Abstract
Background
TGF-β has an important role in the process of wound healing and scar formation. The aim of this study is to determine the effects of ethanolic and methanolic extracts of Calendula officinalis on the expression of TGFβ1 and bFGF in the mouse embryonic fibroblast cells (MEFs).
Methods
Calendula officinalis extract was purchased and different substances defined with gas chromatography and mass spectrometry. MEFs were prepared and after incubating for 15 min, cell viability analyzed. TGF β 1 and bFGF gene expression was evaluated by real-time PCR. TGFβ1 and bFGF protein expression analyzed by ELISA. The statistical analysis of data was done by using SPSS software. Differences were considered significant at (P < 0.05).
Results
The results of the MTT test showed that the concentrations of 5 μg/ml and10 μg/ml were more suitable for cell proliferation. There was an increase in TGF β 1 gene expression in the MEFs. Expression of TGF β 1 gene remains the same after 24 h. Gene expression of bFGF showed a similar pattern with TGF β 1 expression for both solvents. Analysis of TGFβ1 protein expression showed an increase in TGFβ1 gene expression in the MEFs. Protein expression of bFGF in the MEFs increased at different concentrations at 12 and 24 h after treatment (P < 0.05 and P < 0.01 respectively).
Conclusion
Calendula officinalis stimulates proliferation of MEFs. Calendula via increased expression of growth factors (TGFβ1 and bFGF) at the first 12 h and a decrease of these factors at 24 h after treatment may ameliorate function of the MEFs in the during wound healing.
Keywords: Calendula officinalis, bFGF, TGFβ1, Mouse embryonic fibroblasts
Background
Growth of new tissue and scar formation are two important issues in tissue injury. Fibroblasts are the main cells in the wound healing process because they migrate and proliferate to the injury site after 2 or 3 days. Then, the cells produce extracellular matrix especially collagen. Growth factors play important roles in the proliferation, migration, and production of ECM substances by fibroblasts. Recently, researches showed that the growth factor TGF-β has an important role in the process not only in wound healing but also in scar formation. TGF-β applies their effects via the intracellular SMAD pathway. [1, 2]. It has been shown that TGFβ1 activates angiogenesis by stimulating vascular smooth muscle cell migration [3–5]. The importance of this factor in creating and maintaining the vascular system has been proved by numerous studies. TGFβ1 and its receptors lead to fetal death due to impaired vessel formation [6, 7]. TGFβ1 induces the maturation of retinoic acid-inducible gene I (RIGI), therefore, stimulates the formation and intensification of interaction between epithelial cells and the basement membrane of the mural cells [8–10]. Also bFGF, (FGF 2 or FGF-β) contribute to control of endothelial cells and fibroblasts migration, which are responsible for angiogenesis and collagen formation of the epithelial layer [1, 9, 11].
Calendula officinalis L. (Asteraceae) has been traditionally used in the treatment of various diseases such as skin tumors, dermatological lesions, and swellings. Despite a long tradition of use of Calendula officinalis L., the biological aspect of its activity has not been explored properly. Recent research supports the medicinal potential of Calendula officinalis L. Calendula officinalis L. properties need to be investigated to determine their varied biological activities and mechanism of actions. To determine the effectiveness of the Calendula officinalis L. in the treatment of dermatologic disorders, more research is necessary to understand the action mechanism of this plant. Therefore, the aim of this study is to determine the effect of the Calendula officinalis L. extract on proliferation and expression of two important growth factors, TGFβ1 and bFGF, at the MEFs.
Materials and methods
Preparation of Calendula officinalis extract
Twenty grams of dried flowers of Calendula officinalis were collected from Lorestan Agricultural and Natural Resources Research and Education Center and soaked in 120 ml of 50% ethanol or methanol for 72 h in a dark. In the next step, it was centrifuged and passed through the filter and dried at room temperature to yield 8.7% w/w extract. Then the extract was stored at − 20 °C until further use.
GC-MS analysis of Calendula officinalis extract
Different substances within Calendula officinalis extract defined with gas chromatography and mass spectrometry (GC-MS) at the Lorestan University.
