Abstract
Fecal microbiota transplantation (FMT) is becoming part of the treatment algorithms against recurrent Clostridium difficile infection (rCDI) both in adult and pediatric gastroenterology practice. With our increasing recognition of the critical role the microbiome plays in human health and disease, FMT is also being considered as a potential therapy for other disorders, including inflammatory bowel disease (Crohn disease, ulcerative colitis), graft versus host disease, neuropsychiatric diseases, and metabolic syndrome. Controlled trials with FMT for rCDI have not been performed in children, and numerous clinical and regulatory considerations have to be considered when using this untraditional therapy. This report is intended to provide guidance for FMT in the treatment of rCDI in pediatric patients.
Keywords: child, Clostridium difficile, fecal, fecal transplantation, microbiome, microbiota, pediatric
Key observations that the gut microbiome may play a role in health and disease provide a strong basis for developing strategies aimed at gut microbiota normalization. Among others, these strategies include fecal microbiota transplantation (FMT). Despite its increasing application, agreement as to how to define FMT legally or scientifically is lacking. In 2017, a group of experts proposed a definition for microbiota transplantation, which, in addition to fecal, includes vaginal, skin, oral, and nasal microbiota transplantation. Based on considerations that are beyond the scope of this document, microbiota transplantation was defined as “a transfer of biologic material containing a minimally manipulated community of microorganisms from a human donor to a human recipient (including autologous use) with the intent of affecting the microbiota of the recipient” (1). In the case of FMT, fecal material is used. Throughout this manuscript, the term FMT is used. Other terms such as “fecal microbiota transplant,” “stool transplantation or transfer,” “microbial reconstitution therapy (MRT),” or “intestinal microbiota transplant” (IMT) are, however, used interchangeably throughout the literature.
FMT is increasingly being used in the management of recurrent Clostridium difficile infection (rCDI) in adults, with cure rates approaching 90% (2). Although recurrent or severe CDI is increasingly problematic in children, data on the use of FMT in children are scarce. We also recognize that the nomenclature for C difficile is in the process of being modified to Clostridioides difficile (3) but we use the old name of the bacterium in this work because it is still its designation in Bergey’s Manual of Systematic Bacteriology.
This position paper was developed through the collaborative efforts of NASPGHAN and ESPGHAN. The NASPGHAN Clinical Care and Quality Committee approved the outline of this manuscript, and the NASPGHAN and ESPGHAN Society Councils approved the list of authors. In addition to pediatric gastroenterology and infectious disease experts, it included members with an interest in gut microbiota and its applications. The purpose of this position paper is to summarize current evidence and challenges related to the management of pediatric CDI with a focus on FMT as a treatment modality. To identify relevant data, searches of PubMed/MEDLINE databases were performed using terms such as C difficile, children, pediatric, recurrent fecal transplant, refractory fecal transplant, microbiota, microbiome, and microbial. A further aim of this report is to summarize current evidence on FMT in the pediatric population, including its rationale, current recommendations, efficacy, safety, and suggested protocols. For this, searches of PubMed/MEDLINE were performed using terms such as FMT, fecal transplant, faecal transplant, microbiome, and FMT. All searches were performed through December 2017. The PubMed/MEDLINE database also was searched for evidence-based clinical practice guidelines developed by scientific societies related to CDI and FMT.
A draft of this position paper was sent to all members of the NASPGHAN FMT Special Interest Group for review and further comments. All critical feedback were considered and changes were incorporated as necessary. A reviewer grading method was not implemented due to the limited quantity and quality of pediatric data. The conclusions of this document may require revision in the future as new evidence becomes available. For example, currently, fecal material manipulations range from the least manipulated sample of fresh stool transferred from an individual donor to the most manipulated, cultured, bacterial cocktail delivered in oral pill form (1). New stool-based products/procedures are, however, being developed; thus, further progress in fecal material used in FMT is likely. Similarly, new indications for the use of FMT are being studied. An updated revision of this document is planned within 5 years.
C DIFFICILE AND C DIFFICILE INFECTION IN CHILDREN
Epidemiology
C difficile is a spore-forming Gram-positive anaerobe and the most common infectious cause of antibiotic-associated diarrhea (4,5). C difficile pathogenesis is related primarily to the production of toxins. The ability of C difficile to produce resistant spores allows the bacterium to persist in the environment, which enhances transmission (6). Previously identified as a pathogen of significant public health concern in adults, its increasing incidence has been more recently described in children. A large multicenter study of hospitalized patients at 22 children’s hospitals in the United States demonstrated a near doubling in the incidence of CDI between 2001 and 2006 (7). More recent studies from Europe, however, showed stable incidence rates of pediatric CDI in hospitalized patients over 6 years (8). Although classically identified as a healthcare-associated infection, the rate of community-associated CDI has also increased in pediatric patients with 70% to 80% of pediatric cases of CDI identified as community associated (9,10). In fact, in contrast to adults who have a predominance of healthcare-associated infections, community-associated CDI is 3-fold more common than healthcare-associated CDI in children (11).
Risk Factors for Pediatric C difficile Infection and C difficile Infection in Special Populations
The risk factors for pediatric rCDI are slightly different than in adults and include prior antibiotic use, recent surgery, malignancy, solid organ transplantation, presence of a tracheostomy or gastrostomy tube, acid suppression, and concomitant use of non-CDI antibiotics during CDI treatment (12–16). In a large pediatric database including more than 4000 pediatric patients with a diagnosis of CDI, at least 2 of 3 had ≥1 complex chronic condition (17). Adults and children with inflammatory bowel disease (IBD), for example, have rates of CDI that far exceed those seen in the general population (18,19). A statewide database of hospital discharges from 2009 to 2012 demonstrated a prevalence of CDI in children with IBD to be 46 per 1000 versus 4.1 per 1000 in children without IBD (P<0.001) (20). In addition, 25% of pediatric CDI cases occur in children with cancer (21). Cancer has also been demonstrated to be a risk factor for CDI in adults (22). In one study, children with malignancy who developed CDI had longer hospital stays and greater all-cause mortality (relative risk 2.29; 95% confidence interval [CI] 1.47–3.57) compared with children with cancer who did not develop CDI (21).
The increasing rate of community-acquired CDI in children, and increased rates in children with IBD and cancer, has led to a shift in our understanding of the epidemiology of CDI. The previously held view that CDI was a condition that primarily affected adults and hospitalized patients has been replaced with an understanding that nonhospitalized children and children with IBD, cancer, or other risk factors who present with diarrheal illness should be tested for the pathogen.
C difficile Infection Versus Asymptomatic Carriage
The differentiation of CDI from asymptomatic C difficile carriage remains an ongoing challenge for clinicians. Limitations of the currently available C difficile diagnostic tests and aspects unique to pediatric populations must be taken into account when considering the diagnosis of pediatric CDI:
-
C difficile can be a commensal member of the microbiome during infancy and early childhood (23,24). In an animal model of CDI, infant rabbits have been observed to be resistant to C difficile toxin whereby the distal intestinal tract of the infant rabbit was proposed to lack toxin receptors (25). This mechanism of age-dependent expression of toxin receptors was not seen in a hamster model of CDI (26). Human clinical studies appear to be in line with the rabbit model observations, with questionable evidence supporting the ability of C difficile to act as a diarrheal pathogen in infants (27). Consequently, many pediatric infectious diseases and gastroenterology experts, adult CDI experts, and the American Academy of Pediatrics have all suggested that CDI should not be considered in children younger than 1 year and that caution should be exercised when diagnosing CDI in children 1 to 2 years of age (4,28–30). They have also emphasized the common occurrence of coinfections with other pathogenic bacteria in young pediatric patients with diarrhea who also test positive for C difficile. Thus, other infectious etiologies should be considered in children, especially those younger than 2 years (31).
Recurrent or refractory CDI can occur in children 2 years of age or younger.
Notably, the first 2 reported cases of FMT for pediatric rCDI were performed in a 16-month-old patient who developed the first CDI at 11 months of age and a 2-year old (32,33). Therefore, if other more likely infectious and noninfectious causes of diarrhea are not suspected or are ruled out, testing for C difficile may be warranted in children younger than 1 year as well. This is particularly true after previous treatment with antimicrobials and proton pump inhibitors (PPIs) (34).
Similar to adult including elderly patients (35,36), C difficile colonization, transient carriage, or “pass through” rates can be high in children. This is especially true in the healthcare setting (35,37). Children with comorbidities that confer high risk for CDI, including IBD (38), cystic fibrosis (CF) (34), or malignancy (39), also have high rates of asymptomatic carriage. Careful consideration of possible misdiagnosis should be made in a case-specific manner, regardless of the patient’s age. The presence of common CDI comorbidities does not necessarily establish the diagnosis of CDI. Notably, children with cancer and IBD are also at higher risk of asymptomatic carriage with C difficile. A single study demonstrated C difficile stool colonization in 29% of pediatric oncology patients without diarrhea and 55% of asymptomatic pediatric oncology patients with prior CDI (39), but the method of testing for colonization was not clearly enumerated. In addition, children with IBD had an asymptomatic C difficile colonization prevalence of 17% versus 3% of controls (P=0.012) (38). Although testing asymptomatic patients for CDI is not recommended, patients with IBD and cancer have a host of alternative causes for diarrhea. Equally challenging is the fact that the symptoms of CDI and a flare of IBD may be identical. In these patient populations, the presence of a positive test in the setting of diarrhea does not ensure a diagnosis of CDI. To date, this remains one of the largest clinical conundrums for providers.
