Abstract
Activins and inhibins are closely related protein heterodimers with a similar tissue distribution; however, these two complexes have opposing functions in development and disease. Both are secreted cytokine hormones, with activin the primary inducer of downstream signaling cascades and inhibin acting as a rheostat that exquisitely governs activin function. Adding to the complexity of activin signaling, follistatin, a highly glycosylated monomeric protein, binds activin with high affinity and restrains downstream pathway activation but through a mechanism distinct from that of inhibin. These three proteins were first identified as key ovarian hormones in the pituitary–gonadal axis that direct the synthesis and secretion of FSH from the pituitary, hence controlling folliculogenesis. Research during the past 30 years has expanded the roles of these proteins, first by discovering the ubiquitous expression of the trio and then by implicating them in a wide array of biological functions. In concert, these three hormones govern tissue development, homeostasis, and disease in multiple organ systems through diverse autocrine and paracrine mechanisms. In the present study, we have reviewed the actions of activin and its biological inhibitors, inhibin, and follistatin, in mammary gland morphogenesis and cancer.
Activins and inhibins were first isolated as compounds in ovarian extracts that could activate and inhibit the synthesis and secretion of FSH from the pituitary gland (1, 2). Soon after, a third molecule was isolated from porcine follicular fluid that also inhibited the basal secretion of FSH from the pituitary and was termed “follistatin” (FST) to distinguish it from inhibin (3). Further studies determined that this triad also affected expression and secretion of GnRH from hypothalamic neurons and GnRH receptor expression in pituitary gonadotropes, ultimately regulating the secretion of LH and FSH. Together, FSH and LH direct ovarian follicular growth, ovulation, and estrogen and progesterone production. The breast is expressly dependent on estrogen and progesterone for both postnatal and pregnancy-induced development. Thus, the effect of activin, inhibin, and FST on normal and pathological breast function was once thought to be indirect, involving the regulation of circulating levels of ovarian steroidal hormones. It is now well-established that activin, inhibin, and FST are all expressed ubiquitously and control a wide array of physiological processes that range from fetal development to immune response to cancer (4–7). In the present study, we have highlighted the effect of local activin signaling on breast development and cancer and described the mechanisms by which inhibin and FST govern activin signaling to control ductal elongation and branching morphogenesis in the normal gland. We have also outlined the dysregulation of this pathway that occurs during breast tumorigenesis.
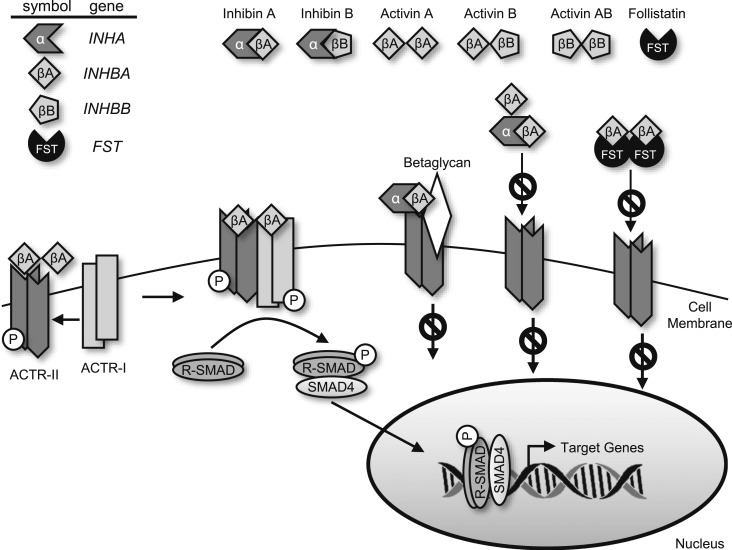
Activins and inhibins are structurally related glycoprotein hormones that are members of the TGF-β superfamily of ligands. Activins consist of disulfide-linked homodimers or heterodimers of primarily two β-subunits, βA and βB, that share 39% amino acid identity (63% in the mouse) and are each transcribed from a different gene, INHBA and INHBB (Fig. 1) (8). Two additional β-subunits, βC and βE, have also been identified; however, little is known concerning their roles in breast development and cancer. Activins have been named according to the dimer composition: activin A is a homodimer of two βA subunits, and activin AB is a heterodimer of βA and βB subunits, and so forth. Similar to TGF-β, canonical activin signaling occurs through activation of the SMAD-signaling pathway. Signaling is initiated on activin binding to one of two constitutively active serine/threonine kinase receptors, activin type II receptor (ACTR-II)A or ACTR-IIB (ACVR2A or ACVR2B) that exist as single-pass transmembrane receptors (9, 10). Binding promotes recruitment and transphosphorylation of the activin type I receptor (ACTR-I), preferentially activin type IB (ACTRIB/ALK4 or ACVR1) and subsequent phosphorylation of the receptor SMADs (R-SMADs), SMAD2 and SMAD3. These active SMADs are released into the cytoplasm where they complex with the co-SMAD, Smad4 and translocate to the nucleus where they bind to DNA and modulate transcription of target genes. In addition to activating canonical SMAD signaling in the breast, activin can also signal through the p38/MAPK, phosphatidylinositol 3-kinase/AKT and WNT/β-catenin pathways, often independently of the SMADs (11–14).
Figure 1.
The activin social network. Activins and inhibins share common β-subunits, each transcribed from a unique gene. Inhibins occur as heterodimers of an α-subunit and one of several β-subunits, and activins exist as homodimers or heterodimers of two β-subunits. Activin signaling occurs through the heteromeric ACTR-II and ACTR-I complex of serine/threonine kinase receptors. This complex phosphorylates and activates SMAD-R signaling proteins, which complex with co-SMAD4 and translocate to the nucleus to modulate transcription of target genes. Activin signaling is limited by inhibin through ligand sequestration or inhibin binding to an ACTR-II/betaglycan complex, thereby inhibiting ACTR-I recruitment and subsequent downstream signaling. FST also impedes activin signaling. Two FST molecules wrap around an activin dimer, abrogating ACTR-II binding and further receptor activation and signaling.
Activin signaling is constrained by two distinct classes of endogenous inhibitors, inhibins and FST. Inhibins are heterodimers of a unique α-subunit (INHA) with one of the β-subunits described previously. Similar to activin, inhibins are named according to their subunit composition: inhibin A is a heterodimer of the inhibin α-subunit and the βA-subunit, and so forth. Inhibins primarily antagonize the actions of activin; however, although both canonical and noncanonical activin signaling is well understood, the mechanisms by which inhibins exert their cellular actions are less clear. The most widely accepted mechanism involves the inhibition of activin signaling through competitive binding of the inhibin β-subunit to the ACTR-II receptors, thereby preventing ACTR-I recruitment and subsequent activation of downstream signaling cascades (15). An alternate mechanism involves inhibin binding to the coreceptor, betaglycan, a type III TGF-β receptor (16). Inhibins can bind betaglycan with high affinity, in contrast to its low affinity for ACTR-II receptors. Inhibin-bound betaglycan then facilitates recruitment of ACTR-II but does not recruit ACTR-I, thereby blocking subsequent downstream signaling. The coreceptor CRIPTO inhibits activin signaling in a similar manner as betaglycan, preventing the association of activin/ACTR-II with ACTR-I in an inhibin-independent fashion (17). Finally, competition exists for forming inhibin or activin owing to their shared β-subunits, revealing an additional level of regulation of these two cytokine hormones. Most importantly, it appears that inhibins do not induce intracellular signaling on their own; their primary role is to suppress the signaling of activins.