Isolation and culture of MEFs
Isolation and culture of mouse embryonic fibroblasts (MEFs) were performed according to the protocol described elsewhere [12]. In the brief, a pregnant mouse at 13 or 14 days post-coitum (d.p.c) was sacrificed by dislocating of cervical vertebrae and uterine horns were dissected and rinsed in 70% (v/v) ethanol and placed into a Falcon tube with PBS buffer, without calcium and magnesium ions. The embryos were separated from its embryonic sac and placenta. Then, 1 ml of 0.05% trypsin/EDTA (Gibco, Invitrogen) containing 100 K units of DNase I per each embryo was added and incubated at the room temperature for 15 min. Cells were dissociated by pipetting each 5 min. Trypsin inactivated by the addition of 1 volume of freshly prepared MEF medium. Then, cells were centrifuged and the cell pellet was washed in warm MEF medium.
MTT assay
To evaluate cell viability, fibroblast cells incubated with different concentrations of Calendula officinalis (5 μg/ml, 10 μg/ml, 20 μg/ml, 40 μg/ml, and 50 μg/ml) for 12, 24, 48, and 72 h. Then, 20 μl of MTT (5 mg/ml, Sigma) in PBS solution added into each of the wells, and the plate was further incubated for 4 h. In the next step, 200 μl of DMSO added into each well, incubated for 15 min to dissolve the formed crystal formation and the light absorption measured using an enzyme-linked immunosorbent assay (ELISA) reader. Cell viability expressed as a percentage of absorbance values in treating cells.
Analysis of TGF β 1 and bFGF gene expression by real-time PCR
MEFs were exposed to different concentrations of ethanolic and methanolic Calendula officinalis extracts (5 μg/ml and 10 μg/ml) and then, cells were collected at 12 and 24 h using trypsin/EDTA. Total RNA isolated and the concentration and purity of RNA determined using biophotometer (Eppendorf, Hamburg, Germany). The concentration and quality of the RNA samples confirmed by electrophoresis on 1% denatured agarose gel.
Following procedures were performed:
Generation of first strand cDNA with 1 μg total RNA using the cDNA Synthesis Kit (Roche Diagnostics GmbH, Mannheim, Germany).
Selection of HPRT as the housekeeping gene.
Real-time quantitative PCR by using Rotor-Gene 6000 and SYBR-Green quantitative PCR (qPCR) kit (Jena Bioscience, Cat No. 311S)
Oligonucleotide sequences of the primers and their characteristics are presented in Table 1.
Table 1.
Sequences of primers for real-time quantitative PCR
| Gene | Primer | Product size | Tm |
|---|---|---|---|
| HPRT | Sense:CCTCCTCAGACCGCTTTTT | 91 | 79.5 |
| Antisense:AACCTGGTTCATCATCGCTAA | |||
| FGF2 | Sense: AACGGCGGCTTCTTCCTG | 133 | 78.9 |
| Antisense:TGGCACACACTCCCTTGATAG | |||
| TGFβ1 | Sense: ATTCCTGGCGTTACCTTGG | 117 | 76.9 |
| Antisense:CCTGTATTCCGTCTCCTTGG |
Analysis of TGFβ1 and bFGF protein expression by ELISA
MEFs were cultured in 6-well plates (105 cell per well) and were exposed to ethanolic and methanolic extract of Calendula officinalis (5 μg/ml, 10 μg/ml) and supernatant were collected at 12 and 24 h. Samples were frozen at − 20 °C. TGF-β1 protein concentration in cell culture supernatants was measured by Ready-Set-GO TGF-β1 cytokine ELISA kit from eBioscience (San Diego, CA, USA, Catalog Number: 88-8350) and FGF ELISA kit (Cat no. ELH-bFGF-001; RayBiotech, Inc., St. Louis, MO).
Statistical analysis
The statistical analysis of data was done by using SPSS software. Differences between the groups were analyzed by using Wilcoxon test. Differences were considered significant at (P < 0.05).
Results
GC-MS analysis
GC-MS analysis showed that the main components present in the Calendula officinalis extract are carvacrol, thymol, ethyl hexadecanoate, and viridiflorene (Table 2).
Table 2.
