Abstract
The quality of the intrauterine environment, in which the placenta plays a critical role, affects birth outcomes and lifelong health. The effect of metal contaminants on the growth and functioning of the placenta have not been widely reported but may provide insights into how metal exposures lead to these outcomes. We examined relationships between placental concentrations of cadmium (Cd), arsenic (As), mercury (Hg) and lead (Pb) and measures of placental growth and functioning (placental weight, placental efficiency (the log ratio of placental weight and birth weight), chorionic disc area and disc eccentricity) as part of the New Hampshire Birth Cohort Study (N=1159). We additionally examined whether these associations were modified by placental concentrations of essential elements zinc (Zn) and selenium (Se). Associations were evaluated using generalized linear models. Multivariable-adjusted differences in placental weight were −7.81g (95% CI: −15.42, −2.48) with every ng/g increase in the Cd concentration of placenta (p-Value = 0.0009). Greater decrements placental weight and efficiency associated with placental Cd were observed for females. For placentae with below median Zn and Se concentrations, decrements in placental weight were −8.81g (95% CI: 16.85, −0.76) and −13.20g (95% CI: −20.70, −5.70) respectively. The Cd concentration of placenta was also associated with reductions in placental efficiency both overall, and in Zn- and Se-stratified models. No appreciable differences were observed with other elements (As, Hg or Pb) and with other placental measures (chorionic disc area and disc eccentricity). In structural equation models, placental weight was a mediator in the relation between placental Cd concentration and reduced birth weight. Our findings suggest a role of interacting essential and contaminant elements on birth weight that may be mediated by changes in the growth and function of the placenta.
Keywords: placenta, weight, efficiency, arsenic, cadmium, zinc, selenium
INTRODUCTION
The quality of the intrauterine environment has a lifelong effect on health and is mediated by the placenta, a temporary organ that plays a critical role in fetal growth and development. The placenta ensures an optimal supply of nutrients and respiratory gases, removes waste products and hormonally regulates the progression of pregnancy. Arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) are ubiquitous environmental contaminants that the Agency for Toxic Substances and Disease Registry lists among its top priority contaminants. All four of these elements pass through the placenta and are known for their developmental toxicity1–4. We have reported previously on the concentrations of these elements in placental specimens collected from women enrolled in the New Hampshire Birth Cohort Study5, 6, an epidemiological study of mother-infant pairs in which we found an association between placental As and household drinking water As concentration and biomarkers of As exposure.
Maternal-fetal transfer of As and Pb are approximately equal, whereas Hg is efficiently transported to the fetus7–10. Cadmium may accumulate in the placenta11–17. Contaminant metal transfer across the placenta is poorly understood, but metals can take advantage of existing transport systems18, 19. This molecular mimicry has been observed in the placenta between Zn and Cd20–24. Cadmium directly interacts with membrane transporters for both iron (Fe) and Zn, and in the placenta Cd may reduce the efficiency of Zn transport to the fetus by stimulating metallothionein production, which bind to and inhibit transport of Zn as well as Cd to the fetus25–28. Transporters for As, Pb and Hg are largely unknown, although rodent studies suggest the MRP (multi-drug resistant proteins) family of transporters may be involved in Hg transport across the placenta29.
In utero exposure to As may contribute to chronic disease risk, such as cardiometabolic disease13, 30. In utero moderate As exposure is also associated with deficits in learning and memory, as well as a host of metabolic and metastatic disorders that can manifest across the lifespan31. Evidence indicates that As can re-program the glucocorticoid receptor system, which impacts adult health32. Cadmium exposure during pregnancy may disturb Zn transfer to the fetus, alter glucocorticoid balance33, 34 and the regulation of insulin-like growth factor-related proteins, as well as inhibit the migration of human trophoblast cells21, 35.
The ability of the placenta to detect and respond to changes in environmental conditions in utero is thought to differ between sexes36. Adverse pregnancy outcomes tend to be sex specific. Much of the prior literature on Cd exposure during pregnancy has observed decreased birth weight in females37, 38.
Nutritional status also plays a pivotal role in toxic metal exposure20, 39. Selenium plays an important role in metal detoxification; functions as an antioxidant that promotes thyroid and immune system functioning and is a constituent of more than two dozen selenoproteins (e.g. glutathione peroxidase) that play critical roles in protection from oxidative damage. Selenium may have a role in mitigating Hg toxicity, although epidemiologic evidence is limited12, 40, 41.
Placental efficiency is commonly defined by birth weight:placental weight ratio, or the weight of fetus produced per weight of placenta42, 43. Placental efficiency is a proxy measure of placental development and/or function, particularly of nutrient transfer to the fetus43. Placental weight is proportional to birth weight to the three-quarters power, and placental weight can be used as a proxy for fetal metabolic rate. The association between placental measurements and health outcomes is established in the placental literature, with a strong association between the growth of the placenta, birth weight and birth outcomes44, 45. Weight and the regularity of the shape (eccentricity) of the placenta are directly related to the placental stress. The weight and regularity of the shape of the placental disc are influenced by placental stresses experienced during growth, whereby shape alterations reflect changes in the vascular architecture of the placenta that occur in response to nutrient limiting conditions46.
The effects of metal contaminants on placental growth and functioning have not been widely reported. Measures of placental functioning can provide information about how environmental stressors affect placental growth and may provide insight into how environmental stressors affect long-term health. These gross measures have implications for health risks associated with poor fetal growth44, 47–50. For example, in a prospective cohort (N=979), irregularity of eccentricity was associated with maternal uteroplacental and fetoplacental vascular pathologies45. Disc shape is established early on in development, and can indicate perturbations or exposures that occur earlier in gestation47. The association between placental measurements and infant health outcomes is well established44, 45. Extremes of either low or high placental efficiency have been associated with cardiovascular disease51. Lower placental weight has been associated with greater risk of pre-eclampsia, induced labor, spontaneous preterm delivery, stillbirth and low birth weight, whereas high placental weight has been associated with a higher risk of C-section, post-term delivery and high birth weight52.
We analyzed concentrations of As, Cd, Hg and Pb as part of the New Hampshire Birth Cohort Study, and the potential interacting role of essential elements Zn and Se as modifiers of these effects. We further evaluated the role of placental growth and functioning as a mediator of the effects of placental metals on birth weight. We also explored sex-specific effects by stratifying our data by the sex of the infant and evaluated the potential role of infant sex as a modifier by including infant sex as an interaction term in our associations.
MATERIALS AND METHODS
New Hampshire Birth Cohort Study
The NHBCS is a prospective study designed to examine the associations of As exposure on fetal growth and development during early childhood53, 54. We used data on the first 1,159 individuals from the ongoing NHBCS, from whom we had collected the elemental profile from placental specimens collected at birth. The NHBCS recruited women with a singleton pregnancy, whose primary residential water source is a private well, and who obtain their prenatal care at clinics in New Hampshire, a state with detectable As concentrations in private well water, which exceeds the current maximum contaminant limit (10 μg/L) in over 10% of these wells. To be eligible for the study, women were currently pregnant, 18–45 years of age, receiving routine prenatal care at one of the study clinics, using a private well that serves less than 15 households or 25 individuals at their place of residence, residing in the same place since their last menstrual period and not planning to move prior to delivery.
Infant length, weight, head circumference, gestational age (weeks), and sex at birth were collected from a medical record review by trained study staff. Women were asked to report their usual weight when not pregnant as part of the enrollment questionnaire. Values were combined with height abstracted from the medical records to compute BMI (kg/m2). Pre-pregnancy weight status was categorized as normal weight (18.5 – 24.9 kg/m2), overweight (≥25 – 29.9 kg/m2) or obese (≥30 kg/m2). Thirty women who were classified as underweight (<18.5 kg/m2) before the current pregnancy were excluded from this analysis.
Demographics, lifestyle and medical history were collected via prenatal and postpartum questionnaires54. Trained study staff conducted a medical record review to collect results of blood pressure measurements, gestational diabetes status, preeclampsia, placenta abnormalities, placenta previa or placental abruption.
The study protocols for the New Hampshire Birth Cohort Study (NHBCS) were approved by the Committee for the Protection of Human Subjects at Dartmouth College. All participants provided written informed consent.
Placental Collection Protocol
After delivery, study coordinators completed a placental documentation form, which collected information on the date and time of delivery, total weight of the placenta recorded to the nearest whole gram, the greater and lesser (cross-section) diameter of the placental disc in centimeters and the location of the cord insertion (central, peripheral or velamentous).