Another mechanism ensuring the correct extent of local activin signaling is the secretion of FST, a heavily glycosylated polypeptide inhibitor of activin. FST exists as two major isoforms with distinct cellular localization: a larger, circulating FST 315 isoform and a smaller splice variant that is membrane-bound, FST 288. A third FST isoform, FST 303, is produced by proteolytic cleavage of the cytoplasmic tail of FST 315. All three isoforms bind activin with affinities in the picomolar range (∼50 to 450 pM). Two FST molecules irreversibly bind an activin dimer, sterically preventing activin from binding to its cognate receptors and, thus, usurping downstream signaling pathways. Activin and FST have overlapping patterns of tissue expression, suggesting that FST finely tunes activin signaling, ensuring homeostasis (18). Structural homologs of FST exist, such as FSTL3, which also bind activin and prevent its interactions with the activin receptor. FST can also bind to and inhibit signaling of myostatin and bone morphogenetic protein, albeit at a lower affinity than activin (19). Thus, activin signaling is regulated in concert by the expression levels of activin and inhibin dimers, binding to the activin and betaglycan receptors, and the levels of the secreted binding protein, FST.
Activin, Inhibin, and FST Expression in the Normal Breast
Assessing the expression patterns of activin and inhibin is complicated by the shared nature of the β-subunits in each dimer. Inhibin α- and β-subunit expression was first discovered within the mammary gland using immunohistochemical (IHC) analyses of tissue from human chorionic gonadotropin–treated Sprague-Dawley rats (20). Although inhibin α- and β-subunit expression was undetectable in the mammary epithelial cells of vehicle-treated rats, human chorionic gonadotropin treatment induced ductal and lobuloalveolar expansion and concomitant expression of cytoplasmic inhibin α- and β-subunits in alveolar epithelial cells but not ductal cells. βB-subunit expression also extended into the mesenchymal compartment. Nearly a decade later, low expression of the inhibin α-subunit protein was reported in the nulliparous mammary epithelia and on the first day of lactation in CD-1 mice, with moderate staining also occurring in the stroma at the onset of lactation. Both βA and βB-subunit mRNA were expressed in different stages of postpubertal mammary gland development: nulliparous, lactation, and involution (21). βB expression was greatest during mid-late lactation, and expression also occurred in the stromal compartment. In contrast, expression of the inhibin α- and βA-subunits was restricted to the epithelial compartment, indicating that activin B is synthesized and secreted from the stroma of the mammary gland. In humans, the IHC findings for activin A or B have supported this finding, with detectable levels in normal human luminal and myoepithelial cells of the breast, with no variation during the menstrual cycle (22–26). Inhibin α is also expressed in luminal epithelial cells but is undetectable in the myoepithelium, and its expression is not altered during menses (23).
Local expression of the activin A and B subunits suggests patent signaling of activin throughout postpubertal mammary gland development. Moreover, mammary epithelial ACTR-IIB, ACTR-IB, betaglycan receptors, and nuclear Smad3 proteins are expressed throughout lactation and involution. However, ACTR-IB was undetectable by IHC in nulliparous mouse mammary epithelial or stroma (21). Given the requirement of ACTR-IB expression for activin signaling, this suggests that such signaling only occurs during terminal differentiation of the mammary epithelium. FST is also expressed in ductal and alveolar mammary epithelia of nulliparous mice and human breast epithelial cells (27, 28), further suggesting that activin signaling is minimal in the prepregnancy gland. However, as the activin and inhibin levels increase during pregnancy and at the onset of lactation, the FST levels appear to decrease. This likely potentiates activin signaling at these stages of development (28). Activin A and FST proteins are also present in human breast milk, further underscoring their expression in the mammary epithelium, although their effect in milk and nursing offspring is not known (29).
Activin, Inhibin, and FST Expression in Breast Cancer
Activin signaling is restrained in normal mammary epithelia by concomitant inhibin α-subunit expression, secretion of inhibins, and relatively higher levels of FST expression compared with activin. However, these expression patterns are reversed in cancerous tissue. The inhibin α-subunit is expressed locally in low-grade ductal carcinoma in situ at similar levels to that of normal tissue but becomes undetectable in invasive breast cancer or metastases (23, 30). Although circulating inhibins are not elevated in patients with postmenopausal breast cancer, tumor resection from cycling, premenopausal women has been reported to decrease serum inhibins, suggesting that breast cancer secretes inhibins at relatively modest levels (26, 31). Low serum inhibin levels in premenopausal women with early-stage breast cancer are predictive of chemotherapy-related amenorrhea. This might have been related to limited prechemotherapy ovarian reserve and reduced the production of inhibins by the ovaries rather than by the breast cancer (32).
In contrast to inhibins, most evidence has suggested an upregulation of local activin A expression in breast cancer tissue compared with normal breast tissue and elevated expression with increasing tumor aggressiveness. Chromosomes harboring the genes of several activin signaling pathway members, including the βB-subunit, are often amplified in breast cancer (33–36), and elevated βA mRNA (INHBA) expression has been reported in breast carcinoma compared with normal breast tissue in multiple studies of small patient cohorts (22, 37, 38). Furthermore, an analysis of three independent publically available gene expression data sets totaling >700 human breast tumors and 70 normal breast tissue samples, revealed that INHBA mRNA expression was increased in most breast tumors compared with normal breast tissue (28). Activin A protein expression has also been shown to be twice as high in breast cancer tissue vs nontumorigenic tissue, and circulating activin A has been elevated in patients with breast cancer, with the levels returning to baseline on tumor excision (39). In a small cohort of patients with breast cancer, activins A and B were also detected in normal, peritumoral, and cancerous breast epithelia by IHC. However, in contrast to activin A, the βB-subunit is expressed in breast carcinoma but its expression decreases in higher grade tumors. Although local activin A expression appears to be elevated within breast cancer, activin receptors and nuclear Smad3 are downregulated with increasing tumor grade (25). This suppression might result from intracellular homeostatic mechanisms aimed at normalizing the extent of activin signaling in aggressive breast cancer. However, most studies did not account for the different subtypes of breast cancer, and it is possible that the activin/inhibin/FST axis might be differentially activated in some subtypes and not others. In a small cohort of patients with breast cancer, INHBA expression was upregulated within breast cancer samples independent of estrogen, progesterone, or HER2 receptor status (22). However, the serum activin A levels were unaffected by HER2 status (40). Additionally, a mouse model of HER2-positive breast cancer demonstrated robust expression of ACTR-1B and nuclear localization of activated/phosphorylated SMAD2, suggesting full activation of the activin signaling pathway in HER2-driven tumors (41). One study reported that serum activin was not associated with tumor grade or lymph node metastases. However, another demonstrated that serum activin A is predictive of bone metastasis in patients with breast cancer (37, 39). In adulthood, serum activin levels remain constant in women, and circulating inhibin B and FST decrease with menopause, the age at which most breast cancer cases are diagnosed, suggesting maximal activin activation (42). Clearly, additional studies with larger patient cohorts are necessary to fully discern the extent of association of circulating activin, inhibin, and FST levels with breast carcinoma progression.