GC-MS analysis showed that the main components present in the Calendula officinalis extract are carvacrol, thymol, ethyl hexadecanoate, and viridiflorene
| t’R(A) | RI(Calc) | RI(STD) | Similarity | Compound name | Area (%) |
|---|---|---|---|---|---|
| 4.092 | 832.5714286 | 800 | 83% | Hexanal | 1.005961 |
| 4.233 | 846 | 830 | 95% | Furfural | 1.415797 |
| 4.375 | 859.5238095 | 851 | 92% | Furfuryl alcohol | 1.453055 |
| 4.958 | 910.5333333 | 899 | 80% | Heptanal | 1.19225 |
| 5.267 | 931.1333333 | 998 | 81% | Octanal | 1.254347 |
| 5.933 | 975.5333333 | 957 | 94% | Furfural 5-methyl | 1.20467 |
| 6.175 | 991.6666667 | 911 | 80% | Amyl acetate | 3.551913 |
| 6.517 | 1010.432692 | 950 | 93% | Glycerin | 19.54794 |
| 7.592 | 1062.115385 | 1112 | 80% | Heptyl acetate | 2.707402 |
| 8.092 | 1086.153846 | 1106 | 77% | Maltol | 1.800795 |
| 10.767 | 1185.402504 | 1171 | 70% | Umbellulone | 0.384998 |
| 12.2 | 1230.191458 | 1228 | 85% | Citronellol | 1.477894 |
| 14.433 | 1295.964654 | 1285 | 80% | Bornyl acetate | 0.471932 |
| 14.642 | 1301.899736 | 1290 | 93% | Thymol | 1.328862 |
| 14.967 | 1310.474934 | 1298 | 95% | Carvacrol | 7.19076 |
| 19.658 | 1432.944162 | 1439 | 82% | Aromadendrene | 0.422255 |
| 20.517 | 1454.746193 | 1458 | 87% | Beta Farnesene | 0.981123 |
| 22.192 | 1497.258883 | 1467 | 83% | Caryophyllene | 0.695479 |
| 22.467 | 1504.249364 | 1493 | 93% | Viridiflorene | 3.154496 |
| 23.433 | 1528.829517 | 1524 | 91% | Delta Cadinene | 1.043219 |
| 24.708 | 1561.272265 | 1549 | 90% | Elemol | 0.34774 |
| 26.142 | 1597.760814 | 1600 | 90% | Hexadecane | 0.471932 |
| 29.125 | 1674.806202 | 1658 | 80% | Eudesmol | 2.309985 |
| 29.275 | 1678.682171 | 1691 | 80% | Juniper Camphor | 0.471932 |
| 29.992 | 1697.209302 | 1700 | 87% | Heptadecane | 0.397417 |
| 39.675 | 1967.424242 | 1959 | 90% | Hexadecanoic acid | 0.894188 |
| 40.633 | 1996.454545 | 1993 | 93% | Ethyl hexadecanoate | 0.558867 |
Effect of Calendula officinalis extract on MEFs viability
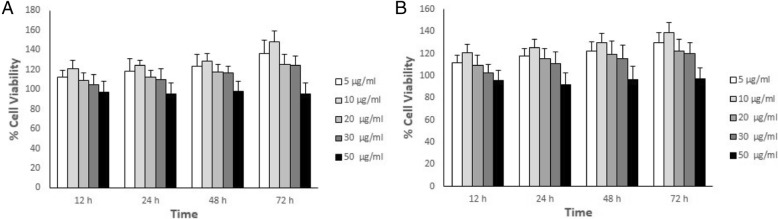
The results of MTT assay to determine if Calendula officinalis ethanolic/methanolic extract affects cell viability of MEFs at different concentration (5 μg/ml, 10 μg/ml, 20 μg/ml, 40 μg/ml) showed that the Calendula officinalis extract using both solvents was non-toxic to the cells. The concentrations of 5 μg/ml and10 μg/ml were more suitable for cell proliferation. Therefore, this study was evaluated in these concentrations (Fig. 1).
Fig. 1.