-
The importance of careful clinical judgment is warranted in the case of a child with suspected CDI. A frequent source of misdiagnosis in possible CDI cases is paradoxical diarrhea in chronically constipated patients (40). This is especially relevant in the setting of central nervous system or neurologic compromise.
Another scenario to consider is toddler’s diarrhea in which concomitant colonization or transient carriage of C difficile can occur. Conversely, it is important to recognize that although rare, the lack of diarrhea in CDI cases can be an ominous sign of evolving toxic megacolon, especially in children with underlying intestinal disease such as CF (41). Similarly to patients with malignancy or IBD, high carriage rates of toxigenic C difficile in adult (42) and pediatric (43) patients with CF can significantly complicate clinical decision making in the setting of predisposing conditions. This has been emphasized in pediatric transplant (6) and oncology (39) patients as well. Again, the importance of careful clinical judgment is warranted in the case of a child with suspected CDI.
Response to C difficile–directed therapy may not be a proof of diagnostic accuracy in suspected CDI, because such treatments may provide symptomatic benefits in chronic constipation (44) or even other missed coinfections in otherwise colonized patients. Conversely, lack of response to CDI antibiotic treatment, particularly vancomycin (45), in a patient with suspected CDI should prompt consideration of other diarrheal etiologies.
C difficile Infection Morbidity and Disease Severity
Fortunately, significant morbidity associated with CDI is less common in children than adults. Severe CDI-related complications, including toxic megacolon, perforation, and the need for a surgical intervention, occurred in fewer than 2% of pediatric patients with CDI (46,47). Although significant morbidity is less common in children, rates of rCDI in pediatric patients mirror those in adults.
DIAGNOSIS OF C DIFFICILE INFECTION: UNIQUE CONSIDERATIONS
As already noted, accurate diagnosis of CDI is not an easy task due to many factors. It is generally recommended that only symptomatic children (defined as 3 or more loose or liquid stools in a 24-hour period (4,48)) older than 1 year be tested once other infectious etiologies of diarrhea are addressed. It should be noted that laboratory tests are only recommended for unformed stool samples (4). Because patients can continue to shed C difficile in their stool without symptoms for weeks to months after diarrhea resolution, and because this persistent shedding does not require additional antibiotic treatment, testing children for “cure”, “clearance” or “colonization” is not recommended (49).
A variety of different tests are available for identifying C difficile in stool, presenting a significant challenge to clinicians. Because these tests differ in their microbiologic targets, the clinical significance of a positive test varies among test types (50). Specifically, 2 primary categories of C difficile tests are available: tests that detect free toxin (ie, C difficile toxins A and B) in stool and tests that detect an organism with the potential to produce toxin in vivo (ie, a toxigenic strain of C difficile) (Table 1). Although we discuss general concepts about CDI diagnosis here, a detailed discussion of diagnostics is out of the scope of this manuscript. Thus, we refer the reader to other references for more detailed information about C difficile diagnostic tests (4,51).
TABLE 1.
Tests for Clostridium difficile infection
| Test | Sensitivity | Cost | Detection | Comment |
|---|---|---|---|---|
| NAAT | High | Medium to high | Toxin gene detection | Highly sensitive and specific for toxigenic C difficile; rapid turn-around-time |
| GDH | High | Low | Detection of common antigens in detection of toxigenic and nontoxigenic C difficile strains | Highly sensitive for C difficile but nonspecific for toxigenic/nontoxigenic strains; rapid turnaround time |
| EIA toxin A/B | Low | Low | Detection of free toxin | Highly specific for toxigenic C difficile but less sensitive than NAAT; rapid turnaround time |
| CCCNA or TC | High | High | Detection of free toxin and culture of a toxigenic C difficile strain, respectively | Significant labor requirements and long turnaround time; primarily limited to research use |
CCCNA = cell culture cytotoxicity neutralization assay; EIA = enzyme immunoassay; GDH = glutamate dehydrogenase; NAAT = nucleic acid amplification test; TC = toxigenic culture.
Cell culture cytotoxicity neutralization assays (CCCNAs; sometimes simply referred to as cytotoxicity assay) and toxin enzyme immunoassays (EIAs) both detect free toxin in stool, indicating a toxigenic strain of C difficile actively producing toxin in vivo at the time of stool collection. Because secreted toxins are responsible for symptoms, these assays are most specific for CDI. CCCNA is, however, time consuming and laborious. Toxin EIA is easy to perform but has questionable sensitivity. Notably, recent studies have suggested that a negative toxin EIA result may NOT accurately rule-out CDI (52,53). On the contrary, toxigenic culture (TC) and nucleic acid amplification tests (NAATs), such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification tests, identify organisms and toxin in the stool that have the potential to produce toxin, but do not determine whether or not the organism is actively producing toxin in vivo. Because toxigenic strains of C difficile can result in either carriage or symptomatic CDI, identifying a toxigenic strain in stool does not differentiate asymptomatic carriage and CDI when the presence of free toxin is not ascertained. Thus, compared to CCCNA and toxin EIA, toxigenic stool culture and NAATs have a lower diagnostic predictive value for CDI.
NAATs, such as PCR, are preferred by many medical centers because of their excellent sensitivity, but results are limited by their inability to differentiate asymptomatic carriage and CDI, particularly when used in patients with low likelihood of CDI and/or high likelihood of carriage. Thus, a positive TC or PCR test should be interpreted with caution. Leibowitz et al (37) reported that as many as 24% of hospitalized children can be positive for CDI by PCR even though asymptomatic, whereas 19% are positive and symptomatic. Interpretation of a positive PCR with such high prevalence of test positivity in asymptomatic patients presents a significant challenge to healthcare providers.
There remains no consensus on a single best test, but in most circumstances, testing for toxin is preferred as it has the greatest specificity for CDI and is less likely to be positive in asymptomatic carriers. Some authorities, such as the American College of Gastroenterology, recommend NAAT (testing for the presence of toxin A and/or B gene, and in some methods even for binary toxin gene) either as a stand-alone test or as part of an algorithm (54). The Infectious Disease Society of America 2017 guidelines suggest that when clinical symptoms are known, testing via NAAT alone may be adequate (4). Alternatively, the toxin assay sensitivity may be optimized with a 2-step testing strategy: glutamate dehydrogenase (highly sensitive for C difficile but does not distinguish toxigenic from nontoxigenic C difficile strains), and if positive, follow-up testing with NAAT as a confirmatory method (4). The European Society of Clinical Microbiology and Infectious Diseases adult guideline recommends 2-step testing: step 1: highly sensitive test such as GDH, or NAAT; step 2: highly specific test toxin A/B EIAs, whereas TC is just optional.
Table 1 lists some of the available testing methods for CDI. It is important to consider the risk factors for CDI, the presence of predisposing conditions such as IBD or cancer, and other potential causes of diarrhea when testing for rCDI. Fecal calprotectin (FC) may be a useful noninvasive marker to screen for IBD in children (with a negative likelihood ratio of 0.03) (55), because pediatric patients with rCDI without underlying chronic intestinal inflammation usually have a normal FC (56). Caution is, however, required in interpreting FC in the context of other potential infectious causes of diarrhea, as the test is sensitive but nonspecific for colitis and can be raised by common gastrointestinal (GI) pathogens (57).
TREATMENT APPROACH TO C DIFFICILE INFECTION/RECURRENT C DIFFICILE INFECTION
A diagnostic and treatment algorithm for CDI and rCDI in adults was recently outlined by Smits et al (58). Although nearly all adult patients will have resolution of symptoms with antibiotic therapy, approximately 20% to 30% of cases will have recurrence of symptoms within a few days to several weeks after cessation of antibiotics. Recurrence of CDI is likely related to persistent dysbiosis, either related to the broad antibiotic spectrum of metronidazole, vancomycin, or other concomitant non-CDI antibiotics; lack of immune response to C difficile toxins; and/or continued C difficile exposures (56). Although several probiotics, including Saccharomyces boulardii, have been studied in the treatment and prevention of rCDI, and preliminary evidence suggests some efficacy with regards to prevention of recurrence, randomized trials supporting these findings are lacking in children.
