Abstract
Background:
Nervous system injuries in mammals often involve transection or segmental loss of peripheral nerves. Such injuries result in functional (behavioral) deficits poorly restored by naturally occurring 1-2mm/d axonal outgrowths aided by primary repair or reconstruction. “Neurorrhaphy” or nerve repair joins severed connective tissues, but not severed cytoplasmic/plasmalemmal extensions (axons) within the tissue.
New Method:
PEG-fusion consists of neurorrhaphy combined with a well-defined sequence of four pharmaceutical agents in solution, one containing polyethylene glycol (PEG), applied directly to closely apposed viable ends of severed axons.
Results:
PEG-fusion of rat sciatic nerves: (1) restores axonal continuity across coaptation site(s) within minutes, (2) prevents Wallerian degeneration of many distal severed axons, (3) preserves neuromuscular junctions, (4) prevents target muscle atrophy, (5) produces rapid and improved recovery of voluntary behaviors compared with neurorrhaphy alone, and (6) PEG-fused allografts are not rejected, despite no tissue-matching nor immunosuppression.
Comparison with existing methods:
If PEG-fusion protocols are not correctly executed, the results are similar to that of neurorrhaphy alone: (1) axonal continuity across coaptation site(s) is not re-established, (2) Wallerian degeneration of all distal severed axons rapidly occurs, (3) neuromuscular junctions are non-functional, (4) target muscle atrophy begins within weeks, (5) recovery of voluntary behavior occurs, if ever, after months to levels well-below that observed in unoperated animals, and (6) allografts are either rejected or not well-accepted.
Conclusion:
PEG-fusion produces rapid and dramatic recovery of function following rat peripheral nerve injuries.
Keywords: Polyethylene glycol fusion, peripheral nerve injury, rat sciatic nerve repair, Wallerian degeneration, neuromuscular junction re-innervation, allograft, neurorrhaphy
1. Introduction
1.1. Terminology
The appropriate use of terms to describe PNS re-innervation by “artificially induced” PEG-fusion versus “naturally occurring” regeneration by outgrowth from surviving proximal axons can help avoid confusion. Re-innervation restores the nerve supply to a denervated structure by any means at any time after denervation. Regeneration restores innervation by slow (1-2mm/d) outgrowth from severed proximal nerve segments that sometimes successfully reinnervate distal denervated structures. PEG-fusion rapidly (seconds to minutes) reinnervates distal denervated structures by the artificial reconnection/joining of many (but not all) cut axonal ends facing each other in severed proximal and distal segments of a nerve produced by the PEG-fusion protocol described herein. n.b.: Axons that are not PEG-fused may also naturally regenerate by outgrowth from surviving proximal stumps. Thus, re-innervation after PEG-fusion can occur by two mechanisms: (1) immediate axonal reconnection by (artificial) PEG-fusion; and/or, (2) by delayed (natural) regeneration via slower axonal outgrowth. Both regeneration and PEG-fusion can produce specific or non-specific re-innervation of a structure. Both PEG-fused axons and axons regenerating by outgrowth can contribute to functional/behavioral recovery, the best measure of PNI repair. Unsuccessful PEG-fusions in the rat sciatic model are PEG-fusions that fail to achieve an SFI recovery at least two standard deviations (p<0.05) better than recovery observed for Negative Control single cuts or allografts, respectively (Ghergherehchi et al., 2016; Mikesh et al., 2018a,b).
1.2. The challenge of peripheral nerve injuries (PNIs)
Transection and ablation (segmental defect) PNIs are the most common nerve traumas in civilian and military populations and significantly burden US health care systems (Birch and Wynn-Parry, 1998; Bittner et al., 2015; Campbell, 2008; Fox and Kreishman, 2010; Stansberry et al., 2007). The proximal axonal segment and perikaryon of a severed axon usually survive because cut axonal ends rapidly seal (Bittner et al., 2016; Schlaepfer and Bunge, 1973; Spaeth et al., 2010). In contrast, severed distal axonal segments in mammals undergo obligatory Wallerian degeneration within 3 days of injury (Brushart, 2011; Green and Wolfe, 2011; Kandel, 2013). Recovery of sensation and voluntary function (behavior) occurs exclusively by natural regeneration at 1-2mm/d that slowly, partially, and non-specifically reinnervate denervated distal target tissues. Surviving Schwann cells and endoneurial sheaths can help guide such outgrowths to reinnervate denervated target tissues that, unfortunately, may irreversibly atrophy before re-innervation occurs (Brushart, 2011; Kandel, 2013). Hence, transection PNIs, especially ablation PNIs, often result in poor, if any, restoration of sensation and especially motor function (Bittner et al., 2015; Campbell, 2008; He et al., 2014; Lee and Wolfe, 2000; Mackinnon et al., 2001).
1.3. Contemporary standards of care for PNI repair
The current strategy to improve outcomes after a single transection PNI is to microsuture the epineuria of the proximal and distal segments of the severed nerve (“neurorrhaphy”) in a process clinically known as a primary repair. Neurorrhaphy rejoins the connective tissue sheath but does not repair individual cells (neurons) with severed cytoplasmic extensions (axons) within the nerve. After ablation PNIs, where tension-free coaptation is not possible across a gap due to a segmental injury, nerve reconstruction is performed (Birch and Wynn-Parry, 1998; Campbell, 2008; Green and Wolfe, 2011). Current repair strategies for segmental ablations include microsuturing (neurorrhaphy) of synthetic conduits, acellularized allografts or expendable nerve segments called autografts that are harvested from other parts of the body to bridge the gap. Stable recovery of sensation and function occurs months to years after repair of single transection or segmental ablation PNIs. Note that all contemporary nerve repair strategies are designed to improve the chance for natural regeneration, but do not prevent Wallerian degeneration or distal muscle atrophy (Brushart, 2011; Isaacs et al., 2016; McAllister et al., 1996). No current strategy repairs severed cellular (i.e., axonal) processes within a nerve tissue (e.g., sciatic nerve).
1.4. Polyethylene glycol (PEG) as an adjunct to primary repair of traumatic PNIs
We have recently developed (Ghergherehchi et al., 2016; Mikesh et al., 2018a,b) a well-defined protocol for the administration of four pharmaceutical agents in solution, one of which contains the plasmalemmal fusogen PEG (Gefter et al., 1977; Lentz and Lee, 1999; Lentz, 2007; Pontecorvo, 1975) as an adjunct to standard neurorrhaphy for primary repair of PNIs. This protocol results in the immediate reconnection (fusion) of the open axonal ends (PEG-fusion) of many axons in closely apposed proximal and distal ends of singly transected nerves (Bittner et al., 2012; Lore et al., 1999; Mikesh et al., 2018a). The same is true for PEG-fused auto- and allografted nerve segments to repair segmental ablation injuries (Bittner et al., 2015; Mikesh et al., 2018b; Riley et al., 2015). In contrast to current tissue repair strategies using neurorrhaphy alone, PEG-fusion as an adjunct to neurorrhaphy also repairs many severed cellular (axonal) processes within the PNS nerve tissue (e.g., rodent sciatic nerve).
In conjunction with neurorrhaphy, PEG-fusion of axons in singly cut PNIs or PEG-fusion of axons in autografts/allografts to repair ablation PNIs in a rat sciatic nerve model produces dramatically improved recovery of coordinated, volitional function as measured by the Sciatic Functional Index (SFI: Bittner et al., 2012; de Medinaceli et al., 1982; Wood et al., 2011). We (Mikesh et al., 2018a,b) have recently demonstrated that PEG-fusion results in: (a) restoration of axolemmal and axoplasmic continuity and action potential through-conduction across the coaptation site(s) within minutes, (b) prevention of Wallerian degeneration for many axonal segments distal to the coaptation site(s), (c) preservation of distal NMJs indefinitely and prevention of distal target muscle atrophy, (d) recovery of voluntary function (behavior) that occurs rapidly (days to weeks), often to levels seen in unoperated animals, and to substantially improved quality compared to animals repaired with neurorrhaphy alone, and (e) non-rejection of living allogenic allografts in outbred rats in the non-protected environment of a sciatic peripheral nerve with no immunosuppressive treatments. Furthermore, recently reported human clinical cases show that the improved speed and quality of sensory recovery of PEG-fused severed digital nerves are very similar to those reported for recovery of function after PEG-fusion of severed sciatic nerves in the rat sciatic injury model (Bamba et al., 2016b).
In contrast, Negative Control single cuts or allografts that receive all PEG-fusion procedures except the PEG solution exhibit phenomena described in 1.2, rather than a-e, described in this Section.
1.5. Brief description of the PEG-fusion protocol
The PEG-fusion protocol, the rationale for each step in the sequence, and the methods of intra- and postoperative evaluation have not yet been extensively described. It is the intent of this report to fully document this PEG-fusion method so that others can easily and accurately reproduce the procedure and results. We herein describe the protocol, rationale and expected results, if appropriately followed. The PEG-fusion technique and methods of assessment and their rationale are presented in the Methods because of important differences compared to conventional nerve repair.
The PEG-fusion protocol consists of sequential administration of four pharmaceutical agents in solution, which are sequentially applied directly to the ends of a severed nerve in conjunction with neurorrhaphy (#3 below). This protocol and its rationale is summarized in Table 1. In contrast to current tissue repair strategies using neurorrhaphy alone, PEG-fusion is a strategy that repairs PNS nerve tissue with neurorrhaphy and also repairs many severed cellular (axonal) processes within the PNS nerve tissue (e.g., sciatic nerve)
Table 1.