Effect of ethanolic (a) and methanolic (b) extract of Calendula on MEFs viability. Cells were treated with different concentration of Calendula for 12, 24, 48, and 72 h and cell viability was measured by MTT assay
TGF β 1 and bFGF gene expression
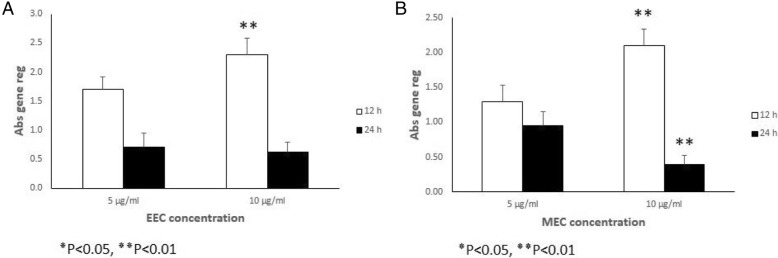
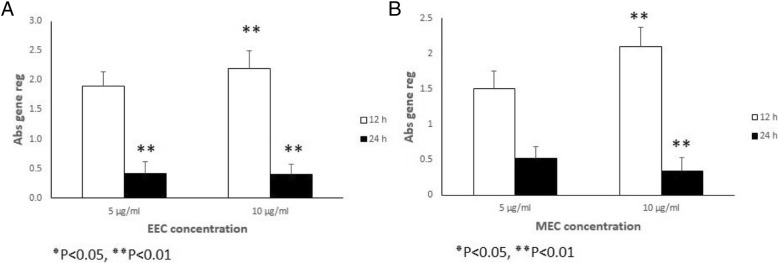
Analysis of TGF β 1 gene expression for the ethanolic extract (Fig. 2a) and the methanolic extract (Fig. 2b), revealed an increase TGF β 1 gene expression in the MEFs at different concentrations (5 μg/ml, 10 μg/ml) at 12 h after treatment (P < 0.05 and P < 0.01 respectively). But the expression of TGF β 1 gene remained the same 24 h at the two concentrations after treatment with ethanolic extract (Fig. 2a and Table 3). The methanolic extract results revealed an increase in the expression for the two extracts at 12 h and a decline in the expression of the gene at 24 h (Fig. 2b and Table 4) (P < 0.05 and P < 0.01 respectively). Gene expression of bFGF in the MEFs indicated a similar pattern with TGF β 1 expression for both solvent, an increase bFGF gene expression in the MEFs at different concentrations (5 μg/ml, 10 μg/ml) at 12 h after treatment (P < 0.05 and P < 0.01 respectively). But the expression of bFGF gene remained the same 24 h at the two concentrations after treatment with ethanolic extract (Fig. 3a and Table 5). The methanolic extract results indicated an increase in the expression for the two extracts at 12 h and a decline in the expression of the gene at 24 h (Fig. 3b and Table 6) (P < 0.05 and P < 0.01 respectively) (Table 2).
Fig. 2.
Relative expression of TGF β 1 gene in the MEFs that were treated with various concentrations of ethanol (a) and methanol (b) extracts of Calendula at different time intervals treatment (12 and 24 h). All comparisons were made compared to the control group. *P < 0.05, **P < 0.01. Abs gene reg: Absolute gene regulation
Table 3.
The total expression ratio of the gene of TGFβ1 in the MEFs treated with various concentrations of ethanol extract of Calendula (5 and 10 μg/ml) relative to control group is presented in each time (12 and 24 h after treatment). The statistic test for significance is randomization re-allocation test, implemented in the relative expression software tool. Significant down or upregulations of the genes highlighted
| 12 h after treatment | 24 h after treatment | |||
|---|---|---|---|---|
| 5 μg/ml | 10 μg/ml | 5 μg/ml | 10 μg/ml | |
| Relative expression | 1.7 | 2.3 | 0.7 | 0.62 |
| Standard error | ± 0.21 | ± 0.28 | ± 0.24 | ± 0.16 |
| P value | 0.235 | 0.001 | 0.490 | 0.359 |
| Fold increase/decrease | + 1.7 | + 2.3 | − 1.4 | − 1.6 |
Table 4.
