Abstract
Background
Many patients with heart failure (HF) with reduced ejection fraction (HFrEF) experience improvement or recovery of left ventricular ejection fraction (LVEF). Data on clinical characteristics, outcomes, and medical therapy in patients with HF with improved ejection fraction (HFiEF) are scarce.
Methods and Results
Of 5625 consecutive patients hospitalized for acute HF in the KorAHF (Registry [Prospective Cohort] for Heart Failure in Korea) study, 5103 patients had baseline echocardiography and 2302 patients had follow‐up echocardiography at 12 months. HF phenotypes were defined as persistent HFrEF (LVEF ≤40% at baseline and at 1‐year follow‐up), HFiEF (LVEF ≤40% at baseline and improved up to 40% at 1‐year follow‐up), HF with midrange ejection fraction (LVEF between 40% and <50%), and HF with preserved ejection fraction (LVEF ≥50%). The primary outcome was 4‐year all‐cause mortality from the time of HFiEF diagnosis. Among 1509 HFrEF patients who had echocardiography 1 year after index hospitalization, 720 (31.3%) were diagnosed as having HFiEF. Younger age, female sex, de novo HF, hypertension, atrial fibrillation, and β‐blocker use were positive predictors and diabetes mellitus and ischemic heart disease were negative predictors of HFiEF. During 4‐year follow‐up, patients with HFiEF showed lower mortality than those with persistent HFrEF in univariate, multivariate, and propensity‐score–matched analyses. β‐Blockers, but not renin–angiotensin system inhibitors or mineralocorticoid receptor antagonists, were associated with a reduced all‐cause mortality risk (hazard ratio: 0.59; 95% CI, 0.40–0.87; P=0.007). Benefits for outcome seemed similar among patients receiving low‐ or high‐dose β‐blockers (log‐rank, P=0.304).
Conclusions
HFiEF is a distinct HF phenotype with better clinical outcomes than other phenotypes. The use of β‐blockers may be beneficial for these patients.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01389843.
Keywords: β‐blockers, heart failure, improved ejection fraction, mortality
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
Among patients with heart failure with reduced ejection fraction, left ventricular ejection fraction improves in a third. Patients with heart failure with improved ejection fraction (HFiEF) have better prognosis than other heart failure phenotypes.
Younger age, female sex, de novo onset, hypertension, atrial fibrillation, and β‐blocker prescription are positive predictors, whereas ischemic heart disease and diabetes mellitus are negative independent predictors of HFiEF.
The use of β‐blockers, but not renin–angiotensin system inhibitors or mineralocorticoid receptor antagonists, is associated with reduced all‐cause mortality among patients with HFiEF.
What Are the Clinical Implications?
HFiEF is a distinct heart failure phenotype with better clinical outcomes than other phenotypes.
β‐Blockers should be continued in HFiEF patients.
Heart failure (HF) is currently classified as HF with reduced ejection fraction (HFrEF), HF with midrange ejection fraction (HFmrEF), or HF with preserved ejection fraction (HFpEF) based on left ventricular ejection fraction (LVEF).1 Although the prognoses for the various HF types appear to be similar, the level of neurohumoral activity and the response to medical therapy differ among HF types, suggesting differences in their underlying pathophysiology.2
Among patients with HFrEF, a subgroup experience the restoration of LVEF with goal‐directed medical therapy (GDMT) and are classified as having HF with improved ejection fraction (HFiEF).3, 4, 5 Data on demographics, etiology, and prognosis remain scarce, especially in Asian patients with HF.
Regarding treatment strategies, drugs targeting the sympathetic nervous system and neurohumoral activation have improved survival in patients with HFrEF6, 7, 8, 9 but not in those with HFpEF.10, 11, 12, 13 It is unknown whether HFiEF would behave like HFrEF or HFpEF in terms of response to GDMT.
KorAHF (Registry [Prospective Cohort] for Heart Failure in Korea) is a prospective, nationwide, multicenter cohort study that consecutively enrolled patients with acute HF (AHF), and every patient was scheduled to undergo echocardiography at baseline and at 1 year after the index admission. Using this registry, we sought to comprehensively investigate the clinical characteristics, outcomes, and response to medical therapy of patients with HFiEF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population and Data Collection
KorAHF was a prospective, multicenter cohort study, and the design and preliminary results have been described elsewhere (ClinicalTrial.gov identifier NCT01389843).14, 15 Briefly, 5625 consecutive patients hospitalized for AHF in 10 tertiary university hospitals in the Republic of Korea were enrolled between March 2011 and December 2014. Patients who had signs or symptoms of HF and lung congestion, objective findings of left ventricular systolic dysfunction, or structural heart disease were included in this study. There were no exclusion criteria.
Each patient was scheduled for follow‐up at least 5 years after the index hospitalization. The mortality data of patients who were lost to follow‐up were collected from National Insurance data or National Death Records.
The institutional review board or ethics committee at each participating hospital approved the study protocol and waived the need for written informed consent. This study complied with the Declaration of Helsinki principles.
Study Variables and Definitions
All echocardiographic studies were performed by cardiologists who were certified by the Korean Society of Echocardiography, using a standard ultrasound machine with a 2.5‐MHz probe. Standard techniques were adopted to obtain M‐mode, 2‐dimensional, and Doppler measurements, in accordance with the American Society of Echocardiography's guidelines.16 LVEF was measured using the Simpson biplane method, unless the Simpson method was not possible. Based on the echocardiography findings at the index AHF hospitalization, patients were classified into those with HFrEF (LVEF ≤40%), HFmrEF (LVEF between 40% and <50%), and HFpEF (LVEF ≥50%). All patients were encouraged to undergo follow‐up echocardiography at 1 year after the index hospitalization. Among patients with HFrEF at the index hospitalization, those whose LVEF improved to >40% were considered to have HFiEF, whereas those with LVEF ≤40% were considered to have persistent HFrEF (Figure 1A).
