Abstract
Background
The prevalence of chronic pain among the elderly is high (estimated at 25–85%) and may adversely affect their everyday functioning, although it is often unrecognized.
Material/Methods
The aim of this study was to assess the prevalence of chronic pain, especially with the neuropathic component, and its effect on overall functioning of elderly patients. We enrolled 145 subjects older than 60 years (nursing home residents, or patients of outpatient geriatric clinic). Information on co-morbidities, functional and mental status, and medications was obtained using a questionnaire. Chronic pain was defined as lasting >3 months and the presence of neuropathic component was detected using the DN4 Questionnaire (Douleur Neuropathique en Questions).
Results
The mean age of patients was 76±9.68 years. Chronic pain was reported by 78% of participants and 32% reported neuropathic pain with neuropathic component (DN4 score ≥4 points). Patients complaining of chronic pain significantly more often presented mood disorders and lower satisfaction with life (particularly those with the highest pain intensity), with no difference in functional status according to the ADL (Activities of Daily Living) tool. Participants with chronic pain were treated with paracetamol (45%), non-steroidal anti-inflammatory drugs (25%), and opioids (24%).
Conclusions
The prevalence of chronic pain, particularly with neuropathic component, in the elderly population seems to be higher than expected based on previous reports, and its treatment appears to be ineffective. This problem requires further research and dissemination of knowledge on the diagnosis and treatment of chronic pain among health care workers caring for elderly patients on a daily basis.
MeSH Keywords: Chronic Pain, Frail Elderly, Neuralgia, Nursing Homes
Background
The worldwide prevalence of chronic pain among the elderly is estimated to be 25–85% [1,2]. Pain is not an attribute of physiological aging; it contributes to functional deterioration, increases the risk of falls and mood disorders, and accelerates dependence on institutional care [1,3]. The problems listed above might be particularly related to chronic pain with the neuropathic component, the etiology of which is related to organic lesions in the nervous system. Prevalence of neuropathic pain in the general population is estimated at 6.5–11.5%, but the exact prevalence of neuropathic pain in the geriatric population is poorly investigated [4]. Treatment of neuropathic pain is based on opioids, anticonvulsants, or antidepressants [5]. Unfortunately, multimorbidity and restricted functional and mental capacity among older adults make it difficult to establish the diagnosis and to introduce proper treatment. Studies on the influence of neuropathic pain on functioning of older adults are scarce and their results are inconsistent [4].
Material and Methods
Aim of the study
The aims of the study were: to evaluate prevalence of chronic pain, particularly of neuropathic etiology and its impact on overall functioning; to assess functional status, mood, and satisfaction with life; and to analyze the current practices in pain management among residents of nursing homes and patients at the Geriatric Outpatient Clinic, aged 60 years and above.
Participants
We included 145 patients of both sexes. Inclusion criteria were: residents of nursing homes and patients of the Geriatric Outpatient Clinic, ≥60 years of age, scored ≥7 points in the Abbreviated Mental Test Score (AMTS) [6], and willing to participate in the study. Non-adherence to the study procedures, co-morbidities causing language problems, and inability to communicate comprised the exclusion criteria. The study protocol was evaluated and accepted by an independent ethics committee, and written informed consent was obtained from all participants.