The total expression ratio of the gene of TGFβ1 in the MEFs treated with various concentrations of methanol extract of Calendula (5 and 10 μg/ml) relative to control group is presented in each time (12 and 24 h after treatment). The statistic test for significance is randomization re-allocation test, implemented in the relative expression software tool. Significant down or upregulations of the genes highlighted
| 12 h after treatment | 24 h after treatment | |||
|---|---|---|---|---|
| 5 μg/ml | 10 μg/ml | 5 μg/ml | 10 μg/ml | |
| Relative expression | 1.3 | 2.1 | 0.7 | 0.62 |
| Standard error | ± 0.23 | ± 0.24 | ± 0.2 | ± 0.13 |
| P value | 0.265 | 0.001 | 0.661 | 0.001 |
| Fold increase/decrease | + 1.3 | + 2.1 | − 1.06 | − 2.5 |
Fig. 3.
Relative expression of bFGF gene in the MEFs that were treated with various concentrations of ethanol (a) and methanol (b) extracts of Calendula at different time intervals treatment (12 and 24 h). All comparisons were made compared to the control group. ٭P < 0.05, ٭٭P < 0.01. Abs gene reg: Absolute gene regulation
Table 5.
The total expression ratio of the gene of bFGF in the MEFs treated with various concentration of ethanol extract of Calendula (5 and 10 μg/ml) relative to control group is presented in each time (12 and 24 h after treatment). The statistic test for significance is randomization re-allocation test, implemented in the relative expression software tool. Significant down or upregulations of the genes highlighted
| 12 h after treatment | 24 h after treatment | |||
|---|---|---|---|---|
| 5 μg/ml | 10 μg/ml | 5 μg/ml | 10 μg/ml | |
| Relative expression | 1.9 | 2.2 | 0.42 | 0.39 |
| Standard error | ±0.24 | ±0.29 | ±0.19 | ±0.18 |
| P- value | 0.256 | 0.001 | 0.001 | 0.001 |
| Fold increase/decrease | + 1.9 | + 2.2 | − 2.4 | −2.5 |
Table 6.
The total expression ratio of the gene of bFGF in the MEFs treated with various concentration of methanol extract of Calendula (5 and 10 μg/ml) relative to control group is presented in each time (12 and 24 h after treatment). The statistic test for significance is randomization re-allocation test, implemented in the relative expression software tool. Significant down or upregulations of the genes highlighted
| 12 h after treatment | 24 h after treatment | |||
|---|---|---|---|---|
| 5 μg/ml | 10 μg/ml | 5 μg/ml | 10 μg/ml | |
| Relative expression | 1.5 | 2.1 | 0.52 | 0.34 |
| Standard error | ± 0.25 | ± 0.27 | ± 0.17 | ± 0.19 |
| P value | 0.231 | 0.001 | 0..275 | 0.001 |
| Fold increase/decrease | + 1.5 | + 2.1 | − 1.9 | − 2.96 |
TGFβ1 and bFGF protein expression
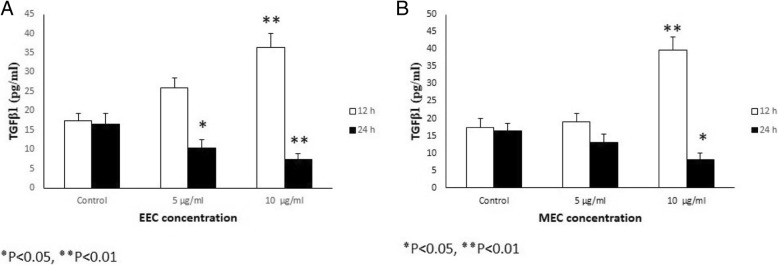
Analysis of TGFβ1 protein expression of the ethanolic extract (Fig. 4a) and the methanolic extract (Fig. 4b) revealed an increase TGFβ1 gene expression in the MEFs at different concentrations (5 μg/ml, 10 μg/ml) at 12 h (P < 0.05 and P < 0.01 respectively). But the expression of TGFβ1 protein was slightly reduced, 24 h at the two concentrations after treatment with ethanolic extract (Fig. 4a). The methanolic extract results indicated a similar pattern (Fig. 4b) (P < 0.05 and P < 0.01 respectively).
Fig. 4.