Placental biopsies were uniformly collected from the fetal side, at the base of the cord insertion avoiding vasculature, and measuring approximately 1 cm deep and 1–2 cm in diameter. The maternal decidua was removed to avoid inclusion of calcium (Ca) deposits and connective tissue. Placental biopsies were stored in trace element-free tubes, which were labeled with a sample barcode ID and stored at −80°C until analysis.
ICP-MS analysis
Prior to analysis placental samples were transferred to a −20°C freezer and then to 4°C for a maximum of 2 days, and then brought to room temperature. 1 ml of HNO3/HCl (Optima™) at a 9:1 ratio was added to samples with up to 500 mg mass, whereas 2 ml was added to samples greater than 500 mg. Samples were digested via microwave (CEM, Microwave Assisted Reaction System) ramping the temperature to 95°C in 15 minutes and holding at this temperature for 45 minutes, after which 0.25 – 0.35 ml H2O2 was added to each tube and the microwave digestion sequence was repeated. Quality control procedures for the digestion included analysis of laboratory-fortified blanks, digestion blanks and standard reference material (NIST 1566b, Oyster tissue). All samples were analyzed by ICP-MS (7700× Agilent, Santa Clara, CA). For analysis quality control, we used a laboratory-prepared reference placental digest prepared from multiple samples of de-identified placental tissue pooled to create a 2L bulk placental digest solution. The pooled sample was mixed, analyzed and an aliquot was included with each batch of placental samples analyzed. Analysis quality control also included the use of internal standard, initial and continuing calibration verification and blanks and analytical duplicates and spikes. Selenium was analyzed in reaction cell mode with hydrogen, and all other elements were analyzed using helium as a collision gas (7700×, Agilent, Santa Clara, CA). NIST 1566b Oyster Tissue was digested and analyzed as a reference material. Quality assurance/quality control parameters for placental elemental analysis are given in Supplemental Table 6.
Placental efficiency measures
Study Outcomes
We used measures of placental weight and size to derive three measures of placental functioning, namely placental efficiency, chorionic plate area and disc eccentricity45. Calculation of placental efficiency (β) from placental weight (PW) and birth weight (BW):
Calculation of chorionic plate area (A) from maximum (a) and minimum (b) radius (diameter/2):
Calculation of disc eccentricity (E) from:
Statistical Analysis
We tested for associations between placental metal concentrations and measures of placental growth and functioning by conducting multivariable linear regressions using generalized linear models (GLM), which are robust and well-suited to variables with arbitrary distributions. We included covariates in our multivariable models based on our a priori knowledge of their associations with exposure and outcomes from our previous work5, 6 (Supplemental Table 5). Models were adjusted for maternal pre-pregnancy body mass index (BMI) (continuous), maternal education level (ordinal, with five categories listed in Table 1, assigned values of 1–5, respectively), gestational age (continuous), parity (ordinal), maternal age at enrollment (continuous) and maternal exposure to cigarette smoke either before or during pregnancy (ordinal, assigned values of 0–2, where 0 is never exposed to cigarette smoke, 1 is exposed before but not during pregnancy and 2 is exposed during pregnancy). We conducted our models with placental metal concentrations both as continuous variables (ng/g) and as quintiles to look for possible non-linear effects. We used a contrast statement in our GLM models to compare Q2 through Q5 with the lowest or control Q1.
Table 1.
Selected characteristics of participants in the New Hampshire Birth Cohort Study. Data are arithmetic mean (standard deviation).
| Characteristics | N (%) | Placental weight (g) | Placental Efficiency | Chorionic Plate Area (cm2) | Disc Eccentricity |
|---|---|---|---|---|---|
| Maternal Age at enrollment | |||||
| <20 | 17 (1.5) | 656 (151) | 0.80 (0.02) | 278.0 (101.1) | 1.16 (0.12) |
| 20–29 | 363 (31.3) | 616 (161) | 0.79 (0.03) | 268.3 (70.0) | 1.20 (0.15) |
| 30–35 | 437 (37.7) | 615 (150) | 0.78 (0.02) | 280.3 (76.4) | 1.19 (0.14) |
| >35 | 342 (29.5) | 606 (145) | 0.78 (0.02) | 277.0 (69.5) | 1.18 (0.13) |
| Maternal Educationa | |||||
| < 11th grade | 8 (0.8) | 670 (210) | 0.80 (0.02) | 297.3 (84.3) | 1.14 (0.05) |
| High school graduate/GED | 117 (11.3) | 613 (173) | 0.79 (0.03) | 276.5 (79.3) | 1.17 (0.12) |
| Junior college/come college | 206 (19.9) | 635 (175) | 0.79 (0.03) | 286.8 (81.8) | 1.19 (0.14) |
| College graduate | 411 (39.8) | 608 (137) | 0.78 (0.02) | 268.6 (64.8) | 1.21 (0.16) |
| Postgraduate schooling | 292 (28.2) | 610 (147) | 0.78 (0.02) | 275.6 (71.5) | 1.18 (0.13) |
| Maternal BMIb | *** | *** | * | ||
| <18.5 | 30 (2.7) | 561 (108) | 0.78 (0.02) | 277.6 (70.1) | 1.17 (0.14) |
| 18.5–24.9 | 553 (49.6) | 592 (142) | 0.78 (0.02) | 268.7 (71.0) | 1.19 (0.14) |
| 25–29.9 | 281 (25.2) | 632 (169) | 0.79 (0.03) | 284.5 (81.3) | 1.20 (0.14) |
| >30 | 252 (22.6) | 643 (146) | 0.79 (0.02) | 281.1 (67.4) | 1.19 (0.15) |
| Previous pregnanciesc | *** | ** | ** | * | |
| 0 | 485 (42.1) | 583 (148) | 0.78 (0.03) | 265.7 (68.7) | 1.21 (0.17) |
| 1 | 430 (37.3) | 632 (154) | 0.79 (0.02) | 284.2 (76.8) | 1.18 (0.12) |
| 2 | 154 (13.4) | 627 (147) | 0.79 (0.02) | 279.8 (78.2) | 1.17 (0.12) |
| ≥3 | 84 (7.3) | 665 (144) | 0.79 (0.02) | 278.9 (61.5) | 1.18 (0.11) |
| Infant Sex | |||||
| Female | 585 (50.5) | 618 (161) | 0.78 (0.02) | 279.1 (73.2) | 1.19 (0.14) |
| Male | 574 (49.5) | 609 (142) | 0.79 (0.02) | 271.9 (72.5) | 1.19 (0.15) |
| Gestational age | ** | * | ** | ** | |
| Pre-term (<37 weeks) | 63 (5.4) | 534 (178) | 0.79 (0.03) | 240.8 (65.5) | 1.25 (0.20) |
| Term (37–40 weeks) | 951 (82.1) | 616 (149) | 0.79 (0.02) | 277.0 (73.3) | 1.19 (0.14) |
| Late (≥41 weeks) | 145 (12.5) | 632 (150) | 0.78 (0.03) | 280.2 (70.1) | 1.18 (0.12) |
| Smoking History | |||||
| History of/currently smoking | 130 (14.9) | 620 (178) | 0.78 (0.02) | 279 (76.5) | 1.19 (0.14) |
| Never smoked | 751 (85.2) | 615 (149) | 0.78 (0.03) | 275 (71.7) | 1.19 (0.14) |
Education data missing for 125 participants
BMI data missing for 43 participants
Parity data missing for 6 participants.
p<0.05
p<0.001
p<0.0001
Concentrations of Pb, Cd, As, Hg, Zn and Se in placenta were normally distributed and continuous concentration data was not transformed for statistical analysis. Elemental values falling below the detection limit were given a value of the limit of detection divided by the square root of 2. This simple substitution approach was used because non-detects constituted less than 10% of the data, and this technique performs better than LOD/2 to estimate the mean of lognormal data with one detection limit55.
All statistical analyses were conducted using the JMP statistical software package, using a complete case approach; wherein participants with missing data were not included in analysis. The number of observations (N) is given alongside results of each analysis. Where the number of missing values was high, we assessed whether the missing population had significantly different covariate characteristics from those with complete data using one-way ANOVAs.