Study procedure
The data were provided by the patients through standardized questionnaires completed by external researchers. Additionally, information was retrieved based on the review of patient files: chronic illnesses were coded using ICD-10 (International Classification of Diseases) and current treatment was coded based on the Anatomical Therapeutic Chemical Classification. All questionnaires used in the survey were validated worldwide as well as in Poland and were widely used in the Comprehensive Geriatric Assessment to assess the functional and mental status of the elderly. Due to mild cognitive impairment and/or mild dementia occurring in some of the studied patients, the validated short forms of available questionnaires were used; e.g., we used a shortened version of the Geriatric Depression Scale (15-item vs. 30-item). To assess functional status of participants, the Activities of Daily Living tool (ADL) was used, in which a score lower than 5 points qualified patients as disabled/dependent on others [7]. A 15-item Geriatric Depression Scale (GDS) was used to assess mood: 0 to 5 points indicates no mood disturbance, 6–10 points indicates moderate depression, and >10 points indicates severe depression [8]. The level of satisfaction with life were assessed using Diener’s Satisfaction with Life Scale (SWLS), in which a higher the score indicates greater satisfaction with life (range, 5–35 points) [9]. This scale is rather rarely used by other researchers to assess the well-being of patients and their satisfaction with life [10]; more often, they use more objective and comprehensive tests such as the McGill Quality of Life Questionnaire, or SF-36 [11,12]. However, in the present study, our intention was to use relatively short and easy-to-use tests. Patients were asked about presence and location of chronic pain (lasting longer than 3 months) using whole-body pain drawings. The intensity of pain was estimated using the Numeric Rating Scale (NRS), in which 0 points indicates no pain and 10 points indicating maximum pain for every pain site. For further analyses, NRS scores were grouped as follows: maximum pain intensity (NRS max), sum of NRS scores for each pain site (NRS total), and mean pain score (NRS average). A verbal scale that assesses pain intensity was chosen due to the high prevalence of vision problems among the elderly, while hearing impairment causing inability to communicate was the criterion of exclusion as opposed to vision problems. Patients also completed the DN4 questionnaire (Douleur Neuropathique en Questions) to assess the neuropathic background of pain on the symptom’s basis, such as: burning, painful cold, electric shocks, tingling, pins and needles, numbness, itching, hypoesthesia to touch, hypoesthesia to prick, and pain sensation while brushing teeth. If the score equaled 4 or higher, the pain was considered as possible neuropathic pain [13]. The decision to use the DN4 questionnaire to assess the neuropathic pain component was quite difficult due to the variety of other scales that can be used to test this. The PainDetect Questionnaire [14], the LANSS (Leeds Assessment of Neuropathic Symptoms and Signs) scale [15], and the Neuropathic Pain Questionnaire (NPQ) [16] are the most frequently used questionnaires for this type of research, but they all require good verbal communication with the patient. The DN4 and LANSS scales, apart from questions about self-assessment of pain, also use elements of a physical examination, which seems to make them more ‘sensitive’ than other scales. Bisaga and other authors who validated neuropathic pain questionnaires in Poland considered the DN4 scale to be the simplest and most sensitive [17].
Statistical analysis
Descriptive statistical analysis of the categorical variables was based on frequencies of the respective values. Quantitative variables were described using the arithmetic mean with standard deviation (SD), median, range, and interquartile range. Normality of the variables’ distributions were tested using the Shapiro-Wilk test. Relationships between qualitative variables were analyzed using frequency tests: Pearson’s chi-square test, or, if the assumptions of this test were not fulfilled, the likelihood ratio (G). The t test was used to analyze the relationship between quantitative and categorical variables with normal distribution; if quantitative variable had more than 2 values, analysis of variance (ANOVA) was used. For variables with distribution other than normal, we used the Mann-Whitney U test and Kruskal-Wallis test. If ANOVA or Kruskal-Wallis test showed significant differences, we also used post hoc analysis: Tuckey test for ANOVA and Steel-Dwass test for non-normally distributed quantitative variables. Relationships between quantitative variables were analyzed using linear regression analysis, while non-normally distributed data were assessed with non-parametric correlation tests.
Results
Characteristics of the study group
Our study included 110 women (76%) and 35 men (24%). No statistically significant differences between the 2 sexes were found. The mean age of the study group was 76 ± 9.68 years (range, 60–93 years). There were 97 subjects who were long-term care facility residents and 48 who were patients of the Geriatric Outpatient Clinic (Table 1). Residents of nursing homes were older and more functionally impaired than Geriatric Outpatient Clinic patients, with a significant difference in ability to perform ADL (p<0.01 for all differences). Most of study participants had several co-morbidities (mean 7±2.79 diseases) (Table 2).
Table 1.
Baseline characteristic of the group according to different types of care.
| Type of care | Gender | Age | Functional status | |||
|---|---|---|---|---|---|---|
| Women | Men | Mean ±SD | Able to walk | Bed bound | Wheelchair bound | |
| Nursing Home 1 | 9 | 1 | 81.8±4.47 | 10 | 0 | 0 |
| Nursing home 2 | 67 | 20 | 78.2±8.41 | 42 | 16 | 29 |
| Geriatric Outpatient Clinic | 34 | 14 | 73.7±6.69 | 48 | 0 | 0 |
Table 2.