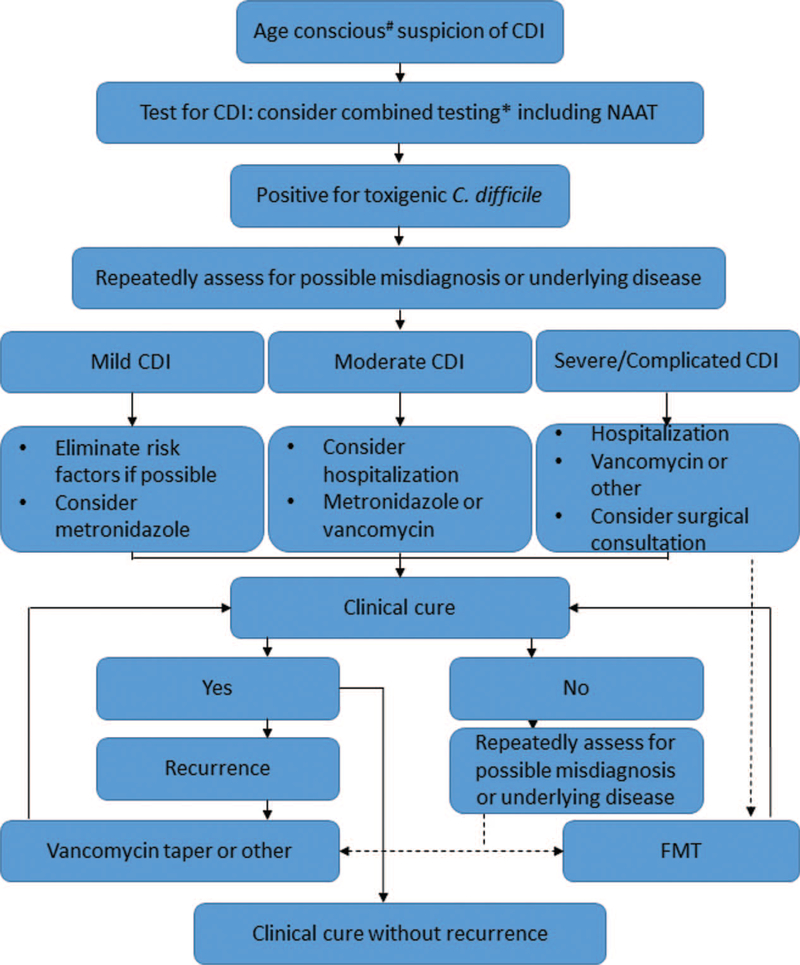
With such considerations, we recommend the diagnostic and treatment approach outlined in Figure 1. Evolving treatments that are either newly commercially available or in phase 2 or 3 clinical trials, including novel antimicrobials (59,60), immunologic agents (ie, monoclonal antibody against toxin B) (56,61), and biotherapeutics (56) (eg, FMT, encapsulated and/or suspensions of microbiota, nontoxigenic strains of C difficile) will be incorporated into the pediatric therapeutic algorithm according to evidence-based findings and pediatric-specific efficacy and safety data. Importantly, FMT, arguably the most effective treatment against rCDI, has become a standard in therapeutic algorithms (58,62). Despite the increasing use of FMT, concerns about this alternative treatment for CDI include unknown long-term consequences (63), especially in children (64). It should be emphasized that repeated and prolonged antibiotic exposures, especially in young children, may also have significant consequences later in life, such as increasing the risk of IBD (65,66) and obesity (67). Antibiotic treatments directed at CDI are not benign in this respect, because these can cause significant perturbation of the mammalian intestinal microbiome (68) and even lead to rCDI themselves (69).
FIGURE 1.
Recommended treatment algorithm for Clostridium difficile infection in pediatric patients. #Patient age should be a significant consideration prior to testing for toxigenic C difficile (see text). *See text for details of testing. Broken arrows indicate routes where fecal microbiota transplantation (FMT) should be considered as treatment. CDI = Clostridium difficile infection.
If testing is pursued, a positive result is obtained, and the test result can be reasonably attributed to CDI (rather than carriage), several treatment options can be considered. Current American Academy of Pediatrics Guidelines recommend oral metronidazole for first episode and first recurrence of mild or moderate CDI (48). Although vancomycin is recommended for second recurrence of mild or moderate CDI, it is reasonable to prescribe vancomycin for the first recurrence in children at high risk for multiple CDI recurrences. Vancomycin can also be considered as first-line agent for moderately ill hospitalized patients, especially with underlying clinical conditions such as IBD (70). Although CDI treatment recommendations are based on CDI severity and laboratory markers (4,71), so far no pediatric-specific systems or guidelines exist for classifying CDI based on severity of illness. Thus, adult CDI severity definitions are used in the pediatric population. Determination of severe and severe-complicated CDI in children using these adult CDI severity definitions is difficult because concomitant medical conditions and other medications can confound such categorization (72). In cases of severe or severe-complicated CDI, combined intravenous metronidazole and high-dose oral vancomycin should be considered. Other antibiotics, such as fidaxomicin (73), may be a feasible option for treatment, but are not yet the Food and Drug Administration (FDA) approved for children younger than 12 years. According to adult consensus guidelines, FMT can be considered a first-line therapy in those not responding to standard treatment for >48 hours or as a treatment for those with ≥3 infections including a 6- to 8-week tapering course of CDI-directed antibiotics (74). Consideration of FMT in pediatric cases of severe CDI has been described (75). In general, we concur with current adult guidelines (74) when considering FMT for the treatment of rCDI in children and propose FMT be considered in children with one of the following:
-
rCDI (recurrence of symptoms within 8 weeks of treatment for CDI) (either a or b)
At least 3 episodes of mild to moderate CDI and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic (eg, rifaximin, nitazoxanide).
At least 2 episodes of severe CDI resulting in hospitalization and associated with significant morbidity.
Moderate CDI not responding to standard therapy (including vancomycin) for at least 1 week. We recommend caution, however, in such cases, with repeated testing for etiologies other than CDI such as IBD.
Severe CDI or fulminant C difficile colitis with no response to standard therapy after 48 hours.
Ongoing research examining CDI and rCDI patterns in children and responses to different treatment regimens will help inform future guidelines regarding the use of FMT in pediatric populations.
FECAL MICROBIOTA TRANSPLANTATION FOR RECURRENT C DIFFICILE INFECTION
We recommend that FMT be performed in established centers with experience in FMT and treating rCDI and where long-term side effects of this procedure can be monitored. Although FMT is technically easy to perform, the following sections of the article will emphasize safety and regulatory considerations for this experimental treatment method. FMT should not be performed by unlicensed healthcare providers or family members. FMT should also not be performed outside of the hospital or medical clinic setting.
FMT originates from ancient Chinese medicine (76). The first reported application in modern times was for the treatment of severe refractory pseudomembranous colitis in 1958. Although Eiseman et al (77) described their experience and approach in 4 patients, their rational for using FMT was not reported. Nevertheless, FMT delivered rapid cure for 4 adult critically ill patients at a time when the infectious organism responsible for pseudomembranous colitis was not even recognized. Since then, and especially over the last 2 decades, retrospective and uncontrolled prospective cohort studies in adult and pediatric patients describe 83% to 100% cure rates by FMT for rCDI patients (78). The first open-label randomized controlled trial (RCT) comparing duodenal delivery of FMT with vancomycin therapy showed significant superiority of FMT (ie, 81% vs. 31% cure rates for FMT vs vancomycin, respectively; P=0.008) (79). More recent controlled trials, however, have provided variable results, which in part may be due to variations in study design, difficulty in differentiating patients with CDI or those with CDI carriage and diarrhea due to other causes, and the processing and delivery of the FMT. A phase 2/3 controlled trial compared 14 days of oral vancomycin followed by a single FMT by enema versus a 6-week course of oral vancomycin taper (“standard of care”) in adult patients experiencing acute recurrence of CDI (80). This trial was terminated due to futility after randomizing 30 patients, with 43.8% and 58.3% symptom resolution in the FMT versus vancomycin taper groups, respectively. The lack of efficacy of FMT compared to vancomycin in this trial was challenged by some authors, however, particularly because of the absence of bowel preparation and the long storage time of stools before FMT (up to 48 hours) (81). In another controlled trial examining adult patients with recurrent CDI following vancomycin taper, comparing FMT by colonoscopy to autologous FMT (patient’s own stool) as control (82), FMT had a significantly greater cure rate (90.9% vs 62.5%; P=0.042). In this latter trial, however, 1 of the 2 recruiting centers had a significantly larger placebo effect than the other (90% vs 42.9%; Fisher exact P=0.0333). A 2017 review of RCT comparing FMT with vancomycin for rCDI found that statistically, FMT was significantly more effective (relative risk=0.41, 95% CI: 0.22–0.74; number needed to treat = 3, 95% CI: 2–7) (81). The results from these controlled trials, however, underscore the need to standardize patient and donor selection, stool preparation, mode of delivery, placebo/control method, and length of follow-up to achieve as reliable a result as possible with respect to FMT as a treatment for rCDI. In addition, patient selection for FMT should take into account unique considerations (83). For example, age, sex, diet, the health, and the past medical history of the donors may be predictive of success rates.
All FMT studies above were performed in adults, and to date no controlled studies have been reported for pediatric rCDI and FMT (84). Small case series and isolated case reports, however, indicate therapeutic success for FMT in pediatric rCDI similar to adults. This has been observed regardless of the mode of delivery and even in immunocompromised children (6,32,33,84,85). Most recently the NASPGHAN Special Interest Group conducted data collection on pediatric patients with rCDI from multiple sites around the USA and reported that FMT was successful in 272 of 336 (81%) patients after a single delivery (Nicholson M et al. Unpublished data; 87). The cumulative success rate approached 90% when patients who received a second FMT were included in the analysis. In general, FMT was safe and well tolerated. Only 5.7% reported minor adverse events (AEs) such as bloating, diarrhea, and pain and even fewer (5%) reported severe AEs. In addition, the investigators found that this treatment was equally effective in children with and without underlying IBD (Nicholson M et al. Unpublished data; 87). The long-term impact of this treatment in these subjects remains unclear.
THE FECAL MICROBIOTA TRANSPLANTATION PROCEDURE: UNIQUE CONSIDERATIONS
Donor Screening and Unique Considerations
Potential FMT donors need to be appropriately screened and selected. However, data describing outcomes of FMT based on donor characteristics are lacking. Many of the published recommendations merely reflect expert opinion.
An understanding of the pathogenesis of CDI may help guide identification of the best potential FMT donors. CDI commonly occurs after antimicrobial therapy or environmental factors perturb the delicate balance of the gut microbiome (86–89). Evaluation of the microbiome before antibiotic treatment demonstrated a unique profile in individuals who developed CDI compared with individuals who did not develop the infection, suggesting premorbid colonization may predispose toward infection (90).