Effect of various concentrations of ethanol (a) and methanol (b) extracts of Calendula the expression of TGFβ1 protein in the MEFs culture supernatants. The MEFs were treated with different concentration of ethanol and methanol extracts of Calendula (5 and 10 μg/ml) at different time intervals treatment (12 and 24 h) and the expression of TGFβ1 protein was assessed by ELISA
Protein expression of bFGF in MEFs revealed an increase in bFGF protein expression at different concentrations (5 μg/ml, 10 μg/ml) of 12 h after treatment (P < 0.05 and P < 0.01 respectively). The same increasing pattern of bFGF protein expression was observed 24 h at the two concentrations after treatment with ethanolic extract. The methanolic extract results indicated a similar pattern with that of the ethanolic extract, as compared with the control (P < 0.05 and P < 0.01 respectively).
When the ethanolic and methanolic extracts of Calendula officinalis were compared, ethanolic extract indicated more effective to stimulate MEFs (Figs. 2, 3, and 4 and Tables 3, 4, 5, and 6).
Discussion
The results of the present study indicated that both methanol and ethanol extracts of Calendula officinalis had non-toxic effects of different concentration. The extracts similar to previous studies increased proliferation of the MEFs [13, 14]. Among different concentrations of Calendula extraction 5 and 10 μg/ml were more suitable for cell proliferation.
Previous investigation revealed that Calendula officinalis can inhibit collagen degradation and matrix metalloproteinase (MMP) activity, and induce neovascularization in the chorioallantoic membrane and skin wound.
Quercetin, one of the active components in Calendula, can decrease the expression of tumor necrosis factor-α (TNF-α), interleukin-1β, IL-6, and IL-8 [14–17].
During the wound healing process, fibroblasts play a crucial role. Then, the cells proliferate and migrate into the wound area, synthesis extracellular matrix (EXM), and the express of thick actin bundles as myofibroblasts [18, 19].
Important cytokines that produce by fibroblast are TGFβ1 and bFGF. TGFβ1 and bFGF impacts on cell division, cell migration, cell differentiation, protein expression, and enzyme production and have the potential ability to heal wounds through stimulation of angiogenesis factors and cellular proliferation which affects the ECM production and degradation through their chemotactic role on inflammatory cells and fibroblasts [20].
Several studies indicated that upregulation of TGFβ1 can stimulate the production of fibrotic disease [21, 22].
In the present study, Calendula officinalis increased TGFβ1 and bFGF at the first 12 h therefore stimulate wound healing. Then, the decrease of these factors at 24 h suppressed the expression of TGFβ1 and bFGF and may inhibit the fibrotic process. The Calendula at first may upregulate the expression of TGFβ1 and bFGF but after that may downregulate the expression of these genes.
Conclusion
Calendula officinalis not only shows no cytotoxicity effects on MEFs but also stimulates proliferation of these cells. Calendula via increased expression of growth factors (TGFβ1 and bFGF) at the first 12 h, and a decrease of these factors at 24 h after treatment may ameliorate function of fibroblasts in the during wound healing.
Acknowledgements
This work was supported by Lorestan University of Medical Science, Khorramabad, Iran.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- bFGF
Basic fibroblast growth factor
- cDNA
Complementary deoxyribonucleic acid
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- HPRT
Hypoxanthine phosphoribosyltransferase
- MEF
Mouse embryonic fibroblast
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- nm
Nanometer
- PBS
Phosphate-buffered saline
- Pg/ml
Picograms per milliliter
- Real-time PCR
Real-time polymerase chain reaction
- TGFβ1
Transforming growth factor β1
- Tm
Melting temperature
- TMB
3, 3′, 5, 5′-Tetramethylbenzidine
Authors’ contributions
MH, AB, MRG, and AMG devised the study concept and design. MH and AB collected the data. MG and AMG drafted the manuscript. All authors have seen and approved the manuscript.
Ethics approval and consent to participate
All of the experimental manuals on animals were conducted with agreement of protocols of the laboratory animal care. These principles were approved by Animal Ethics Committee of Lorestan University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Hormozi, Email: takhim55@yahoo.com.
Mohammadreza Gholami, Email: rezagholami57@gmail.com.
Ayda Babaniazi, Email: salimeh65@yahoo.com.
Anneh Mohammad Gharravi, Phone: 009823-32395054, Email: annehgh@yahoo.com, Email: gharravi@shmu.ac.ir.