Prevalence of chronic diseses among patients wih possible impact in neuropathic pain occurence.
| Disease (according to ICD-10) | Number of patients |
|---|---|
| C, D (Neoplasms)* | 38 |
| E11, E10 (Diabetes) | 43 |
| F32 (Major depressive disorder) | 18 |
| F (Mental, Behavioral and Neurodevelopmental disorders) | 67 |
| F00–F03 (Dementia) | 18 |
| G (Diseases of the nervous system) | 50 |
| I60–I69 (Cerebrovascular diseases) | 35 |
| I70 (Atherosclerosis) | 58 |
| M (Diseases of the musculoskeletal system and connective tissue) | 77 |
Active neoplasms and neoplasms in the past.
Prevalence of chronic pain and its relationship with comprehensive geriatric assessment
There were 113 participants (78%) who reported chronic pain and 46 participants (32%) reported possible neuropathic pain. Chronic pain, including possible neuropathic pain, occurred in men and women with similar frequency. There was no relationship between age and type of pain reported. Possible neuropathic pain was significantly more common among nursing home residents than in Geriatric Outpatient Clinic patients (p<0.01).
No significant relationship was found between chronic pain (and possible neuropathic pain) and cognitive (AMTS) and functional (ADL) status of the participants. However, patients with chronic pain more often manifested low mood; among participants experiencing chronic pain, we found higher depression scale (GDS) scores (p=0.02). There was no statistically significant relationship between GDS and frequency of possible neuropathic pain.
In contrast, a significant relationship was found between chronic pain with neuropathic component and satisfaction with life. Among patients reporting chronic pain (p=0.03) as well as possible neuropathic pain (p<0.01; Figure 1), satisfaction with life measured using the SWLS tool was significantly lower.
Figure 1.
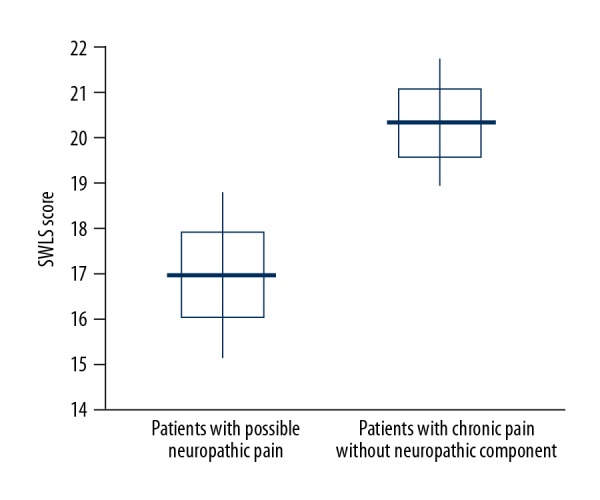
SWLS score in patients with possible neuropathic pain and with chronic pain without neuropathic component.
The Tuckey post hoc test revealed that patients with possible neuropathic pain presented significantly lower satisfaction with life than patients with less than 4 points on the DN4 scale.
Chronic pain intensity
Overall, the subjects were suffering from severe pain; the mean value on the NRS was 5.7±1.89 points (Table 3).
Table 3.
Chronic pain intensity in NRS.
| Mean ±SD | Median | Min. | Max. | Lower quartile | Higher quartile | ||
|---|---|---|---|---|---|---|---|
| Pain intensity | NRS max | 5.7±1.89 | 5.0 | 1.0 | 10.0 | 5.0 | 7.0 |
| NRS average | 5.3±1.61 | 5.0 | 1.0 | 10.0 | 4.0 | 6.3 | |
| NRS total | 12±6.36 | 12.0 | 1.0 | 28.0 | 6.0 | 17.0 |
A significant relationship between GDS score and NRS max was found; participants with low mood reported higher pain intensity (p=0.02). Post hoc analysis demonstrated that subjects with possible severe depression (GDS score ranging between 10 and 15 points) reported greater pain level as measured with NSR-max than those without mood disturbances (Figure 2). The same relationship was observed for the NRS – average (p=0.04).
Figure 2.