Decreased microbial diversity is associated with a high risk of CDI recurrence (91,92). CDI is associated with a microbiome rich in facultative anaerobes and deficient in Bifidobacteria and Bacteroides (93,94). In children, a microbiome rich in Ruminococcus gnavus and Klebsiella pneumoniae was permissive for C difficile colonization, and a microbiome abundant in Bifidobacteria was associated with colonization resistance (95) with low levels of this genus seen in infected children (96). Therefore, a donor microbiome rich in Bifidobacteria and Bacteroides would theoretically be ideal. Recent data, however, suggest that the mechanism underlying FMT’s success extends beyond bacteria and could also depend on viral/bacteriophage transfer and colonic bile acid composition (97–99). Such conclusions, however, should be made with caution, because bacteriophage composition inherently depends on the bacteriome.
Donor Characteristics
Age
In general, it has been suggested that donors should be restricted to adults older than 18 years for medicolegal considerations. It has previously been shown, however, that the gut microbial profile of adolescents and younger children is quite distinct from that of adults (100,101). Therefore, although short-term data on the gut microbiome in adult studies after FMT can be helpful, the gut microbiome of pediatric subjects may behave differently in response to transplant. Differences in the pediatric microbiome may affect its ability to tolerate or resist a transplanted microbiome and enable mucosal healing. Furthermore, both safety and efficacy take on a new meaning when applied to children with 20 to 30 additional life-years compared to adults. In a letter to the FDA, the Joint Society Consensus Recommendation (JSCR) (102) states that “children could also potentially serve as donors as long as both parental consent and child assent are obtained.” Further research on age-matched donors is warranted.
Sex
Some investigators have speculated that men may be preferred donors, as women may harbor a microbiome that may make them more susceptible to developing irritable bowel syndrome (IBS) (103). Currently no evidence, however, shows that donor sex can affect the outcome of FMT or that asymptomatic healthy women harbor a microbiome that predisposes them to developing IBS.
Health Screening and Questionnaires
There is a consensus among authorities that all donors should undergo health screening. The FDA regulation on screening continues to be in flux, and as such it is recommended that FMT providers routinely check the FDA for the most up-to-date guidance (http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm). Potential donors should undergo initial screening using a questionnaire analogous to those provided to blood donors (AABB Donor History Questionnaire Documents are available at http://www.aabb.org/tm/questionnaires/Pages/dhqaabb.aspx). Donors with responses to these questionnaires indicating a risk factor or illness that could potentially be transmitted by FMT should be excluded. It is also recommended that a follow-up questionnaire or other means of assessment be performed at the time of donation, to screen for interval change in inclusion or exclusion criteria (74,79,102,104,105). Other exclusion criteria related to conditions that may influence the gut microbiome have been suggested in prior screening recommendations, including neurologic, neuropsychiatric, metabolic, immune diseases, GI disorders, obesity, chronic PPI use, malignancy, and recent antibiotic use.
Screening Laboratory Tests
Recommended testing from the JSCR to the FDA (102) can be found in Table 2.
TABLE 2.
Recommended stool donor screening
| Serum testing* | Stool testing* |
|---|---|
| HAV-IgM | C difficile toxin B (preferably by PCR) |
| HbsAg | Culture for enteric pathogens |
| Anti-HCV-Ab | Ova and parasites, if travel history |
| HIV-EIA | |
| Rapid plasma reagin |
Additional testing may be indicated and should be considered accordingly.
Performed within 4 weeks of donation.
Other authorities have made additional recommendations for donor testing. A useful review on the topic was published in 2017 (106).
Universal Donors and Stool Banks
The FDA and the 2013 JSCR have suggested that fecal donors should be known to the recipient or treating physician. This draft guidance was, however, never approved, and many centers find it difficult to organize internal programs for stool donor selection, screening, and fecal processing given the time, logistics, and financial costs. Therefore, use of banked stool for FMT has become increasingly common in research and clinical practice settings.
In general, resorting to a universal donor may have some advantages, as families or close acquaintances of children with C difficile may have a higher risk of exposure and infection with C difficile and confidentiality (such as Health Insurance Portability and Accountability Act of 1996) concerns may exist during screening (107,108). Therefore, the donor gut microbiome may potentially be impacted by prior infection, colonization, or therapy that may render a suboptimal microbiome for transfer. Studies also suggest that universal donors may yield better outcomes than individual donors (109), although recent work does not support this notion (110). Universal donor FMT use is easier, faster, and more cost-effective. In addition, stool from banked donors may also be safer due to more comprehensive screening and biobanking of samples for any additional testing. For individuals with underlying IBD who require FMT, the use of a nonrelated donor or stool from a bank may be preferable due to the shared familial risk factors for IBD in related donors (111).
Recipient Preparation
Studies vary with regards to antibiotic use, cleanout, PPI use, and diet in preparation for FMT.
Antibiotic use before FMT: Some studies advocate treatment with metronidazole, oral vancomycin, or fidaxomicin for at least 3 days before FMT. Most recommendations, however, conclude that antibiotics should be stopped 12 to 48 hours before FMT. This appears to be a strong recommendation that is supported by several published studies (74,79,112,113). It is also important to note that no studies directly compare antibiotic versus no antibiotic preconditioning on the efficacy of FMT in eradicating CDI. Additional questions remain regarding which antibiotic is superior, how many days are needed for optimal effect of antibiotics, and when to discontinue relative to FMT.
Bowel lavage: Evidence in the literature suggests good outcomes with (79) and without lavage in adult patients (114). Retrospective pediatric data from a multicenter collaboration suggested improved outcomes in the patients who had undergone a cleanout before FMT (115).
Acid suppression: Some studies advocate for the use of PPIs and other antacids especially with the use of upper GI delivery of FMT (85). No studies compare acid suppression versus nonacid suppression. Caution is advised with PPI use because it has been recognized as a risk factor for rCDI, albeit with chronic and not incidental use (15,16,34,116).
Fecal Microbiota Transplantation Preparation and Delivery Variation
Because of the lack of evidence, FMT preparation is more of an art than a science. Donor stool should be prepared to a consistency that allows for easy infusion via enema, a biopsy channel, gastrostomy tube, jejuenostomy tube, nasogastric (NG), nasoduodenal (ND), or nasojejunal (NJ) tube. Published reports have described mixing varying amounts of stool with either saline or water, mixing the stool in solution with a spoon, blender or similar device, and filtering the solution though gauze pads, sieves, or coffee filters. Recommended volumes range from 50 to 100 g of stool diluted in 300 to 700 mL of solution. The typical volume recommended for upper GI tract delivery is 30 to 100 mL (79,113,117,118), with smaller volumes recommended in children.
Environmental conditions during FMT preparation may affect microbiota composition and subsequent efficacy of the FMT. Recent data suggest that exposure to oxygen during the fecal homogenization process alters the composition of the fecal microbiota and may affect efficacy as well (119). It is important to recognize that a stool sample is a live microbial ecosystem and not biologically static. Environmental changes may rapidly and significantly influence the composition and viability of the donor stool microbiome.
Because of potential aspiration risk, practitioners may consider keeping their patients nil per os for 1 to 2 hours before FMT delivery via NG/NJ. One case of aspiration during delivery of FMT via endoscope into the duodenum resulted in the death of an 80-year-old patient with rCDI (120). An additional reported case of aspiration leading to death occurred during sedation for the FMT, before any fecal material was delivered to the patient (82).
If there is concern for an underlying inflammatory condition, practitioners should consider FMT via colonoscopy, as it allows for visualization of colonic mucosa and the concurrent diagnosis of concomitant diseases via tissue biopsy. A meta-analysis including both adult and pediatric patients has not shown any significant differences in outcomes when comparing FMT for rCDI via colonoscopy versus NG tube (121). In a recent multicenter review, FMT via colonoscopy was, however, found to be significantly more effective than FMT delivered via other routes in pediatric patients (odds ratio 2.6, 95% CI [1.22, 4.48]) (115).
In adult patients, FMT via enema has a similar success rate to colonoscopic delivery (113,122). Adequate patient preparation and compliance is often necessary for successful delivery in the pediatric population. No clinical trials, even in adults, have, however, studied the duration of time necessary for stool retention when FMT is delivered via either colonoscopy or enema to reach the most beneficial effect. Some centers use Foley catheter balloons in the rectum or antimotility agents such as loperamide to increase retention time post-FMT. Specific studies or clinical data on the utility of these practices are lacking.
FMT via capsules appears to be as efficacious as other means of delivery. In a prospective study of 180 patients, aged 7 to 95 years, 82% achieved cure after a single course of 15 capsules over 2 days, and 91% of patients were cured after a second treatment course. The primary side effects were headache, abdominal pain, and nausea (123). Capsule FMT should be considered in patients who can accept and tolerate that route of delivery and where colonoscopic evaluation at the time of treatment is unwarranted. Unfortunately, due to the number and size of the capsule required for FMT, it is particularly challenging or currently not feasible in younger children.
A randomized trial of fresh versus frozen stool used for FMT showed no significant difference in the cure rates (113). Therefore, based on current knowledge, frozen-thawed fecal preparations can be used with similar success as freshly prepared stool.
Recent clinical observations have indicated that fecal matter devoid of live bacteria may be used successfully in treating rCDI (98). In addition, defined live bacterial and spore combinations to replace whole stool for FMT are being actively tested in clinical trials with variable success (124). Advancement in this field is ongoing. Most experts believe that FMT in its current form simply represents our first incarnation of microbial therapeutics.