PEG-fusion protocol
| Step # | Technique | Purpose(s) |
|---|---|---|
| Preparation | Trim nerve ends |
|
| 1 - Priming | Irrigation of the surgical field with hypotonic Ca2+-free saline for 1-2min |
|
| 2 - Protection | Administration of 1% methylene blue (MB; an antioxidant) in distilled water for 1-2 min to the opened cut ends |
|
| 3 - Coapt cut peripheral nerve ends | Perform neurorrhaphy |
|
| 4 - PEG-fuse many axons | Apply 50% w/w 3.35kDa PEG in distilled water for 1-2min to the coaptation site |
|
| 5 - Complete membrane repair | Irrigation of the coaptation site with excess volume of isotonic Ca2+-containing saline |
|
2. Materials, Equipment, Methods, Assays and their Rationale
2.1. Surgical Solutions
Sterile Plasmalyte A (Baxter, Deerfield, IL), Calcium free 290mOsmol/L (mM). This is an isotonic solution for rats and humans for which isotonic solutions range from 270-310Mm (millimolar).
Sterile isotonic Lactated Ringers (Dechra, Overland Park, KS), containing calcium 273 mOsmol/L.
Sterile Hypotonic Plasmalyte A: Plasmalyte A diluted to ~90% with distilled water to produce a 250mOsmol/L (mM) solution.
Methylene Blue (Acros Organics, Morris Plains, NJ) 1% in sterile distilled water.
PEG solution: 3.35kDa polyethylene glycol (Sigma Aldrich, St. Louis) 50% w/w in distilled water aka 500mm (millimolal). This mixture is about 400mM.
2.2. Materials
10-0 microsutures (Ethicon, Somerville, NJ)
5-0 microsutures (Ethicon, Somerville, NJ)
9 mm wound clips (Stoelting Co., Wood Dale, IL)
Texas-red dextran (Molecular Probes, Eugene, OR)
Carprofen Sterile Injectable Solution (Putney Inc., Portland, ME)
Hard Plus Resin 812 (Electron Microscopy Sciences, Hatfield, PA)
Diamond knife (DDK, Wilmington, DE)
Formvar-coated Synaptek grids (Electron Microscopy Sciences, Hatfield, PA)
2.3. Equipment
Powerlab 4/30 data acquisition system (ADInstruments, Colorado Springs, CO)
Small mammal isoflurane inhaled anesthetic system (Handlebar Anesthesia, Austin, TX)
Wilde operating scope model MS-C
Leica SP2 AOBS confocal microscope
Technai Spirit electron microscope (Hillsboro, OR)
AMT Advantage HR 1kX1k digital camera
2.4. Animals
All experimental procedures are approved by standards set forth by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Sprague Dawley rats, an outbred genetically variable strain (Churchill et al., 1990; Rogers et al., 1998; Baud et al. 2013; Gileta et al., 2018), of the same sex are housed 2-3/cage and maintained on a 12hr:12hr reverse light:dark cycle with food and water given ad libitum. Surgical and behavioral procedures are performed in the active cycle. Animals used for behavioral assessments are handled and trained for behavioral testing (see section 2.4 below) for at least a week prior to surgery.
2.5. Surgical and electrophysiological procedures to produce and assess successful PEG-fusions
2.5.1. Procedures prior to a single transection
Rats weighing 225-300g (females) or 300-350g (males) are anesthetized with a small mammal isoflurane inhaled anesthetic system (Handlebar Anesthesia, Austin, TX). After initial induction using a 4% isoflurane/oxygen mixture at 1.5 liters/min, animals are maintained by a 1.5–2% mixture at 1liter/min for the duration of the surgery. Surgeries are typically performed on the lateral side of the left hindlimb trimmed of fur and disinfected with 10% iodine/povidone and 70% ethanol. For a single transection of the sciatic nerve, a 2-3cm incision is made through the skin and the biceps femoris, parallel to its muscle fibers; muscle fibers are then separated to expose the sciatic nerve. The connective tissue immediately surrounding the sciatic nerve is carefully trimmed away with microscissors to fully expose the nerve.
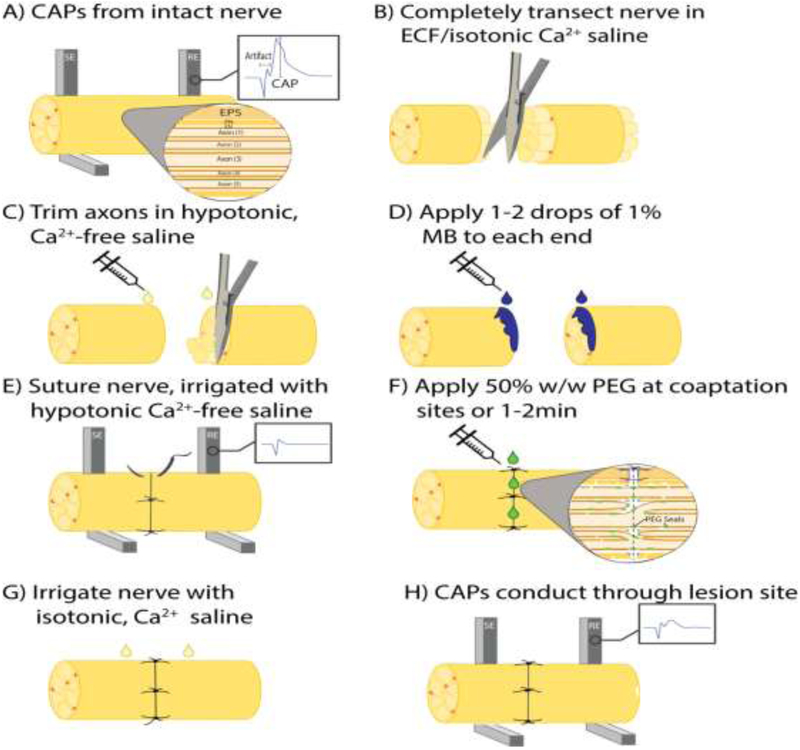
A PEG-fusion procedure cannot be assumed to have succeeded on any given animal. It is essential to demonstrate initial intraoperative success to produce axolemmal and axoplasmic continuity and function of some axons. The easiest and most direct way of doing this is to show that some axons conduct action potentials from proximal to distal before nerve transection and after PEG-fusion, but not after completely transecting the nerve. Hence, prior to any transection, compound action potentials (CAPs) are extracellularly stimulated and recorded by placing stimulating and recording electrodes at least 1cm apart across the site of intended transection to confirm that the sciatic nerve is initially intact and electrically viable (Fig. 1A). The well-spaced electrodes can be placed on top or beneath the sciatic nerve that is always kept moist with its own extracellular fluid and/or with calcium-containing Lactated Ringers. CAPs are a “yes/no” (binary) method of evaluating axonal continuity because CAP amplitude and time course depend on many variables such as electrode shape, construction and placement, the microfluid environment surrounding the electrodes, and sciatic axons having different numbers, diameters and myelin wrappings (Ghergherehchi et al., 2016).
Figure 1.
Schematic diagram showing the stepwise protocol of PEG-fusion. (A) As an initial test of nerve viability, extracellular CAPs are recorded from the intact nerve. (B) The nerve is completely severed in physiological, isotonic Ca2+ containing saline. (C) Nerve axons are carefully trimmed with sharp microscissors in hypotonic, Ca2+-free saline. (D) 1-2 drops of 1% MB dissolved in double-distilled H2O is applied to each end of the nerve. (E) The nerve ends are carefully sutured with minimal damage to the proximal and distal stumps. Additional drops of hypotonic Ca2+-free saline are applied as needed to prevent dehydration of the nerve during the surgical repair. (F) A 50% w/w solution of PEG is applied directly to the coaptation site(s) for 1-2min. (G) The nerve is irrigated with isotonic, Ca2+-containing saline. (H) CAPs are taken as a positive control to confirm initial success of the PEG-fusion procedure. EPS: epineural sheath; PN: perineurium; EN: endoneurium; CAPs: compound action potentials; ECF: extracellular fluid; MB: methylene blue; PEG: polyethylene glycol.
Compound muscle action potentials (CMAPs) are also often stimulated by electrodes placed on the sciatic nerve as described for CAPs and recorded by monopolar needle electrodes placed within a muscle well distal to the planned lesion site, typically the tibialis anterior or the gastrocnemius muscle. Muscle twitches are also associated with this stimulation. CMAPs and muscle twitches are a binary measure of PEG-fusion success due to many uncontrolled variables, including electrode placement and muscle fiber properties (Bittner et al., 2017a; Mikesh et al., 2018a,b).
2.5.2. Procedures for single transection repair
Complete transections of the entire sciatic nerve are made in calcium-containing isotonic extracellular fluid and/or Lactated Ringers by a single stroke of very sharp dissection scissors to completely sever all axons as well as their endo-, peri-, and epineural sheaths (Fig. 1B). Cut axonal ends and sheaths separate by 1-3mm. Bundles of axons sometimes swell out of the epineural sheath at the cut ends. Proximal and distal cut axonal ends partially collapse; a calcium-induced accumulation of membrane-bound single- and multi-layered vesicles accumulate and interact to restrict influx and efflux of substances in the extracellular and intracellular fluids. Complete sealing of cut axonal ends takes 5-15min in isotonic calcium-containing solutions with diffusion of higher molecular weight dyes blocked sooner than lower molecular weight dyes or hydrated ions (Bittner et al., 2016; Spaeth et al., 2012b, 2010).