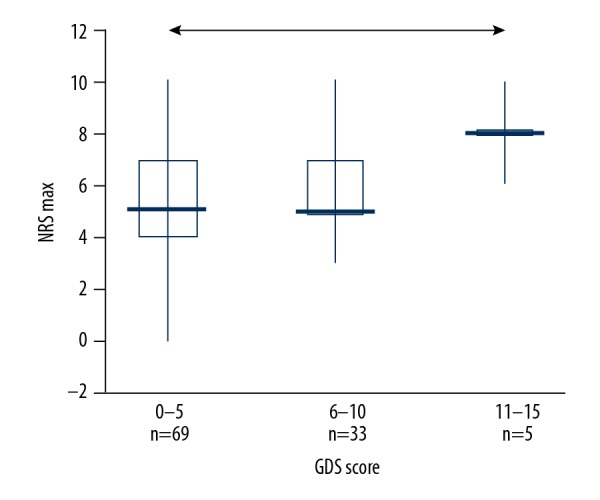
The relationship between pain intensity (NRS max) and GDS score (arrow indicates difference confirmed/proved with post hoc tests)
Pain locations
Patients suffering from possible neuropathic pain reported 4±2.23 pain sites (median, 4 sites), as compared to those with chronic pain without neuropathic component (mean, 3±2.22, median 2 pain sites; p=0.01).
Possible neuropathic pain characteristics
The probable causes of symptoms were established based on patients’ medical files. Results are shown in the Table 4.
Table 4.
The most common causes of possible neuropathic pain*.
| Cause | Number of patients N=46 |
|---|---|
| Diabetic neuropathy | 13 |
| Post-stroke neuropathic pain | 10 |
| Post-surgery/injury peripheral neuropathic pain | 8 |
| Non-diabetic neuropathy | 6 |
| Low back pain | 5 |
| Post-injury/post-surgery of CNS, brain tumor | 3 |
| Post-surgery peripheral neuropathic pain | 3 |
| Amputation | 3 |
| Others (carpal tunnel syndrome, neuropathy due to ischaemia, alcoholism) | 5 |
There were more than one possible causes at one case.
According to the DN4 questionnaire, the most common complaints were: numbness, tingling, electric shocks, burning, hypoesthesia to touch, and hypoesthesia to prick. Most patients reported 4/10 complaints in the DN4 questionaire, but 10 patients reported 6–7 complaints. The most common localizations of possible neuropathic pain were the feet, hands, and lower limbs.
Chronic pain and co-morbidities
Chronic pain and possible neuropathic pain were more common among patients who had endocrine, nutritional, and metabolic diseases (ICD 10: code E) compared to those with other diseases (p=0.01). There was a higher prevalence of type 2 diabetes (borderline significant) in patients with chronic pain, particularly those with possible neuropathic pain (p=0.08), but possible neuropathic pain was reported less frequently by subjects with a diagnosis of depression (ICD 10: code F-32).
Chronic pain and current treatment
Generally, the number of drugs used in study participants ranged between 0 and 22 (mean 8.7±3.98), and was significantly higher among patients with chronic pain (median 7 vs. 6 drugs, respectively, p=0.03). Seventy-three participants (65% of patients suffering from chronic pain) were receiving analgesics; 46% of patients with chronic pain were treated with paracetamol, 25% with non-steroidal inflammatory drugs (NSAIDs), and 24% with opioids (Table 5). Twenty-three participants with severe pain (who scored more than 4 points in NRS) received no analgesics (Figure 3). Most of the residents declared that they receive analgesics from nursing staff PRN (pro re nata, ‘as needed’) in case of pain, and 4 patients reported than no medications had been given to them ‘as needed’ in case of pain.
Table 5.
Selected analgesics and medicines interacting with nervous system function received by patients with and without possible neuropathic pain.
| Groups of medicines (according to ATC code) | Chronic pain without neuropathic component (N= 64)*,** | Possible neuropathic pain (N= 46)*,** | Statistical significance |
|---|---|---|---|
| N02A Opioids# | 11 (17%) | 15 (33%) | p= 0.01 |
| N02BE01 Paracetamol | 29 (45%) | 22 (48%) | p= NS |
| M01A Non steroidal antyinflammatory drugs## | 17 (27%) | 10 (22%) | |
| N03A Antiepileptics | 7 (11%) | 7 (15%) | |
| N05B Anxiolytic drugs | 7 (11%) | 8 (17%) | |
| N06A Antidepressants | 20 (31%) | 8 (17%) | |
| N05C Hypnotic and sedatives drugs | 16 (25%) | 9 (20%) | |
| N02BB02 Metamizole | 1 (2%) |
Percentages do not sum up to 100%; one participant might have received two or more drugs from different groups;
in brackets: number of patients with available information about drug received;
among patients receiving opioids: 23 received tramadol, 1 received dihydrocodeine, 2 received buprenorphine;
among patients receiving NSAID: 10 patients received ketoprofen, 9 patients received diclofenac, 3 patients received naproxen.