In contrast to activin, FST mRNA is expressed in normal mammary epithelial cells and is significantly decreased in human breast cancer and the surrounding stroma, supporting maximal activin signaling in tumor epithelia and stroma (28). This pattern of expression is mirrored at the protein level in normal and breast cancer cell lines, with FST expression highest in the nontransformed human mammary epithelial cell lines, MCF10A and MCF12A, and undetectable in several breast cancer cell lines, independent of the breast cancer subtype (28). Several studies have assessed FST protein expression in normal and malignant breast tissue, with conflicting results. Zabkiewicz et al. (27) reported that cytoplasmic and nuclear FST expression was identified in normal human breast epithelia and decreased in human breast cancer specimens with increasing invasiveness using a cohort of 93 breast cancer and 30 normal breast samples. In contrast, two separate studies from the same group found no changes in FST expression with breast cancer invasiveness or tumor grade in two smaller patient cohorts. In one study, cytoplasmic and nuclear FST expression was unchanged when comparing normal (n = 8), benign [fibroadenoma (n = 17) and florid hyperplasia with atypia (n = 17)], and malignant tissue [intraductal carcinoma in situ (n = 10) and infiltrating ductal carcinoma (n = 15)] (43). However, the staining intensity for FST was increased in the stroma of fibroadenoma samples. In a more recent study involving a larger patient cohort of 154 samples, no association in FST protein expression was found with tumor grade, menopausal status, disease stage, or progesterone expression. However, FST staining intensity was significantly lower in estrogen receptor (ER)-positive tumors compared with those lacking ER expression (44). Many possible reasons exist for the differences in the outcomes from these studies. The immunostaining approaches used to examine protein expression in breast cancer samples used different antibodies, which could have been a major contributor to the divergent results. In addition, the limited cohort sizes for these analyses could have limited the extent of conclusions that could be drawn. Addressing these potential shortcomings, a meta-analysis of >2800 patients with breast cancer (all subtypes) revealed that elevated FST mRNA expression was associated with extended recurrence-free survival compared with low FST expression, independent of breast cancer subtype (28). These data were confirmed in three additional data sets and further showed that FST mRNA expression was especially prognostic of reduced recurrence of breast cancer in the brain and lungs but not in bone. Furthermore, increased FST expression correlated with increased overall survival. Although it is possible that the mRNA and protein expression for FST could be divergent, these data did align with the study by Zabkiewicz et al. (27), suggesting that FST is likely to be suppressed in breast cancer. The finding that activin A exhibits elevated expression in human breast cancers in concert with decreased FST expression and that decreased FST expression is prognostic of recurrence imply that activin signaling is at its maximal levels in the absence of suppression provided by FST or inhibin. Moreover, these data suggest that FST and inhibin might be suppressors of breast tumorigenesis and metastasis.
Functional Insights From Genetically Engineered Mouse Models
Discerning the function of the activin signaling pathway components has been hindered by their essential role in fetal development [reviewed by Namwanje and Brown (5)]. Deletion of the ACTR-1B (Acvr1b−/−) receptor in mice leads to disruption of the primitive streak and embryonic lethality (45). Disruption of the ACTR-IIA (Acvr2a−/−) receptor results in ∼25% rate of perinatal lethality, and ACTR-IIB–null mice (Acvr2b−/−) are viable and fertile. As the type II activin receptors have largely redundant functions, the loss of both receptors in mice results in embryonic lethality with arrested growth at the egg cylinder stage (46, 47).
Regarding the ligands, homozygous loss of the inhibin α-subunit in mice (Inha−/−, leading to loss of all inhibins) results in adrenal and ovarian tumor formation and elevated activin expression, suggesting that inhibin has tumor suppressor functions that restrict protumorigenic activin signaling (48). Additionally, increased activin signaling in these mice promotes cancer-like muscle wasting or cachexia (49). The genetic loss of the βA-subunit in mice (Inhba−/−, leading to the loss of activin A, activin AB, and inhibin A) results in death shortly after birth (50). In contrast to the postnatal lethality with βA disruption, βB (Inhbb−/−, leading to loss of activin AB, activin B, and inhibin B) null mice are viable but have defects in eyelid development, no or modest impairment of female reproduction, and a failure to nurse their pups (50, 51). Inhibin-null mice do not display these phenotypes, suggesting activin, not inhibin, is essential for eyelid development and lactation. FST-null mice (Fst−/−) will die shortly after birth of craniofacial defects and respiratory failure (52). Its loss does not phenocopy inhibin (Inha) disruption, suggesting some nonoverlapping functions exist for these two endogenous activin inhibitors that might be due to their differing spatiotemporal expression patterns (48).
The divergent phenotypes of the β-subunit–null mice have demonstrated that βA- and βB-subunits have independent functions, with βA playing an essential role in organogenesis and βB in lactation. The failure of Inhbb-null mice to nurse their pups could be due to either a mammary epithelial cell-intrinsic or a mammary epithelial cell-extrinsic defect. Mouse mammary gland development closely mimics that of humans, and the use of genetically manipulated mouse models in conjunction with syngeneic transplants of mammary tissues are excellent resources to discern gene function (53). Just as in humans, mouse mammary gland morphogenesis consists of three stages: embryonic, pubertal, and adult/reproductive. In the mouse, embryonic mammary development begins at embryonic day 10 with the formation of paired placodes that form along the milk line. By embryonic day 12.5, the cells within each placode invaginate and invade into the mesenchyme, giving rise to a nascent mammary anlage. By embryonic day 18.5, a minimally branched rudimentary gland has formed that remains developmentally quiescent until puberty. Until this point, activin signaling is not required for mammary gland development. However, the Inhbb-null mouse phenotype suggests that activin is essential for ductal elongation and branching morphogenesis in the postnatal gland. Adult mammary glands from female Inhbb-null mice (lacking activin B, activin AB, and inhibin B) exhibit arrested development of the ductal tree, with stunted ductal extension into the fat pad and underdeveloped lobuloalveolar structures during pregnancy and postpartum (54). Although βB-null glands express milk protein genes, the glands are not fully capable of terminal differentiation during pregnancy/lactation, as evidenced by a lack of milk production. βB mRNA is expressed in the mammary epithelia throughout all postnatal developmental stages, with levels greater than those in most other tissues and comparable to βB expression in the ovary. Expression is further increased during lactation and extends into the surrounding stroma (21). Thus, it was unclear whether βB regulation of branching morphogenesis was conveyed in the epithelium or stroma, or both. ER-α–null mice exhibit a breast developmental defect similar to that of Inhbb-null mice, suggesting that alterations in ovarian hormones might contribute to the phenotype (55). To assess the potential contribution of ovarian estrogen and progesterone to the Inhbb-null mammary defect, intact wild-type (WT) mammary glands (anlagen that included both mammary epithelia and stroma) were transplanted into the cleared fat pads of Inhbb-null hosts. Although transplanted WT glands developed normally in Inhbb-null hosts, intact Inhbb-null glands transplanted into WT hosts remained underdeveloped, indicating systemic endocrine effects were not responsible for the mammary gland defect (56). To determine whether mammary epithelial or stromal inhibin-βB expression was necessary for the mammary gland defect, orthotopic transplantation of Inhbb−/− mammary epithelia (without surrounding stroma) into the cleared mammary fat pads of WT-recipient mice was performed. Analysis of mammary outgrowth demonstrated that the transplanted mutant epithelia developed into a mammary ductal tree to the same extent as the contralateral WT gland, indicating that intrinsic loss of Inhbb (resulting in loss of activin B, activin AB, and inhibin B) in mammary epithelia is not required for ductal elongation and alveolar development. In contrast, WT epithelia transplanted into the cleared fat pads of Inhbb-null mice failed to fully form, indicating that βB secreted from the stroma of the host promoted mammary gland epithelial branching morphogenesis and terminal differentiation. Furthermore, these data revealed that epithelially derived βB cannot compensate for the loss of Inhbb from the stroma. Finally, it is likely that homodimers or heterodimers of activin B (or AB) are responsible for this process, rather than inhibins, because the developmental defect was not revealed in inhibin α-null mice. Exogenous activin A inhibits hepatocyte growth factor-induced tubule formation of human mammary epithelial organoids in vitro, suggesting a role for activin A in constraining branching morphogenesis (24). This contrasts with the mammary gland phenotype observed in the Inhbb−/− mice described previously. Rather than indicating an inhibitory role for activins, the results from tissue recombination techniques have suggested stromal-derived activin B promotes ductal elongation and alveolar development. This discrepancy likely resulted from the different activin heterodimers examined, differing experimental paradigms used, and different timing of activin treatment. It also underscores the complex crosstalk that occurs between the stroma and epithelia, which is governed, in part, by activin signaling. To address whether phenotypic differences of the βA- and βB-subunit–null mice resulted from altered spatiotemporal expression patterns or differences in ligand/receptor effects, Brown et al. (8) generated mice with replacement of the Inhba coding region with the protein coding region of Inhbb. Insertion of Inhbb rescued the neonatal lethality and craniofacial phenotypes of the βA-null mice. However, the mice died prematurely, and the effect on mammary gland development was not explored. Together, these data indicate that the perinatal lethality of the Inhba-null mice can be rescued with appropriate spatiotemporal expression of Inhbb to support fetal organ development but that Inhba and Inhbb expression are not fully compensatory. Whether Inhba and Inhbb are redundant for mammary gland development/differentiation when expressed in the same spatiotemporal manner remains to be determined.