2.5.3. Application of hypotonic calcium-free Plasmalyte and rationale
The transection site and adjacent 1-2mm of proximal and distal sciatic nerve is flushed with sterile hypotonic Plasmalyte A (Baxter, Deerfield, IL) for at least 1-2min while the nerve endings are carefully trimmed and maintained in hypotonic saline (Fig. 1C). The hypotonic solution opens cut ends by osmotically-driven intracellular hydrostatic pressure and expels many calcium-induced intracellular vesicles that form to seal cut axonal ends. Sealed axons have a greatly reduced ability to be successfully PEG-fused (Bittner et al., 2016; Lore et al., 1999; Spaeth et al., 2012b).
2.5.4. Application of 1% Methylene Blue (MB) in (hypotonic) distilled water and rationale
The severed and opened nerve ends are treated for 1-2min with 1% MB (Fig. 1D), an antioxidant dissolved in ddH2O that continues to keep axonal ends open and prevents formation of additional intracellular vesicles (Bittner et al., 2016, 2012; Spaeth et al., 2010). Collapsed axonal ends and/or vesicles at cut axonal ends decreases the probability of successful PEG-fusion in 2.5.6 below.
2.5.5. Neurorrhaphy and rationale
Axonal ends and epineural sheaths are carefully trimmed so that the cut ends form smooth flat planes that can be closely apposed with minimal gaps or axonal protrusions (Fig. 1E). The cut proximal and distal nerve segments are closely apposed with 10-0 microsutures, carefully passing the needle through the epineurial sheath and minimizing damage to the proximal and distal nerve ends. A minimum of 4 sutures are placed, leaving enough space between sutures to allow close positioning of a micropipette for diffusion of sterile solutions of PEG, antioxidants, etc., to the lesion site (Fig. 1F). Microsutures through the connective tissue of the epineurium or perineurium that closely appose the severed cut ends provide mechanical strength at the transection site to prevent the repaired axolemma of a PEG-fused axon from pulling apart. That is, axonal (or other cell-type) plasmalemmas have minimal tensile strength (Bittner et al., 2017a, 2015, 2012; Ghergherehchi et al., 2016; Lore et al., 1999) to resist stretching. PEG-fusion repair requires delicate handling of the nerve stumps to avoid damage during surgical repair. Nerve axons must be carefully trimmed to provide clean, uniform apposition. The coaptation site must be kept clean of blood and other debris that can create a physical barrier to the fusion repair.
Animals are randomly assigned to PEG-fused or Negative Control groups after neurorrhaphy to eliminate bias in surgical procedures. After transection, neurorrhaphy and MB application, CAPs are again stimulated immediately post-repair, but should not be recordable distal to the transection site, nor should CMAPs, as confirmation that transection is complete (Fig. 1E). The stimulus current must be carefully checked to avoid direct ephaptic activation of muscles distal to the transection site. Stimulation of a transected sciatic nerve may also rarely evoke CMAPs or twitches in distal muscle masses by collateral branches that can arise proximal to the proximal stimulating electrodes (Rupp et al., 2007). These CMAPs are no longer observed once the collateral branch is subsequently transected. Ephaptic activation of CAPs is avoided by use of low stimulating current (Bittner et al., 2017a, 2017b, 2016, 2012; Britt et al., 2010; Ghergherehchi et al., 2016; Riley et al., 2015).
2.5.6. Application of PEG
For PEG-fused animals, after neurorrhaphy, a sterile hypotonic solution of 50% w/w 3.35kDa PEG in distilled water is applied to the repair site for 1-2min to non-specifically repair/join/fuse closely apposed open cut axonal ends (Fig. 1F). Continuity of axoplasm and axolemma across the lesion site after PEG-fusion is confirmed by demonstrating that CAPs are conducted across the coaptation site. In contrast, CAPs do not conduct across the transection site immediately if the microsutured nerve is not PEG-fused.
Negative Control animals receive the same procedures and solution applications as do PEG-fused animals, except for the addition of the PEG solution. The cut and trimmed ends of Negative Controls are apposed by microsutures, the “gold standard” for clinical repair (Campbell, 2008; Green and Wolfe, 2010; Isaacs et al., 2016; McAllister et al., 1996). Additional negative control protocols consist of PEG application to cut nerves that are either not microsutured or the cut ends are not closely apposed by microsutures. In these cases, PEG-fusion is not successful and PEG causes the cut axonal ends to collapse and the collapsed leaflets to fuse, sealing off the cut ends within microseconds rather than minutes (aka “PEG-sealing”; Bittner et al., 2016; Spaeth et al., 2012b).
2.5.7. Application of isotonic, calcium-containing saline
The microsutured ends of both PEG-fused and Negative Control nerves are gently irrigated several times with sterile isotonic Lactated Ringers containing calcium (Fig. 1G) to repair axolemmal holes with calcium-induced vesicles (Bittner et al., 2016; Lore et al., 1999; Spaeth et al., 2010).
After this irrigation step, recording of CAPs and CMAPs are again always attempted (Fig. 1H) as described in 2.5.1 to evaluate restoration of electrophysiological continuity as evidence of restored axonal integrity. CAP or CMAP through-conduction immediately after PEG-fusion is essential to confirm successful PEG-fusion of any axons whose axolemmal and cytoplasmic continuity is restored at the coaptation site. Without confirmation, data generated from post-operative samples collected over time cannot be stated to be from PEG-fused nerves.
In successful PEG-fusions, CAPs and CMAPs recorded distal to the transection site are associated with twitches of muscles innervated by axons distal to the lesion site, indicating that some axons have been fused and are functional across the lesion site; NMJs distal to the lesion site must also be reinnervated by PEG-fused axons. In our experience, if an intraoperative CAP is not obtained after an attempted PEG-fusion, then successful behavioral recovery does not occur within 4-6 weeks. However, successful behavioral recovery sometimes does not occur even if an intraoperative CAP is obtained, possibly because free ambulation of the animal during recovery may exert tension at the coaptation site causing PEG-fused axons to pull apart (Bittner et al., 2015; Ghergherehchi et al., 2016). To further demonstrate success immediately after PEG-fusion, we and others have also used dye diffusion in a parallel set of animals (see 2.5.9).
After testing for CAPs and/or CMAPs, the muscle incision is closed with 5-0 sutures, and the skin is stapled with wound clips. The animal is allowed to recover on heated pads and returned to standard housing. All animals to be tested for behavioral recovery receive a 5mg/kg subcutaneous injection of carprofen during recovery from anesthesia.
2.5.8. Procedures for double cut (ablation) repair
Procedures for repair of ablation (segmental) PNIs are the same as described for single cut repairs described in 2.5.1 - 2.5.7 above with the following modifications. A 25-35mm long incision is made to expose the sciatic nerve that then has a 6-8mm segment ablated in mid-thigh with fine surgical scissors, leaving an 8-10mm gap between cut axonal ends in the proximal and distal stumps of the host nerve. Because intact nerves are under tension, an ablation produces a gap that is several mm longer than the removed segment.
Host autografts (isogenetic tissue) are obtained from peroneal nerve segments in the contralateral or ipsilateral hind limb. Two segments of peroneal nerve are used to construct a cable graft that has a smaller diameter than the host sciatic nerve. Donor allografts that match the diameter of the host nerve are obtained from the left or right sciatic nerve of another outbred Sprague Dawley rat that is neither tissue matched nor immunosuppressed in either the donor or the host. Autografts or allografts are 1-3mm longer than the gap created by the ablated segment of the host nerve. Autografts and allografts are stored in calcium-free, hypotonic saline (Plasmalyte A) at 2°C for 30min – 6h before use. See Sections 2.3.3 – 2.3.5. The sciatic nerve and ablation sites in the host rat are washed with hypotonic Plasmalyte A and 1% MB and all axonal ends carefully trimmed. The identical procedure for primary repair is performed sequentially for the proximal and distal ends of the auto/allograft coaptation sites. Stimulating and recording electrodes are placed just proximal and just distal to the autograft or allograft and CAPs/CMAPs are recorded to assess whether axonal continuity is re-established through the graft; e.g., across both coaptation sites. As with single cut PEG-fusion repairs, CAP conduction across a successful PEG-fused graft will evoke twitches of muscles innervated distal to the graft.
2.5.8. Behavioral analyses
Stable behavioral recovery after a traumatic injury is the only measure of a successful repair protocol for long-term recovery that parallels clinical success. Hence, a behavioral assay is essential for determining the success of any PEG-fusion protocol. Any validated behavioral measure could be used; we use the Sciatic Functional index (SFI). The SFI is a common and reliable test to assess function and return of behaviors mediated by the sciatic nerve in rats and mice (de Medinaceli et al., 1982; Geuna, 2015; Wood et al., 2011). The SFI score is highly dependent upon foot flexion and toe spread, movements controlled by lower leg and foot muscles innervated by the sciatic nerve, e.g., tibialis anterior, soleus, or gastrocnemius muscles. Any recovery is typically associated with gradual decreases in the absolute value of negative SFI scores as axons regenerating by outgrowth from severed surviving proximal axonal stumps that increasingly reinnervate more denervated distal muscle fibers.
Animals are handled for acclimation and trained in the testing procedure for at least one week prior to surgery. For SFI tests run by testers blinded to their experimental group, both hind paws are marked with red or blue ink, right side unoperated and left injured, respectively. Rats are placed upon an inclined 100mm wide board, 5ft long, lined with paper strips. After a rat runs back to its home cage across the paper-lined board, footprints are measured by testers blinded to treatment. Three variables are measured for both normal and experimental footprints: footprint length, total toe spread, and intermediate toe spread, as previously reported (Carlton and Goldberg, 1986). The average of the two runs is recorded as the SFI score for the day. Animals are first tested 3d after surgery, then weekly for at least 42d post-operatively (PO). Data are recorded in spreadsheets and graphs prepared in Graphpad Prism 7.