Fecal Microbiota Transplantation Follow-up
Patients who have a successful FMT typically have resolution of symptoms within 2 to 3 days. Instructions should be given to families to monitor for signs of serious AEs, including but not limited to fevers, severe abdominal pain, and vomiting. A follow-up phone call is recommended within 1 week after the procedure to confirm resolution (lack of liquid or loose stools) and to monitor for AEs. Although recommendations vary on specific timelines, FMT is generally considered successful if symptoms of CDI do not recur within 2 to 3 months postprocedure (33,121).
If symptoms recur shortly after the initial FMT, the procedure can be repeated. The need for repeated patient and donor screening before repeat FMT is patient- and site-specific and should be determined by the treating provider/team. Although there is no evidence to support these recommendations, the authors recommend the following:
Phone follow-up by physician or nurse approximately 1 week after FMT to document any AEs and response to FMT.
Follow-up by a pediatric gastroenterologist within 2 to 3 months of FMT to document clinical cure and any AEs. Testing for C difficile in asymptomatic patients is NOT recommended following FMT.
An additional follow-up visit at 1-year post-FMT may be considered to assess for potential long-term AEs. These side effects may include, but are not limited to, fluctuations in weight, development of metabolic disease, and worsening course of IBD or other underlying disease. Monitoring of late AEs and long-term effects of FMT is important and another one reason why the authors recommend performing FMT in established centers where long-term monitoring systems and safety registries are in place. Long-term multicenter follow-up studies, which are currently in development, will help elucidate these potential complications.
Family members should be advised to contact the provider post-FMT with any potential AEs.
REVIEW OF FECAL MICROBIOTA TRANSPLANTATION SAFETY
In addition to its overall safety in the general population, FMT has been shown to be relatively safe in immunocompromised adult patients, including solid organ transplant recipients and patients with IBD (82,125,126). In a recent multicenter pediatric FMT review analyzing the safety of FMT in 336 patients, the overall occurrence of serious AEs was only 5%. The most serious complications involved aspiration pneumonia following upper GI delivery of FMT (in 1 patient) and worsening IBD symptoms requiring hospitalization following FMT (115). No death has been reported following FMT in pediatric patients.
Reported serious AEs in adults only have been related to aspiration occurring during colonoscopy for FMT delivery and aspiration of fecal content after ND tube delivery (120). A case of post-FMT colitis leading to death (127) occurred in a 68 year-old man who developed pneumoperitoneum and sepsis within 3 days of FMT. Clear causality, however, is difficult to establish based on the case description. A recent pediatric case series (42 patients, 47 FMTs, median age of 9 years) utilizing a nurse-led intragastric FMT procedure only reported vomiting as postprocedural complication (13%) (118). In all cases, the vomiting was a single, self-limited episode that did not require medical treatment.
Common reported side effects of FMT include bloating, diarrhea, abdominal pain, constipation, and transient fever. A systematic review of FMT case reports documented a 0.6% incidence of worsening IBD symptoms following FMT (128), whereas a more recent study suggested worsening symptoms in 13% of adult patients with IBD post-FMT (129). Rates of flare in pediatric IBD patients may be lower than in adults, as was noted in the multicenter pediatric FMT cohort. Subsequent bacterial and viral infections have been reported as well, although causality is difficult to establish (130,131). Additional case reports describe medical conditions that developed post-FMT and include idiopathic thrombocytopenic purpura, Sjogren syndrome, peripheral neuropathy, and rheumatoid arthritis (117), but clear causation has not been established.
Long-term safety of FMT still needs to be established, as the long-term impact of microbiome manipulation is unknown. For example, FMT may modulate the propensity to develop obesity and metabolic syndrome. Alang and Kelly reported a case of significant increase in BMI in a 32-year-old woman following FMT where her 16-year-old daughter with elevated BMI was the donor (132). The case was complicated by clearance of Helicobacter pylori infection, which is also associated with weight gain.
Interestingly, in 2012, Vrieze et al (133) found that FMT from lean donors delivered via ND tube increased insulin sensitivity in males with metabolic syndrome, suggesting that FMT could be used to treat metabolic disease. A follow-up controlled study on FMT from lean donors into obese individuals showed a transient improvement in insulin sensitivity in those recipients who had lower microbiome diversity at baseline (responders) (118).
Again, we recommend performing FMT in established centers where long-term side effects can be monitored. Long-term prospective multicenter follow-up studies, which are ongoing, will help elucidate these and other potential complications.
CURRENT REGULATIONS AND REGULATORY RECOMMENDATIONS/CONSIDERATIONS
In the United Sates, the FDA has placed the study and use of FMT under the guidance of the Center for Biologics Evaluation and Research (CBER). This is the same section that monitors vaccines, blood products, and gene therapy. As such, they view FMT as both a biologic and as a drug with the applicable regulations in respect to procedures and monitoring. Following a workshop at the FDA in May 2013, the FDA published a Guidance for Industry in 2016 (http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm) that stated that they will “exercise enforcement discretion” with regard to FMT for rCDI not responding to standard therapies. Practically, this means that for now, they will not mandate applications for an investigational new drug (IND) for performing FMT for rCDI. Minimal guidance was provided, but included the requirement for informed consent, which states that the procedure is “investigational.”
Per FDA guidelines, an IND must be obtained for all clinical uses of FMT other than rCDI and for research purposes. For immediate non-rCDI needs, emergency and single-patient INDs, providers should use the resources on the FDA Web site (https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm597130.htm) or contact the FDA directly for guidance.
In Canada, Health Canada (HC) oversees the use of FMT through its Biologics and Genetic Therapies Directorate. HC considers FMT to be an investigational new biologic drug that must be studied under an authorized clinical trial. Following a 6-month consultation period with key stakeholders, a revised Guidance Document was, however, published in August 2016 (https://www.canada.ca/en/healthcanada/services/drugs-health-products/biologics-radiopharmaceuticals-genetictherapies/applicationssubmissions/guidance-documents/regulation-fecal-microbiotatherapy-treatment-difficile-infections.html) that allows greater access to FMT for the treatment of CDI in recognition of the “very encouraging” research conducted to date. HC recognizes rCDI as the only indication for which FMT has demonstrated safety and efficacy, and offers an exception to the standard regulatory provisions of an investigational clinical trial. Health care practitioners are permitted to treat patients suffering from CDI not responsive to conventional therapies with FMT, provided the following conditions are met:
Informed consent is obtained from the patient recipient.
Donor feces are obtained from a single donor only, who is known to the patient or health care practitioner.
Donors are screened for relevant transmissible diseases, as suggested in the Guidance Document.
Records are kept of both the donor and recipient to facilitate a trace-back program in case of disease transmission.
Further suggestions include ensuring the donor is healthy by using a health history/lifestyle screening questionnaire and considering a rationalized periodic retesting protocol of the donor. Health care practitioners were also advised to submit a “Notification of Fecal Microbiota Therapy Used in the Treatment of C difficile
Infection Not Responsive to Conventional Therapies Form” (https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhpmps/alt_formats/pdf/brgtherap/applic-demande/guides/notification-declaration-eng.pdf) to facilitate communication by the Biologics and Genetic Therapies Directorate if necessary. This notification can be filed at any time and does not require advanced HC approval before providing FMT treatment for CDI.
For the time being, the European Medicines Agency has not yet regulated FMT in a standardized fashion for the European Union (134). At the “Tissues and Cells Competent Authorities” meeting in June 2014, the Commission concluded that for the purposes of FMT, feces as a “combined substance” cannot be regulated under the European Union Tissues and Cells Directive. Nevertheless, a legal mandate exists for the potential future regulation of FMT. Consequently, one has to adhere to regulations of their own National Authority in respect to FMT. For instance, in France, the National Agency for the Safety of Medicine and Health Products states that FMT is considered a drug and released guidelines for the use of FMT in clinical trials (http://ansm.sante.fr/S-informer/Points-dinformation-Points-d-information/La-transplantation-de-microbiote-fecal-et-sonencadrement-dans-les-essais-cliniques-Point-d-Information2). In addition, the French Group of Fecal Transplantation published guidelines for FMT in rCDI (135). In other countries including the United Kingdom, Belgium, and the Netherlands, FMT is considered as a tissue, whereas in many other countries no regulation for FMT exists at all.
A European consensus recommends implementation of FMT centers for the treatment of CDI and outlines guidelines of technical, regulatory, administrative, and laboratory requirements (134). Knowledge and experience resulting from using FMT to treat CDI needs to be centralized to increase the possibility of translating the experience into scientific information, technical expertise, and knowledge dissemination to other research areas, with the ultimate goal of understanding the role that FMT may play in other clinical conditions (134).
FECAL MICROBIOTA TRANSPLANTATION BEYOND RECURRENT CLOSTRIDIUM DIFFICILE INFECTION
The success of FMT in rCDI within the context of an exploding interest in the gut microbiome across human diseases has prompted consideration of its clinical utility in many other conditions. It is, however, beyond the scope of this position paper to provide a detailed overview of FMT as a potential therapeutic intervention in disorders other than rCDI. Current data on FMT for IBD and other conditions are insufficient to make general clinical recommendations for children. Importantly, existing knowledge about FMT from rCDI studies is not transferable to other conditions, and each will require consideration of particular key aspects before the therapy is optimized for use (Supplementary Table 1 Supplemental Digital Content 1, http://links.lww.com/MPG/B527). These can be considered on a broad sense as similar to those seen in bone marrow transplantation in terms of their potential complexity. Here we provide a brief, nonexhaustive summary of diseases in which FMT is being considered as a therapeutic modality. Supplementary Table 2 (Supplemental Digital Content 1, http://links.lww.com/MPG/B527) provides an overview of active trials in children.