In addition to gene disruption studies, the effect of activin A (Inhba) overexpression has been examined in transgenic mice. Overexpression of activin A in the skin leads to hyperkeratosis and susceptibility to skin tumorigenesis and promotes malignant progression (57, 58). These data suggest that activin A, and possibly the other activins, could be classified as skin oncogenes. It has not yet been determined whether activin A can also promote tumorigenesis in other tissues. Moreover, whether activins are sufficient to initiate tumor formation and metastasis in any tissue has not been directly tested in vivo. Assessing the requirement for activins in breast cancer initiation and progression has been further hampered by the shared nature of the subunits between activins and inhibins, the redundant functions of the activin receptors, and the lack of specificity of small molecule inhibitors to the activin receptors, which also target TGF-β receptors with similar affinity. To overcome these limitations, overexpression of FST, the naturally occurring high-affinity inhibitor of activin, has been used to indirectly assess the role of activin signaling in autochthonous mammary tumor growth and metastasis. In a mouse model of HER2/Neu-induced metastatic breast cancer [mouse mammary tumor virus (MMTV)-Neu], ACTR-IB expression was associated with elevated phosphorylated-SMAD2 at the tumor–stromal interface, in the absence of detectible TGF-β receptor I (ALK5) (41). Given that βA-subunit (INHBA) mRNA and activin A protein are increased in breast cancer, these data from the mouse suggest that SMAD signaling can be active in breast cancer in the absence of TGF-β signaling. Finally, the presence of phosphorylated-SMAD2 at the tumor–stromal interface underscores the paracrine actions of stromally derived activin in tumor epithelial cells, further supporting the complex crosstalk between the tumor and its microenvironment. Similar to the expression pattern of FST mRNA in human breast cancer, Fst mRNA is decreased dramatically and FST protein is undetectable in MMTV-HER2 mouse mammary tumors compared with normal tissue, further indicating that maximal activin signaling can occur in the mammary tumor microenvironment (28). To block activin signaling in this mouse model, Fst expression was restored in the mammary epithelium using the mammary-targeting promoter of the MMTV. Enforced FST expression did not alter tumor size or latency in the MMTV-HER2/Neu mouse model, suggesting that sustained activin signaling is unnecessary for tumor formation or growth, at least in this model. In contrast, FST overexpression completely blocked metastatic progression to the lung (28), revealing that FST can act as a potent metastasis suppressor. Because the primary role of FST is blockade of activin signaling, these data also support a role for activin as a metastasis promoter. Reinforcing this possibility, inhibition of activin signaling with a soluble ACTR-IIA fusion protein (ACTRIIA.muFc) decreased breast cancer metastasis to the bone in a mouse xenograft model of experimental metastasis involving the triple-negative breast cancer cell line, MDA-MB-231, which lacks estrogen, progesterone, and HER2 receptors (59). Similar to FST inhibition of activin signaling in the HER2 mouse model, treatment with the ACTR-IIA fusion protein did not alter the proliferation of breast cancer cells in vitro or in vivo. Further indicating a prometastatic role for activin, the INHBA gene is one of the most predictive genes within a core metastasis-associated expression signature of multiple cancers (60). Selected subtypes of breast cancer will preferentially metastasize to the bone, and elevated serum activin expression in patients with breast cancer can predict for bone metastasis. Activin A is highly expressed in the bone and, in concert with FST, regulates osteoblast/osteoclast function and bone remodeling (61, 62). Together, these data suggest that breast carcinoma seeding and outgrowth in the bone might occur because of the highly favorable activin-mediated interactions between the tumor and the bone microenvironment (37).
Whether activin promotes or inhibits growth of the primary tumor appears to be either model or breast cancer subtype dependent. The tumorigenesis and metastasis studies described examined HER2-positive and triple-negative breast cancer models. In contrast to these models, which displayed no change in tumor growth with activin inhibition, activin A overexpression in the ER-positive breast cancer cell line, MCF7, promoted tumor growth when the cells were subcutaneously injected into nude mice (22). This contrasts with the results from in vitro studies, which demonstrated activin A-induced cell cycle arrest in the ER-positive breast cancer cell line, T47D, and suggests that the autocrine/paracrine actions of activin on tumor cell growth might depend on the cell microenvironment (63). Moreover, activin overexpression did not increase metastatic nodules of MCF7 cells in the liver, although the lesion size was increased. Tumors also demonstrated increased expression of α-smooth muscle actin and vascular endothelial growth factor and decreased E-cadherin (CDH1) expression, markers indicative of epithelial-to-mesenchymal transition (EMT). In addition to evaluating activin function in ER-positive breast cancer cells, these studies were restricted to overexpression of activin A. In contrast, FST overexpression should block signaling from all activins. Thus, differences in primary tumor growth vs metastatic progression in the different studies could also have resulted from the unique actions of the different activins. One study directly compared the effect of activin and FST overexpression. In that study, activin or FST was overexpressed in the R30C human breast cancer cell line (ER-negative), and their individual effects on tumor growth were assessed using flank xenografts in severe combined immunodeficiency mice. In these studies, activin expression increased, and FST decreased, the tumor growth rates. The proliferation marker, Ki-67, was not altered among the mock, activin, or FST-overexpressing tumors. However, FST-overexpressing tumors did demonstrate an increase in TUNEL-positive staining, indicating that FST overexpression promoted tumor cell death. In this model, the investigators attributed the changes in cell viability to altered vascularization of the tumors (64). Angiogenesis is required for tumor cell growth. Activin overexpression decreased angiogenesis in tumors, and FST promoted vascularization. However, these data are inconsistent with the tumor growth phenotypes observed. In addition, the specific isoform of activin that was expressed in that model was not disclosed. Taken together, the results from these studies suggest that activin signaling has a substantial effect on breast cancer progression (Table 1), although the specific stages affected are less clear.
Table 1.
Mouse Models of Activin Social Network Function in Breast
| Activin Network Member | Model | Manipulation | Outcome | Investigator |
|---|---|---|---|---|
| Inhbb | Knockout mouse | Inhbb−/− mouse | Reduced ductal and alveolar development, lactation failure | Matzuk et al. (49), 1994; Schrewe et al. (51), 1994 |
| Inhbb | Knockout mouse | Inhbb−/− mouse | Lactation failure, retarded ductal and alveolar morphogenesis, absence of secreted milk | Robinson et al. (54), 1997 |
| Inhbb | Knockout mouse | Inhbb−/− mouse and syngeneic transplant of mammary tissue | WT epithelia transplanted into Inhbb−/− stroma failed to develop | Robinson et al. (54), 1997 |
| Activin A | Heterotopic xenograft in mice | Activin A overexpression in human luminal breast cancer cells (MCF7) xenografted into the flanks of nude mice | Increased tumor growth associated with increased expression of SMA, VEGF, and decreased CDH1 | Bashir et al. (22), 2015 |
| Activin A | Heterotopic xenograft in mice | Tail-vein injection of activin A overexpression in human luminal breast cancer cells (MCF7) | Increased size, not number, of liver metastases | Bashir et al. (22),2015 |
| Activin (?) | Heterotopic xenograft in mice | Activin overexpression in R30C human breast cancer cell line (ER-negative) subcutaneously xenografted into flanks of mice | Increased tumor size; decreased tumor angiogenesis | Krneta et al. (64), 2006 |
| ACTR-IIA | Heterotopic xenograft in mice | Subcutaneous or intracardiac xenograft of DA-MB-231-luc-D3H2LN human triple-negative breast cancer cells and treatment with soluble activin receptor type IIA fusion protein by subcutaneous injection 2×/wk | No change in tumor burden with subcutaneous xenografted cells; reduced osteolytic disease and metastasis to bone of metastatic outgrowth xenografted cells | Chantry et al. (59), 2010 |
| FST | Compound hemizygous mice | Overexpression of follistatin in transgenic mouse model of HER2/Neu-induced metastatic breast cancer | No change in tumor growth or latency, complete abrogation of metastatic progression to lung | Seachrist et al. (28), 2017 |
| FST | Heterotopic xenograft in mice | FST overexpression in R30C human breast cancer cell line (breast cancer subtype unknown) subcutaneously xenografted into flanks of mice | Decreased tumor size, increased tumor angiogenesis, increased TUNEL+ cells | Krneta et al. (64), 2006 |
The activin dimer is unknown.