Figure 1.
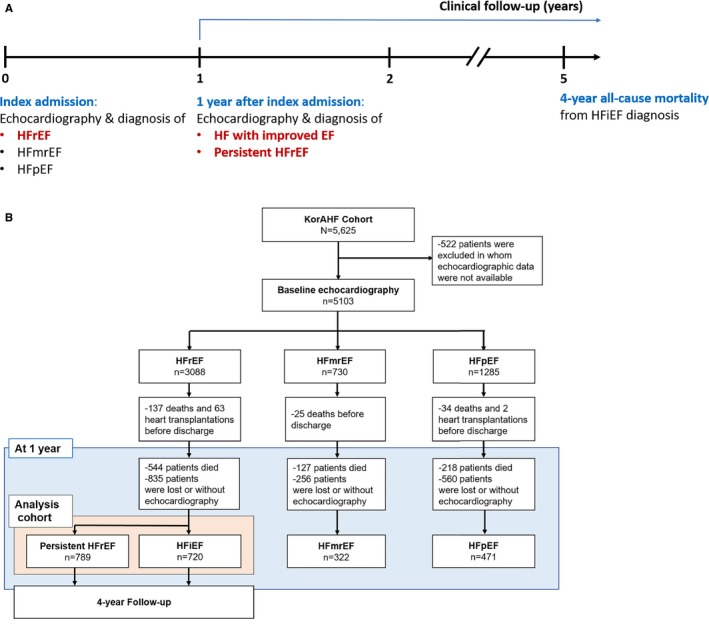
Study population. A, Flowchart of the study. B, Patients demographics according to the flowchart. EF indicates ejection fraction; HF, heart failure; HFiEF, heart failure with improved ejection fraction; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KorAHF, Registry (Prospective Cohort) for Heart Failure in Korea.
In terms of medication, the use of β‐blockers for HF treatment was defined as a prescription for carvedilol, metoprolol, bisoprolol, or nebivolol, according to the recommendation of the current guidelines.1, 4 Use of renin–angiotensin system (RAS) inhibitors was defined as the administration of either an angiotensin‐converting enzyme inhibitor or an angiotensin II receptor blocker. The β‐blocker name and dose were evaluated in the year following diagnosis of HFiEF. Low‐ and high‐dose β‐blockers were defined as those with 1% to 49% and ≥50% of the target dose, respectively. The target dose of the β‐blockers was based on the clinical guideline.1, 17 Medication history at admission, during admission, at discharge, and during follow‐up (at 1, 3, 6, and 12 months) was recorded in the KorAHF registry.
The primary outcome was 4‐year all‐cause mortality from time of HFiEF diagnosis.
Statistical Analysis
The data are presented as number and frequency for categorical variables and as mean±SD for continuous variables. For comparison between groups, the χ2 test (or Fisher exact test when any expected cell count was <5 for a 2×2 table) was used for categorical variables and the unpaired Student t test was used for continuous variables. The chronological trends of the outcomes were expressed as Kaplan–Meier estimates and compared by β‐blocker use. The log‐rank test was performed for comparison of the differences in the clinical outcomes. A multivariable Cox proportional hazards regression model was used to determine the independent predictors of all‐cause mortality. Variables associated with mortality with a P<0.05 were included as confounding variables in the multivariate analysis. As a sensitivity analysis, we performed both propensity‐score–matched (PSM) and inverse‐probability treatment‐weighted (IPTW) analysis. The propensity score was calculated using multivariable logistic regression analysis, and the PSM population was created using the nearest neighbor method without replacement in a 1:1 ratio (the following variables were included for matching: age, sex, body mass index, previous history of heart failure, hypertension, diabetes mellitus, ischemic heart disease, valvular heart disease, chronic obstructive pulmonary disease, cerebrovascular disease, atrial fibrillation, malignancy, New York Heart Association functional class, and medication history of β‐blockers, renin–angiotensin system inhibitors, and mineralocorticoid receptor antagonists). Considering reduction of participants during PSM analysis, the IPTW analysis was also performed to account for confounders. Success of PSM and IPTW analyses was assessed by calculating standardized differences in the baseline characteristics (Tables S1 and S2). We used the “MatchIt” package for R programming for PSM analysis and the “Twang” package for IPTW analysis.
A 2‐sided P<0.05 was considered statistically significant. The statistical tests were performed using IBM SPSS v23 (IBM Corp) and R v3.1.0 (R Foundation for Statistical Computing).
Results
Demographic and Clinical Characteristics
Among 5625 patients included in the KorAHF registry, 5103 patients underwent baseline echocardiographic evaluation. Based on LVEF, 3088 (61%) patients were classified as having HFrEF, 730 (14%) as having HFmrEF, and 1285 (25%) as having HFpEF. During the following year, 889 had died and 1651 were either lost to follow‐up or did not undergo 1‐year follow‐up echocardiography; therefore, the data of 2302 patients were available for this analysis. Of these patients, 789 (34%) were finally diagnosed with persistent HFrEF, 720 (31%) with HFiEF, 322 (14%) with HFmrEF, and 471 (20%) with HFpEF (Figure 1B).
