Wang TY. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2014;3:e000849 DOI: 10.1161/JAHA.114.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bueno H, Sinnaeve P, Annemans L, Danchin N, Licour M, Medina J, Pocock S, Sanchez‐Covisa J, Storey RF, Jukema JW, Zeymer U, Van de Werf F; EPICOR Investigators . Opportunities for improvement in anti‐thrombotic therapy and other strategies for the management of acute coronary syndromes: insights from EPICOR, an international study of current practice patterns. Eur Heart J Acute Cardiovasc Care. 2016;5:3–12. [DOI] [PubMed] [Google Scholar]
- 8. Angiolillo DJ, Bernardo E, Sabate M, Jimenez‐Quevedo P, Costa MA, Palazuelos J, Hernandez‐Antolin R, Moreno R, Escaned J, Alfonso F, Banuelos C, Guzman LA, Bass TA, Macaya C, Fernandez‐Ortiz A. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541–1547. [DOI] [PubMed] [Google Scholar]
- 9. Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello‐Montoliu A, Rollini F, Franchi F, Ueno M, Darlington A, Desai B, Moser BA, Sugidachi A, Guzman LA, Bass TA. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64:1005–1014. [DOI] [PubMed] [Google Scholar]
- 10. Erlinge D, Varenhorst C, Braun OO, James S, Winters KJ, Jakubowski JA, Brandt JT, Sugidachi A, Siegbahn A, Wallentin L. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol. 2008;52:1968–1977. [DOI] [PubMed] [Google Scholar]
- 11. Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez‐Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. [DOI] [PubMed] [Google Scholar]
- 12. Gremmel T, Muller M, Steiner S, Seidinger D, Koppensteiner R, Kopp CW, Panzer S. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28:2116–2122. [DOI] [PubMed] [Google Scholar]
- 13. Baber U, Bander J, Karajgikar R, Yadav K, Hadi A, Theodoropolous K, Gukathasan N, Roy S, Sayeneni S, Scott SA, Kovacic JC, Yu J, Sartori S, Mehran R, Uribarri J, Badimon JJ, Muntner P, Moreno P, Kini AS, Sharma SK. Combined and independent impact of diabetes mellitus and chronic kidney disease on residual platelet reactivity. Thromb Haemost. 2013;110:118–123. [DOI] [PubMed] [Google Scholar]
- 14. Franchi F, Rollini F, Angiolillo DJ. Defining the link between chronic kidney disease, high platelet reactivity, and clinical outcomes in clopidogrel‐treated patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8:e002760. [DOI] [PubMed] [Google Scholar]
- 15. Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez‐Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez‐Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. [DOI] [PubMed] [Google Scholar]
- 16. Engwenyu LR, Franchi F, Rollini F, Cho JR, DeGroat C, Bhatti M, Alobaidi Z, Ferrante E, Jakubowski JA, Sugidachi A, Zenni M, Bass TA, Angiolillo DJ. Impact of chronic kidney disease on platelet P2Y12 receptor signalling in patients with type 2 diabetes mellitus. Thromb Haemost. 2017;117:201–203. [DOI] [PubMed] [Google Scholar]
- 17. Mangiacapra F, Cavallari I, Barbato E, Ricottini E, Patti G, Vizzi V, D'Ambrosio A, De Bruyne B, Wijns W, Di Sciascio G. Impact of chronic kidney disease on platelet reactivity and outcomes of patients receiving clopidogrel and undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113:1124–1129. [DOI] [PubMed] [Google Scholar]
- 18. Tello‐Montoliu A, Ferreiro JL, Kodali MK, Ueno M, Tomasello SD, Rollini F, Capodanno D, Darlington A, Patel R, Desai B, Guzman LA, Bass TA, Angiolillo DJ. Impact of renal function on clopidogrel‐induced antiplatelet effects in coronary artery disease patients without diabetes mellitus. J Thromb Thrombolysis. 2013;36:14–17. [DOI] [PubMed] [Google Scholar]
- 19. Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Xu K, Parise H, Brodie BR, Stuckey TD, Stone GW. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug‐Eluting Stents registry. Circ Cardiovasc Interv. 2015;8:e001683. [DOI] [PubMed] [Google Scholar]
- 20. Baber U, Farkouh ME, Arbel Y, Muntner P, Dangas G, Mack MJ, Hamza TH, Mehran R, Fuster V. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J. 2016;37:3440–3447. [DOI] [PubMed] [Google Scholar]
- 21. Drexler H, Zanolin D, Vonbank A, Rein P, Saely CH. Impaired kidney function is a diabetes risk equivalent in patients with established coronary artery disease. Diabetes. 2014;63(supplement 1):A3 [Abstract]. [Google Scholar]
- 22. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators , Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 23. James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, Maya J, Nicolau JC, Spinar J, Storey RF, Stevens SR, Wallentin L; PLATO Study Group . Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31:3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, Lewis BS, Parikh K, Storey RF, Szummer K, Wojdyla D, Wallentin L. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122:1056–1067. [DOI] [PubMed] [Google Scholar]