Abbreviations: SMA, smooth muscle actin; VEGF, vascular endothelial growth factor.
Mechanistic Insights of Activin Action in the Breast
Our understanding of the effects of activin signaling on mammary gland biology has continued to evolve. This is largely because of the complex nature of activin dimers in conveying both positive and negative effects on mammary gland proliferation and branching morphogenesis. In addition, activins and inhibins are secreted cytokines that can act in cell-intrinsic and cell-extrinsic manners. Because of these complexities, the mechanisms of action of activins in mammary gland proliferation and tumorigenesis have not yet been fully elucidated.
Functional studies of activin signaling in normal breast have often been extrapolated from experimental paradigms using human breast cancer cell lines and transgenic mouse models (Tables 1 and 2). In mice, stromal activin B promotes ductal and alveolar development and differentiation into patent milk-forming units and is necessary for lactation. However, similar to its cousin, TGF-β, ectopic activin A inhibits proliferation of luminal breast cancer cell lines in vitro (24). Activin-induced G1 cell cycle arrest is mediated by SMAD-dependent inhibition of cyclin A1 expression. This results in reduced phosphorylation of Rb protein and subsequent inhibition of the cell cycle (63). Activin treatment also activates the p38/MAPK signaling cascades, leading to phosphorylation of the transcription factor ATF2. Both this and the SMAD pathways are required for activin-induced repression of proliferation of T47D luminal breast cancer cells that express ERs (11, 63). The activin signaling pathway also intersects with estrogen signaling to mutually repress one another, with estrogen stimulating proliferation and activin repressing growth. Activin inhibits estrogen-induced expression of Trefoil factor-1 (TFF1) and transcriptional activity of promoters that are dependent on estrogen response elements. Estrogen, in turn, inhibits activin-induced signal transduction by blocking SMAD signaling. Estrogen also suppresses expression of the βB-subunit and activin B secretion, ensuring termination of activin signaling (65). Activin-induced expression of plasminogen activator inhibitor-1 (PAI-1) is also repressed in the presence of estrogen. Low PAI-1 expression has been associated with poor patient outcomes in ER-positive breast cancer and the ability of activin to suppress PAI-1 expression might explain the highly invasive nature of ER-negative breast malignancies (71). The proliferation of ER-positive (luminal) breast cancer cell lines but not triple-negative breast cancer cells can be suppressed by treatment with CDK4/6 inhibitors, such as palbociclib, which prevent degradation of the cell cycle repressor, Rb. These drugs are currently in clinical use for ER-positive disease. Adding activin A to palbociclib can cooperatively inhibit proliferation of ER-positive breast cancer cells, independently of Rb status, indicating intersections between the SMAD and CDK4/6 pathways in activin-regulated growth (72). Thus, although indirect, estrogen can be added to the list of activin inhibitors, in addition to inhibin and FST, which keep activin signaling in check. Given the ability of estrogen to repress the actions of activin, the loss of serum ovarian estrogens that occurs with menopause might promote activin signaling in the patient population and lead to breast cancer initiation or progression, despite the absence of changes in the serum activin levels with age (42). Furthermore, circulating inhibin and FST levels decline with age in women, suggesting maximal activin signaling. However, most cases of postmenopausal breast cancer are ER- and/or progesterone receptor–positive and dependent on estrogen signaling. This suggests a complex interplay between signaling by activin and estrogen in postmenopausal breast cancer. More studies are required to dissect the complexity of the crosstalk between estrogen and activin signaling pathways in both breast development and cancer.
Table 2.
Mechanisms of Activin Social Network Function in Breast
| Activin Network Member | Model | Outcome | Investigator |
|---|---|---|---|
| Activin A | Activin A treatment of human mammary epithelial organoids | Inhibits HGF-induced tubule formation | Liu et al. (24), 1996 |
| Activin A | Activin A treatment of luminal breast cancer cell lines | Induces G1 cell cycle arrest dependent on activin-induced SMAD and p38/MAPK signaling | Burdette et al. (63), 2005 |
| Activin A | Activin A treatment of luminal breast cancer cell lines | Inhibits estrogen-induced transcription activity and estrogen inhibits activin B expression and SMAD signaling | Burdette and Woodruff (65), 2007 |
| Activin A | Genotoxic stress of mammary epithelia and stromal fibroblasts | Increased activin A leading to COX-2 and cytokine induction, promotes protumorigenic cancer-associated fibroblasts and desmoplasia | Fordyce et al. (66), 2012 |
| Activin A | Activin A treatment of mammary epithelial cells | Represses CD36 expression in adjacent fibroblasts contributing to dense breasts and increased breast cancer risk | DeFilippis et al. (67), 2012; DeFilippis et al. (68), 2014 |
| Activin/Nodal | Activin/nodal inhibition of Luminal and basal breast cancer cell lines | Interconversion of differentiated breast cancer cells into cancer stem cells | Meyer et al. (69), 2009 |
| Activin A | Activin A overexpression in luminal or knockdown in basal breast cancer cell lines | Activin A promotes and is necessary to maintain the CD44+/CD24− cancer stem cell population | Bashir et al. (22), 2015 |
| Activin A | Overexpression or treatment with activin A of breast cancer cell lines | Activin A overexpression or treatment promotes migration, invasion and select EMT characteristics in breast cancer cell lines | Bashir et al. (22), 2015; Seachrist et al. (28), 2017; Neel and Lebrun (70), 2013 |
Abbreviation: HGF, hepatocyte growth factor.
Although one report demonstrated that activin A does not alter cell cycle progression in primary human luminal and myoepithelial cells, the weight of evidence has suggested that activin A induces cell cycle arrest in epithelial cells. Data suggesting that activin A has no effect on human mammary epithelial cell proliferation could be limited to the use of human primary epithelial cells that have undergone numerous passages but retain active p16/Rb signaling (24, 73). Under normal conditions, human mammary epithelial cells undergo senescence when subjected to DNA damage or telomere erosion. These genetic insults induce expression and secretion of activin A, resulting in upregulation of the proinflammatory enzyme, cyclooxygenase-2 (COX-2), which is dependent on the p38 signaling pathway (74). Activin-induced SMAD activation reduces Cyclin A expression, leading to reduced phosphorylation of Rb. When DNA surveillance checkpoints are in place, such insults result in growth arrest or senescence of mammary epithelial cells. In contrast, loss of an intact p16/Rb tumor suppressor pathway can enable epithelial cells to bypass senescence checkpoints and become hyperproliferative, predisposing them to genomic instability and oncogenic transformation (75, 76).
Activin A can also act in a paracrine fashion to foster a protumorigenic microenvironment in the mammary gland. Activin A promotes polarization and a proinflammatory phenotype of macrophages and alterations in adjacent fibroblasts (77). Under genotoxic stress, epithelial-derived activin A is secreted into the microenvironment, inducing COX-2 expression by undamaged epithelial cells and cancer-associated fibroblasts and macrophages (66, 77, 78). COX-2 is one of the two rate-limiting steps in the synthesis and secretion of prostaglandins, powerful tumor promoting cytokines that stimulate proliferation, invasion, angiogenesis, and immune evasion (79). Fibroblasts exposed to epithelial-derived activin A have increased expression of cytokines and growth factors and extracellular matrix deposition. They also transition to aerobic glycolysis, phenocopying cancer-associated fibroblasts and a desmoplastic phenotype. In addition, increased cytokine and growth factor production from fibroblasts promotes migration of mammary epithelial cells (66).