Tables 1 and 2 present clinical characteristics of patients with HFrEF at the index admission and at 1 year after index admission. In brief, patients with HFiEF had more favorable baseline characteristics: they were younger, showed a preponderance of de novo HF, and had less hypertension, diabetes mellitus, ischemic heart disease, and chronic obstructive lung disease. Change of LVEF from index admission to 1‐year follow‐up was 13.7±15.1% in all, 2.7±7.6% in persistent HFrEF, and 25.7±11.6% in HFiEF. The clinical information of other HF phenotypes is presented in Table S3.
Table 1.
Clinical Characteristics According to HF Phenotypes at the Index Admission
| All HFrEF (n=1509) | Persistent HFrEF (n=789) | HFiEF (n=720) | P Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, y | 62.4±15.2 | 65.0±14.1 | 59.5±15.8 | <0.001 |
| Men | 937 (62.1) | 516 (65.4) | 421 (58.5) | <0.001 |
| BMI, kg/m2 | 23.7±3.8 | 23.6±3.5 | 23.7±4.1 | 0.507 |
| De novo HF | 833 (55.2) | 354 (44.9) | 479 (66.5) | <0.001 |
| Past medical history | ||||
| Hypertension | 757 (50.2) | 409 (51.8) | 348 (48.3) | <0.001 |
| Diabetes mellitus | 495 (32.8) | 319 (40.4) | 176 (24.4) | <0.001 |
| Ischemic heart disease | 378 (25.0) | 267 (33.9) | 111 (15.4) | <0.001 |
| Valvular heart disease | 131 (8.7) | 60 (7.6) | 71 (9.9) | 0.120 |
| COPD | 127 (8.4) | 72 (9.1) | 55 (7.6) | 0.008 |
| Cerebrovascular disease | 167 (11.1) | 100 (12.7) | 67 (9.3) | 0.037 |
| Atrial fibrillation | 326 (21.6) | 163 (20.7) | 163 (22.6) | <0.001 |
| Malignancy | 123 (8.2) | 55 (7.0) | 68 (9.4) | 0.079 |
| Current smoking | 341 (22.6) | 176 (22.3) | 165 (22.9) | 0.777 |
| NYHA functional class | ||||
| II | 240 (15.9) | 124 (15.7) | 116 (16.1) | 0.532 |
| III | 595 (39.4) | 302 (38.3) | 293 (40.7) | |
| IV | 674 (44.7) | 363 (46.0) | 311 (43.2) | |
| Physical examination | ||||
| SBP, mm Hg | 127.7±28.2 | 125.4±25.7 | 130.3±30.5 | 0.001 |
| DBP, mm Hg | 80.1±18.8 | 77.6±16.4 | 82.8±20.7 | <0.001 |
| HR, beats/min | 94.7±24.7 | 92.5±23.5 | 97.1±25.7 | <0.001 |
| Laboratory examination | ||||
| Hemoglobin, mg/dL | 13.1±2.3 | 13.0±2.2 | 13.2±2.3 | 0.032 |
| Sodium, mmol/L | 137.9±4.5 | 137.9±4.4 | 137.9±4.5 | 0.772 |
| Potassium, mmol/L | 4.4±0.6 | 4.4±0.6 | 4.3±0.6 | 0.021 |
| BUN, mg/dL | 24.5±14.8 | 25.6±15.2 | 23.2±14.2 | 0.002 |
| Creatinine, mg/dL | 1.4±1.4 | 1.4±1.3 | 1.4±1.5 | 0.692 |
| BNP, pg/mL | 980.5 (533.3–1856.5) | 927.0 (508.5–1685.0) | 1063.0 (545.0–2078.0) | 0.090 |
| NT‐proBNP, pg/mL | 4688.0 (2363.5–10 491.2) | 4785.0 (2419.0–11 784.0) | 4453.0 (2336.0–9531.5) | 0.221 |
| Troponin I, ng/mL | 0.06 (0.04–0.20) | 0.06 (0.04–0.18) | 0.06 (0.03–0.24) | 0.198 |
| Echocardiography | ||||
| LAD, mm | 47.7±9.0 | 48.3±8.7 | 47.0±9.3 | 0.004 |
| LVEDD, mm | 62.3±9.1 | 64.5±9.0 | 60.0±8.7 | <0.001 |
| LVESD, mm | 53.0±9.9 | 55.3±9.8 | 50.5±9.5 | <0.001 |
| E/e′ | 21.8±11.1 | 22.8±11.7 | 20.6±10.3 | 0.001 |
| RVSP, mm Hg | 43.4±14.3 | 44.1±14.8 | 42.5±13.6 | 0.083 |
| LVEF, % | 26.2±7.4 | 25.3±7.1 | 27.3±7.6 | <0.001 |
| Medication | ||||
| Β‐Blocker | 906 (60.0) | 453 (57.4) | 453 (62.9) | 0.029 |
| RASi | 1186 (78.6) | 622 (78.8) | 564 (78.3) | 0.813 |
| MRA | 840 (55.7) | 472 (59.8) | 368 (51.1) | 0.001 |
Data are shown as n (%), mean±SD, or median (interquartile range). BMI indicates body mass index; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HF, heart failure; HFiEF, heart failure with improved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal proB‐type natriuretic peptide; NYHA, New York Heart Association; RASi, renin‐angiotensin system inhibitor; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure.
Table 2.