In order to obtain reliable results, we have used several criteria for acceptable SFI tapes. Animals must run for three consecutive steps for each hindlimb without stops or hesitation, which can create artificially long footprints (Varejao et al., 2001). We also ensure that the animal does not step with the dorsum of its paw, which creates an artificially short footprint. We have also observed that the contralateral hindlimb sometimes compensates for the injured limb by increasing weight bearing, which increases the print length of the unoperated limb. This compensation rarely significantly affects the SFI score. Animals are excluded from the study if they exhibit signs of autotomy, which results in the complete removal of digits in the affected hind limb. When these specific selection criteria are followed, we find the SFI to be a reliable measure of functional return of sciatic mediated behaviors.
2.5.9. Intra-axonal dye diffusion
As an alternate or additional measure of morphological/anatomical continuity across a severance site, intra-axonal diffusion of dye in 2-4cm lengths of sciatic nerve excised immediately following any operative procedure can be assessed ex vivo (Bittner et al., 2012; Ghergherehchi et al., 2016; Riley et al., 2015). In a Petri dish, a 1-2cm long nerve segment proximal to the injury site is mounted inside a watertight ring of petroleum jelly (Vaseline) containing 50μl of an uncharged fluorescent dye (e.g., 3kDa Texas red dextran: Molecular Probes, Eugene, OR) in hypotonic Ca2+-free saline. The injury site and the 1-2cm long segment of nerve distal to the injury site extends outside the well and are bathed in isotonic Ca2+-containing saline. The nerves are maintained for 12-24h at 4°C to allow intra-axonal diffusion of the dye throughout the entire length of nerve. Sciatic nerves are examined for the presence of fluorescent dye anywhere along their length using a microscope capable of detecting emitted fluorescence.
2.5.10. Use of delivery device
We have also used a delivery device (Neuraptive Therapeutics, Lafayette CO) for the PEG administration step of the protocol as described in 2.3.6. The device establishes a fluid containment field around the coaptation site to allow PEG to be more focally administered and easily removed by aspiration. The device also protects the surrounding tissues from unnecessary exposure to PEG. Although not required for successful PEG-fusion of smaller diameter (1-2mm) nerves, the device may be helpful for PEG-fusing larger diameter nerves and for more complex procedures in the clinic. Removal of the device places no tension at the coaptation site.
2.6. Other methods used to assess data obtained using our PEG-fusion protocols
2.6.1. Morphological analyses of sciatic axons and nerves
For detailed procedures, see Mikesh et al., 2018 a and b. Briefly, nerve tissue is dissected from sacrificed animals and fixed overnight at room temperature, followed by en bloc post-staining in osmium-ferrocyanide, washes in water, and aqueous uranyl acetate. Care is taken to ensure that sampling sites are equivalent in all nerves relative to suture placement.
Tissues are then dehydrated and embedded in Hard Plus Resin 812 (Electron Microscopy Sciences, Hatfield, PA). Sections (silver-gold, 65nm) are cut on a diamond knife (DDK, Wilmington, DE) and mounted on formvar-coated Synaptek grids (Electron Microscopy Sciences, Hatfield, PA) prior to viewing with an electron microscope (Hillsboro, OR) fitted with a digital camera. Images are analyzed for axon morphology (diameter and g ratio) and area composition (Mikesh et al., 2018a,b).
2.6.2. Immunohistochemical (IHC) analyses
Muscles are taken from unoperated rats and the operated sides of PEG-fused or Negative Control rats 7, 21, or 42d PO. Muscles are labeled with antibodies specific to nerve proteins (SV2 and 2H3, DSHB) and fluorescent secondary antibodies, and conjugated bungarotoxin to label acetylcholine receptors (Kang et al., 2007; Mikesh et al., 2018a,b). The superficial muscle fibers are dissected and mounted to slides with Fluoromount mounting medium (Sigma). Labeled acetylcholine receptors are then assessed for the presence or absence of innervating axons.
2.6.3. Retrograde axon tracers
To determine the specificity of connections made by PEG-fused maintained axons versus axons regenerating by outgrowth, we unilaterally singly-cut or ablate a segment of a sciatic nerve and repair it by neurorrhaphy alone (Negative Control) or with PEG-fusion of single cut or a graft following an ablation lesion. After 0d-160d PO, horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP; 0.5μl, 0.2% in distilled water) is injected bilaterally into the tibialis anterior or other muscles to label spinal cord motoneurons by fast retrograde transport. At this volume and concentration, BHRP is specifically taken up by distal motor axons and does not label dorsal root ganglion cells that lack GM1 ganglioside to actively transmembrane transport BHRP (Lappi et al., 2014; Yu et al., 2015). Two days after BHRP injection, spinal cords are processed to visualize BHRP labeling of motoneurons and their 3D dendritic morphology. Measures of soma size and motoneuron counts are also performed.
2.6.4. Statistical Analyses
Excel is used to calculate means, linear regressions and t-test comparisons. For axons, Welch’s t-test allows for heteroscedastic data, where N = the total number of axons in a treatment group, and N-2 = the degrees of freedom. A two-tailed Student’s t-test, a test for homoscedastic data, is used to compare mean SFI scores for each treatment group on a given post-operative day. Two-way ANOVAs analyze means and standard errors of SFI scores (Bittner et al., 2012; Ghergherehchi et al., 2016; Mikesh et al., 2018a; Riley et al., 2015).
3. Results
3.1. Behavior, axonal and NMJ structure/function after unoperated or sham protocols
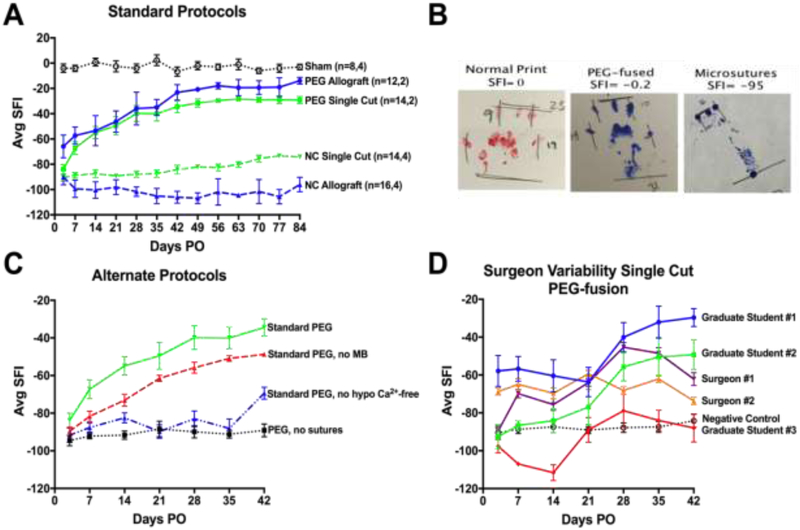
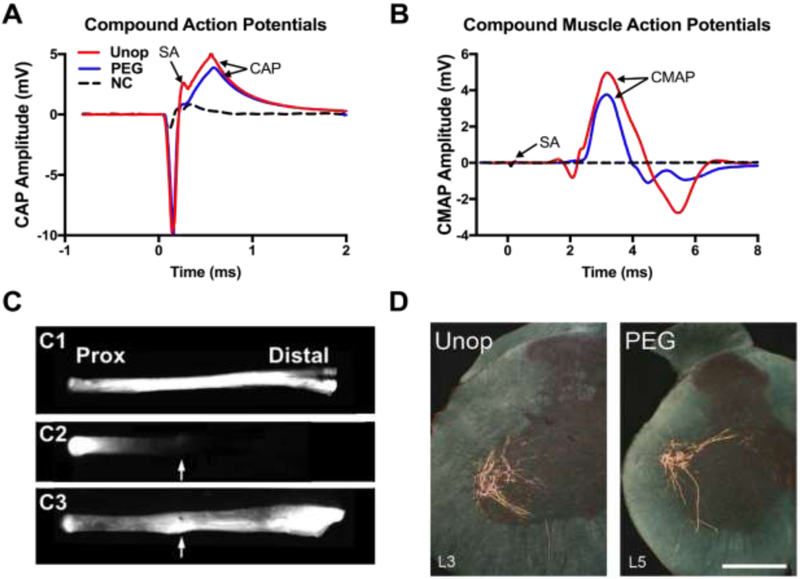
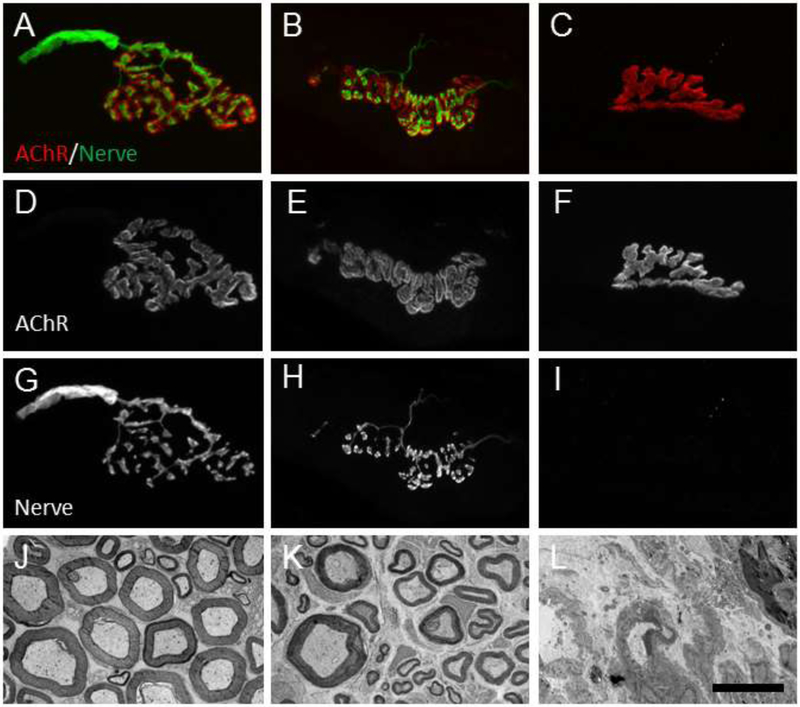
At any time they are measured, unoperated or sham control rats having sciatic nerves exposed but not lesioned, SFI scores (a measure of voluntary behavior) typically range from ±10 (Fig. 2A, Table 2). CAPs and CMAPs are always recordable, intra-axonal dye diffuses the entire length of the nerve (Fig. 3A-C), and retrograde transported tracers or diffusible dyes injected into a muscle always label a well-characterized set of motoneurons in a given spinal segment (Fig. 2D). Unoperated or sham axons have an average diameter of about 3.9μm in the proximal or distal thigh, g ratio (axon diameter/diameter of axon + myelin sheath) of 0.62, and a density of about 200 axons/10,000μm2 Fig. 4, Table 2). Muscles are innervated by labeled nerve terminals at their NMJ (100% muscle fiber innervation (MFI): Fig. 4, Table 2).