The human microbiome has probably been most studied in IBD, so it is of little surprise that several trials on FMT in IBD have been reported. These fit within a growing area of microbial therapeutics in IBD that may change the landscape of treatment for these chronic conditions (134). The vast majority of FMT studies in IBD are uncontrolled observational cohort studies or case series, summarized in a systemic review by Paramsothy et al in 2017 (134). Several studies have been published to date using FMT for ulcerative colitis (UC): 3 RCTs and 1 trial published in abstract form (136–139). A recent systematic review pooled these results to demonstrate an efficacy of achieving clinical remission in 28% by FMT against 9% by placebo (140), suggesting moderate efficacy. Methodology and results, however, varied considerably among trials, and much work remains to be done before FMT in UC becomes a recommended therapy. Interestingly, a microbial signal of success linked to particular Clostridium clusters associated with butyrate production has started to emerge from these early RCTs, reigniting an interest in butyrate in UC first proposed as important in disease pathophysiology approximately 40 years ago (140). Pouchitis is a microbially driven disease seen in a physiologically abnormal postsurgical state, and is probably the variant of IBD most amenable to antibiotic and probiotic therapies. FMT is therefore of considerable interest as a therapeutic option (141,142); however, to date only case series have been published and have conflicting results (143,144). Two adult pouchitis studies are actively recruiting in the USA (ClinicalTrials.gov Identifiers: NCT02782325 and NCT02049502) but currently active pediatric studies are lacking. In Crohn’s disease no RCTs investigating FMT have been published and therefore, the role of FMT remains uncertain. Nonetheless, several pediatric studies of FMT in IBD are currently ongoing (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/MPG/B527).
Functional GI diseases, particularly IBS and chronic idiopathic constipation, are being actively explored as therapeutic avenues for FMT. A double-blind placebo-controlled RCT was recently published of single colonoscopic FMT versus placebo in 83 adults with IBS (by intention-to-treat), demonstrating 65% versus 43% clinical response at 3 months (P=0.049) (145). A placebo-controlled RCT of NJ tube FMT in 60 adults with slow transit constipation suggested a cure rate of 37% versus 13% (P=0.04) and a clinical improvement rate in 53% versus 20% (P=0.009) with 12 weeks follow-up (1,146). The same authors recently reported loss of efficacy with time, although 33% were still meeting the primary endpoint of 3 spontaneous bowel movements per week by 24 weeks (2,147). Further studies are clearly needed. Three studies are listed as actively recruiting on ClinicalTrials.gov for IBS (NCT03125564, NCT02651740, NCT02847481) and 3 for constipation (NCT03018613, NCT02676388, NCT02526849), but none include children (as of December 2017).
No trial evaluating FMT as a treatment option for Primary Sclerosing Cholangitis has been published, but 1 study is ongoing in adults (ClinicalTrials.gov Identifier: NCT02424175).
In allogeneic hematopoietic stem cell transplantation, graft-versus-host disease–related mortality is closely associated with use of broad-spectrum antibiotics and loss of microbial diversity, in particular anaerobic Clostridiales (148,149). Early results from case series on FMT after allogeneic hematopoietic stem cell transplantation show promising clinical response in patients with treatment-refractory graft-versus-host disease including complete resolution of all GI symptoms in few individuals (150–152).
As mentioned above, in adult patients with metabolic syndrome, FMT from lean individuals resulted in improved glucose metabolism after 6 weeks, but this effect was only transient and at 12 weeks no significant effect on insulin resistance was observed (133,153).
One area of potential interest that is emerging in the FMT field is the possibility of using FMT to drive out colonization with potentially harmful multidrug-resistant organisms (MDRO), presumably by direct ecological competition. Although no RCTs exist for this application, 1 adult case report demonstrated a reduction from 12 MDRO culture isolates to 4 in a single-patient post-FMT for C difficile (154). Other case reports and a case series have explored this phenomenon since (155–157). It is likely that data on MDRO alterations will emerge as a secondary outcome in FMT trials for other indications, but with increasing antibiotic resistant rates worldwide, novel strategies will gain increasing importance.
Autism has been examined as a potential disorder where FMT may provide benefits (158), although this treatment modality has not been tested in an RCT to date. Taking the gut-microbiome axis into consideration, FMT may be studied for the treatment of various central nervous system disorders.
For conditions other than rCDI, the use of FMT is not yet evidence based, and FMT should be performed within the research setting only.
FUTURE STEPS/KNOWLEDGE GAPS
FMT is still in its infancy, although its investigational and clinical applications are expanding rapidly. Numerous knowledge gaps exist, and collaborative research to advance the field, particularly in pediatrics, is much needed. Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/MPG/B527) lists a number of these tasks. As our FMT knowledge matures, we hope to advance our understanding of the role of the microbiome in health and disease. In addition, we will use this knowledge to move away from the shot-gun approach of using whole stool FMT to develop more targeted, refined, safe, and effective microbial therapeutics that will have the potential to treat a broader range of pediatric diseases.
ESPGHAN DISCLAIMER
ESPGHAN is not responsible for the practices of physicians and provides guidelines and position papers as indicators of best practice only. Diagnosis and treatment is at the discretion of physicians.
NASPGHAN DISCLAIMER
The NASPGHAN practice guidelines are evidence-based decision-making tools for managing health conditions. Practice Guidelines include Clinical Practice Guidelines, clinical reports, technical reports, and position statements. They are authorized by the NASPGHAN Executive Council, peer reviewed, and periodically updated.
They are not to be construed as standards of care and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. All decisions regarding the care of a patient should be made by the health care team, patient, and family in consideration of all aspects of the individual patient’s specific medical circumstances.
Although NASPGHAN makes every effort to present accurate and reliable information, these guidelines are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with the guidelines or reliance on the information presented.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the guidance and support of Dr Andrew Grossman in developing, drafting, and revising this article. The authors also gratefully acknowledge the following: Cures Within Reach (PI: Kahn), The Neil and Anna Rasmussen Foundation (PI: Kahn), and the Hamel Family (PI: Kahn), CTSA KL2 award, No.UL1 TR000445 (PI Hartmann), Wagner Family led Gutsy Kids Fund (PI: Kellermayer), and the Klaasmeyer family funds for Primary Sclerosing Cholangitis research (PI: Kellermayer).
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Hoffmann DE, Palumbo FB, Ravel J, et al. A proposed definition of microbiota transplantation for regulatory purposes. Gut Microbes 2017;8:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013;108:500–8. [DOI] [PubMed] [Google Scholar]
- 3.Lawson PA, Citron DM, Tyrrell KL, et al. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016;40:95–9. [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr 2013;167:567–73. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson MR, Osgood CL, Acra SA, et al. Clostridium difficile infection in the pediatric transplant patient. Pediatr Transplant 2015;19:792–8. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Smathers SA, Prasad P, et al. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics 2008; 122:1266–70. [DOI] [PubMed] [Google Scholar]
- 8.van Dorp SM, Smajlović E, Knetsch CW, et al. Clinical and microbiological characteristics of Clostridium difficile infection among hospitalized children in the Netherlands. Clin Infect Dis 2017;64:192–8. [DOI] [PubMed] [Google Scholar]
- 9.Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a populationbased study. Clin Infect Dis 2013;56:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014;133:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson MR, Thomsen IP, Slaughter JC, et al. Novel risk factors for recurrent Clostridium difficile infection in children. J Pediatr Gastroenterol Nutr 2015;60:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kociolek LK, Palac HL, Patel SJ, et al. Risk factors for recurrent Clostridium difficile infection in children: a nested case-control study. J Pediatr 2015;167:384–9. [DOI] [PubMed] [Google Scholar]
- 14.Schwab EM, Wilkes J, Korgenski K, et al. Risk factors for recurrent Clostridium difficile infection in pediatric inpatients. Hosp Pediatr 2016;6:339–44. [DOI] [PubMed] [Google Scholar]
- 15.Nylund CM, Eide M, Gorman GH. Association of Clostridium difficile infections with acid suppression medications in children. J Pediatr 2014;165:979.e1–84.e1. [DOI] [PubMed] [Google Scholar]
- 16.Freedberg DE, Lamousé-Smith ES, Lightdale JR, et al. Use of acid suppression medication is associated with risk for C. difficile infection in infants and children: a population-based study. Clin Infect Dis 2015;61:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samady W, Pong A, Fisher E. Risk factors for the development of Clostridium difficile infection in hospitalized children. Curr Opin Pediatr 2014;26:568–72. [DOI] [PubMed] [Google Scholar]
- 18.Hourigan SK, Sears CL, Oliva-Hemker M. Clostridium difficile infection in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2016;22:1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mir SAV, Kellermayer R. Clostridium difficile infection in newly diagnosed pediatric inflammatory bowel disease in the mid-southern United States. J Pediatr Gastroenterol Nutr 2013;57:487–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hourigan SK, Oliva-Hemker M, Hutfless S. The prevalence of Clostridium difficile infection in pediatric and adult patients with inflammatory bowel disease. Dig Dis Sci 2014;59:2222–7. [DOI] [PubMed] [Google Scholar]