Clinical Characteristics According to HF Phenotypes 1 Year After Index Admission (ie, at HFiEF diagnosis)
| All HFrEF | Persistent HFrEF (n=789) | HFiEF (n=720) | P Value | |
|---|---|---|---|---|
| Physical examination | ||||
| SBP, mm Hg | 118.2±18.7 | 114.7±17.9 | 121.8±18.9 | <0.001 |
| DBP, mm Hg | 70.6±12.7 | 68.4±12.0 | 72.8±12.9 | <0.001 |
| HR, bpm | 78.2±15.6 | 78.4±16.1 | 78.1±15.2 | 0.767 |
| Laboratory examination | ||||
| Hemoglobin, mg/dL | 12.7±2.1 | 12.8±2.1 | 12.7±2.0 | 0.371 |
| Sodium, mmol/L | 139.1±3.3 | 138.8±3.2 | 139.4±3.5 | 0.006 |
| Potassium, mmol/L | 4.5±0.5 | 4.5±0.5 | 4.5±0.5 | 0.072 |
| BUN, mg/dL | 24.5±14.5 | 25.9±15.2 | 22.8±13.5 | 0.001 |
| Creatinine, mg/dL | 1.5±1.6 | 1.6±1.5 | 1.5±1.6 | 0.423 |
| Echocardiography | ||||
| LAD, mm | 44.4±8.8 | 46.9±8.4 | 41.6±8.4 | <0.001 |
| LVEDD, mm | 57.7±10.0 | 63.6±8.8 | 51.2±6.7 | <0.001 |
| LVESD, mm | 44.8±12.3 | 53.6±9.6 | 35.6±7.0 | <0.001 |
| E/e′ | 16.7±10.2 | 19.8±11.4 | 13.5±7.5 | <0.001 |
| RVSP, mm Hg | 36.8±31.6 | 40.3±24.2 | 32.3±38.6 | <0.001 |
| LVEF, % | 39.9±14.8 | 28.0±7.4 | 53.0±8.4 | <0.001 |
| ΔLVEF from index admission, % | 13.7±15.1 | 2.7±7.6 | 25.7±11.6 | <0.001 |
| Medications | ||||
| Β‐Blocker | 878 (63.3) | 443 (60.9) | 443 (65.8) | 0.058 |
| RASi | 981 (70.7) | 535 (74.9) | 446 (66.3) | <0.001 |
| MRA | 612 (44.1) | 373 (52.2) | 239 (35.5) | <0.001 |
Data are shown as n (%) or mean±SD. BUN indicates blood urea nitrogen; DBP, diastolic blood pressure; E/e′, the ratio between early mitral inflow velocity and mitral annular early diastolic velocity; HF, heart failure; HFiEF, heart failure with improved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MRA, mineralocorticoid receptor antagonist; RAS, renin‐angiotensin system inhibitor; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure.
Predictors of HFiEF
The etiology and aggravating factors for AHF by HF phenotype are presented in Figure 2A and 2C. Compared with patients with persistent HFrEF, patients with HFiEF had less ischemic but more tachycardia‐induced cardiomyopathy.
Figure 2.
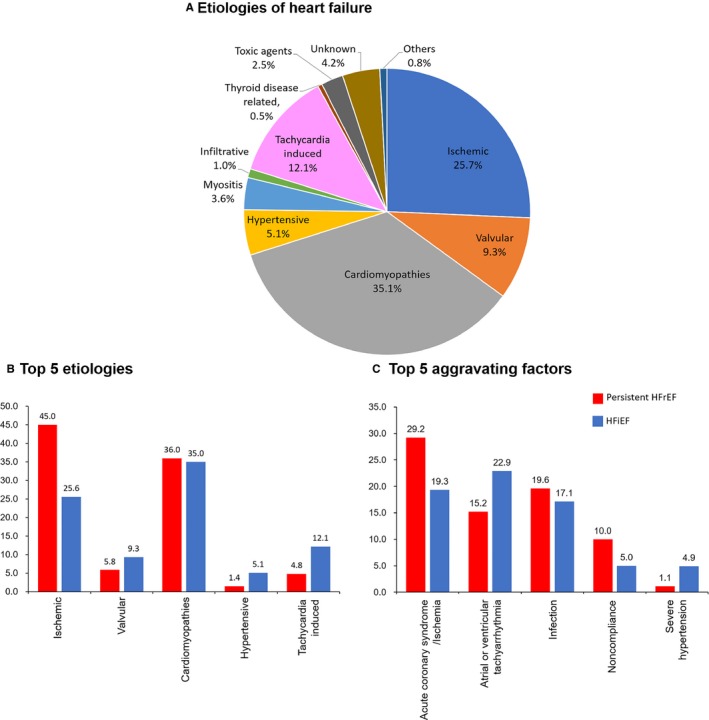
Etiology and aggravating factors according to HF phenotypes. A, Proportion of HF etiology. B, Top 5 etiologic causes according to the HF phenotypes. C, Five most common aggravating factors of acute HF according to the HF phenotypes. HF indicates heart failure; HFiEF, heart failure with improved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
We investigated independent predictors of HFiEF in patients who were initially diagnosed as having HFrEF at baseline (Table 3). In the multivariable analysis, younger age, female sex, de novo HF, hypertension, atrial fibrillation, and use of β‐blockers were positive independent predictors. In contrast, diabetes mellitus, ischemic heart disease, and mineralocorticoid receptor antagonist (MRA) prescription at discharge were inversely associated with an HFiEF diagnosis.
Table 3.