We first examined associations between placental growth measures (placental weight, placental efficiency, chorionic plate area and disc eccentricity) and maternal-infant characteristics, including maternal age at enrollment, maternal education (as an indicator of socioeconomic status), maternal exposure to smoking, infant sex, gestational age, maternal BMI and parity, using generalized linear models.
We examined associations between placental metal concentrations with infant and placental growth measures overall (unstratified) and stratified by the sex of the infant (sex-specific). Additional stratification was performed on the basis of the median placental concentration of essential elements, Zn (9.8 μg/g) or Se (263 ng/g). In addition, to test whether infant sex was a modifier we also conducted three-way interaction models for each individual element, which considered infant sex, placental Zn and Se status for each individual measure of placenta functioning.
For elements for which we observed a statistically significant associations, we used a mediation approach56 to test the mediation effect of placental weight in the causal pathway between metal concentrations in the placenta and birth weight, with additional stratification by infant sex. The mediation effect is an estimate of the indirect effect of the metal concentration of the placenta on reduced birth weight through a change in placental weight. The confounders in other models were also used for adjustment of the mediation analysis. The Sobel Z test was used to estimate and test the mediation effect57.
Sensitivity Analysis
We conducted sensitivity analysis by running models with and without the inclusion of smoking variables, and by excluding participants that had smoked tobacco either before or during their pregnancy. Because smoking is a major potential source of Cd exposure to the general population, we also added an interaction term between maternal exposure to cigarette smoke during pregnancy and placental Cd in our models of the effects of Cd on placental growth and function. Additionally, we tested the effects on the models of excluding participants who reported a diagnosis of hypertension, gestational diabetes and pre-eclampsia during pregnancy.
RESULTS
Maternal-infant characteristics
The study population was predominantly white, with an average maternal age of 31.2 years (10th – 90th percentile range of 24.9 – 37.5) and a mean pre-pregnancy BMI of 26 kg/m2 (20.2 – 33.9) (Table 1). Overall 82% of participants gave birth to full-term infants (⩾37– 40.9 weeks’ gestation). There was an equal male/female distribution (574 males and 585 females) and no reported cases of pre-eclampsia in the NHBCS as of this analysis. Delivery reports noted placental abnormalities in a total 4% participants (N = 8 cases of placental abnormalities and n = 5 cases of placental abruption), and these were excluded from our analyses. While demographic information was obtained from medical record review, education data was not available for 125 participants, BMI data was missing for 43 participants and parity information was not available for 6 participants. Additionally, placental weight data was missing for 164 participants, measures of minimum and/or maximum placental diameter (used to calculate plate area and eccentricity) were missing for 166 participants, and birthweight measures (used to calculate placental efficiency) were missing for 21 participants. The final number of observations for placental measures was: 995 placental weights, 977 placental efficiency, 993 plate area and 993 for eccentricity. Using one-way ANOVA, we compared maternal age at enrollment, maternal BMI, parity, gestational age and maternal education between the population missing placental measures data and those for whom data was complete and found no statistically significant differences in these characteristics.
Parity was positively associated with placental weight. After adjustment for maternal pre-pregnancy BMI and age at enrollment, we found an average increase in placental weight of 25g (95% CI: 15.3 – 34.6) (p<0.0001). Also, placental efficiency increased by 0.003 for every previous pregnancy; and placental disc symmetry was also greater (p = 0.02) among women with a higher number of previous pregnancies (Supplemental Table 5). We observed a positive relationship between placental efficiency and maternal pre-pregnancy BMI, with an increase of 0.001 with every unit increase in BMI (p<0.0001). As expected, placental disc area, eccentricity and efficiency all varied as a function of gestational age: the placental efficiency was lower for term than pre-term infants, and even lower for late-delivery (≥ 41 weeks) infants. A lower gestational age was associated with a less symmetrical placental shape.
Placental metal concentrations
Detection rates (percentage of samples with concentrations higher than the instrument detection limit) were 92% for As and Hg, 99% for Cd, and 100% for Pb, Se and Zn. The average placental concentrations were 1.21 (range of 0.006 – 18.35) ng/g for As, 3.46 (range of 0.07– 22.3) ng/g for Cd, 2.32 (range of 0.002 – 35.35) ng/g for Hg; 2.31 (range of 0.007 – 53.6) ng/g for Pb; 269.7 (range of 46.0 – 883.9) ng/g for Se and 10.26 (range of 0.55 –48.2) μg/g for Zn (Supplemental Table 1).
Cadmium concentrations differed between placentae of male and female infants: with males having an average Cd concentration of 3.31 (± SD 1.97) ng/g in the placenta, and females 3.61 (± SD 2.47) ng/g (t = −2.23, p = 0.0259). Cadmium concentrations of the placenta were also associated with maternal age at enrollment: for every year older the Cd concentration of the placenta was estimated to increase by 0.1 ng/g. Zinc concentrations of the placenta also differed between male and female placentae: with males having 9.99 (± SD 4.13) μg/g and females having 10.51 (± SD 4.30) μg/g (t = −2.85, p = 0.0044). Placental Zn concentrations varied as a function of maternal pre-pregnancy BMI, with lower Zn concentrations associated with higher BMI (ß = −44.23, p = 0.0059). Likewise, similar significant trends were observed for both Cd and Pb, where women with a higher pre-pregnancy BMI had lower concentrations of these elements in their placenta (Supplemental Table 1).
Placental growth and functioning measures
We observed that lower placental weight and placental efficiency were both associated with higher Cd concentrations in placenta (Table 2). In our adjusted models, placental weight decrements were −7.80 g for every ng/g of Cd in placenta. Every ng/g increase in Cd concentrations in placenta were also associated with a reduction in placental efficiency of −0.0012. Analysis of placental concentrations as quintiles did not uncover any non-linear effects in any of the metals, however they did support our findings for Cd (Supplemental Table 4).
Table 2.
Adjusted parameter estimates for the change in placental growth measures (weight, efficiency, chorionic plate area and eccentricity) with each unit increase in term placental biopsy metal concentration (ng/g), stratified by infant sex and placental zinc and selenium concentrations.