Desmoplastic features are present in normal breast tissue with high mammographic density (67, 80), and women with dense breasts are approximately five times more likely to develop invasive breast cancer (81, 82). The stroma of cancer-free dense breasts is similar to the stroma surrounding cancerous lesions, with chronic inflammation and elevated extracellular matrix deposition, concomitant with reduced adiposity (83). Tissue undergoing wound repair also displays these features, and cancer has often been referred to as a “wound that will not heal” (84). Activin is a critical orchestrator of wound repair and is both necessary and sufficient for this process in the skin (85, 86). The epithelial compartment of dense breasts also differs from low-density breast epithelia by displaying shorter telomeres and increased DNA-damage signaling, which leads to elevated expression and secretion of activin A. Moreover, mammary epithelial cells transiently exposed to physiological levels of activin A will induce durable repression of CD36 gene expression in adjacent fibroblasts over several passages (68). In chronic wounds and in dense normal and malignant breast stroma, CD36 is downregulated, making its absence a marker of reactive stroma. DeFilippis et al. (67, 68) further found that CD36 is both necessary and sufficient to recapitulate the desmoplastic response. Together, these data suggest that even transient DNA damage in mammary epithelia will result in short bursts of activin A secretion that may be sufficient to induce lasting gene expression changes in adjacent fibroblasts and promote a protumorigenic environment, especially in breast tissue with high mammographic density. These findings also suggest that activin A might be a therapeutic target to remediate high-density breast tissue with the goal of reducing the breast cancer risk in these women.
Cancer stem cells are considered the seeds of metastatic disease. Inflammation and wound healing pathways enhance cancer stem cell properties and are associated with breast cancer metastasis (87). Dysregulated activin signaling is also associated with wound healing and breast cancer metastasis, suggesting that activin might control the formation or maintenance of breast cancer stem cells (37). Supporting this possibility, the addition of activin A to culture media sustained pluripotency and self-renewal of embryonic stem cells (74, 88). In breast cancer, activin/nodal signaling is required for interconversion of differentiated breast cancer cells into cancer stem cells (69). Furthermore, activin A overexpression in luminal breast cancer cells leads to enrichment of a population of cells with the CD44+/CD24− cancer stem cell phenotype, and silencing activin decreases this population (22). The gene expression signature of stem cells overlaps with that of cells that have undergone EMT and are migratory and invasive (89). Activin induces migration and invasion of breast cancer cells in vitro (22, 28, 70) and can induce the expression of select EMT markers in breast cancer, albeit to a more modest extent compared with its family member, TGF-β, a highly potent inducer of EMT (22, 90, 91).
Conclusion
Activin regulation of biological processes is not only complex, but also incredibly diverse. Thus, it should be unsurprising that the role of activin in breast development and cancer is equally broad. Local activin signaling can either promote or inhibit mammary growth, depending on the state of the epithelial cells and other factors within the microenvironment (Table 2). In addition, systemic activin can affect the levels of circulating ovarian steroids, which also profoundly affect the breast. The local effects of activins are manifest by gene expression changes in epithelial cells, supportive fibroblasts, vascular endothelium, and immune and mammary stem cells. This has a clear effect on mammary gland morphogenesis, for which paracrine activin signaling is essential for terminal differentiation and milk production. In addition, the triad of activin, inhibin, and FST within the microenvironment has been associated with breast cancer outcomes. We are just beginning to understand the effect of these proteins in metastatic progression. The complex crosstalk between epithelial-derived activin signaling molecules and the supportive microenvironment is dysregulated when combined with genotoxic stressors such as telomere erosion or DNA damage, promoting oncogene-induced transformation and metastasis. Thus, inhibition of activin signaling has been suggested as a viable target for the treatment of breast cancer (28, 92). Whether selective small molecule inhibitors of activin signaling can be generated remains to be determined. However, the results from current studies suggest that FST could be a useful biological agent to inhibit metastatic progression. Additionally, inhibition of activin signaling through activin type II receptors would have the added benefit of abrogating cachexia in cancer patients by promoting muscle regeneration and bone growth (93–95). Selective modified soluble antagonists of either ACTR-IIA or ACTR-IIB have been developed and have shown efficacy in several disease models (96, 97). However, given the ubiquitous role of activin signaling in multiple physiological processes, the effect of activin inhibition on wound repair, the immune response, and ovarian function should be weighed against its benefits as a therapeutic target in breast cancer. Fully discerning the mechanisms of action of activin in breast development, transformation, and cancer progression will likely be necessary before targeting this pathway as a therapeutic approach for breast cancer.
Acknowledgments
FinancialSupport: The present study was supported by the National Institutes of Health (Grants R01CA206505, R01CA213843, and P30CA043703, all to R.A.K.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACTR-I
activin type I receptor
- ACTR-II
activin type II receptor
- COX-2
cyclooxygenase-2
- EMT
epithelial-to-mesenchymal transition
- ER
estrogen receptor
- FST
follistatin
- IHC
immunohistochemical
- MMTV
mouse mammary tumor virus
- PAI-1
plasminogen activator inhibitor-1
- SMAD-R
receptor SMAD
- WT
wild-type
References
- 1. Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321(6072):776–779. [DOI] [PubMed] [Google Scholar]
- 2. Rivier J, Spiess J, McClintock R, Vaughan J, Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985;133(1):120–127. [DOI] [PubMed] [Google Scholar]
- 3. Ueno N, Ling N, Ying SY, Esch F, Shimasaki S, Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci USA. 1987;84(23):8282–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ball EMA, Risbridger GP. Activins as regulators of branching morphogenesis. Dev Biol. 2001;238(1):1–12. [DOI] [PubMed] [Google Scholar]
- 5. Namwanje M, Brown CW. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb Perspect Biol. 2016;8(7):a021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aleman-Muench GR, Soldevila G. When versatility matters: activins/inhibins as key regulators of immunity. Immunol Cell Biol. 2012;90(2):137–148. [DOI] [PubMed] [Google Scholar]
- 7. Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. In: Gerald L, ed. Vitamins & Hormones. Vol 85. Cambridge, MA: Academic Press; 2011:255–297. [DOI] [PubMed] [Google Scholar]
- 8. Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet. 2000;25(4):453–457. [DOI] [PubMed] [Google Scholar]
- 9. Attisano L, Wrana JL, Montalvo E, Massagué J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 1996;16(3):1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson TB, Woodruff TK, Jardetzky TS. Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. EMBO J. 2003;22(7):1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cocolakis E, Lemay S, Ali S, Lebrun J-J. The p38 MAPK pathway is required for cell growth inhibition of human breast cancer cells in response to activin. J Biol Chem. 2001;276(21):18430–18436. [DOI] [PubMed] [Google Scholar]