Independent Predictors of HFiEF Among Patients With HFrEF at the Index Admission
| OR | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.98 | 0.97–0.99 | <0.001 |
| Male | 0.65 | 0.52–0.81 | <0.001 |
| De novo onset | 2.23 | 1.77–2.80 | <0.001 |
| Hypertension | 1.31 | 1.05–1.65 | 0.020 |
| Diabetes mellitus | 0.55 | 0.43–0.70 | <0.001 |
| Ischemic heart disease | 0.58 | 0.45–0.76 | <0.001 |
| Atrial fibrillation | 1.77 | 1.36–2.32 | <0.001 |
| Β‐Blocker at discharge | 1.28 | 1.03–1.59 | 0.024 |
| MRA at discharge | 0.59 | 0.47–0.73 | <0.001 |
ORs have been adjusted for age, sex, de novo heart failure, previous history of hypertension, diabetes mellitus, ischemic heart disease, chronic obstructive pulmonary disease, cerebrovascular accident, atrial fibrillation and malignancy, New York Heart Association functional class, β‐blocker at discharge, renin–angiotensin system inhibitor at discharge, and MRA at discharge. HFiEF indicates heart failure with improved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; OR, odds ratio.
Clinical Outcomes
The treatment and outcomes during the index hospitalization are displayed in Table S4.
During 4‐year follow‐up, 116 (16%) patients with HFiEF died, all of whom had more unfavorable characteristics, as expected (Table S5). Patients with HFiEF showed better prognosis (log‐rank, P<0.001) than those with persistent HFrEF in crude, PSM, and IPTW cohorts (Figure 3, Tables S1 and S2). Clinical outcomes of other HF phenotypes are presented in Figure S1. Briefly, those with HFiEF had the lowest mortality (116 deaths, 16.1%) compared with those with persistent HFrEF (270 deaths, 34.2%), HFmrEF (214 deaths, 33.5%), and HFpEF (149 deaths, 31.6%).
Figure 3.
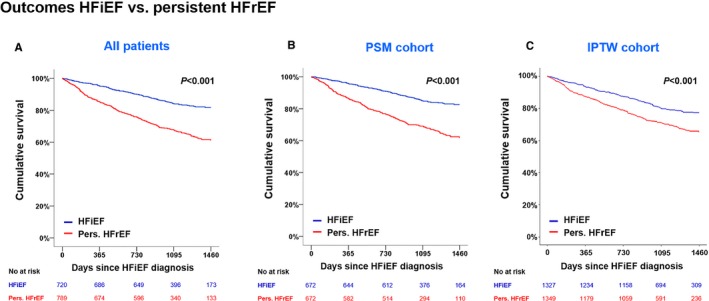
Clinical outcomes according to HFiEF and persistent HFrEF. A, Kaplan–Meier survival curves for 4‐year mortality according to HF phenotypes. As sensitivity analyses, the PSM cohort (B) and the IPTW cohort (C) were also analyzed. The curves are left‐truncated at 4 years after index admission. HFiEF, heart failure with improved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IPTW, inverse‐probability treatment weighted; PSM, propensity score matching.
GDMT in HFiEF
Regarding the effect of GDMT in HFiEF, patients with β‐blockers had lower 4‐year all‐cause mortality in crude, PSM and IPTW populations (Figure 4, Table S6, Figure S2).
Figure 4.
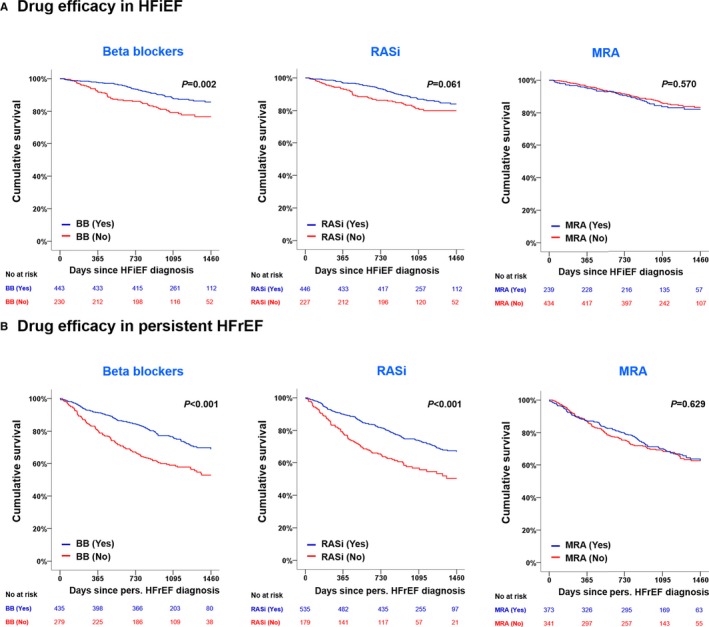
Impact of GDMT on 4‐year mortality in HFiEF patients (A) and persistent HFpEF patients (B). GDMT indicates goal‐directed medical therapy; HFrEF, heart failure with reduced ejection fraction; HFiEF, heart failure with improved ejection fraction; MRA, mineralocorticoid receptor antagonists; RASi, renin–angiotensin system inhibitor.
In multivariate analysis, only the use of β‐blockers was associated with a 41% reduced risk of mortality (hazard ratio: 0.59; 95% CI, 0.40–0.87; P=0.007), whereas the effect of RAS inhibitor and MRA use on mortality appeared to be neutral (Table 4).
Table 4.