| Model Stratification | Element | Placenta weight (g) | Placenta efficiency | Chorionic disc area | Eccentricity | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | ||
| NONE | As | −2.80 (−9.35, 3.75) | 0.4018 | −0.0004 (−0.001, 0.001) | 0.5183 | 1.83 (−1.39, 5.04) | 0.2645 | −0.0018 (−0.0081, 0.0045) | 0.5755 |
| (N=868) | Cd | −7.80 (−15.42, −2.48) | 0.0009 | −0.0012 (−0.0020, −0.0004) | 0.0026 | −1.94 (−4.21, 0.34) | 0.0946 | 0.0023 (−0.0022, 0.0067) | 0.3213 |
| Hg | −1.80 (−5.65, 2.20) | 0.1495 | −0.0002 (−0.0006, 0.0002) | 0.2554 | −0.25 (−1.45, 0.96) | 0.6846 | −0.0002 (−0.0026, 0.0022) | 0.8683 | |
| Pb | 2.49 (−1.74, 6.69) | 0.1083 | 0.0005 (0.0000, 0.0010) | 0.0498 | 0.28 (−1.21, 1.78) | 0.7123 | −0.0026 (−0.0055, 0.0004) | 0.0865 | |
| Infant sex: | As | −8.68 (−19.20, 1.84) | 0.1058 | −0.0012 (−0.003, 0.000) | 0.1633 | −0.31 (−5.35, 4.72) | 0.9023 | −0.0004 (−0.0101, 0.0094) | 0.9433 |
| Female (N=495) | Cd | −8.95 (−15.42, −2.48) | 0.0068 | −0.0013 (−0.0023, −0.0003) | 0.0140 | −2.25 (−5.37, 0.86) | 0.1560 | 0.0052 (−0.0007, 0.0112) | 0.0861 |
| Hg | −1.73 (−5.65, 2.20) | 0.3880 | −0.0003 (−0.0009, 0.0003) | 0.3103 | −0.43 (−2.31, 1.45) | 0.6527 | −0.0005 (−0.0041, 0.0031) | 0.7820 | |
| Pb | 2.48 (−1.74, 6.69) | 0.2486 | 0.0005 (−0.0002, 0.0011) | 0.1706 | −0.48 (−2.49, 1.53) | 0.6401 | −0.0024 (−0.0063, 0.0015) | 0.2330 | |
| Male (N=498) | As | 2.43 (−5.78, 10.63) | 0.5616 | 0.0003 (−0.001, 0.002) | 0.7016 | 3.73 (−0.42, 7.88) | 0.0780 | −0.0023 (−0.0106, 0.0060) | 0.5893 |
| Cd | −6.09 (−12.80, 0.06) | 0.0751 | −0.0009 (−0.0021, 0.0003) | 0.1490 | −2.20 (−5.61, 1.21) | 0.2045 | −0.0004 (0.0072, 0.0064) | 0.9046 | |
| Hg | −1.56 (−4.66, 1.43) | 0.3212 | −0.0001 (−0.0007, 0.0004) | 0.6500 | −0.12 (−1.69, 1.46) | 0.8851 | 0.0003 (−0.0028, 0.0034) | 0.8490 | |
| Pb | 2.33 (−2.16, 6.83) | 0.3075 | 0.0006 (−0.0002, 0.0014) | 0.1519 | 1.47 (−0.81, 3.74) | 0.2049 | −0.0034 (−0.0079, 0.0012) | 0.1474 | |
| Median Zn: | As | −0.97 (−10.18, 8.25) | 0.8366 | 0.0001 (−0.001, 0.002) | 0.8477 | 2.12 (−2.55, 6.75) | 0.3701 | 0.0001 (−0.0092, 0.0095) | 0.9777 |
| Below (N=501) | Cd | −8.81 (−16.85, −0.76) | 0.0320 | −0.0017 (−0.0031, −0.0004) | 0.0124 | −0.15 (−4.23, 3.93) | 0.9407 | 0.0057 (−0.0024, 0.0138) | 0.1702 |
| Hg | −0.60 (−4.22, 3.03) | 0.7460 | 0.0001 (−0.0005, 0.0007) | 0.8171 | −0.36 (−2.18, 1.46) | 0.6983 | −0.0003 (−0.0047, 0.0041) | 0.2546 | |
| Pb | 2.42 (−2.63, 7.47) | 0.3468 | 0.0006 (−0.0002, 0.0014) | 0.1581 | 0.55 (−2.00, 3.09) | 0.6731 | −0.0025 (−0.0066, 0.0015) | 0.6502 | |
| Above (N=502) | As | −3.60 (−13.06, 5.86) | 0.4545 | −0.0008 (−0.002, 0.001) | 0.3173 | 1.94 (−2.58, 6.47) | 0.3987 | −0.0022 (−0.0108, 0.0064) | 0.6202 |
| Cd | −5.14 (−11.99, 1.71) | 0.141 | −0.0008 (−0.0019, 0.0002) | 0.1336 | −3.10 (−6.05, −0.15) | 0.0398 | 0.0012 (−0.0044, 0.0069) | 0.6755 | |
| Hg | −3.06 (−6.43, 0.31) | 0.0754 | −0.0005 (−0.0011, 0.0001) | 0.1046 | −0.33 (−1.95, 1.29) | 0.6870 | −0.0003 (−0.0047, 0.0041) | 0.4005 | |
| Pb | 2.96 (−0.95, 6.86) | 0.1375 | 0.0006 (−0.0001, 0.0012) | 0.1064 | 0.10 (−1.78, 1.97) | 0.9171 | −0.0025 (−0.0066, 0.0015) | 0.1529 | |
| Median Se: | As | −1.38 (−9.90, 7.14) | 0.7507 | 0.0001 (−0.013, 0.001) | 0.9228 | 1.54 (−2.82, 5.91) | 0.4874 | −0.0001 (−0.0091, 0.0088) | 0.9763 |
| Below (N=505) | Cd | −13.20 (−20.70, −5.70) | 0.0006 | −0.0022 (−0.0034, −0.0009) | 0.0008 | −1.00 (−4.89, 2.88) | 0.6117 | 0.0008 (−0.0072, 0.0087) | 0.8514 |
| Hg | −2.67 (−5.59, 0.25) | 0.0726 | −0.0003 (−0.0008, 0.0002) | 0.2114 | −0.25 (−1.75, 1.25) | 0.7448 | −0.0011 (−0.0042, 0.0020) | 0.4854 | |
| Pb | 0.32 (−3.48, 4.12) | 0.8691 | 0.0001 (−0.0056, 0.0007) | 0.8680 | −0.33 (−2.27, 1.62) | 0.7424 | −0.0029 (−0.0069, 0.0011) | 0.1602 | |
| Above (N=506) | As | −6.04 (−16.41, 4.34) | 0.2533 | −0.0012 (−0.003, 0.001) | 0.2100 | 1.60 (−3.16, 6.37) | 0.5083 | −0.0039 (−0.0126, 0.0048) | 0.3766 |
| Cd | −5.12 (−11.26, 1.03) | 0.1023 | −0.0008 (−0.0019, 0.0003) | 0.1411 | −1.67 (−4.51, 1.17) | 0.1814 | 0.0025 (−0.0027, 0.0077) | 0.3515 | |
| Hg | −0.38 (−4.94, 4.17) | 0.6880 | −0.0002 (−0.0010, 0.0006) | 0.6194 | 0.12 (−1.97, 2.21) | 0.9104 | 0.0004 (−0.0034, 0.0042) | 0.8321 | |
| Pb | 6.50 (1.39, 11.60) | 0.0127 | 0.0013 (0.0004, 0.0022) | 0.0032 | 1.33 (−1.03, 3.68) | 0.2689 | −0.0017 (−0.0060, 0.0026) | 0.4357 | |
Models adjusted for maternal pre-pregnancy BMI (continuous), maternal education level (ordinal, with five categories listed in Table 1, assigned values of 1–5 respectively, gestational age (continuous), parity (continuous), maternal age at enrollment (continuous) and maternal exposure to cigarette smoke before or during pregnancy (ordinal, with three categories, where 0 is never exposed; 1 is exposed before pregnancy and 2 is continued to smoke during pregnancy) p<0.05 in bold type. Placental weight data was missing for 164 participants, and measures of maximum placental diameter were missing for 163 participants, measures of minimum and maximum placental diameter were missing for 166 participants and birthweight measures were missing for 21 participants
Stratified analyses
We found that associations between Cd concentrations of the placenta and both placental weight and placental efficiency were stronger among female than males, and stronger in infants with below-median Zn concentrations than above (Supplemental Table 2). However, three-way interaction terms between placental Cd concentration, infant sex and either Zn or Se were not statistically significant (Table 3). We found associations between placental efficiency and Cd concentration (Supplemental Table 2), particularly among female placentae (p-Value = 0.0014, Table 2), which was not significant in male infants (p = 0.149).
Table 3.
p-Values for the three-way interaction terms between infant sex, placental growth measures and either placental zinc or selenium status (N=992).
| Nutrient | Element | Placenta Weight (g) | Efficiency | Disc Area | Disc Eccentricity |
|---|---|---|---|---|---|
| Zn | As | 0.9887 | 0.8228 | 0.9667 | 0.4776 |
| Cd | 0.2134 | 0.3137 | 0.3388 | 0.8500 | |
| Hg | 0.6108 | 0.7958 | 0.7521 | 0.9341 | |
| Pb | 0.4334 | 0.1354 | 0.9310 | 0.6544 | |
| Se | As | 0.8038 | 0.9774 | 0.8398 | 0.7505 |
| Cd | 0.7356 | 0.662 | 0.5641 | 0.5912 | |
| Hg | 0.4498 | 0.9209 | 0.5148 | 0.7186 | |
| Pb | 0.7595 | 0.8788 | 0.9715 | 0.7468 | |
When we stratified data by median Zn concentration of the placenta, the decrements in placental weight associated with Cd concentrations were larger in placentae with below-median Zn concentrations: −8.81g (95% CI −16.85, −0.76; p = 0.032) compared to −6.97g (95% CI −13.09, −0.84; p = 0.026) in placentae with above-median Zn concentrations. This was also observed for Se; decrements in placental weight were larger when placental Se concentrations were lower (−13.20 g, 95% CI −20.70, −5.70; p = 0.0006) than when placental Se concentrations were above median (−5.11g, 95% CI −11.26, 1.03; p = 0.1023).
Stratifying the data by both infant sex and placental Zn status showed significant associations between placental Cd and reduction in placental weight and efficiency for females regardless of placental Zn concentrations. Stratifying by infant sex and placental Se showed significant associations between placental weight in both females and with low Se. The highest placental weight decrement observed in this study was in the placental weight of males with low Se in response to placental Cd.