Figure 3.
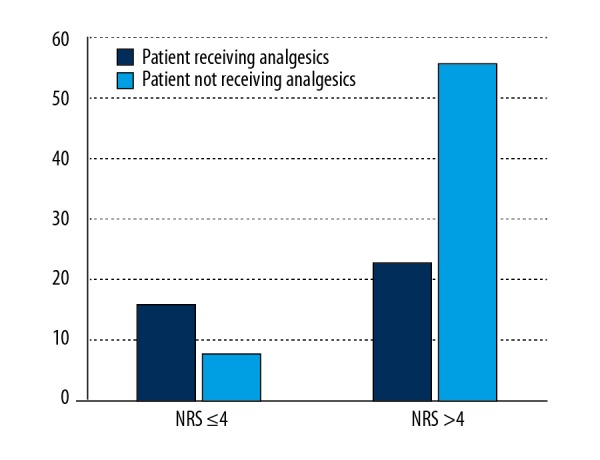
The number of patients with severe pain (NRS >4 points) receiving analgesics.
Finally, we analyzed the relationship between presence of possible neuropathic pain and current pain treatment guidelines (e.g., treatment with opioids, anticonvulsants, and antidepressants). It was found that participants with possible neuropathic pain were significantly more likely to receive opioids than were other participants (p= 0.01), but a similar relationship was not found for antiepileptic and antidepressant drugs.
Discussion
Difficulties in diagnosis and treatment of chronic pain may be related to the belief of physicians and patients (particularly the oldest) that pain is an inherent attribute of old age. Some scientific reports support this attitude, showing that perception of pain among older adults is lower than among younger patients, for many reasons, such as structural and biochemical changes in the nervous system [18]. However, other reports suggest that pain is not a normal part of aging, showing that pain perception depends on the type of stimulus, the duration of the pain, and the nature of the co-morbidities [19]. The present study showed a high prevalence of chronic pain among people over 60 years old, as well as possible neuropathic pain, in accordance with the literature [2,20]. Chronic pain is reported more often by assisted living community residents than by seniors living independently [18]. Ferrell et al. demonstrated that 71–80% of assisted living community residents in the United States reported chronic pain symptoms [21]. In our data, no difference in prevalence of chronic pain was found between residents and outpatient clinic patients. The prevalence of neuropathic pain in the general population worldwide is also high, ranging from 6.5% in Germany [22] to 11.5% in Canada [23], but data on the prevalence of neuropathic pain among the elderly are scarce, perhaps due to difficulties in conducting questionnaire-based studies among patients with visual and hearing disorders, as well as mental problems (especially dementia) [24]. Our data suggest that the prevalence of neuropathic pain among older adults is high (32% of the subjects), but this is not entirely consistent with the published literature. For example, a similar prevalence of painful neuropathic disorders (35%) was reported by Mailis-Gagnon et al., who conducted a survey among older patients with chronic pain and who were referred to a tertiary pain clinic in Canada [25], but a survey of nursing home residents in The Netherlands found only a 10.9% prevalence [26]. In our study, long-term care facility patients more frequently complained of possible neuropathic pain; this may be explained, at least in part, by the higher incidence of neurodegenerative disorders present in this population. The most frequent causes of neuropathic pain in the elderly include: painful diabetic neuropathy and post-herpetic neuralgia [25], followed by other disorders of the nervous system; for example, sequelae of stroke, as demonstrated by Aprile et al., who reported that 32% of stroke survivors scored ≥4 points in the DN4 questionnaire [27]. In present study, the most common cause of neuropathic pain was diabetic neuropathy, followed by neuropathic pain in post-stroke patients. Interestingly, we found no patients with post-herpetic neuropathy. We also found that the prevalence of possible neuropathic pain was lower in older adults with diagnosed depression; this might be linked to use of antidepressants, which are also the drugs of choice for treatment of neuropathic pain [5]. No link between chronic pain and disability (ADL scale) was found in our study, whereas such a relationship was clearly demonstrated by other authors [21,23]. However, a clear relationship was observed between pain complaints and low mood as well as low level of satisfaction with life, particularly among patients with severe pain and possible neuropathic pain, which agrees with results of a survey by similarly Aprile et al. [27]. Our data also revealed multiple pain locations, with significantly more among patients with possible neuropathic pain. This finding was also reported in other studies conducted among elderly people, who usually pointed more than 3 pain sites [2].