- 12. Do T-V, Kubba LA, Antenos M, Rademaker AW, Sturgis CD, Woodruff TK. The role of activin A and Akt/GSK signaling in ovarian tumor biology. Endocrinology. 2008;149(8):3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirota M, Watanabe K, Hamada S, Sun Y, Strizzi L, Mancino M, Nagaoka T, Gonzales M, Seno M, Bianco C, Salomon DS. Smad2 functions as a co-activator of canonical Wnt/beta-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cell Signal. 2008;20(9):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki K, Kobayashi T, Funatsu O, Morita A, Ikekita M. Activin A induces neuronal differentiation and survival via ALK4 in a SMAD-independent manner in a subpopulation of human neuroblastomas. Biochem Biophys Res Commun. 2010;394(3):639–645. [DOI] [PubMed] [Google Scholar]
- 15. Lebrun JJ, Vale WW. Activin and inhibin have antagonistic effects on ligand-dependent heteromerization of the type I and type II activin receptors and human erythroid differentiation. Mol Cell Biol. 1997;17(3):1682–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404(6776):411–414. [DOI] [PubMed] [Google Scholar]
- 17. Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA. 2003;100(9):5193–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling: a paradigm for the new millennium. Exp Biol Med (Maywood). 2002;227(9):724–752. [DOI] [PubMed] [Google Scholar]
- 19. Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147(7):3586–3597. [DOI] [PubMed] [Google Scholar]
- 20. Alvarado MV, Russo J, Russo IH. Immunolocalization of inhibin in the mammary gland of rats treated with hCG. J Histochem Cytochem. 1993;41(1):29–34. [DOI] [PubMed] [Google Scholar]
- 21. Jeruss JS, Santiago JY, Woodruff TK. Localization of activin and inhibin subunits, receptors and SMADs in the mouse mammary gland. Mol Cell Endocrinol. 2003;203(1-2):185–196. [DOI] [PubMed] [Google Scholar]
- 22. Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer. 2015;1(1):15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Loreto C, Reis FM, Cataldi P, Zuiani C, Luisi S, Beltrami CA, Petraglia F. Human mammary gland and breast carcinoma contain immunoreactive inhibin/activin subunits: evidence for a secretion into cystic fluid. Eur J Endocrinol. 1999;141(2):190–194. [DOI] [PubMed] [Google Scholar]
- 24. Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L. Inhibitory effects of activin on the growth and morpholgenesis of primary and transformed mammary epithelial cells. Cancer Res. 1996;56(5):1155–1163. [PubMed] [Google Scholar]
- 25. Jeruss JS, Sturgis CD, Rademaker AW, Woodruff TK. Down-regulation of activin, activin receptors, and Smads in high-grade breast cancer. Cancer Res. 2003;63(13):3783–3790. [PubMed] [Google Scholar]
- 26. Reis FM, Luisi S, Carneiro MM, Cobellis L, Federico M, Camargos AF, Petraglia F. Activin, inhibin and the human breast. Mol Cell Endocrinol. 2004;225(1-2):77–82. [DOI] [PubMed] [Google Scholar]
- 27. Zabkiewicz C, Resaul J, Hargest R, Jiang WG, Ye L. Increased expression of follistatin in breast cancer reduces invasiveness and clinically correlates with better survival. Cancer Genomics Proteomics. 2017;14(4):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seachrist DD, Sizemore ST, Johnson E, Abdul-Karim FW, Weber Bonk KL, Keri RA. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res. 2017;19(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luisi S, Calonaci G, Florio P, Lombardi I, De Felice C, Bagnoli F, Petraglia F. Identification of activin A and follistatin in human milk. Growth Factors. 2002;20(3):147–150. [DOI] [PubMed] [Google Scholar]
- 30. Mylonas I, Jeschke U, Shabani N, Kuhn C, Friese K, Gerber B. Inhibin/activin subunits (inhibin-alpha, -betaA and -betaB) are differentially expressed in human breast cancer and their metastasis. Oncol Rep. 2005;13(1):81–88. [PubMed] [Google Scholar]
- 31. Robertson DM, Stephenson T, Pruysers E, McCloud P, Tsigos A, Groome N, Mamers P, Burger HG. Characterization of inhibin forms and their measurement by an inhibin α-subunit ELISA in serum from postmenopausal women with ovarian cancer. J Clin Endocrinol Metab. 2002;87(2):816–824. [DOI] [PubMed] [Google Scholar]
- 32. Anders C, Marcom PK, Peterson B, Gu L, Unruhe S, Welch R, Lyons P, Behera M, Copland S, Kimmick G, Shaw H, Snyder S, Antenos M, Woodruff T, Blackwell K. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26(3):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JKV, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. [DOI] [PubMed] [Google Scholar]
- 34. Bièche I, Khodja A, Driouch K, Lidereau R. Genetic alteration mapping on chromosome 7 in primary breast cancer. Clin Cancer Res. 1997;3(6):1009–1016. [PubMed] [Google Scholar]
- 35. Bièche I, Lidereau R. Genetic alterations in breast cancer. Genes Chromosomes Cancer. 1995;14(4):227–251. [DOI] [PubMed] [Google Scholar]
- 36. Aubele MM, Cummings MC, Mattis AE, Zitzelsberger HF, Walch AK, Kremer M, Hofler H, Werner M. Accumulation of chromosomal imbalances from intraductal proliferative lesions to adjacent in situ and invasive ductal breast cancer. Diagn Mol Pathol. 2000;9(1):14–19. [DOI] [PubMed] [Google Scholar]
- 37. Leto G, Incorvaia L, Badalamenti G, Tumminello FM, Gebbia N, Flandina C, Crescimanno M, Rini G. Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin Exp Metastasis. 2006;23(2):117–122. [DOI] [PubMed] [Google Scholar]
- 38. Wang J, Jiang H, Huang Z, Liu R, Huang Q, Wu J, Liu R.The expression status of INHBA as a prognostic marker for human breast cancer. Int J Clin Exp Pathol. 2016;9(11):11334–11342. [Google Scholar]
- 39. Reis FM, Cobellis L, Tameirão LC, Anania G, Luisi S, Silva IS, Gioffrè W, Di Blasio AM, Petraglia F. Serum and tissue expression of activin a in postmenopausal women with breast cancer. J Clin Endocrinol Metab. 2002;87(5):2277–2282. [DOI] [PubMed] [Google Scholar]
- 40. Jueckstock J, Burkhardt N, Kuhn C, Blankenstein T, Mahner S, Schindlbeck C, Janni W, Rack B, Mylonas I. Expression of activin during and after chemotherapy in peripheral blood of patients with primary breast cancer. Anticancer Res. 2016;36(5):2153–2159. [PubMed] [Google Scholar]
- 41. Landis MD, Seachrist DD, Montañez-Wiscovich ME, Danielpour D, Keri RA. Gene expression profiling of cancer progression reveals intrinsic regulation of transforming growth factor-beta signaling in ErbB2/Neu-induced tumors from transgenic mice. Oncogene. 2005;24(33):5173–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reame NE, Lukacs JL, Olton P, Ansbacher R, Padmanabhan V. Differential effects of aging on activin A and its binding protein, follistatin, across the menopause transition. Fertil Steril. 2007;88(4):1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bloise E, Couto HL, Massai L, Ciarmela P, Mencarelli M, Borges LE, Muscettola M, Grasso G, Amaral VF, Cassali GD, Petraglia F, Reis FM. Differential expression of follistatin and FLRG in human breast proliferative disorders. BMC Cancer. 2009;9(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Couto HL, Dela Cruz C, Buzelin MA, Toppa NH, Wainstein AJ, Reis FM. Follistatin expression in human invasive breast tumors: pathologic and clinical associations. Appl Immunohistochem Mol Morphol. 2018;26(2):108–112. [DOI] [PubMed] [Google Scholar]
- 45. Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev. 1998;12(6):844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213(1):157–169. [DOI] [PubMed] [Google Scholar]
- 47. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356–360. [DOI] [PubMed] [Google Scholar]
- 48. Matzuk MM, Finegold MJ, Su J-GJ, Hsueh AJW, Bradley A. α-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360(6402):313–319. [DOI] [PubMed] [Google Scholar]
- 49. Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A. 1994;91(19):8817–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995;374(6520):354–356. [DOI] [PubMed] [Google Scholar]
- 51. Schrewe H, Gendron-Maguire M, Harbison ML, Gridley T. Mice homozygous for a null mutation of activin βB are viable and fertile. Mech Dev. 1994;47(1):43–51. [DOI] [PubMed] [Google Scholar]
- 52. Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374(6520):360–363. [DOI] [PubMed] [Google Scholar]
- 53. McNally S, Stein T. Overview of mammary gland development: a comparison of mouse and human. In: Martin F, Stein T, Howlin J, eds. Mammary Gland Development: Methods and Protocols. New York: Springer New York; 2017:1–17. [DOI] [PubMed] [Google Scholar]
- 54. Robinson GW, Hennighausen L. Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development. 1997;124(14):2701–2708. [DOI] [PubMed] [Google Scholar]
- 55. Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–186. [PubMed] [Google Scholar]