Sensitivity Analysis
Sensitivity analysis of statistically significant models between placental Cd concentration and the weight or efficiency of the placenta excluding women who had reported smoking either before or during their pregnancy found that these models were robust (Supplemental Table 3). Sensitivity analysis of statistically significant models between placental Cd and placental weight or placental efficiency, excluding women who reported having hypertension during their pregnancy (N=74); women who were diagnosed with gestational diabetes (N=71), or women who were diagnosed with pre-eclampsia (N=46) showed that these associations were robust. Only one association did not remain statistically significant with these exclusions; between placental Cd concentration and placental weight in placenta with above median Zn: β= −5.14 (−11.99, 1.71) p-Value = 0.141.
We did not observe associations between the concentration of other metals of interest (As, and Pb) and measures of placental weight and functioning.
Interactions
In the unstratified models the interaction term with maternal smoking during pregnancy was significant in measures of placental weight and efficiency (Table 4). In sex-stratified analysis, the interaction term was significant for both placental weight and efficiency in females only. When the population was stratified by Zn or Se, interaction terms with maternal smoking were only significant in the above median strata. When we applied both layers of stratification, the interaction term with maternal smoking was significant only in females with above median placental concentrations of Zn and Se.
Table 4.
p-Values for the model interaction term maternal smoking x placental cadmium concentration on measures of placental growth and functioning.
| Model Stratification | Placenta weight (g) | Placenta Efficiency | Chorionic Disc Area |
|---|---|---|---|
| None | 0.0154 | 0.0024 | 0.4145 |
| Infant Sex: | |||
| Female | 0.0093 | 0.0016 | 0.294 |
| Male | 0.8747 | 0.5218 | 0.9133 |
| Median Zn: | |||
| Below | 0.302 | 0.1073 | 0.8931 |
| Above | 0.001 | 0.0011 | 0.0436 |
| Infant sex and Zn: | |||
| Female, below | 0.4197 | 0.1686 | 0.9785 |
| Female, above | 0.0008 | 0.0005 | 0.0253 |
| Male, below | 0.5141 | 0.3215 | 0.5932 |
| Male, above | 0.9276 | 0.7789 | 0.6361 |
| Median Se: | |||
| Below | 0.997 | 0.2382 | 0.2071 |
| Above | 0.0069 | 0.0046 | 0.1814 |
| Infant sex and Se: | |||
| Female, below | 0.2686 | 0.071 | 0.0386 |
| Female, above | 0.0027 | 0.4267 | 0.0567 |
| Male, below | 0.2816 | 0.2608 | 0.8229 |
| Male, above | 0.9189 | 0.388 | 0.5632 |
Placental weight data was missing for 164 participants, and measures of maximum placental diameter were missing for 163 participants, measures of minimum and maximum placental diameter were missing for 166 participants and birthweight measures were missing for 21 participants
Mediation Analysis
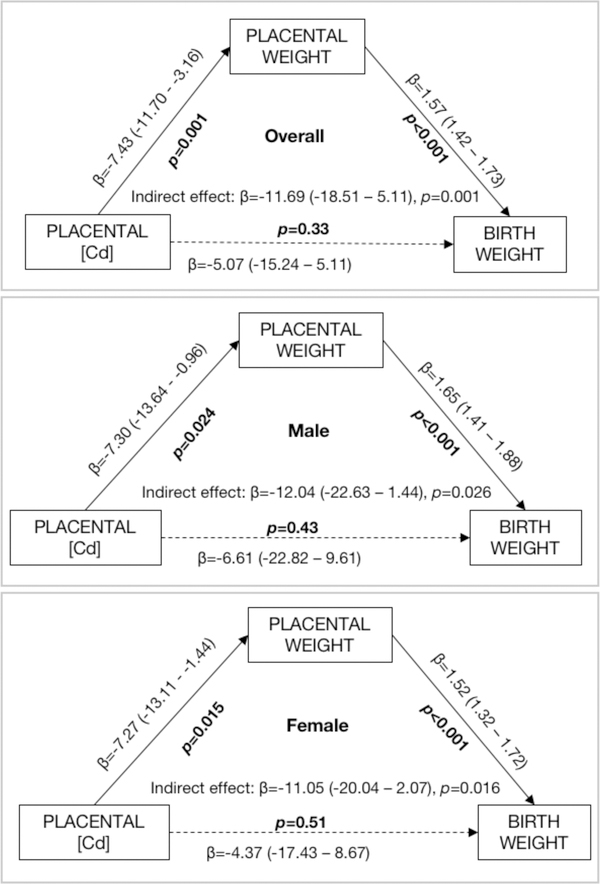
Placental weight was found to be a mediator (β = −11.69, p = 0.001) in the association between Cd concentration of the placenta and infant birth weight. This is shown in Figure 1, which represents the relationships between placental Cd concentration and placental weight; placental weight and birth weight, and placental Cd and birth weight and their statistical significance. A one ng/g increase in Cd exposure was estimated to be associated with a 11.69g decrease in birth weight by reducing the placental weight. The direct effect of Cd exposure on birth weight is not statically significant (β = −5.07, p = 0.33) in the mediation analysis. We performed this mediation analysis stratifying for female and male placentae separately, and effect sizes were similar in males and females (Figure 1).
Figure 1.
Results of Structural Equation Modeling to examine whether placental weight is a significant mediator in the relationship between the measured placental Cd concentration and infant birth weight. The model was adjusted for maternal age (continuous), smoking (a binary variable), parity (continuous), gestational age (continuous) and maternal educational level (ordinal, as described in Table 1). Models are for the overall population and stratified by infant sex.
DISCUSSION
The concentrations of metals in placental tissue and their association with birth outcomes have been assessed in several large comparative reviews58–60. Associations between placental Cd and cord length and placental thickness were noted in a cross-sectional study of 1578 women in Saudi Arabia, a study which also saw an association between placental Hg and infant head circumference58. In a study of 1869 newborns, prenatal exposure to Hg was associated with reduced placental and fetal growth59. We did not observe significant associations with birth outcomes in the NHBCS, which included birth weight, length and head circumference. As we discuss in our previous investigations of placental metals, the concentrations of metals measured in NHBCS placental tissue fall at the lower end of the range reported concentrations for other populations, many of which included participants who were recruited from heavily industrialized areas5, 6, 11. In our population, the concentration of Hg is particularly low, and has been attributed to the low consumption of seafood in the region.
We found significant interactions between placental Cd concentration and smoking behavior: participants who had smoked either before or during their pregnancy showed positive associations between placental weight and efficiency in comparison to the significant negative associations observed in participants who reported never having smoked. We hypothesize that these significant interactions might indicate that there are other aspects of smoking that influence placental growth and function besides Cd exposure. While we did not observe associations with other metals also found in cigarette smoke (e.g. As and Pb), it is widely known that cigarette smoke also contains numerous organic contaminants as well as exerting additional influence on placental growth and functioning through changes in blood pressure or other cardiovascular effects. There may also be other, dietary sources of Cd, such as rice, other cereal grains, potatoes and other vegetables61. However, given the small number of participants reporting a history of smoking in this population (N=127), these findings should be confirmed in a larger study.
In this study, higher Cd concentrations in placenta were associated with decrements in placental weight and placental efficiency, and these decrements were larger among those with lower placental concentrations of essential elements Zn and Se. We did not observe that placental metal concentrations were associated with chorionic disc area or disc eccentricity in this population, nor did we find placental As, Hg and Pb concentrations associated with any measures of placental growth or function. Our findings suggest an indirect relationship between placental Cd and birth weight mediated by placental weight. To our knowledge this is the largest study to have evaluated the mediating role of placental growth and function on this relationship.
Maternal nutrition, maternal pre-pregnancy BMI as well as infant sex may all influence placental weight and efficiency36, 50, 52, 62–64. Our observation of significant associations between placental Pb, Cd and Zn concentrations and pre-pregnancy BMI (Supplemental Table 1) may be explained by the dilution effect of the larger placental weight of women with higher BMI (Table 1). Wallace et al, 52 observed that placental efficiency was negatively associated with maternal pre-pregnancy BMI. However, we found an increase in placental efficiency with increasing maternal pre-pregnancy BMI. Our study had far fewer participants than Wallace et al.’s study, which included over 55,000 pregnancies recruited retrospectively from the Aberdeen Maternity and Neonatal Databank. Other potentially confounding factors could explain the differences between the present study and those that observed a negative association between BMI and placental efficiency. For instance, we saw that a higher number of previous pregnancies resulted in a more efficient placenta, and in our cohort maternal pre-pregnancy BMI and parity were positively correlated. Others also have observed positive associations between placental weight and parity, as well as maternal pre-pregnancy BMI and gestational weight gain65.