Cox Regression Analysis for 4‐Year Mortality From HFiEF Diagnosis
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Age | 1.06 | 1.04–1.07 | <0.001 | 1.05 | 1.03–1.06 | <0.001 |
| Male | 1.28 | 0.88–1.87 | 0.198 | |||
| De novo onset | 0.41 | 0.28–0.59 | <0.001 | 0.53 | 0.35–0.79 | 0.002 |
| Hypertension | 1.99 | 1.36–2.90 | <0.001 | 0.96 | 0.60–1.52 | 0.852 |
| Diabetes mellitus | 2.41 | 1.67–3.48 | <0.001 | 1.39 | 0.90–2.16 | 0.140 |
| Ischemic heart disease | 2.93 | 1.98–4.33 | <0.001 | 1.56 | 0.99–2.46 | 0.055 |
| COPD | 1.01 | 0.51–2.00 | 0.971 | |||
| Cerebrovascular disease | 3.21 | 2.07–4.96 | <0.001 | 2.09 | 1.29–3.38 | 0.003 |
| Atrial fibrillation | 0.78 | 0.52–1.18 | 0.234 | |||
| Malignancy | 1.52 | 0.88–2.62 | 0.130 | |||
| NYHA functional class | ||||||
| II | 1 | Reference | 0.079 | |||
| III | 1.22 | 0.67–2.24 | ||||
| IV | 1.74 | 0.97–3.10 | ||||
| Β‐Blocker at HFiEF diagnosis | 0.54 | 0.37–0.80 | 0.002 | 0.59 | 0.40–0.87 | 0.007 |
| RASi at HFiEF diagnosis | 0.69 | 0.46–1.02 | 0.063 | |||
| MRA at HFiEF diagnosis | 1.12 | 0.75–1.67 | 0.570 | |||
Adjusted hazard ratios were adjusted for variables that showed P<0.05 in univariate analysis. COPD indicates chronic obstructive pulmonary disease; HFiEF, heart failure with improved ejection fraction; MRA, mineralocorticoid antagonist; NYHA, New York Heart Association; RASi, renin–angiotensin system inhibitor.
Effect of the Dose and Timing of Initiation of β‐Blockers
Among patients with HFiEF who took β‐blockers, most received carvedilol (216 patients, 48.8%) or bisoprolol (201 patients, 45.4%) whereas nebivolol (24 patients, 5.4%) and metoprolol (2 patients, 0.5%) were rarely used. There was no difference between carvedilol and bisoprolol; however, because of the small number of patients taking metoprolol and nebivolol, a definite conclusion could not be drawn. Stratified by β‐blocker dose, patients who received either high‐ or low‐dose β‐blockers at the time of diagnosis of HFiEF showed better 4‐year mortality than those who did not; however, there was no difference between the patients who received low‐ and high‐dose β‐blockers (log‐rank, P=0.304; Figure S3).
Because the status of β‐blocker prescription changed between discharge from the index hospitalization and the time of HFiEF diagnosis, we further categorized the patients into 4 groups according to β‐blocker use at discharge and at HFiEF diagnosis. In the Kaplan–Meier analysis, patients who were on β‐blockers at the time of HFiEF diagnosis had similar prognoses, regardless of β‐blocker use at discharge from the index hospitalization (log‐rank, P=0.497; Figure S3).
Subgroup Analysis
We performed exploratory subgroup analyses that included age, sex, ischemic versus nonischemic etiology, HF onset (de novo versus acute decompensated HF [ADHF]), chronic kidney disease, diabetes mellitus, RAS inhibitor use, MRA use, and changes in LVEF. There was no significant interaction between the β‐blocker effect and subgroups, and β‐blocker use was consistently associated with reduced risk for 4‐year all‐cause mortality across all subgroups (Figure S4).
Next, we stratified the patients by rhythm. Patients with a β‐blocker had better survival than patients without among those with sinus rhythm but not among those with atrial fibrillation (Figure S5).
Regarding the onset of HF, 55% of the patients had de novo HF and 45% had ADHF. Patients with HFiEF had better survival than those with persistent HFrEF among both de novo HF and ADHF patients (Figure S6). Regarding GDMT, β‐blocker use was associated with improved survival of both de novo HF and ADHF patients. In Kaplan–Meier analysis, β‐blockers showed a therapeutic implication for de novo HF (log‐rank, P=0.016) but attenuated improvement in ADHF (log‐rank, P=0.089). After adjusting for covariates, both de novo HFiEF (hazard ratio: 0.73; 95% CI, 0.54–1.00; P=0.049) and acute decompensated HFiEF (hazard ratio: 0.57; 95% CI, 0.33–0.98, P=0.041) showed a benefit of β‐blockers. In contrast, the effect of RAS inhibitors and MRAs appeared to be neutral in both de novo HF and ADHF patients (Figures S7 and S8).
Discussion
In this comprehensive analysis of HFiEF, we investigated the clinical characteristics, predictors, and prognostic outcomes of patients with HFiEF in comparison with persistent HFrEF. Younger age, de novo onset, and β‐blocker prescription were positive predictors; in contrast, ischemic heart disease and diabetes mellitus were negative independent predictors of HFiEF among patients with HFrEF at index admission. Compared with persistent HFrEF, patients with HFiEF had better prognosis, and the use of β‐blockers was associated with improved survival in these patients.
Clinical Characteristics and Predictors of HFiEF
Understanding the clinical characteristics and predictors of HFiEF provides important information and can be used for risk stratification and guidance of therapy in patients with HF. In this study, we showed that younger age and de novo HF were independent predictors of HFiEF. Previous studies also found patients with LVEF improvement to be younger.18 Conversely, ischemic heart disease was a strong negative predictor, in accordance with a report indicating that patients with HFiEF had less coronary artery disease.6 Patients with ischemic cardiomyopathy have been found to have less viable myocardium and more scarring; in addition, owing to its irreversible nature, the extent of the myocardial scar was found to correlate inversely with LVEF improvement.19, 20
Prognosis of Patients With HFiEF
The principal finding of this study pertains to mortality, and patients with HFiEF had better prognosis compared not only with HFpEF but also with other HF phenotypes (Figure 3, Figure S1), with a remarkably reduced risk of 4‐year all‐cause mortality. Our findings are consistent with previous studies reporting the superior long‐term clinical prognosis of patients with HFiEF compared with the other HF phenotypes.3, 5
Notably, patients with HFiEF required more catecholamines and mechanical circulatory support device assistance during the index admission, indicating a more serious in‐hospital course in contrast to the ultimately favorable long‐term outcomes. This implies that in patients with HFrEF who survive the first year, the more serious in‐hospital course does not necessarily equate to grave long‐term postdischarge outcomes.