References
- 1.Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadava EE, Krpata DM, Gao Y, Rosen MJ, Novitsky YW. Wound healing process and mediators: implications for modulations for hernia repair and mesh integration. J Biomed Mater Res A. 2014;102(1):295–302. doi: 10.1002/jbm.a.34676. [DOI] [PubMed] [Google Scholar]
- 3.Atiba A, Nishimura M, Kakinuma S, Hiraoka T, Goryo M, Shimada Y, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am J Surg. 2011;201(6):809–818. doi: 10.1016/j.amjsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219(2):449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma J, Wang Q, Fei T, Han J-DJ, Chen Y-G. MCP-1 mediates TGF-β–induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109(3):987–994. doi: 10.1182/blood-2006-07-036400. [DOI] [PubMed] [Google Scholar]
- 6.Larsson J, Goumans MJ, Sjöstrand LJ, van Rooijen MA, Ward D, Levéen P, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J. 2001;20(7):1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SI, Kwak JH, Na H-J, Kim JK, Ding Y, Choi ME. TGF-β1 activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-β receptor kinase activity in mesangial cells. J Biol Chem. 2009;284(33):22285–22296. doi: 10.1074/jbc.M109.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Elsner T, Botella LM, Velasco B, Corbí A, Attisano L, Bernabéu C. Synergistic cooperation between hypoxia and transforming growth factor-β pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276(42):38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 9.Mason JC, Lidington EA, Ahmad SR, Haskard DO. bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction. Am J Phys Cell Phys. 2002;282(3):C578–CC87. doi: 10.1152/ajpcell.00339.2001. [DOI] [PubMed] [Google Scholar]
- 10.Ponce CC, Chauffaille MLLF, Ihara SSM, Silva MRR. Increased angiogenesis in primary myelofibrosis: latent transforming growth factor-β as a possible angiogenic factor. Rev Bras Hematol Hemoter. 2014;36(5):322–328. doi: 10.1016/j.bjhh.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hom DB, Unger GM, Pernell KJ, Manivel JC. Improving surgical wound healing with basic fibroblast growth factor after radiation. Laryngoscope. 2005;115(3):412–422. doi: 10.1097/01.mlg.0000157852.01402.12. [DOI] [PubMed] [Google Scholar]
- 12.Jozefczuk J, Drews K, Adjaye J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J Vis Exp. 2012;(64). 10.3791/3854. [DOI] [PMC free article] [PubMed]
- 13.Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol. 2009;126(3):463–467. doi: 10.1016/j.jep.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Saini P, Al-Shibani N, Sun J, Zhang W, Song F, Gregson KS, et al. Effects of Calendula officinalis on human gingival fibroblasts. Homeopathy. 2012;101(2):92–98. doi: 10.1016/j.homp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56(5):210–215. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 16.Parente LM, Andrade MA, Brito LA, Moura VM, Miguel MP, Lino-Junior Rde S, et al. Angiogenic activity of Calendula officinalis flowers L. in rats. Acta Cir Bras. 2011;26(1):19–24. doi: 10.1590/S0102-86502011000100005. [DOI] [PubMed] [Google Scholar]
- 17.Patrick K, Kumar S, Edwardson P, Hutchinson J. Induction of vascularisation by an aqueous extract of the flowers of Calendula officinalis L. the European marigold. Phytomedicine. 1996;3(1):11–18. doi: 10.1016/S0944-7113(96)80004-3. [DOI] [PubMed] [Google Scholar]
- 18.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 19.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 20.Ganapathy N, Venkataraman SS, Daniel R, Aravind RJ, Kumarakrishnan VB. Molecular biology of wound healing. J Pharm Bioallied Sci. 2012;4(Suppl 2):S334–S337. doi: 10.4103/0975-7406.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaka YTM, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-beta 1 antisense oligode-oxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000;58:1885–1892. doi: 10.1111/j.1523-1755.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Tian Y, Xu Z-J, Zhang C-P. Adrenaline inhibited cell proliferation and regulated expression of TGF-beta1 and bFGF in cultured human hypertrophic scar fibroblasts via alpha-receptor. EXCLI J. 2008;7:1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.