Figure 2.
Voluntary-behavior analyses (SFI scores) of PEG-fusion. (A) Our historical mean±SEM SFI scores for Sham Controls, PEG-fused Allografts, PEG-fused Single Cuts, and Negative Control Allografts and Single Cuts with neurorrhaphy and all Standard PEG solutions except PEG. The two n values in the key for each curve gives the number of animals sampled at 3-42d PO, and the number sampled 3d-84d PO, respectively. Negative Control Allografts show no significant recovery at any time up to 84d PO. Animals with Negative Control Single Cuts show some functional recovery by 63-84d PO (p<0.05). PEG-fused Single-Cuts recover within 14d PO (p<0.05), increase recovery by 21-28d PO (p<0.01), and plateau around 42d PO (p>0.001). PEG-fused Allografts show significant recovery by 3-7d PO (p<0.01), and recover to near baseline by 42d PO (p>0.001). (B) Representative footprints at 42d PO for Sham, PEG-fused, and Negative Control animals after single cut repair. (C) SFI data showing decreased success of PEG-fusion by altering or omitting steps in the standard protocol (Standard PEG). Omission of MB (Standard PEG, no MB) or hypotonic Ca2+ free saline (Standard PEG, no hypotonic Ca2+-free) produces significant behavioral recovery compared to Negative Controls, but less recovery compared to the Standard Protocol. Animals receiving the Standard PEG protocol without microsutures (PEG, no sutures) show no behavioral recovery at any PO time. (D) Surgical variability exhibited by different personnel all using the Standard PEG-fusion protocol. Students with no prior surgical background sometimes attain successful PEG-fusions (p<0.05) after 0-30 practice animals during which they are mostly not successful; trained surgeons typically attain success after 0-10 practice animals.
Table 2.
Summary of single cut and allograft axonal morphometric data at 7-42d PO.
| Treatment | SFI | Mean axon diameter (μm) |
Mean g ratio |
Axons/10,000 μm2 |
MFA (μm2) |
MFI (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| prox | graft | distal | prox | graft | distal | prox | graft | distal | ||||
| Unoperated | ||||||||||||
| Unoperated mean | −4 | 3.95 | [3.88] | 3.82 | 0.62 | [0.62] | 0.61 | 210 | [201] | 191 | 2710 | 100% |
| 7d PO (7-14d PO) | ||||||||||||
| PEG mean | −78 (−90) | 3.88 | 3.41 (2.69) | 0.67 | 0.64 (0.66) | 154 | 226 (196) | 71% (80%) | ||||
| NC mean | −85 (−98) | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 1370 | 0% (0%) | |||
| 21d PO | ||||||||||||
| PEG mean | −64 (70) | 3.56 | 2.62 | 2.01 (3.24) | 0.62 | 0.71 | 0.62 (0.71) | 192 | 307 | 448 (150) | 620 | 91% (78%) |
| NC mean | −90 (−87) | 2.7 | 2.24 | 1.35 (1.72) | 0.73 | 0.8 | 0.78 (0.76) | 150 | 18 | 3 (116) | 0% (0%) | |
| 42d PO | ||||||||||||
| PEG mean | −8, (−22) | 3.33 (3.82) | 2.87 | 2.96 (2.98) | 0.64 (0.64) | 0.65 | 0.67 (0.68) | 198 (190) | 264 | 236 (218) | 2890 (1780) | 99% (98%) |
| NC mean | −106, (−83) | 3.27 (3.37) | 2.07 | 1.59 (1.90) | 0.64 (0.66) | 0.72 | 0.63 (0.65) | 175 (199) | 160 | 151 (237) | 790 (1440) | 8% |
Table 2 lists mean allograft ablation data (black font) versus single cut data (red font) for sciatic nerve lesions in the mid-thigh for each protocol and PO times, as given in the 1st column. N= 2-6 nerves sampled for each mean. These protocols are: Unoperated (Unop), PEG-fused (PEG: yellow highlighted) and Negative Control (NC). Each PO time is 7-14d, 21d, or 42d. For each protocol at each PO time Table 1 lists in sequential columns: SFI scores, axon diameter (μm), g ratio (axon diameter/fiber diameter), axon density (per 10,000μm2), average fiber diameter and the average of the ten largest fibers (MAX10), muscle fiber cross sectional area (MFA), and % of muscle fibers having innervated muscle fibers (MFI%).
Figure 3.
Axolemmal and axoplasmic continuity is restored within minutes after PEG-fusion repairs and axonal transport is restored within days for single transections and within 2-3 weeks for segmental ablations repaired with PEG-fusion. (A and B) Electrophysiological confirmation of sciatic nerve continuity after single cut PEG-fusion. (A) CAP (mV) recordings of intact nerves (Unop: solid red line) and after transection without (Negative Control: dashed black line) or with PEG-fusion (solid blue line). SA = stimulus artifact. CAP arrow: peak amplitude. (B) CMAP recordings using same protocols as CAPS. (C) Morphological evidence of axonal continuity only after PEG-fusion. Intra-axonal dye diffusion of Texas Red at 1d PO in an Unoperated (C1), Negative Control (C2) or PEG-fused (C3) sciatic nerve. Arrow: points to repair site. Dye diffuses from the proximal segment of the nerve into the distal segment after PEG-fusion, but does not cross the repair site in Negative Controls. (D) Transverse spinal hemisections of labeled motoneurons of an intact control animal (Unop), and an animal with a PEG-fused sciatic nerve at 5d PO after injection of BHRP into the anterior tibialis muscle. Scale bar = 500μm.
Figure 4.
NMJs and nerve fibers in Unoperated, PEG-fused and Negative Control (NC) animals. Unoperated animals (A,D,G) have NMJs whose innervating axons are not fragmented and whose shape mirrors that of the muscle acetylcholine receptor (AChR). Unoperated nerve fibers (J) have electron-lucid axoplasm and distinct electron-dense myelin sheaths. A 7d PO PEG-fused allograft NMJ (B,E,H) is also clearly innervated by a non-fragmented axons. Nerve fibers from a 14d PO PEG-fused allograft distal host nerve (K) have more extracellular space than unoperated nerves, but also have intact myelin sheaths and electron-lucid axoplasm with little or no signs of Wallerian degeneration. In contrast, a 7d PO Negative Control allograft has no axon innervating the AChR (C,F,I). A 14d PO singly cut NC (L) nerve has no myelinated nerve fibers and shows extracellular debris characteristic of Wallerian degeneration. Scale bar= 20μm for A-I, 10μm for J-L.
Table 2 compares morphometric data at three different PO times for PEG-fusion and Negative Control neurorrhaphy repair of single-cut versus ablation-allograft lesions. At any given PO time, the PEG-fused single cut and allograft data are very similar and the Negative Control single cut versus allograft data are very similar. In contrast, at any given PO time, the PEG-fused single cut and allograft data versus the Negative Control single cut and allograft data are very different. Table 2 shows that: (1) Animals with PEG-fused single cuts or allografts recover lost functions more rapidly and completely compared to Negative Controls as assayed by the SFI in column 2. (2) Animals with PEG-fused sciatic nerves maintain axons, NMJs, and muscle fibers at all PO times while Negative Control preparations undergo Wallerian degeneration, denervation, and muscle fiber atrophy, as assessed by morphometric measures in columns 3-13. (3) Negative Control allografts have small diameter axons that regenerate into the distal stump by 21d PO by 42d PO. (4) Measures of g ratios and axon numbers have greater variation and do not correlate well (p>0.05) with SFI scores, as reported for regeneration by axonal outgrowth from proximal stumps (Brushart, 2011).