GDMT in HFiEF
Another principal finding was related to the effect of GDMT in patients with HFiEF. We found that the use of β‐blockers, but not the use of RAS inhibitors or MRAs, was associated with improved survival. This finding is crucial and has important clinical implications: In patients with HFrEF, β‐blockers should be continued even after the restoration of LVEF. Interestingly, there was no difference in mortality between the patients with high‐ and low‐dose β‐blockers in our study. Considering the similar prognoses for those taking low‐ or high‐dose β‐blockers, careful dose reduction of β‐blockers may be possible for patients with HFiEF who do not tolerate β‐blockers well. Furthermore, we showed that β‐blocker use at HFiEF diagnosis was associated with improved survival regardless of the prescription of β‐blockers at hospital discharge. This finding suggests that all patients with HFiEF could benefit from β‐blocker use. The reasons for the lack of effect of RAS inhibitors and MRAs are not clear.
Strengths and Limitations
This study has several limitations. First, because this study is a post hoc analysis of a prospective cohort study, albeit a large one, as opposed to a randomized controlled trial, there could be unmeasured confounding factors. Second, we enrolled only patients who underwent echocardiographic assessment at 1 year after index admission, and this approach may have led to selection and lead‐time biases, possibly favoring less ill patients or those with better compliance, in this substudy (Table S7). Third, because the participants comprise only East Asian patients, it is unknown whether the results can be extrapolated to other ethnicities and countries. In addition, we assessed left ventricular systolic function by LVEF, but even patients with “normal” LVEF might have impaired left ventricular systolic function.21 In addition, β‐blocker, RAS inhibitor, and MRA administration may have been altered, and other factors could be related to medication during the follow‐up period. Although we evaluated the therapeutic implications of GDMT including β‐blockers, RAS inhibitors, and MRAs, further studies are necessary to validate the prognostic value of sacubitril or valsartan in patients with HFiEF. Digoxin and loop diuretics have been prevalently prescribed to manage patients with AHF, but these patients did not show significant prognostic improvement (Figure S9). In addition, we defined de novo HF based on medical history of HF.22, 23, 24 Last, we did not perform core laboratory analysis of the echocardiographic measurement of LVEF.
This study also has specific strengths. The KorAHF registry is a well‐designed, nationwide, prospective cohort study in which every patient was scheduled to undergo echocardiography at baseline and 1 year after index admission and to be followed up for at least 5 years after index hospitalization. This design facilitates a definitive diagnosis of HFiEF, the identification of predictors, and the demonstration of its natural history; thanks to the prospective design and follow‐up schedule, the KorAHF registry could identify more patients with HFiEF than previously reported.3, 5, 25 Furthermore, we were also able to investigate the effect of GDMT in patients with HFiEF for the first time. Considering that LVEF improvement by GDMT was often observed between 6 and 12 months after the initiation of therapy,26, 27 echocardiographic assessment of LVEF at 1 year may be the appropriate timing for the detection of HFiEF. To minimize bias by indication, we performed several sensitivity analyses, and the protective relationship between β‐blocker use and clinical outcomes was consistent in the univariate, multivariate, PSM and IPTW analyses. Despite the strengths of this study, a randomized clinical trial is necessary to rigorously evaluate the effect of GDMT in patients with HFiEF.
Conclusions
HFiEF is a unique disease entity that has superior clinical outcomes. Younger age, de novo HF, nonischemic heart disease, and a β‐blocker prescription are independent predictors of HFiEF.
Sources of Funding
This work was supported by Research of Korea Centers for Disease Control and Prevention (2010‐E63003‐00, 2011‐E63002‐00, 2012‐E63005‐00, 2013‐E63003‐00, 2013‐E63003‐01, 2013‐E63003‐02, and 2016‐ER6303‐00) and by the Seoul National University Bundang Hospital Research Fund (grant no 14‐2015‐029, 16‐2017‐003).
Disclosures
None.
Supporting information
Table S1. Clinical Characteristics in Propensity Score–Matched Population
Table S2. Clinical Characteristics in Inverse Probability Treatment Weighted–Adjusted Population
Table S3. Clinical Characteristics According to Heart Failure Phenotypes at the Index Admission
Table S4. In‐Hospital Treatment During Index Hospitalization According to Heart Failure Phenotypes
Table S5. Clinical Characteristics of Patients With Heart Failure with Improved Ejection Fraction (HFiEF) According to 4‐Year All‐Cause Mortality From HFiEF Diagnosis
Table S6. Baseline Characteristics According to β‐Blocker Medication at the Diagnosis of Heart Failure with Improved Ejection Fraction
Table S7 Clinical Characteristics of Patients With Heart Failure with Reduced Ejection Fraction According to Presence of 1‐Year Follow‐up Echocardiography
Figure S1. Clinical outcomes according to heart failure phenotypes. HFiEF indicates heart failure with improved ejection fraction; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Figure S2. β‐Blockers in heart failure with improved ejection fraction after adjustment.