We saw indications that differences in the response of the placenta to Cd varied by infant gender, with associations stronger in female than male babies, and with on average higher Cd in female placentae than male. Inverse associations between gestational exposure to Cd and offspring size at birth have been reported previously, whereas no association or inconsistent associations across biomarkers of Cd exposure (umbilical cord blood, maternal blood, placenta, umbilical cord serum, urine) have also been reported38, 58, 66–76. The effects of Cd may be sex-specific, with smaller birth sizes being observed among female neonates exposed to greater Cd during gestation37, 38, 77–80. In a Bangladeshi population, Kippler et al. observed decreasing birth weight among female infants only with higher maternal urinary Cd38. An association between maternal blood Cd concentrations during pregnancy and lower birth weight percentiles among girls was reported in a South African cohort77. Wang et al.78 found an adverse association between maternal serum Cd concentrations with the baby being small for gestational age (SGA) in 3,254 mother-infant pairs in China, and Taylor et al.79 found a negative association between maternal blood Cd concentration and birth weight in girls in a cohort of 4191 mother-infant pairs. Another study based in China saw that higher first-trimester maternal urinary Cd concentrations were associated with reduced size at birth among female newborns 80. Although not statistically significant, Romano et al. also saw associations between lower birth weight among girls with increasing maternal urinary Cd during early pregnancy, but not in boys in a US based cohort37. While distinct mechanisms remain to be found for the role of Cd in fetal growth, current evidence points toward sex-specific alterations in the epigenetic regulation of genes involved in growth81–83. Sex-specific differences in Cd-related placental DNA methylation changes have previously been observed, with high Cd exposures associated with hypomethylation of sites related to cell damage response among female placentas, and with hypermethylation of the body of the piezo type mechanosensitive ion channel 1 (PIEZ01) gene among male placentas83,84.
Among all of the four toxic metalloids considered here, Cd is the only element on which there is evidence for placental accumulation11, 13, 14. Although correlated with both maternal plasma and erythrocyte Cd11, Cd concentrations of placenta tend to be much higher11, 17. Inverse associations between Cd concentration of placenta and size at birth have been observed in prior studies though others observed no association 66, 68, 74–76, 85.
Although few, if any studies to date have investigated the cellular distribution of Cd in the human placenta, analysis of Cd-exposed rat placentae using laser ablation inductively coupled plasma mass spectroscopy found Cd associated with the trophoblasts in the labyrinth zone, with concurrent swelling and vacuolar degeneration86. This study also found unusually high metallothionein expression in the trophoblastic septa of the labyrinth zone, lending further credence to our consideration of Zn (which is strongly bound by metallothionein) as potentially influencing the effects of Cd on the placenta. The labyrinth zone in this study is broadly comparable to the trophoblast cells covering the villi in the human placenta. Further studies of the human placenta, particularly those using spatially resolved analysis (imaging) of Cd are warranted.
In our study, we observed greater decrements in placental weight and placental efficiency in relation to Cd concentrations of placenta according to the Zn and Se concentrations of the placentae. These observations were consistent with the mechanistic literature, where higher concentrations of essential elements can help to ameliorate the negative effects of toxic metal exposure 20–24. Studies have shown that intestinal absorption of Cd is greater among women with Zn, Ca and Fe deficiencies 87. The findings that placental efficiency is less affected by the presence of Cd when essential elements Zn and Se are higher agrees with the theory of nutritional resistance to toxic metal exposure, an observation made in numerous metal-exposed biological organisms and systems88, 89.
The strengths of our study include a relatively large population on whom we performed a multi-elemental analysis enabling us to consider the concentrations of other elements thought to be involved in the maternal-fetal transfer of Cd, such as Zn and Se. A potential limitation of our work is that the placental measures used here (namely placental efficiency) have limited sensitivity as indicators of impairment of placental function, and problems have been noted recently with the use of ratios90. Further mechanistic data on the effect of Cd on placental DNA oxidation, lipid peroxidation and nitrotyrosine production would help to elucidate our findings. Further, there may be residual confounding in our models from factors not considered that may affect placental growth and functioning measures.
In conclusion, our study adds to the body of evidence Cd that may impact birth weight by affecting the growth and functioning of the placenta.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIGMS P20 GM104416 (to TP and MRK), NIH/NIEHS P42 ES007373 (to BPJ, TP and MRK), NIH/NCI P30CA023108 (to BPJ), NIH/NIGMS R01GM123014 (to ZL), and NIGMS P20GM104416–06 (to MER).
Footnotes
The authors declare they have no actual or potential competing financial interests.
BIBLIOGRAPHY
- 1.ATSDR, Toxicological Profile for Arsenic. 2007, Agency for Toxic Substances and Disease Registry: Atlanta, GA. [PubMed] [Google Scholar]
- 2.ATSDR, Toxicological Profile for Cadmium, A.f.T.S.D. Registry, Editor. 2012, US Department of Health and Human Services: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15. [Google Scholar]
- 3.ATSDR, Toxicological Profile for Mercury, A.f.T.S.D. Registry, Editor. 1999, US Department of Health and Human Services: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=115&tid=24. [Google Scholar]
- 4.ATSDR, Toxicological Profile for Lead, A.f.T.S.a.D. Registry, Editor. 2007, US Department of Health and Human Services: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22. [Google Scholar]
- 5.Punshon T, Davis MA, Marsit CJ, Theiler SK, Baker ER, Jackson BP, Conway DC, and Karagas M, Placenta arsenic concentration in relation to both maternal and infant biomarkers of exposure in a US cohort. Journal of Exposure Science and Environmental Epidemiology, 2015. 25(6): p. 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, and Karagas MR, Placental Metal Concentrations in Relation to Maternal and Infant Toenails in a U.S. Cohort. Environmental Science & Technology, 2016. 50(3): p. 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karp WB and Robertson AF, Correlation of human placental enzymatic activity with trace metal concentration in placentas from three geographical locations. Environ Res, 1977. 13(3): p. 470–7. [DOI] [PubMed] [Google Scholar]
- 8.Baglan RJ, Brill AB, Schulert A, Wilson D, Larsen K, Dyer N, Mansour M, Schaffner W, Hoffman L, and Davies J, Utility of placental tissue as an indicator of trace element exposure to adult and fetus. Environ Res, 1974. 8(1): p. 64–70. [DOI] [PubMed] [Google Scholar]
- 9.Gundacker C, Frohlich S, Graf-Rohrmeister K, Eibenberger B, Jessenig V, Gicic D, Prinz S, Wittmann KJ, Zeisler H, Vallant B, Pollak A, and Husslein P, Perinatal lead and mercury exposure in Austria. The Science of the Total Environment, 2010. 408(23): p. 5744–9. [DOI] [PubMed] [Google Scholar]
- 10.Gundacker C and Hengstschlager M, The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr, 2012. 162(9–10): p. 201–6. [DOI] [PubMed] [Google Scholar]
- 11.Esteban-Vasallo MD, Aragones N, Pollan M, Lopez-Abente G, and Perez-Gomez B, Mercury, cadmium, and lead levels in human placenta: a systematic review. Environmental Health Perspectives, 2012. 120(10): p. 1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima S, Tohyama C, Lu C, and Matsumoto N, Placental transfer and body distribution of methylmercury and selenium in pregnant mice. Toxicol Appl Pharmacol, 1978. 44(1): p. 143–6. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas-Acien A, Guallar E, and Wang X, Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol, 2014. 24(5): p. 537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchiya H, Mitani K, Kodama K, and Nakata T, Placental transfer of heavy metals in normal pregnant Japanese women. Archives of Environmental Health 1984. 39(1): p. 11–7. [DOI] [PubMed] [Google Scholar]