- 56. Robinson GW, Accili D, Hennighausen L. Rescue of mammary epithelium of early lethal phenotypes by embryonic mammary gland transplantation as exemplified with insulin receptor null mice. In: Ip MM, Asch BB, eds. Methods in Mammary Gland Biology and Breast Cancer Research. Boston, MA: Springer; 2000:307–316. [Google Scholar]
- 57. Antsiferova M, Huber M, Meyer M, Piwko-Czuchra A, Ramadan T, MacLeod AS, Havran WL, Dummer R, Hohl D, Werner S. Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response. Nat Commun. 2011;2(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans LW, Huylebroeck D, Balling R, Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 1999;18(19):5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud’huin M, Coulton L, Evans H, Abdul N, Werner ED, Bouxsein ML, Key ML, Seehra J, Arnett TR, Vanderkerken K, Croucher P. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res. 2010;25(12):2633–2646. [DOI] [PubMed] [Google Scholar]
- 60. Kim H, Watkinson J, Varadan V, Anastassiou D. Multi-cancer computational analysis reveals invasion-associated variant of desmoplastic reaction involving INHBA, THBS2 and COL11A1. BMC Med Genomics. 2010;3(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ogawa Y, Schmidt DK, Nathan RM, Armstrong RM, Miller KL, Sawamura SJ, Ziman JM, Erickson KL, de Leon ER, Rosen DM, Seyedin SM, Glaser CB, Chang RJ, Corrigan AZ, Vale W Bovine bone activin enhances bone morphogenetic protein-induced ectopic bone formation. J Biol Chem. 1992;267(20):14233–14237. [PubMed] [Google Scholar]
- 62. Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HA, van Leeuwen JP. The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J. 2007;21(11):2949–2960. [DOI] [PubMed] [Google Scholar]
- 63. Burdette JE, Jeruss JS, Kurley SJ, Lee EJ, Woodruff TK, Activin A. Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells. Cancer Res. 2005;65(17):7968–7975. [DOI] [PubMed] [Google Scholar]
- 64. Krneta J, Kroll J, Alves F, Prahst C, Sananbenesi F, Dullin C, Kimmina S, Phillips DJ, Augustin HG. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 2006;66(11):5686–5695. [DOI] [PubMed] [Google Scholar]
- 65. Burdette JE, Woodruff TK. Activin and estrogen crosstalk regulates transcription in human breast cancer cells. Endocr Relat Cancer. 2007;14(3):679–689. [DOI] [PubMed] [Google Scholar]
- 66. Fordyce CA, Patten KT, Fessenden TB, DeFilippis R, Hwang ES, Zhao J, Tlsty TD. Cell-extrinsic consequences of epithelial stress: activation of protumorigenic tissue phenotypes. Breast Cancer Res. 2012;14(6):R155–R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen Y-Y, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, Marx JJ, Tjoe JA, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty TD. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2(9):826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DeFilippis RA, Fordyce C, Patten K, Chang H, Zhao J, Fontenay GV, Kerlikowske K, Parvin B, Tlsty TD. Stress signaling from human mammary epithelial cells contributes to phenotypes of mammographic density. Cancer Res. 2014;74(18):5032–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meyer MJ, Fleming JM, Ali MA, Pesesky MW, Ginsburg E, Vonderhaar BK. Dynamic regulation of CD24 and the invasive, CD44posCD24neg phenotype in breast cancer cell lines. Breast Cancer Res. 2009;11(6):R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Neel J-C, Lebrun J-J. Activin and TGFβ regulate expression of the microRNA-181 family to promote cell migration and invasion in breast cancer cells. Cell Signal. 2013;25(7):1556–1566. [DOI] [PubMed] [Google Scholar]
- 71. Corte MD, Vérez P, Rodríguez JC, Roibás A, Domínguez ML, Lamelas ML, Vázquez J, García Muñiz JL, Allende MT, González LO, Fueyo A, Vizoso F. Tissue-type plasminogen activator (tPA) in breast cancer: relationship with clinicopathological parameters and prognostic significance. Breast Cancer Res Treat. 2005;90(1):33–40. [DOI] [PubMed] [Google Scholar]
- 72. Harada M, Morikawa M, Ozawa T, Kobayashi M, Tamura Y, Takahashi K, Tanabe M, Tada K, Seto Y, Miyazono K, Koinuma D. Palbociclib enhances activin-SMAD-induced cytostasis in estrogen receptor-positive breast cancer. Cancer Sci. 2019;110(1):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409(6820):633–637. [DOI] [PubMed] [Google Scholar]
- 74. Xiao L, Yuan X, Sharkis SJ, Activin A. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24(6):1476–1486. [DOI] [PubMed] [Google Scholar]
- 75. Fordyce C, Fessenden T, Pickering C, Jung J, Singla V, Berman H, Tlsty T. DNA damage drives an activin a-dependent induction of cyclooxygenase-2 in premalignant cells and lesions. Cancer Prev Res (Phila). 2010;3(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gauthier ML, Berman HK, Miller C, Kozakeiwicz K, Chew K, Moore D, Rabban J, Chen YY, Kerlikowske K, Tlsty TD. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12(5):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sierra-Filardi E, Puig-Kröger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, Bernabéu C, Vega MA, Corbí AL. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117(19):5092–5101. [DOI] [PubMed] [Google Scholar]
- 78. Nüsing RM, Barsig J. Induction of prostanoid, nitric oxide, and cytokine formation in rat bone marrow derived macrophages by activin A. Br J Pharmacol. 1999;127(4):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–386. [DOI] [PubMed] [Google Scholar]
- 80. Guo Y-P, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10(3):243–248. [PubMed] [Google Scholar]
- 81. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. [DOI] [PubMed] [Google Scholar]
- 82. Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103(15):1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1(1):119–150. [DOI] [PubMed] [Google Scholar]
- 84. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. [DOI] [PubMed] [Google Scholar]
- 85. Sulyok S, Wankell M, Alzheimer C, Werner S. Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol Cell Endocrinol. 2004;225(1-2):127–132. [DOI] [PubMed] [Google Scholar]
- 86. Wankell M, Munz B, Hübner G, Hans W, Wolf E, Goppelt A, Werner S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001;20(19):5361–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis. 2015;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A, Activin A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23(4):489–495. [DOI] [PubMed] [Google Scholar]
- 89. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brown KA, Aakre ME, Gorska AE, Price JO, Eltom SE, Pietenpol JA, Moses HL. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004;6(3):R215–R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Valcourt U, Kowanetz M, Niimi H, Heldin C-H, Moustakas A. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16(4):1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal. 2009;7(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Latres E, Mastaitis J, Fury W, Miloscio L, Trejos J, Pangilinan J, Okamoto H, Cavino K, Na E, Papatheodorou A, Willer T, Bai Y, Hae Kim J, Rafique A, Jaspers S, Stitt T, Murphy AJ, Yancopoulos GD, Gromada J. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8:15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lotinun S, Pearsall RS, Davies MV, Marvell TH, Monnell TE, Ucran J, Fajardo RJ, Kumar R, Underwood KW, Seehra J, Bouxsein ML, Baron R. A soluble activin receptor type IIA fusion protein (ACE-011) increases bone mass via a dual anabolic-antiresorptive effect in Cynomolgus monkeys. Bone. 2010;46(4):1082–1088. [DOI] [PubMed] [Google Scholar]
- 95. Pearsall RS, Canalis E, Cornwall-Brady M, Underwood KW, Haigis B, Ucran J, Kumar R, Pobre E, Grinberg A, Werner ED, Glatt V, Stadmeyer L, Smith D, Seehra J, Bouxsein ML. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci USA. 2008;105(19):7082–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dong F, He XJI. Activin A: a potential therapeutic target for characterizing and stopping joint pain early in rheumatoid arthritis patients. Inflammation. 2014;37(1):170–176. [DOI] [PubMed] [Google Scholar]
- 97. Chen JL, Walton KL, Al-Musawi SL, Kelly EK, Qian H, La M, Lu L, Lovrecz G, Ziemann M, Lazarus R, El-Osta A, Gregorevic P, Harrison CA. Development of novel activin-targeted therapeutics. Mol Ther. 2015;23(3):434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]