3.2. Recovery, axonal and NMJ structure/function after neurorrhaphy protocols
Immediately after neurorrhaphy of a single cut or ablation PNI, behaviors mediated by the sciatic nerve are lost as are electrical and intra-axonal continuity at the lesion site(s), i.e., CAPs, CMAPs and dye diffusion initiated proximal to the lesion are not observed distal to a lesion (Figs. 1, 2A-C). Axonal, myelin and NMJ structure and/or function are obviously degraded beginning 1-3d PO and by 7-14d PO are no longer structurally or functionally intact, i.e., Wallerian degeneration is complete (Fig. 4, Table 2). Behavioral recovery greater than obtained for single transections without neurorrhaphy (SFI scores of −75, p<0.05) has been sometimes observed to occur about 42d PO following single transections repaired by neurorrhaphy, but very rarely occurs even after 84d PO for 5-10mm ablations repaired by allograft neurorrhaphy. Allografts that are neither tissue-matched nor immunosuppressed are rejected within days (Mackinnon et al., 2001; Churchill et al., 1990; Rogers et al., 1998; Baud et al., 2013). Small diameter regenerating axons with a thin myelin sheath (higher g ratio) are observed distally at 21d PO for single cuts and/or ablations, but do not yet re-establish NMJs (Fig. 4, Table 2). At 42d PO, some NMJ are reinnervated following both types of PNIs. However, some single transections exhibit significant behavioral recovery only to −75 SFI (p<0.05), and such recoveries do not significantly correlate with the number of regenerating axons (Mikesh et al., 2018a).
3.3. Recovery, axonal and NMJ structure/function after standard PEG-fusion protocol
After PEG-fusion of a single cut or ablation PNI, behaviors mediated by the sciatic nerve are not immediately restored (Fig. 2) even though electrical and intra-axonal continuity at the lesion site(s) are immediately restored, i.e., CAPs, CMAPs and dye diffusion initiated proximal to the lesion are always observed distal to a lesion (Fig. 3A-C). Although axonal, myelin and NMJ structure and/or function are altered for many axons, successfully PEG-fused axons more closely anatomically resemble axons in Unoperated or Sham-operated nerves than Negative Control nerves. From 1-14d PO, many axons are structurally and functionally intact, i.e., Wallerian degeneration is prevented for many PEG-fused axons and MFI is 70-80% (Fig. 4, Table 2). MFI for PEG-fused axons increases to 75-90% at 21d PO and 98-99% at 42d PO (Table 2). Some small diameter, thinner myelinated axons that are presumably axons regenerating by outgrowth from the axonal stump are seen distal to a PEG-fused lesion site or within a graft at 21d Po (Mikesh et al., 2018a,b).
Significant behavioral recovery often occurs beginning at 3-21d PO following single transections or ablations repaired by PEG-fusion and plateaus at a maximum value of −50 to +10 by 42d PO (Fig. 2A, D). Compared to negative controls, significant recovery at p<0.05 or p<0.01 for single cuts are SFI scores of −75 and −59 and for allografts are −80 and −65, respectively. On average, SFI scores for ablations repaired by PEG-fused allografts are usually significantly better (p<0.05) than SFI scores for PEG-fused single transections at all PO times (Fig. 2a), probably because nerve axons are not under tension (stretched) in allograft repairs that use oversized grafts (Mikesh et al., 2018b). PEG-fused allografts appear to not be rejected, even though the donor and host are neither tissue-matched nor immunosuppressed (Fig. 4B,E,H,K). At 42d PO, some NMJs are reinnervated following both types of PNIs, but only some single transections show some significant behavioral recovery to −75 SFI (p<0.05).
3.4. Recovery, axonal and NMJ structure/function after unsuccessful PEG-fusion or altered PEG-fusion protocols
The standard PEG-fusion protocol occasionally (5-10%) produces unsuccessful PEG-fusion results by surgeons who consistently have success and by trainees (see 3.5 below). Lack of successful fusion is usually immediately exhibited intraoperatively (Fig. 1H) by lack of CAP through-conduction. Subsequent recutting of the nerve and repeating the PEG-fusion protocol sometimes results in a successful PEG-fusion. In other cases, intraoperative success is demonstrated by CAP through-conduction, however functional recovery as demonstrated by the SFI initially recovers to −75 or better within 2-3w, then falls to −80 or lower, and never recovers. In still other cases, even though a CAP is initially recorded after a PEG-fusion, the SFI never exceeds −75. In all failure modes, recovery of function as well as axonal, myelin, and NMJ morphologies resemble that described for standard neurorrhaphy seen in Negative Controls described in 3.2 above (Figs. 2, 3, and 4; Table 2).
Altered PEG-fusion protocols often greatly reduce or prevent successful restoration of CAPs/CMAPs and/or functional recovery after PEG-fusion. Figure 2C shows some examples tested to date such as using isotonic rather than hypotonic saline to prepare the nerve for neurorrhaphy, poor trimming, sutures to tight or too loose, stretching the nerve, and dissolving PEG in isotonic or hypotonic saline. Other published examples include using PEG of <1kDa or >5kDa (Lore et al., 1999), altering PEG concentration (Lore et al., 1999), and delaying up to 24h after a transection to PEG-fuse (Bamba et al., 2016a).
3.5. Behavioral recovery per individual surgeon
Figure 2D shows SFI curves for PEG-fusions exhibited by some of the undergraduates, graduate students and surgeons who have trained in our laboratory. In our experience, with practice, using CAP and behavioral feedback from positive controls, 30% of all undergraduates (n=10), 50% of all graduate students and postdoctoral fellows (n=6) and 67% of all surgeons previously trained to perform neurorrhaphy (n=6) produce successful PEG-fusions of singly cut peripheral nerves, usually after 5-30 failures. Only one surgeon and one graduate student have produced successful PEG-fusions on their first and most subsequent trials.
4. Discussion
4.1. Summary of PEG-fusion Methods and Results
PEG-fusion results for both sexes are very similar for CAP restoration, dye diffusion, muscle fiber morphology, and SFI recovery (Bittner et al., 2017a, 2017b, 2016, 2012; Britt et al., 2010; Ghergherehchi et al., 2016; Riley et al., 2015; Sexton et al., 2012). The only differences between sexes noted to date are that muscle masses and muscle fibers are larger in males. A group size of (n) = of 4-6 each at 2-6 time points provide statistical power to detect differences of p≤0.05 of ≥ 20% between an experimental and a control group. Our results at UT Austin have shown good reproducibility when replicated elsewhere (Bamba et al., 2016a; Riley et al., 2015; Sexton et al., 2012) using procedures described in 2.5.
If protocols are not followed as described in Methods Sections 2.5.1 – 2.5.8, or surgeons not appropriately trained as described in Results Section 3.5 and Figure 2D, behavioral recovery is much poorer and usually closely resembles outcomes obtained for neurorrhaphy without PEG-fusion. Our results also confirm that PEG-fusion studies must incorporate: (1) Sham-operated and Negative Controls; (2) Intraoperative methods to assess that PEG-fusion has indeed initially succeeded (CAPs, CMAPs); and (3) Postoperative behavioral assays as the ultimate, and often only valid, measure of PEG-fusion success. In fact, behavioral assays are the only valid measure of success of any protocol designed to increase the (often poor) outcome of natural regeneration by axonal outgrowths because, as previously reported (Brushart, 2011; Mikesh et al., 2018a,b), other measures such as number of regenerating axons, levels of myelination, etc., typically do not correlate well with behavioral outcomes as assessed by the SFI or other assays. Our data also demonstrate that most board-certified surgeons, as well as undergraduate, graduate or postdoctoral students, need practice and feedback from behavioral assays before they consistently achieve successful PEG-fusions.
We (Bittner et al., 2015; Britt et al., 2010; Ghergherehchi et al., 2016) and others (Brushart, 2011; Nguyen et al., 2002; Wood et al., 2011) have observed that 1-3 mm long micro-crush injuries made with fine forceps do not completely disrupt axonal endoneurial sheaths. Subsequent behavioral recovery is often very good since axonal outgrowths can be guided to their appropriate original targets by surviving sheaths containing viable Schwann cells PEG-fusion without neurorrhaphy may slightly increase the rate of behavioral recovery for 1-2mm micro-crushes of rat sciatic nerves in mid-thigh (Bittner et al., 2012; Britt et al., 2010), but traumatic micro-crushes do not occur naturally in larger mammals, including humans. In contrast, nerve transections that completely disrupt nerve continuity result in significantly less functional recovery compared with crush injuries. SFI scores 6 weeks post-operatively show that transections of rat sciatic nerve in the mid-thigh often result in poor (−84.2±3.4) return of original functions, and ablation injuries that produce a segmental loss of nerve tissue typically result in even worse (−106±4.7) recovery. PEG-fusion with neurorrhaphy greatly increases the rate and extent of functional recovery after single transection (−34.4±4±4.6) or ablation and allograft repair (−23.1±6.1) after 6 weeks.
4.2. Proper interpretation of PEG-fusion protocols
Incorrect understandings of the mechanism of action of PEG-fusion, how to evaluate intra- and post-operative success, and unwarranted conclusions about negative outcomes are problems that must be addressed as they could impede progress and innovation of a potentially important advance in clinical management of patients with peripheral nerve injuries. Problems with misunderstandings or not following the standard protocol (especially intraoperative testing to confirm an initial successful PEG-fusion), its rationale, and subsequent misinterpretation of tracer labels are illustrated by two recent papers by Robinson and Madison (2016) and Brown et al. (2018).
Robinson and Madison (2016) report inappropriate specificity of outgrowth regeneration after two-tracer-labeling of a motor branch of a PEG-fused femoral nerve at 8w (56d) PO. They attribute all inappropriate motoneuron labeling to regenerating axons without confirming the success or failure of PEG fusion intraoperatively or performing behavioral recovery at any time, i.e., without positive controls. Their motoneuron labeling data resemble ours at PO times when re-innervation by outgrowth has occurred, but they do not distinguish re-innervation by PEG-fused versus regenerating axons. Additionally, their method of loading dyes distal to the terminal nerve branch does not examine “re-innervation” accuracy to NMJs, but rather examines regeneration accuracy to the correct nerve branch that innervates several muscles. Whether the labeled axons ever reinnervated the correct or incorrect muscle is never assessed.