- 15.Yoon M, Nong A, Clewell HJ 3rd, Taylor MD, Dorman DC, and Andersen ME, Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol Sci, 2009. 112(1): p. 44–58. [DOI] [PubMed] [Google Scholar]
- 16.Hubermont G, Buchet JP, Roels HA, and Lauwerys R, Placental Transfer of Lead, Mercury and Cadmium in Women Living in a Rural Area: Importance of Drinking Water in Lead Exposure. International Archives of Occupational and Environmental Health, 1978. 41: p. 117–124. [DOI] [PubMed] [Google Scholar]
- 17.Roels HA, Hubermont G, Buchet JP, and Lauwerys R, Placental transfer of lead, mercury, cadmium and carbon monoxide in women: III. Factors influencing the accumulation of heavy metals in the placenta and the relationship between metal concentration in the placenta and in maternal and cord blood. Environmental Research, 1977. 16(1–3): p. 236–247. [DOI] [PubMed] [Google Scholar]
- 18.Simmons-Willis TA, Koh AS, Clarkson TW, and Ballatori N, Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J, 2002. 367(Pt 1): p. 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballatori N, Transport of toxic metals by molecular mimicry. Environ Health Perspect, 2002. 110 Suppl 5: p. 689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhao Y, Wang J, Zhu H, Liu Q, Fan Y, Wang N, Liu A, Liu H, Ou-Yang L, Zhao J, and Fan T, Effects of zinc, copper, and selenium on placental cadmium transport. Biol Trace Elem Res, 2004. 102(1–3): p. 39–49. [DOI] [PubMed] [Google Scholar]
- 21.Turgut S, Kaptanoglu B, Turgut G, Emmungil G, and Genc O, Effects of cadmium and zinc on plasma levels of growth hormone, insulin-like growth factor I, and insulin-like growth factor-binding protein 3. Biol Trace Elem Res, 2005. 108(1–3): p. 197–204. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Wang Y, Bo QL, Ji YL, Liu L, Hu YF, Chen YH, Zhang J, Zhao LL, and Xu DX, Maternal cadmium exposure reduces placental zinc transport and induces fetal growth restriction in mice. Reprod Toxicol, 2016. 63: p. 174–82. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnert BR, Kuhnert PM, and Zarlingo TJ, Associations between placental cadmium and zinc and age and parity in pregnant women who smoke. Obstet Gynecol, 1988. 71(1): p. 67–70. [PubMed] [Google Scholar]
- 24.Lin YS, Ho WC, Caffrey JL, and Sonawane B, Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ Res, 2014. 134: p. 33–8. [DOI] [PubMed] [Google Scholar]
- 25.Fukase Y, Tsugami H, Nakamura Y, Ohba K, and Ohta H, The role of metallothionein and metal transporter on cadmium transport from mother to fetus. Yakugaku Zasshi, 2014. 134(7): p. 801–4. [DOI] [PubMed] [Google Scholar]
- 26.Boadi WY, Yannai S, Urbach J, Brandes JM, and Summer KH, Transfer and accumulation of cadmium, and the level of metallothionein in perfused human placentae. Archives of Toxicology, 1991. 65: p. 318–323. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Ohba K, Suzuki K, and Ohta H, Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. The Journal of Toxicological Sciences, 2012. 37(1): p. 149–56. [DOI] [PubMed] [Google Scholar]
- 28.Tekin D, Kayaaltι Z, Aliyev V, and Söylemezoğlu T, The effects of metallothionein 2A polymorphism on placental cadmium accumulation: is metallothionein a modifiying factor in transfer of micronutrients to the fetus? Journal of Applied Toxicology, 2011. 32: p. 270–275. [DOI] [PubMed] [Google Scholar]
- 29.Bridges CC, Joshee L, and Zalups RK, Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: role of Mrp2. Reprod Toxicol, 2012. 34(4): p. 628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyer RA, Transplacental transport of lead. Environmental Health Perspectives, 1990. 89: p. 101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell KE, Labrecque MT, Solomon BR, Ali A, and Allan AM, Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol Teratol, 2015. 47: p. 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunton PJ and Russell JA, Neuroendocrine control of maternal stress responses and fetal programming by stress in pregnancy. Prog Neuropsychopharmacol Biol Psychiatry, 2011. 35(5): p. 1178–91. [DOI] [PubMed] [Google Scholar]
- 33.Kippler M, Hoque AM, Raqib R, Ohrvik H, Ekstrom EC, and Vahter M, Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett, 2009. 192(2): p. 162–8. [DOI] [PubMed] [Google Scholar]
- 34.Stasenko S, Bradford EM, Piasek M, Henson MC, Varnai VM, Jurasovic J, and Kusec V, Metal in human placenta: focus on the effects of cadmium on steroid hormones and leptin. Journal of Applied Toxicology, 2010. 30(3): p. 242–253. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez MM and Chakraborty C, Cadmium inhibits motility factor-dependent migration of human trophoblast cells. Toxicol In Vitro, 2011. 25(8): p. 1926–33. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld CS, Sex-Specific Placental Responses in Fetal Development. Endocrinology, 2015. 156(10): p. 3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano ME, Enquobahrie DA, Simpson C, Checkoway H, and Williams MA, Maternal body burden of cadmium and offspring size at birth. Environ Res, 2016. 147: p. 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, and Vahter M, Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect, 2012. 120(2): p. 284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kordas K, Queirolo EI, Mañay N, Peregalli F, Hsiao PY, Lu Y, and Vahter M, Low-level arsenic exposure: nutritional and dietary predictors in first-grade Uruguayan children. Environ Res, 2016. 147: p. 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry MJ and Ralston NV, Mercury toxicity and the mitigating role of selenium. Ecohealth, 2008. 5(4): p. 456–9. [DOI] [PubMed] [Google Scholar]
- 41.Parizek J, Ostadalova I, Kalouskova J, Babicky A, Pavlik L, and Bibr B, Effect of mercuric compounds on the maternal transmission of selenium in the pregnant and lactating rat. J Reprod Fertil, 1971. 25(2): p. 157–70. [DOI] [PubMed] [Google Scholar]
- 42.Wilson ME and Ford SP, Comparative aspects of placental efficiency. Reprod Suppl, 2001. 58: p. 223–32. [PubMed] [Google Scholar]
- 43.Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, and Dilworth MR, Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio? Front Physiol, 2016. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salafia CM, Misra DP, Yampolsky M, Charles AK, and Miller RK, Allometric metabolic scaling and fetal and placental weight. Placenta, 2009. 30(4): p. 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, Eucker B, and Thorp J, Placental surface shape, function, and effects of maternal and fetal vascular pathology. Placenta, 2010. 31(11): p. 958–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, and Thorp J, Modeling the variability of shapes of a human placenta. Placenta, 2008. 29(9): p. 790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salafia CM, Charles AK, and Maas EM, Placenta and fetal growth restriction. Clin Obstet Gynecol, 2006. 49(2): p. 236–56. [DOI] [PubMed] [Google Scholar]
- 48.Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, Sun W, and Maas EM, Placental characteristics and birthweight. Paediatric and Perinatal Epidemiology, 2008. 22(3): p. 229–238. [DOI] [PubMed] [Google Scholar]
- 49.Salafia CM, Zhang J, Miller RK, Charles AK, Shrout P, and Sun W, Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol, 2007. 79(4): p. 281–8. [DOI] [PubMed] [Google Scholar]
- 50.Misra DP, Salafia CM, Miller RK, and Charles AK, Non-linear and gender-specific relationships among placental growth measures and the fetoplacental weight ratio. Placenta, 2009. 30(12): p. 1052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yampolsky M, Salafia CM, Misra DP, Shlakhter O, and Gill JS, Is the placental disk really an ellipse? Placenta, 2013. 34(4): p. 391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace JM, Horgan GW, and Bhattacharya S, Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta, 2012. 33(8): p. 611–8. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, and Karagas MR, Rice consumption raises a health concern: evidence from U.S. pregnant women. Proceedings of the National Academy of Sciences, 2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, and Karagas MR, Relation between in Utero Arsenic Exposure and Birth Outcomes in a Cohort of Mothers and Their Newborns from New Hampshire. Environmental Health Perspectives, 2016. 124(8): p. 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanford RF, Pierson CT, and Crovelli RA, An Objective Replacement Method for Censored Geochemical Data. Mathematical Geology, 1993. 25(1): p. 59–80. [Google Scholar]