- 21.De Blank P, Zaoutis T, Fisher B, et al. Trends in Clostridium difficile infection and risk factors for hospital acquisition of Clostridium difficile among children with cancer. J Pediatr 2013;163:699.e1–705.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hefazi M, Patnaik MM, Hogan WJ, et al. Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin Proc 2017; 92:1617–24. [DOI] [PubMed] [Google Scholar]
- 23.Kellermayer R Burdening questions about Clostridium difficile in pediatric inflammatory bowel diseases. J Pediatr Gastroenterol Nutr 2015;60:421–2. [DOI] [PubMed] [Google Scholar]
- 24.Kubota H, Makino H, Gawad A, et al. Longitudinal investigation of carriage rates, counts, and genotypes of toxigenic Clostridium difficile in early infancy. Appl Environ Microbiol 2016;82:5806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest 1992;90:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolfe RD. Binding kinetics of Clostridium difficile toxins A and B to intestinal brush border membranes from infant and adult hamsters. Infect Immun 1991;59:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leffler DA, Lamont JT. Clostridium difficile infection. Longo DL, editor. N Engl J Med 2015;372:1539–48. [DOI] [PubMed] [Google Scholar]
- 28.Faust SN, Wilcox MH, Banaszkiewicz A, et al. Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin Infect Dis 2015;60:912–8. [DOI] [PubMed] [Google Scholar]
- 29.Schutze GE, Willoughby RE. Clostridium difficile infection in infants and children. Pediatrics 2013;131:196–200. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson MR, Freswick PN, Di Pentima MC, et al. The use of a computerized provider order entry alert to decrease rates of Clostridium difficile testing in young pediatric patients. Infect Control Hosp Epidemiol 2017;38:542–6. [DOI] [PubMed] [Google Scholar]
- 31.De Graaf H, Pai S, Burns DA, et al. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis 2015;34:1281–7. [DOI] [PubMed] [Google Scholar]
- 32.Russell G, Kaplan J, Ferraro M, et al. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics 2010;126:e239–42. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SA, Young S, Rubin DT. Colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection in a child. Am J Gastroenterol 2012;107:1930–1. [DOI] [PubMed] [Google Scholar]
- 34.Adams DJ, Eberly MD, Rajnik M, et al. Risk factors for community-associated Clostridium difficile infection in children. J Pediatr 2017;186:105–9. [DOI] [PubMed] [Google Scholar]
- 35.Truong C, Schroeder LF, Gaur R, et al. Clostridium difficile rates in asymptomatic and symptomatic hospitalized patients using nucleic acid testing. Diagn Microbiol Infect Dis 2017;87:365–70. [DOI] [PubMed] [Google Scholar]
- 36.Rea MC, O’Sullivan O, Shanahan F, et al. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol 2012;50:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leibowitz J, Soma VL, Rosen L, et al. Similar proportions of stool specimens from hospitalized children with and without diarrhea test positive for Clostridium difficile. Pediatr Infect Dis J 2015;34:261–6. [DOI] [PubMed] [Google Scholar]
- 38.Hourigan SK, Chirumamilla SR, Ross T, et al. Clostridium difficile carriage and serum antitoxin responses in children with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2744–52. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez SR, Dolan SA, West K, et al. High colonization rate and prolonged shedding of Clostridium difficile in pediatric oncology patients. Clin Infect Dis 2014;59:401–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linton A Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv reports 2014;3:pii: u201903.w1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binkovitz LA, Allen E, Bloom D, et al. Atypical presentation of Clostridium difficile colitis in patients with cystic fibrosis. AJR Am J Roentgenol 1999;172:517–21. [DOI] [PubMed] [Google Scholar]
- 42.Burke DG, Harrison MJ, Fleming C, et al. Clostridium difficile carriage in adult cystic fibrosis (CF); implications for patients with CF and the potential for transmission of nosocomial infection. J Cyst Fibros 2017;16:291–8. [DOI] [PubMed] [Google Scholar]
- 43.Peach SL, Borriello SP, Gaya H, et al. Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol 1986;39:1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celik AF, Tomlin J, Read NW. The effect of oral vancomycin on chronic idiopathic constipation. Aliment Pharmacol Ther 1995;9:63–8. [DOI] [PubMed] [Google Scholar]
- 45.Zar FA, Bakkanagari SR, Moorthi KMLST, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302–7. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J 2012;31:134–8. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz K, Darwish I, Richardson S, et al. Severe clinical outcome is uncommon in Clostridium difficile infection in children: a retrospective cohort study. BMC Pediatr 2014;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutze GE, Willoughby RE. Committee on Infectious Diseases, American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics 2013;131:196–200. [DOI] [PubMed] [Google Scholar]
- 49.Avila MB, Avila NP, Dupont AW. Recent advances in the diagnosis and treatment of Clostridium difficile infection. F1000Res 2016:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kociolek LK. Strategies for optimizing the diagnostic predictive value of Clostridium difficile molecular diagnostics. J Clin Microbiol 2017;55:1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnham C-AD, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 2013;26:604–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multi-centre diagnostic validation study of C difficile infection. Lancet Infect Dis 2013;13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015;175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108:478–98 quiz 499. [DOI] [PubMed] [Google Scholar]
- 55.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:637–45. [DOI] [PubMed] [Google Scholar]
- 56.Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol 2016;13:150–60. [DOI] [PubMed] [Google Scholar]
- 57.Chen C-C, Huang J-L, Chang C-J, et al. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr 2012;55:541–7. [DOI] [PubMed] [Google Scholar]
- 58.Smits WK, Lyras D, Lacy DB, et al. Clostridium difficile infection. Nat Rev Dis Prim 2016;2:16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile associated diarrhoea in adults In: Nelson RL, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2017:p. CD004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007;44:846–8. [DOI] [PubMed] [Google Scholar]
- 61.Péchiné S, Janoir C, Collignon A. Emerging monoclonal antibodies against Clostridium difficile infection. Expert Opin Biol Ther 2017;17:415–27. [DOI] [PubMed] [Google Scholar]
- 62.Bakken JS, Polgreen PM, Beekmann SE, et al. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe 2013;24:20–4. [DOI] [PubMed] [Google Scholar]
- 63.Marra F, Ng K. Controversies around epidemiology, diagnosis and treatment of Clostridium difficile infection. Drugs 2015;75:1095–118. [DOI] [PubMed] [Google Scholar]
- 64.Johnson S Evidence-based approach to Clostridium difficile infection. Gastroenterol Hepatol (N Y) 2017;13:238–41. [PMC free article] [PubMed] [Google Scholar]
- 65.Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014;109:1728–38. [DOI] [PubMed] [Google Scholar]
- 67.Azad MB, Moossavi S, Owora A, et al. Early-life antibiotic exposure, gut microbiota development, and predisposition to obesity. Nestle Nutr Inst Workshop Ser 2017;88:67–79. [DOI] [PubMed] [Google Scholar]
- 68.Lewis BB, Buffie CG, Carter RA, et al. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole. J Infect Dis 2015;212:1656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khanafer N, Vanhems P, Barbut F, et al. , CDI01 Study group. Factors associated with Clostridium difficile infection: a nested case-control study in a three year prospective cohort. Anaerobe 2017;44:117–23. [DOI] [PubMed] [Google Scholar]
- 70.Horton HA, Dezfoli S, Berel D, et al. Antibiotics for treatment of Clostridium difficile infection in hospitalized patients with inflammatory bowel disease. Antimicrob Agents Chemother 2014;58:5054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nomura K, Fujimoto Y, Yamashita M, et al. Absence of pseudomembranes in Clostridium difficile-associated diarrhea in patients using immunosuppression agents. Scand J Gastroenterol 2009;44:74–8. [DOI] [PubMed] [Google Scholar]
- 72.Kociolek LK, Patel SJ, Shulman ST, et al. Concomitant medical conditions and therapies preclude accurate classification of children with severe or severe complicated Clostridium difficile infection. J Pediatric Infect Dis Soc 2015;4:e139–42. [DOI] [PubMed] [Google Scholar]
- 73.Arends S, Defosse J, Diaz C, et al. Successful treatment of severe Clostridium difficile infection by administration of crushed fidaxomicin via a nasogastric tube in a critically ill patient. Int J Infect Dis 2017;55:27–8. [DOI] [PubMed] [Google Scholar]
- 74.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pant C, Sferra TJ, Deshpande A, et al. Clinical approach to severe Clostridium difficile infection: update for the hospital practitioner. Eur J Intern Med 2011;22:561–8. [DOI] [PubMed] [Google Scholar]
- 76.Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 2012;107:1755author reply 1755–1756. [DOI] [PubMed] [Google Scholar]
- 77.Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–9. [PubMed] [Google Scholar]
- 78.Chapman BC, Moore HB, Overbey DM, et al. Fecal microbiota transplant in patients with Clostridium difficile infection: a systematic review. J Trauma Acute Care Surg 2016;81:756–64. [DOI] [PubMed] [Google Scholar]
- 79.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407–15. [DOI] [PubMed] [Google Scholar]
- 80.Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an openlabel, randomized controlled trial. Clin Infect Dis 2017;64:265–71. [DOI] [PubMed] [Google Scholar]
- 81.Galpérine T, Sokol H, Guery B. Fecal microbiota transplantation: do we need harmonization? Clin Infect Dis 2017;64:1292. [DOI] [PubMed] [Google Scholar]
- 82.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med 2016;165: 609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahn SA, Rubin DT. When subjects violate the research covenant: lessons learned from a failed clinical trial of fecal microbiota transplantation. Am J Gastroenterol 2016;111:1508–10. [DOI] [PubMed] [Google Scholar]
- 84.Hourigan SK, Oliva-Hemker M. Fecal microbiota transplantation in children: a brief review. Pediatr Res 2016;80:2–6. [DOI] [PubMed] [Google Scholar]
- 85.Kronman MP, Nielson HJ, Adler AL, et al. Fecal microbiota transplantation via nasogastric tube for recurrent Clostridium difficile infection in pediatric patients. J Pediatr Gastroenterol Nutr 2015; 60:23–6. [DOI] [PubMed] [Google Scholar]