Brown et al. (2018) conclude that “polyethylene glycol fusion has shown efficacy in the surgical repair of spinal nerve injuries, but this finding was not replicated in facial nerve injury repair.” However, Brown et al. (2018) almost-certainly did not induce PEG-fusion repair because of technical issues including use of: 1) 500mM PEG solution rather than a 50% w/w (500millimolal) solution, 2) isotonic rather than hypotonic conditioning solutions, and/or 3) an unstated solution of MB. Furthermore, they performed no intraoperative assays to demonstrate successful PEG-fusion, i.e., to confirm that they successfully PEG-fused proximal and distal ends of some axons at the lesion site. Their results are almost-certainly due to unsuccessful PEG-fusions that produce results not significantly different from their Negative Controls, i.e., neurorrhaphy without PEG application. Hence, they observed no surviving axons at 7d PO almost-certainly because no axons were PEG-fused.
4.3. Other uses of PEG in traumatic nerve repair not involving PEG-fusion
Other uses of PEG reported to increase behavioral recoveries after traumatic nerve injuries (usually for spinal injuries) are not studies of PEG-fusion in which a set of well-specified solutions, one containing PEG, are directly and briefly applied to transected and closely apposed axons. For example, some have reported that systemic injections of PEG have a neuroprotective effect. PEG use in synthetic hydrogels or in >15kDa polymers may have some neuroprotective effects by unknown mechanisms (Kwon et al., 2009). Lower molecular weight PEG polymers may have some neuroprotective effects due to PEG-sealing, but if so, not by PEG-fusion. The term PEG-sealing is also used to describe intravenous injection of PEG or micelles/nano-particles associated with PEG (reviewed by Jin, 2014). The latter “PEG-sealing” in vivo procedures typically produce low concentrations of PEG in body fluids associated with modest increases in behavioral recovery many weeks post injury, presumably by neuroprotective effects that rescue damaged neurons from cell death, rather than by fusing transected axons thus preventing much Wallerian degeneration (Jin, 2014).
4.4. PEG-fusion repair shows knowledge gaps in some common assumptions
The results of PEG-fused transection and allograft repair contradict some current commonly held concepts in Neuroscience (Kandel, 2013) and/or Immunology (Murphy and Weaver, 2016). Specifically: (1) Distal stumps of severed axons are often assumed to undergo obligatory Wallerian degeneration within days. (2) Re-innervation after transection is often assumed to exclusively occur by outgrowths slowly regenerating from proximal stumps that rarely produce good recovery of lost behaviors after loss of a more proximal segment (ablation) of major peripheral nerve such as the sciatic. And, (3) Allografts, like other tissues in a non-protected immune environment, are expected to be quickly rejected in the absence of tissue matching and immunosuppression. In contrast, our results show that: (1) Distal segments of PEG-fused axons can survive indefinitely. (2) PEG-fusion induces rapid re-innervation by non-selectively connecting proximal, graft and/or distal axons to immediately and non-selectively reinnervate denervated target tissues, that often produce very good behavioral recovery within 4-6 weeks by extensive peripheral and/or CNS plasticities. And, (3) PEG-fused allografts are not in an immune-privileged environment, but appear to not be rejected despite the lack of tissue matching or immunosuppression. We do not yet know the cellular/molecular/ systems mechanisms responsible for these phenomena well-documented in our recent papers (Riley et al., 2015; Bittner et al., 2017a; Mikesh et al., 2018a,b).
4.5. Clinical applications of PEG-fusion repair
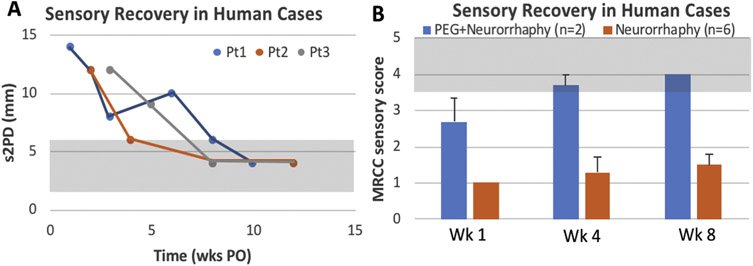
PEG-fusion has potential to benefit patients with traumatic injuries by significantly increasing the speed and magnitude of functional recovery. To date, limited clinical case studies suggest that PEG-fusion indeed produces very favorable outcomes for single transection injuries (Bamba et al., 2016b). That is, PEG-fusion rapidly restores sensation in singly cut human digital nerves as assessed by static two-point discrimination (Fig. 6A) and British Medical Research Council Classification sensory recovery score (Fig. 6B). (Level of Evidence: Therapeutic study, level IV). In two independent clinical proof-of-concept studies, the efficacy of PEG-fusion was evaluated in humans and retrospectively compared to recovery from standard nerve repair (Bamba et al., 2016). One study evaluated two patients (one male, one female) and the second study evaluated one female patient. All had acute traumatic lacerations involving digital nerves (the second study case is excluded from statistical evaluation because control patients were not included in that study).
Figure 6.
Recovery of Sensation in PEG-treated clinical cases. (A) PEG-therapy group demonstrated statistically significant recovery at 1, 4, and 8 weeks PO (p < 0.05) by static 2-point discrimination (s2PD), shaded area represents normal range; and (B) Medical Research Council Classification (MRCC) for Sensory Recovery Scale, shaded area represents good clinical recovery.
Patients were treated within 24 hours of injury with PEG-fusion in conjunction with standard neurorrhaphy. Sensory assessments after injury were performed over 12 weeks PO using static two-point discrimination (s2PD) and Semmes-Weinstein monofilament testing (data not shown). The British Medical Research Council Classification (MRCC) for Sensory Recovery Scale was used to evaluate the level of injury. The PEG-fused patient group was compared to six patient-matched controls treated with standard nerve repair whose data were retrospectively collected. Note that the male patient had three digital nerves lacerated, each of which was repaired with PEG-fusion. PEG-fusion nerve repair improves outcomes and speed of nerve recovery in the clinical setting as assessed by average MRCC score in week 1 (2.8 vs. 1.0, p = 0.03). At four weeks, MRCC scores remained superior in the PEG-fusion group (3.8 vs. 1.3, p = 0.01). At 8 weeks, there was improvement in both groups with the PEG-fusion cohort remaining statistically better (4.0 vs. 1.7, p = 0.01, Fig. 6B).
5. Conclusions
We describe a standard PEG-fusion protocol, methods of evaluation and some common sources of misunderstanding and confusion. When properly executed, PEG-fusion of rodent peripheral nerves produces rapid and more complete repair after transection or ablation injuries. Limited clinical case studies suggest that PEG-fusion indeed produces improved outcomes for single transection injuries (compared to recovery from standard neurorrhaphy repair) by surgeons trained to properly perform the procedure. PEG-fusion of allografts may have even wider application and greater benefits compared to currently available treatments.
Highlights.
PEG-fusion: neurorrhaphy and pharmaceutical solutions, including polyethylene glycol (PEG)
PEG-fusion immediately re-establishes axonal continuity across lesion sites
Wallerian degeneration is prevented for axons that are successfully PEG-fused
PEG-fusion restores lost behavioral functions much better than neurorrhaphy alone PEG-fusion protocols must be appropriately followed to produce significant recovery
Acknowledgements
Supported by grants from the Lone Star Paralysis Foundation and NIH R01 NS081063 to GDB
Abbreviations
- Ca2+
calcium ion
- CAP
compound action potential
- CMAP
compound muscle action potential
- D
day
- MB
methylene blue
- MFI
muscle fiber innervation
- NMJ
neuromuscular junction
- PEG
polyethylene glycol
- PEG-fusion
term describing a technology to connect (join) severed axonal ends using PEG
- PO
post-operative
- PNI
peripheral nerve injury
- PNS
peripheral nervous system
- SFI
Sciatic Functional Index (behavioral text for hind-leg function)
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Dr. Jackson is CEO of Neuraptive Therapeutics. Neuraptive has exclusively licensed the PEG-fusion patent estate from the University of Texas at Austin invented by, and based on, research performed by Dr. Bittner. Dr. Bittner has assigned all of his economic interests in the licensed PEG-fusion patent estate to a third party. Neither potential conflict has affected in any way any data analyses or text in this manuscript.