Figure S3. β‐Blockers in heart failure with improved ejection fraction according to dose and duration.
Figure S4. Association between the 4‐year all‐cause mortality and β‐blocker use in the subgroups of patients with heart failure with improved ejection fraction.
Figure S5. β‐Blockers in heart failure with improved ejection fraction according to rhythm.
Figure S6. Outcomes according to onset of heart failure.
Figure S7. Drug efficacy in de novo heart failure with improved ejection fraction.
Figure S8. Drug efficacy in acute decompensated heart failure with improved ejection fraction.
Figure S9. Impact of digoxin and loop diuretics on 4‐year mortality in patients with heart failure with improved ejection fraction.
(J Am Heart Assoc. 2019;8:e011077 DOI: 10.1161/JAHA.118.011077.)
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Hogg K, McMurray J. Neurohumoral pathways in heart failure with preserved systolic function. Prog Cardiovasc Dis. 2005;47:357–366. [DOI] [PubMed] [Google Scholar]
- 3. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 5. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 9. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 10. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 11. Lund LH, Benson L, Dahlstrom U, Edner M, Friberg L. Association between use of beta‐blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–2018. [DOI] [PubMed] [Google Scholar]
- 12. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 13. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, Kim MS, Kim JJ, Hwang KK, Chae SC, Seo SM, Baek SH, Kang SM, Oh IY, Choi DJ, Yoo BS, Ahn Y, Park HY, Cho MC, Oh BH. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. [DOI] [PubMed] [Google Scholar]
- 15. Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, Jeon ES, Kim MS, Kim JJ, Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Park HY, Cho MC, Oh BH. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017;47:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 17. Kim MS, Lee JH, Kim EJ, Park DG, Park SJ, Park JJ, Shin MS, Yoo BS, Youn JC, Lee SE, Ihm SH, Jang SY, Jo SH, Cho JY, Cho HJ, Choi S, Choi JO, Han SW, Hwang KK, Jeon ES, Cho MC, Chae SC, Choi DJ. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Groote P, Fertin M, Duva Pentiah A, Goeminne C, Lamblin N, Bauters C. Long‐term functional and clinical follow‐up of patients with heart failure with recovered left ventricular ejection fraction after beta‐blocker therapy. Circ Heart Fail. 2014;7:434–439. [DOI] [PubMed] [Google Scholar]
- 19. Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta‐blocker therapy. Circulation. 2003;108:1945–1953. [DOI] [PubMed] [Google Scholar]
- 20. Schleman KA, Lindenfeld JA, Lowes BD, Bristow MR, Ferguson D, Wolfel EE, Abraham WT, Zisman LS. Predicting response to carvedilol for the treatment of heart failure: a multivariate retrospective analysis. J Card Fail. 2001;7:4–12. [DOI] [PubMed] [Google Scholar]
- 21. Merken J, Brunner‐La Rocca HP, Weerts J, Verdonschot J, Hazebroek M, Schummers G, Schreckenberg M, Lumens J, Heymans S, Knackstedt C. Heart failure with recovered ejection fraction. J Am Coll Cardiol. 2018;72:1557–1558. [DOI] [PubMed] [Google Scholar]
- 22. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 23. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L; EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology . EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 24. Sato N, Kajimoto K, Asai K, Mizuno M, Minami Y, Nagashima M, Murai K, Muanakata R, Yumino D, Meguro T, Kawana M, Nejima J, Satoh T, Mizuno K, Tanaka K, Kasanuki H, Takano T; ATTEND Investigators . Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 2010;159:949–955. [DOI] [PubMed] [Google Scholar]
- 25. Givertz MM, Mann DL. Epidemiology and natural history of recovery of left ventricular function in recent onset dilated cardiomyopathies. Curr Heart Fail Rep. 2013;10:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia‐New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol. 1997;29:1060–1066. [DOI] [PubMed] [Google Scholar]
- 27. Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long‐term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–2581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Characteristics in Propensity Score–Matched Population
Table S2. Clinical Characteristics in Inverse Probability Treatment Weighted–Adjusted Population
Table S3. Clinical Characteristics According to Heart Failure Phenotypes at the Index Admission
Table S4. In‐Hospital Treatment During Index Hospitalization According to Heart Failure Phenotypes
Table S5. Clinical Characteristics of Patients With Heart Failure with Improved Ejection Fraction (HFiEF) According to 4‐Year All‐Cause Mortality From HFiEF Diagnosis
Table S6. Baseline Characteristics According to β‐Blocker Medication at the Diagnosis of Heart Failure with Improved Ejection Fraction
Table S7 Clinical Characteristics of Patients With Heart Failure with Reduced Ejection Fraction According to Presence of 1‐Year Follow‐up Echocardiography
Figure S1. Clinical outcomes according to heart failure phenotypes. HFiEF indicates heart failure with improved ejection fraction; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Figure S2. β‐Blockers in heart failure with improved ejection fraction after adjustment.
Figure S3. β‐Blockers in heart failure with improved ejection fraction according to dose and duration.
Figure S4. Association between the 4‐year all‐cause mortality and β‐blocker use in the subgroups of patients with heart failure with improved ejection fraction.
Figure S5. β‐Blockers in heart failure with improved ejection fraction according to rhythm.
Figure S6. Outcomes according to onset of heart failure.
Figure S7. Drug efficacy in de novo heart failure with improved ejection fraction.
Figure S8. Drug efficacy in acute decompensated heart failure with improved ejection fraction.
Figure S9. Impact of digoxin and loop diuretics on 4‐year mortality in patients with heart failure with improved ejection fraction.