- 56.MacKinnon DP, Introduction to Statistical Mediation Analysis (Multivariate Applications Series) Multivariate Application Aries. 2008, New York, NY: 10016: Taylor & Francis Group. [Google Scholar]
- 57.Sobel ME, Some new results on indirect effects and their standard errors in covariance structure. Sociological Methodology, 1986. 16: p. 159–186. [Google Scholar]
- 58.Al-Saleh I, Shinwari N, Mashhour A, and Rabah A, Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. International Journal of Hygiene and Environmental Health, 2014. 217(2–3): p. 205–18. [DOI] [PubMed] [Google Scholar]
- 59.Murcia M, Ballester F, Enning AM, Iniguez C, Valvi D, Basterrechea M, Rebagliato M, Vioque J, Maruri M, Tardon A, Riano-Galan I, Vrijheid M, and Llop S, Prenatal mercury exposure and birth outcomes. Environ Res, 2016. 151: p. 11–20. [DOI] [PubMed] [Google Scholar]
- 60.Rahman A, Vahter M, Ekstrom EC, and Persson LA, Arsenic Exposure in Pregnancy Increases the Risk of Lower Respiratory Tract Infection and Diarrhea during Infancy in Bangladesh. Environmental Health Perspectives, 2011. 119(5): p. 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, and Moore MR, A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett, 2003. 137(1–2): p. 65–83. [DOI] [PubMed] [Google Scholar]
- 62.Che L, Yang ZG, Xu MM, Xu SY, Che LQ, Lin Y, Fang ZF, Feng B, Li J, Chen DW, and Wu D, Maternal nutrition modulates fetal development by inducing placental efficiency changes in gilts. BMC Genomics, 2017. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace JM, Bhattacharya S, Campbell DM, and Horgan GW, Inter-pregnancy weight change impacts placental weight and is associated with the risk of adverse pregnancy outcomes in the second pregnancy. BMC Pregnancy Childbirth, 2014. 14: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clifton VL, Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta, 2010. 31 Suppl: p. S33–9. [DOI] [PubMed] [Google Scholar]
- 65.Roland MCP, Friis CM, Godang K, Bollerslev J, Haugen G, and Henriksen T, Maternal Factors Associated with Fetal Growth and Birthweight Are Independent Determinants of Placental Weight and Exhibit Differential Effects by Fetal Sex. PLOS ONE, 2014. 9(2): p. e87303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kippler M, Hoque AM, Raqib R, Ohrvik H, Ekstrom EC, and Vahter M, Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett, 2010. 192(2): p. 162–8. [DOI] [PubMed] [Google Scholar]
- 67.Shirai S, Suzuki Y, Yoshinaga J, and Mizumoto Y, Maternal exposure to low-level heavy metals during pregnancy and birth size. Journal of Environmental Science and Health Part A-Toxic/Hazardous Substances & Environmental Engineering, 2010. 45(11): p. 1468–1474. [DOI] [PubMed] [Google Scholar]
- 68.Llanos MN and Ronco AM, Fetal growth restriction is related to placental levels of cadmium lead and arsenic but not with antioxidant activities. Reproductive Toxicology, 2009. 27: p. 88–92. [DOI] [PubMed] [Google Scholar]
- 69.Tian LL, Zhao YC, Wang XC, Gu JL, Sun ZJ, Zhang YL, and Wang JX, Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol Trace Elem Res, 2009. 132(1–3): p. 51–9. [DOI] [PubMed] [Google Scholar]
- 70.Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Wang NF, Zhao JH, Liu HS, Ou-Yang L, Liu AP, and Fan TQ, Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J Environ Sci Health A Tox Hazard Subst Environ Eng, 2004. 39(9): p. 2507–15. [DOI] [PubMed] [Google Scholar]
- 71.Nishijo M, Tawara K, Honda R, Nakagawa H, Tanebe K, and Saito S, Relationship between newborn size and mother’s blood cadmium levels, Toyama, Japan. Arch Environ Health, 2004. 59(1): p. 22–5. [DOI] [PubMed] [Google Scholar]
- 72.Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, and King W, Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ Res, 2015. 140: p. 430–9. [DOI] [PubMed] [Google Scholar]
- 73.Tang M, Xu C, Lin N, Liu K, Zhang Y, Yu X, and Liu W, Lead, mercury, and cadmium in umbilical cord serum and birth outcomes in Chinese fish consumers. Chemosphere, 2016. 148: p. 270–5. [DOI] [PubMed] [Google Scholar]
- 74.Klapec T, Cavar S, Kasac Z, Rucevic S, and Popinjac A, Selenium in placenta predicts birth weight in normal but not intrauterine growth restriction pregnancy. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements, 2008. 22(1): p. 54–8. [DOI] [PubMed] [Google Scholar]
- 75.Odland JO, Nieboer E, Romanova N, and Thomassen Y, Elements in placenta and pregnancy outcome in arctic and subarctic areas. Int J Circumpolar Health, 2004. 63(2): p. 169–87. [DOI] [PubMed] [Google Scholar]
- 76.Osman K, Akesson A, Berglund M, Bremme K, Schutz A, Ask K, and Vahter M, Toxic and essential elements in placentas of Swedish women. Clinical Biochemistry, 2000. 33(2). [DOI] [PubMed] [Google Scholar]
- 77.Rollin HB, Kootbodien T, Channa K, and Odland JO, Prenatal Exposure to Cadmium, Placental Permeability and Birth Outcomes in Coastal Populations of South Africa. PLoS One, 2015. 10(11): p. e0142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Liu L, Hu YF, Hao JH, Chen YH, Su PY, Fu L, Yu Z, Zhang GB, Wang L, Tao FB, and Xu DX, Maternal serum cadmium level during pregnancy and its association with small for gestational age infants: a population-based birth cohort study. Sci Rep, 2016. 6: p. 22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor CM, Golding J, and Emond AM, Moderate Prenatal Cadmium Exposure and Adverse Birth Outcomes: a Role for SexSpecific Differences? Paediatr Perinat Epidemiol, 2016. 30(6): p. 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng L, Zhang B, Zheng T, Hu J, Zhou A, Bassig BA, Xia W, Savitz DA, Buka S, Xiong C, Braun JM, Zhang Y, Zhou Y, Pan X, Wu C, Wang Y, Qian Z, Yang A, Romano ME, Shi K, Xu S, and Li Y, Critical Windows of Prenatal Exposure to Cadmium and Size at Birth. Int J Environ Res Public Health, 2017. 14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kippler M, Engstrom K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, Raqib R, Vahter M, and Broberg K, Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics, 2013. 8(5): p. 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vidal AC, Semenova V, Darrah T, Vengosh A, Huang Z, King K, Nye MD, Fry R, Skaar D, Maguire R, Murtha A, Schildkraut J, Murphy S, and Hoyo C, Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol Toxicol, 2015. 16: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohanty AF, Farin FM, Bammler TK, MacDonald JW, Afsharinejad Z, Burbacher TM, Siscovick DS, Williams MA, and Enquobahrie DA, Infant sex-specific placental cadmium and DNA methylation associations. Environ Res, 2015. 138: p. 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Everson TM, Punshon T, Jackson BP, Hao K, Lambertini L, Chen J, Karagas MR, and Marsit CJ, Cadmium-Associated Differential Methylation throughout the Placental Genome: Epigenome-Wide Association Study of Two U.S. Birth Cohorts. Environ Health Perspect, 2018. 126(1): p. 017010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ronco AM, Arguello G, Munoz L, Gras N, and Llanos MN, Metals content in placentas from moderate cigarette consumers: correlation with newborn birth weight. Biometals, 2005. 18(3): p. 233–241. [DOI] [PubMed] [Google Scholar]
- 86.Yamagishi Y, Furukawa S, Tanaka A, Kobayashi Y, and Sugiyama A, Histopathological localization of cadmium in rat placenta by LA-ICP-MS analysis. J Toxicol Pathol, 2016. 29(4): p. 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kippler M, Ekstrom EC, Lonnerdal B, Goessler W, Akesson A, El Arifeen S, Persson LA, and Vahter M, Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol Appl Pharmacol, 2007. 222(2): p. 221–6. [DOI] [PubMed] [Google Scholar]
- 88.Reeves PG and Chaney RL, Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ Res, 2004. 96(3): p. 311–22. [DOI] [PubMed] [Google Scholar]
- 89.Hammad TA, Sexton M, and Langenberg P, Relationship between blood lead and dietary iron intake in preschool children. A cross-sectional study. Ann Epidemiol, 1996. 6(1): p. 30–3. [DOI] [PubMed] [Google Scholar]
- 90.Christians JK, Grynspan D, Greenwood SL, and Dilworth MR, The problem with using the birthweight: placental weight ratio as a measure of placental efficiency. Placenta, 2018. 68: p. 52–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.