Concerning the current treatment, our data show the problem of polypharmacy among patients with chronic pain; they received significantly more prescription drugs than subjects without pain symptoms. Unfortunately, medical practitioners are often negligent in failing to appropriately administer analgesics to elderly patients with severe pain, and in the present study we found that 4 residents of assisted living communities declared that they did not receive pain medication ‘as needed’. Similar evidence was obtained by Podczaska et al. in their survey among assisted living community residents in Poland, finding that only 1 of 3 of them received analgesics, mostly NSAIDs, and only 2 persons out of 113 were taking paracetamol [28]. In the present study, more patients received analgesics with higher paracetamol and opioids consumption, but NSAID use was also common, which is against the chronic pain management guidelines [1]. Weak opioids were used more frequently among patients with possible neuropathic pain, which might have been related to higher intensity of pain. Unfortunately, this may indicate treatment failure, since patients were still in pain. There was no relationship between possible neuropathic pain prevalence and treatment with co-analgesics (antidepressants and anticonvulsants). The analgesic efficacy of those drugs may explain this finding – participants receiving them did not present symptoms of possible neuropathic pain [5,29].
Furthermore, Podczaska et al. and other authors have pointed out other worrisome trend – people with symptoms of dementia were less likely to receive analgesics than were other older adults [28]. In this study, no relationship between current treatment and AMTS tool score was discovered, but people with severe dementia were not included.
Conclusions
The prevalence of chronic pain, particularly with neuropathic component, in the elderly population seems to be higher than previously expected and observed in published studies, and its treatment seems to be ineffective. The unrecognized neuropathic component of pain may contribute to underdiagnosis and undertreatment. Patients suffering from chronic pain significantly more often presented mood disorders and lower satisfaction with life (especially those with the highest pain intensity), with no difference in functional status according to the ADL tool. This problem requires further research and education on the diagnosis and treatment of chronic pain in the elderly among medical practitioners who deal with elderly patients on a daily basis.
Acknowledgements
The study was conducted among residents of the Cracow Nursing Home, Poland, the “Na Wzgórzu” Nursing Home, Głogoczów, Poland, and the and Med-All Geriatric Outpatient Clinic, Cracow, Poland
Footnotes
Source of support: This project was supported by funding from the National Committee for Scientific Research (Cracow, Poland – grant No. K/ZDS/003786)
References
- 1.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 2.Kozak-Szkopek E, Broczek K, Slusarczyk P, et al. Prevalence of chronic pain in the elderly Polish population – results of the PolSenior study. Arch Med Sci. 2017;13(5):1197–206. doi: 10.5114/aoms.2015.55270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ. 2015;350:h532. doi: 10.1136/bmj.h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: Results from a cross-sectional survey. Eur J Pain. 2006;10(2):127–35. doi: 10.1016/j.ejpain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Pickering G, Marcoux M, Chapiro S, et al. An algorithm for neuropathic pain management in older people. Drugs Aging. 2016;33(8):575–83. doi: 10.1007/s40266-016-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Malladi S. Screening and case finding tools for the detection of dementia. Part I: Evidence-based meta-analysis of multidomain tests. Am J Geriatr Psychiatry. 2010;18(9):759–82. doi: 10.1097/JGP.0b013e3181cdecb8. [DOI] [PubMed] [Google Scholar]
- 7.Arnau A, Espaulella J, Serrarols M, et al. Risk factors for functional decline in a population aged 75 years and older without total dependence: A one-year follow-up. Arch Gerontol Geriatr. 2016;65:239–47. doi: 10.1016/j.archger.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Ferrell BA, Stein WM, Beck JC. The geriatric pain measure: Validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48(12):1669–73. doi: 10.1111/j.1532-5415.2000.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujita F, Diener E. Life satisfaction set-point: Stability and change. J Person Social Psychol. 2005;1(88):158–64. doi: 10.1037/0022-3514.88.1.158. [DOI] [PubMed] [Google Scholar]