6. References
- Bamba R, Riley DC, Kelm ND, Does MD, Dortch RD, Thayer WP, 2016a. A novel technique using hydrophilic polymers to promote axonal fusion. Neural Regen. Res 11(4), 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba R, Waitayawinyu T, Nookala R, Riley DC, Boyer RB, Sexton KW, Boonyasirikool C, Niempoog S, Kelm ND, Does MD, Dortch RD, Shack RB, Thayer WP, 2016b. A novel therapy to promote axonal fusion in digital nerves. J. Trauma Acute Care Surg 81, S177–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch R, Wynn-Parry CB, 1998. Clinical aspects of nerve injury Surgical Disorders of the Peripheral Nerves. New York, Churchill Livingstone. [Google Scholar]
- Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T, 2012. Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue and polyethylene glycol after completely cutting rat sciatic nerves. J. Neurosci. Res 90, 967–980. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Sengelaub DR, Ghergherehchi CL, 2017a. Conundrums and confusions regarding how PEG-fusion produces excellent behavioral recovery after peripheral nerve injuries. Neural Regen. Res 13(1), 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner GD, Sengelaub DR, Trevino RC, Ghergherehchi CL, Mikesh M, 2017b. Robinson and Madison have published no data on whether polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J. Neurosci. Res 95, 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP, 2015. The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J. Neurosci. Res 94, 207–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner GD, Spaeth CS, Poon AD, Burgess ZS, McGill CH, 2016. Repair of traumatic plasmalemmal damage to neurons and other eukaryotic cells. Neural Regen. Res 11, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD, 2010. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J. Neurophysiol 104(2), 695–703. [DOI] [PubMed] [Google Scholar]
- Brown LB, Asante T, Welch HR, Sandelski MM, Drejet SM, Shah K, Runge EM, Shipchandler TZ, Jones KJ, Walker CL 2018. Functional and anatomical outcomes of facial nerve injury with application of polyethylene glycol in a rat model. JAMA Facial Plastic Surg. doi: 10.1001/jamafacial.2018.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, 2011. Nerve repair. New York, Oxford University Press. [Google Scholar]
- Campbell WW, 2008. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol 119(9), 1951–1965. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Goldberg NH, 1986. Quantitating integrated muscle function following reinnervation. Surg. Forum 37, 611–612. [Google Scholar]
- Churchill M, Kline R, Schwartz M, Bidani A, Churchill P 1990. Kidney transplants in cyclosporine-treated sprague-dawley rats. Transplantation. 49, 8–13. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ, 1982. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol 77(3), 634–643. [DOI] [PubMed] [Google Scholar]
- Fox LC, Kreishman MP, 2010. High-energy trauma and damage control in the lower limb. Semin. Plast. Surg 24(1), 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter ML, Margulies DH, Scharff MD, 1977. A simple method for polyethylene glycol promoted hybridization of mouse myeloma cells. Somatic Cell Genetics. 3, 231–236. [DOI] [PubMed] [Google Scholar]
- Geuna S, 2015. The sciatic nerve injury model in pre-clinical research. J. Neurosci. Methods 243, 39–46. [DOI] [PubMed] [Google Scholar]
- Ghergherehchi CL, Bittner GD, Hastings RL, Mikesh M, Riley DC, Trevino RC, Schallert T, Thayer WP, Sunkesula SR, Ha TN, Munoz N, Pyarali M, Bansal A, Poon AD, Mazal AT, Smith TA, Wong NS, Dunne PJ, 2016. Effects of extracellular calcium and surgical techniques on restoration of axonal continuity by polyethylene glycol fusion following complete cut or crush severance of rat sciatic nerves. J. Neurosci. Res 94, 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DP, Wolfe SW, 2011. Green's Operative Hand Surgery, sixth ed. Churchill Livingstone, Philadelphia. [Google Scholar]
- He B, Zhu Z, Zhu W, Zhou X, Zheng C, Li P, Zhu S, Liu X, Zhu J, 2014. Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res. 9(6), 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WH, Huber GC, 1893. A physiological, histological, and clinical study of the degeneration and regeneration in peripheral nerve fibres after severance of their connections with the nerve centres. J. Physiol 14, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Liu D, Zhang YP, Shen ZY, Gu TW, Gu XS, Gu JH, 2013. Neurological function following intra-neural injection of fluorescent neuronal tracers in rats. Neural Regen. Res 8, 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J, Safa B, Evans PJ, Greenberg J, 2016. Technical assessment of connector-assisted nerve repair. J. Hand Surg. Am 41, 760–766. [DOI] [PubMed] [Google Scholar]
- Jin X, 2014. Membrane resealing as a promising strategy for early treatment of neurotrauma. Neural Regen. Res 9, 1876–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, 2013. Principles of Neural Science, fifth ed. McGraw-Hill, New York. [Google Scholar]
- Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, Ponomareva O, Mignone J, Enikolopov G, Rimer M, Thompson W, 2007. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J. Neurosci 27, 5948–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS, 2009. Magnesium chloride in a polyethylene glycol formulation as a neuro-protective therapy for acute spinal cord injury: preclinical refinement and optimization. J. Neurotrauma 26, 1379–1393. [DOI] [PubMed] [Google Scholar]
- Lappi D, Feldman J, Sengelaub D, McGaughy J, 2014. Nervous system research with RIP conjugates: From determination of function to therapy, in: Stirpe F, Lappi D (Eds.), Ribosome-inactivating Proteins: Ricin and Related Proteins. Wiley-Blackwell, New York, pp 253–269. [Google Scholar]
- Lee SK, Wolfe SW 2000. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 8, 243–252. [DOI] [PubMed] [Google Scholar]
- Lentz BR, 2007. PEG as a tool to gain insight into membrane fusion. Eur. Biophys. J 36(4-5), 315–326. [DOI] [PubMed] [Google Scholar]
- Lentz BR, Lee JK, 1999. Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: a mechanism in common with viral fusion and secretory vesicle release? Mol. Membr. Biol 16(4), 279–296. [DOI] [PubMed] [Google Scholar]
- Lewis GM, Kucenas S, 2014. Perineurial glia are essential for motor axon regrowth following nerve injury. J. Neurosci 34(38), 12762–12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore AB, Hubbell JA, Bobb DS Jr., Ballinger ML, Loftin KL, Smith JW, Smyers ME, Garcia HD, Bittner GD, 1999. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J. Neurosci 19(7), 2442–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon SE, Doolabh VB, Novak CB, Trulock EP, 2001. Clinical outcome following nerve allograft transplantation. Plast. Reconstr. Surg 107(6), 1419–1429. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Gilbert SE, Calder JS, Smith PJ, 1996. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J. Hand Surg. Br 21(1), 4–13. [DOI] [PubMed] [Google Scholar]
- Mikesh M, Ghergherehchi CL, Hastings RL, Ali A, Rahesh S, Jagannath K, Sengelaub DR, Trevino RC, Jackson DM, Bittner GD, 2018a. Polyethylene glycol solutions rapidly restore and maintain axonal continuity, neuromuscular structures and behaviors lost after sciatic nerve transections in female rats. J. Neurosci. Res 96, 1223–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesh M, Ghergherehchi CL, Rahesh S, Jagannath K, Ali A, Sengelaub DR, Trevino RC, Jackson DM, Bittner GD, 2018b. Polyethylene glycol treated allografts not tissue matched nor immunosuppressed rapidly repair sciatic nerve gaps, maintain neuromuscular functions, and restore voluntary behaviors in female rats. J. Neurosci. Res 96, 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AM, MacEwan M, Santosa KB, Chenard KE, Ray WZ, Hunter DA, Mackinnon SE, Johnson PJ, 2011. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve 44(2), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Weaver C, 2016. Janeway's Immunobiology, ninth ed. Garland Publishing, New York. [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW, 2002. Pre-existing pathways promote precise projection patterns. Nat. Neuroscience 5(9), 861–867. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G, 1975. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genetics. 1, 397–400. [DOI] [PubMed] [Google Scholar]
- Raffe MR, 1985. Principles of peripheral nerve repair, in: Newton CD, Nunamaker DM (Eds.), Textbook of Small Animal Orthopedics. International Veterinary Information Service, Ithaca. [Google Scholar]
- Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, Bhupanapadu Sunkesula SR, Ha TN, Hall BT, Poon AD, Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T, Thayer WP, 2015. Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J. Neurosci. Res 93, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD, 2016. Polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J. Neurosci. Res 94(7), 636–644. [DOI] [PubMed] [Google Scholar]
- Rogers SA, Lowell JA, Hammerman NA, Hammerman MR, 1998. Transplantation of developing metanephroi into adult rats. Kidney International. 54, 27–37. [DOI] [PubMed] [Google Scholar]
- Rupp A, Dornseifer U, Fischer A, Schmahl W, Rodenacker K, Jütting U, Gais P, Biemer E, Papadopulos N, Matiasek K, 2007. Electrophysiologic assessment of sciatic nerve regeneration in the rat: surrounding limb muscles feature strongly in recordings from the gastrocnemius muscle. J. Neurosci. Methods. 166, 266–77. [DOI] [PubMed] [Google Scholar]
- Savastano LE, Laurito SR, Fitt MR, Rasmussen JA, Gonzalez PA, Patterson SI, 2014. Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J. Neurosci. Methods. 227, 166–180. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW, Bunge RP, 1973. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 59, 456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP, 2012. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J. Surg. Res 177, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth CS, Boydston EA, Figard LR, Zuzek A, Bittner GD, 2010. A model for sealing plasmalemmal damage in neurons and other eukaryotic cells. J. Neurosci 30(47), 15790–15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth CS, Fan JD, Spaeth EB, Robison T, Wilcott RW, Bittner GD, 2012a. Neurite transection produces cytosolic oxidation which enhances plasmalemmal repair. J. Neurosci. Res 90, 945–954. [DOI] [PubMed] [Google Scholar]
- Spaeth CS, Robison T, Fan JD, Bittner GD, 2012b. Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue J. Neursosci. Res 90, 955–966. [DOI] [PubMed] [Google Scholar]
- Stansbury LG, Branstetter JG, Lalliss SJ, 2007. Amputation in military trauma surgery. J. Trauma 63(4), 940–944. [DOI] [PubMed] [Google Scholar]
- Tsao JW, George EB, Griffin JW, 1999. Temperature modulation reveals three distinct stages of Wallerian degeneration. J. Neurosci 19(12), 4718–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varejao ASP, Meek MF, Ferreira AJA, Patricio, Cabrita AMS, 2001. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J. Neurosci. Methods. 68(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Wood MW, Kemp SWP, Weber C, Borschel GH, Gordon T, 2011. Outcome measures of peripheral nerve regeneration. Ann. Anat 193, 321–333. [DOI] [PubMed] [Google Scholar]
- Yu YL, Li HY, Zhang PX, Yin XF, Han N, Kou YH, Jiang BG, 2015. Comparison of commonly used retrograde tracers in rat spinal motor neurons. Neural Regen. Res 10, 1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]