- 25. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- 26. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueno M, Ferreiro JL, Tomasello SD, Capodanno D, Tello‐Montoliu A, Kodali M, Seecheran N, Dharmashankar K, Alissa R, Capranzano P, Desai B, Charlton RK, Bass TA, Angiolillo DJ. Functional profile of the platelet P2Y(1)(2) receptor signalling pathway in patients with type 2 diabetes mellitus and coronary artery disease. Thromb Haemost. 2011;105:730–732. [DOI] [PubMed] [Google Scholar]
- 28. Chang H, Yanachkov IB, Michelson AD, Li Y, Barnard MR, Wright GE, Frelinger AL III. Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res. 2010;125:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jankowski V, Gunthner T, Herget‐Rosenthal S, Zidek W, Jankowski J. Dinucleoside polyphosphates and uremia. Semin Dial. 2009;22:396–399. [DOI] [PubMed] [Google Scholar]
- 30. Leblond F, Guevin C, Demers C, Pellerin I, Gascon‐Barre M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12:326–332. [DOI] [PubMed] [Google Scholar]
- 31. Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4:1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jankowski J, Hagemann J, Yoon MS, van der Giet M, Stephan N, Zidek W, Schluter H, Tepel M. Increased vascular growth in hemodialysis patients induced by platelet‐derived diadenosine polyphosphates. Kidney Int. 2001;59:1134–1141. [DOI] [PubMed] [Google Scholar]
- 33. Bruck K, Stel VS, Gambaro G, Hallan S, Volzke H, Arnlov J, Kastarinen M, Guessous I, Vinhas J, Stengel B, Brenner H, Chudek J, Romundstad S, Tomson C, Gonzalez AO, Bello AK, Ferrieres J, Palmieri L, Browne G, Capuano V, Van Biesen W, Zoccali C, Gansevoort R, Navis G, Rothenbacher D, Ferraro PM, Nitsch D, Wanner C, Jager KJ; European CKD Burden Consortium . CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–1226. [DOI] [PubMed] [Google Scholar]
- 35. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 36. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. [DOI] [PubMed] [Google Scholar]
- 37. Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon CP, Becker RC, Wallentin L. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56:1456–1462. [DOI] [PubMed] [Google Scholar]
- 38. Sweeny JM, Angiolillo DJ, Franchi F, Rollini F, Waksman R, Raveendran G, Dangas G, Khan ND, Carlson GF, Zhao Y, Teng R, Mehran R. Impact of diabetes mellitus on the pharmacodynamic effects of ticagrelor versus clopidogrel in troponin‐negative acute coronary syndrome patients undergoing ad hoc percutaneous coronary intervention. J Am Heart Assoc. 2017;6:e005650 DOI: 10.1161/JAHA.117.005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clavijo LC, Maya J, Carlson G, Angiolillo DJ, Teng R, Caplan R, Price MJ. Platelet inhibition with ticagrelor versus clopidogrel in Hispanic patients with stable coronary artery disease with or without diabetes mellitus. Cardiovasc Revasc Med. 2015;16:450–454. [DOI] [PubMed] [Google Scholar]
- 40. Deharo P, Pankert M, Quilici J, Bonnet G, Bassez C, Verdier V, Morange P, Alessi MC, Bonnet JL, Cuisset T. Chronic kidney disease has a significant impact on platelet inhibition of new P2Y12 inhibitors. Int J Cardiol. 2015;184:428–430. [DOI] [PubMed] [Google Scholar]
- 41. Barbieri L, Pergolini P, Verdoia M, Rolla R, Nardin M, Marino P, Bellomo G, Suryapranata H, De Luca G; Novara Atherosclerosis Study Group . Platelet reactivity in patients with impaired renal function receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Vascul Pharmacol. 2016;79:11–15. [DOI] [PubMed] [Google Scholar]
- 42. Vilahur G, Gutierrez M, Casani L, Varela L, Capdevila A, Pons‐Llado G, Carreras F, Carlsson L, Hidalgo A, Badimon L. Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2016;134:1708–1719. [DOI] [PubMed] [Google Scholar]
- 43. Baber U, Chandrasekhar J, Sartori S, Aquino M, Kini AS, Kapadia S, Weintraub W, Muhlestein JB, Vogel B, Faggioni M, Farhan S, Weiss S, Strauss C, Toma C, DeFranco A, Baker BA, Keller S, Effron MB, Henry TD, Rao S, Pocock S, Dangas G, Mehran R. Associations between chronic kidney disease and outcomes with use of prasugrel versus clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a report from the PROMETHEUS study. JACC Cardiovasc Interv. 2017;10:2017–2025. [DOI] [PubMed] [Google Scholar]
- 44. Desai RJ, Spoendlin J, Mogun H, Gagne JJ. Contemporary time trends in use of antiplatelet agents among patients with acute coronary syndrome and comorbid diabetes mellitus or chronic kidney disease. Pharmacotherapy. 2017;37:1322–1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The members of PLATO Investigators.