- 10.Kimmel PL, Emont SL, Newmann JM, et al. ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis. 2003;42(4):713–21. doi: 10.1016/s0272-6386(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 11.Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1(3):277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 12.Rapo-Pylkkö S, Haanpää M, Liira H. A one-year follow-up study of chronic pain in community-dwelling older adults with and without neuropathic pain. BMC Geriatr. 2017;17(1):152. doi: 10.1186/s12877-017-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attal N, Perrot S, Fermanian J, Bouhassira D. The neuropathic components of chronic low back pain: A prospective multicenter study using the DN4 Questionnaire. J Pain. 2011;12(10):1080–87. doi: 10.1016/j.jpain.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Freynhagen R, Baron R, Gockel U, et al. PainDetect: A new screening questionnaire to detect neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 15.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. J Pain. 2005;6:149–58. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Backonja MM, Krause SJ. Neuropathic Pain Questionnaire – short form. Clin J Pain. 2003;19:315–16. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Bisaga W, Dorazil M, Dobrogowski J, Wordliczek J. A comparison of the usefulness of selected neuropathic pain scales in patients with chronic pain syndromes: A short communication. Advances in Palliative Medicine. 2010;9(4):117–22. [Google Scholar]
- 18.Zyczkowska J, Szczerbińska K, Jantzi MR, Hirdes JP. Pain among the oldest old in community and institutional settings. Pain. 2007;129(1–2):167–76. doi: 10.1016/j.pain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Cole LJ, Farrell MJ, Gibson SJ, Egan GF. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol Aging. 2010;31(3):494–503. doi: 10.1016/j.neurobiolaging.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Miller A, Sanderson K, Bruno R, et al. The prevalence of pain and analgesia use in the Australian population: Findings from the 2011 to 2012 Australian National Health Survey. Pharmacoepidemiol Drug Saf. 2017;26(11):1403–10. doi: 10.1002/pds.4301. [DOI] [PubMed] [Google Scholar]
- 21.Stein WM, Ferrell BA. Pain in the nursing home. Clin Geriatr Med. 1996;12(3):601–13. [PubMed] [Google Scholar]
- 22.Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res. 2012;46(4):444–50. doi: 10.1016/j.jpsychires.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.VanDenKerkhof EG, Mann EG, Torrance N, et al. An epidemiological study of neuropathic pain symptoms in Canadian adults. Pain Res Manag. 2016;2016 doi: 10.1155/2016/9815750. 9815750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett A, Husebo BS, Achterberg WP, et al. The importance of pain management in older people with dementia. Br Med Bull. 2014;111(1):139–48. doi: 10.1093/bmb/ldu023. [DOI] [PubMed] [Google Scholar]
- 25.Mailis-Gagnon A, Nicholson K, Yegneswaran B, Zurowski M. Pain characteristics of adults 65 years of age and older referred to a tertiary care pain clinic. Pain Res Manag. 2008;13(5):389–94. doi: 10.1155/2008/541963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kollenburg EG, Lavrijsen JC, Verhagen SC, et al. Prevalence, causes, and treatment of neuropathic pain in Dutch nursing home residents: A retrospective chart review. J Am Geriatr Soc. 2012;60(8):1418–25. doi: 10.1111/j.1532-5415.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 27.Aprile I, Briani C, Pazzaglia C, et al. Pain in stroke patients: Characteristics and impact on the rehabilitation treatment. A multicenter cross-sectional study. Eur J Phys Rehabil Med. 2015;51(6):725–36. [PubMed] [Google Scholar]
- 28.Neumann-Podczaska A, Nowak T, Suwalska A, et al. Analgesic use among nursing homes residents, with and without dementia, in Poland. Clin Interv Aging. 2016;11:335–40. doi: 10.2147/CIA.S101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callegari C, Ielmini M, Bianchi L, et al. Antiepileptic drug use in a nursing home setting: A retrospective study in older adults. Funct Neurol. 2016;31(2):87–93. doi: 10.11138/FNeur/2016.31.2.087. [DOI] [PMC free article] [PubMed] [Google Scholar]