- 86.Jernberg C, Löfmark S, Edlund C, et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1:56–66. [DOI] [PubMed] [Google Scholar]
- 87.Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216–23. [DOI] [PubMed] [Google Scholar]
- 88.Löfmark S, Jernberg C, Jansson JK, et al. Clindamycin-induced enrichment and long-term persistence of resistant Bacteroides spp. and resistance genes. J Antimicrob Chemother 2006;58:1160–7. [DOI] [PubMed] [Google Scholar]
- 89.Reeves AE, Theriot CM, Bergin IL, et al. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes 2011;2:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De La Cochetière MF, Durand T, Lalande V, et al. Effect of antibiotic therapy on human fecal microbiota and the relation to the development of Clostridium difficile. Microb Ecol 2008;56: 395–402. [DOI] [PubMed] [Google Scholar]
- 91.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile—associated diarrhea. J Infect Dis 2008;197:435–8. [DOI] [PubMed] [Google Scholar]
- 92.Skraban J, Dzeroski S, Zenko B, et al. Gut microbiota patterns associated with colonization of different Clostridium difficile ribotypes. PLoS One 2013;8:e58005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 2002;51:448–54. [DOI] [PubMed] [Google Scholar]
- 94.Louie TJ, Emery J, Krulicki W, et al. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother 2009;53:261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rousseau C, Levenez F, Fouqueray C, et al. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol 2011;49:858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fallani M, Rigottier-Gois L, Aguilera M, et al. Clostridium difficile and Clostridium perfringens species detected in infant faecal microbiota using 16S rRNA targeted probes. J Microbiol Methods 2006; 67:150–61. [DOI] [PubMed] [Google Scholar]
- 97.Zuo T, Wong SH, Lam K, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018;67:634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017;152:799.e7–811.e7. [DOI] [PubMed] [Google Scholar]
- 99.Weingarden AR, Dosa PI, DeWinter E, et al. Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control Clostridium difficile germination and growth. PLoS One 2016;11:e0147210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agans R, Rigsbee L, Kenche H, et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol 2011;77:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hollister EB, Riehle K, Luna RA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Relman D, Rustgi A, Bousvaros A, et al. Current consensus guidance on donor screening and stool testing for FMT. Infectious Disease Society of America, Arlington, VA: https://www.naspghan.org/files/documents/Joint_Scty_Sign-on_FDA%20FMT_final%207.15.13%20(1).pdf. [Google Scholar]
- 103.Gwee K, Read N, Graham J, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 1996;347:150–3. [DOI] [PubMed] [Google Scholar]
- 104.Paramsothy S, Borody TJ, Lin E, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis 2015;21:1600–6. [DOI] [PubMed] [Google Scholar]
- 105.Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015;149:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woodworth MH, Neish EM, Miller NS, et al. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Microbiol 2017; 55:1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pépin J, Gonzales M, Valiquette L. Risk of secondary cases of Clostridium difficile infection among household contacts of index cases. J Infect 2012;64:387–90. [DOI] [PubMed] [Google Scholar]
- 108.Barfield E, Small L, Navallo L, et al. Going to the bank: fecal microbiota transplantation in pediatrics. Clin Pediatr (Phila) 2017;57:481–3. [DOI] [PubMed] [Google Scholar]
- 109.Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 2012;107:761–7. [DOI] [PubMed] [Google Scholar]
- 110.Khanna S, Vazquez-Baeza Y, González A, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 2017;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial translplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis 2015;21:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015;41:835–43. [DOI] [PubMed] [Google Scholar]
- 113.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA 2016;315:142. [DOI] [PubMed] [Google Scholar]
- 114.Link A, Lachmund T, Schulz C, et al. Endoscopic peroral jejunal fecal microbiota transplantation. Dig Liver Dis 2016;48:1336–9. [DOI] [PubMed] [Google Scholar]
- 115.Nicholson M, Alexander E, Bartlett M, et al. Fecal microbiota transplantation in pediatric Clostridium difficile infection, a multicenter study [Abstract]. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Annual Meeting; November 1–4, 2017; Las Vegas, NV Abstract nr 481. [Google Scholar]
- 116.Oshima T, Wu L, Li M, et al. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol 2018;53:84–94. [DOI] [PubMed] [Google Scholar]
- 117.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012;107:1079–87. [DOI] [PubMed] [Google Scholar]
- 118.Brumbaugh DE, De Zoeten EF, Pyo-Twist A, et al. An intragastric fecal microbiota transplantation program for treatment of recurrent Clostridium difficile in children is efficacious, safe, and inexpensive. J Pediatr 2018;194:123.e1–7.e1. [DOI] [PubMed] [Google Scholar]
- 119.Chu ND, Smith MB, Perrotta AR, et al. Profiling living bacteria informs preparation of fecal microbiota transplantations. PLoS One 2017;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baxter M, Ahmad T, Colville A, et al. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis 2015;61:136–7. [DOI] [PubMed] [Google Scholar]
- 121.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection 2012;40:643–8. [DOI] [PubMed] [Google Scholar]
- 122.Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis 2014;33:1425–8. [DOI] [PubMed] [Google Scholar]
- 123.Youngster I, Mahabamunuge J, Systrom HK, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med 2016;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ratner M Seres’s pioneering microbiome drug fails mid-stage trial. Nat Biotechnol 2016;34:1004–5. [DOI] [PubMed] [Google Scholar]
- 125.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther 2012;36:503–16. [DOI] [PubMed] [Google Scholar]
- 126.Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr 2013; 56:597–601. [DOI] [PubMed] [Google Scholar]
- 127.Solari PR, Fairchild PG, Noa LJ, et al. Tempered enthusiasm for fecal transplant. Clin Infect Dis 2014;59:319–1319. [DOI] [PubMed] [Google Scholar]
- 128.Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One 2016;11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fischer M, Kao D, Kelly C, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:2402–9. [DOI] [PubMed] [Google Scholar]
- 130.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013;108:1367–11367. [DOI] [PubMed] [Google Scholar]
- 131.Hohmann EL, Ananthakrishnan AN, Deshpande V. Case 25–2014: a 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med 2014;371:668–75. [DOI] [PubMed] [Google Scholar]
- 132.Neha A, Colleen RK. Weight gain after fecal microbiota transplantation. Open Forum Infectious Diseases 2015;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913.e7–6.e7. [DOI] [PubMed] [Google Scholar]
- 134.Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis 2017;11:1180–99. [DOI] [PubMed] [Google Scholar]
- 135.Sokol H, Galperine T, Kapel N, et al. Faecal microbiota transplantation in recurrent Clostridium difficile infection: recommendations from the French group of faecal microbiota transplantation. Dig Liver Dis 2016;48:242–7. [DOI] [PubMed] [Google Scholar]
- 136.Costello SP, Waters O, Bryant RV, et al. Short duration, low intensity, pooled fecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomised controlled trial. Gastroenterology 2017;152:S198–9. [Google Scholar]
- 137.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017; 389:1218–28. [DOI] [PubMed] [Google Scholar]
- 138.Rossen NG, Fuentes S, Van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110.e4–8.e4. [DOI] [PubMed] [Google Scholar]
- 139.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102.e6–9.e6. [DOI] [PubMed] [Google Scholar]
- 140.Costello SP, Soo W, Bryant RV, et al. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017; 46:213–24. [DOI] [PubMed] [Google Scholar]
- 141.Ghouri YA, Richards DM, Rahimi EF, et al. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 2014;7: 473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pardi DS, D’Haens G, Shen B, et al. Clinical guidelines for the management of pouchitis. Inflamm Bowel Dis 2009;15:1424–31. [DOI] [PubMed] [Google Scholar]
- 143.Stallmach A, Lange K, Buening J, et al. Fecal microbiota transfer in patients with chronic antibiotic-refractory pouchitis. Am J Gastroenterol 2016;111:441–3. [DOI] [PubMed] [Google Scholar]
- 144.Landy J, Walker AW, Li JV, et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci Rep 2015;5:12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 2018;3:17–24. [DOI] [PubMed] [Google Scholar]
- 146.Tian H, Ge X, Nie Y, et al. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS One 2017;12:e0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding C, Fan W, Gu L, et al. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol Rep 2018;6:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weber D, Jenq RR, Peled JU, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017;23:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016;8:339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Webb BJ, Brunner A, Ford CD, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis 2016;18:628–33. [DOI] [PubMed] [Google Scholar]
- 151.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versushost disease of the gut. Blood 2016;128:2083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spindelboeck W, Schulz E, Uhl B, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host disease. Haematologica 2017;102:e210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 2017;26: 611.e6–9.e6. [DOI] [PubMed] [Google Scholar]
- 154.Crum-Cianflone NF, Sullivan E, Ballon-Landa G. Fecal microbiota transplantation and successful resolution of multidrug-resistant-organism colonization. J Clin Microbiol 2015;53:1986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bilinski J, Grzesiowski P, Sorensen N, et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single center study. Clin Infect Dis 2017;65:364–70. [DOI] [PubMed] [Google Scholar]
- 156.Davido B, Batista R, Michelon H, et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J Hosp Infect 2017;95:433–7. [DOI] [PubMed] [Google Scholar]
- 157.Millan B, Park H, Hotte N, et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin Infect Dis 2016;62:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kang D-W, Adams JB, Gregory AC, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.