Abstract
Transcatheter aortic valve replacement (TAVR) is a minimally-invasive approach for treating severe aortic stenosis. All clinically-used TAVR valves to date utilize chemically-fixed xenograft as the leaflet material. Inherent limitation of the tissue (e.g., calcific degeneration) motivates the search for alternative leaflet material. Here we introduce a novel polymeric TAVR valve that was designed to address the limitations of tissue-valves. In this study, we experimentally evaluated the hemodynamic performance of the valve and compared its performance to clinically-used valves: a gold standard surgical tissue valve, and a TAVR valve. Our comparative testing protocols included: (i) baseline hydrodynamics (ISO:5840–3), (ii) complementary patient-specific hydrodynamics in a dedicated system, and (iii) thrombogenicity. The patient-specific testing system facilitated comparing TAVR valves performance under more realistic conditions. Baseline hydrodynamics results at CO 4–7 L/min showed superior effective orifice area (EOA) for the polymer valve, most-notably as compared to the reference TAVR valve. Regurgitation fraction was higher in the polymeric valve, but within the ISO minimum requirements. Thrombogenicity trends followed the EOA results with the polymeric valve being the least thrombogenic, and clinical TAVR being the most. Hemodynamic-wise, the results strongly indicate that our polymeric TAVR valve can outperform tissue valves.
Keywords: TAVI, TAVR, Aortic stenosis, Heart valve, Prosthetic heart valve, Valve hydrodynamics, Thrombogenicity, Medical device
INTRODUCTION
Calcific aortic valve disease (CAVD) is a chronic pathological disease where calcific deposits build up and accumulate in the aortic root and valve. In the progressive stages of the disease, also known as aortic stenosis (AS), the aortic valve leaflets are heavily calcified, lose their natural flexibility, and cannot fully open and close. Transcatheter Aortic Valve Replacement (TAVR) is a minimally invasive approach intended for high risk AS patients who may not tolerate a surgical valve replacement, where a stented prosthetic TAVR tissue valve is introduced via a catheter into the aortic annulus and deployed over the native diseased valve leaflets without surgically removing the valve. Being much less invasive than the surgical alternative (surgical aortic valve replacement, or SAVR), recovery from TAVR is much faster with discharge as short as 1-day post procedure.17 The concept of TAVR device design includes a flexible leaflets material that form a three-leaflet valve, and a metal stent that provides the device its structure and also anchoring to the native aortic root.
Common to all of the currently FDA and CE approved TAVR devices is leaflets material that is made of chemically-fixed pericardium bovine or porcine tissue. Persistent complications that are associated with the tissue-based TAVR devices in the current cohort of high-risk surgical patients may impede the current expansion to lower-risk patients.12 Large randomized studies are currently underway for low-risk surgical patients, with small studies showing TAVR and SAVR equivalence. However, long term effects concerning long term durability and potential complications specific to younger patients (i.e. structural valve deterioration)3are still unknown, and longer follow up clinical studies will be required. Such complications are thrombosis,22 calcific degeneration,28 paravalvular leaks (PVL),20 and limited durability.36 In addition, recent evidence indicate that the tissue leaflets endure mechanical damage already during the crimping and deployment stages, even before their deployed duty cycle,1,19 raising concerns as for their longer term durability and other ensuing complications. Improvements in performance of the latest generation of FDA-approved TAVR devices, the SAPIEN3 (Edwards Lifesciences, Irvine, CA, USA) and the Evolut PRO (Medtronic, Minneapolis, MN, USA), have gradually opened the door to expanding TAVR utility to younger and lower risk patients,34 though raising a major concern regarding their longer term performance.
Polymers provide better design freedom to overcome many of the aforementioned limitations. Additionally, it allows mass production at high reproducibility and lower costs. However, all previous attempts to develop a viable polymeric aortic valve have failed.18 Many of these valves had promising early results, yet none had the characteristics needed for successful overall performance: (a) optimal hydrodynamic performance (per ISO 5840–3), (b) low thrombogenicity, (c) hemocompatibility, (d) durability (per ISO 5840–3), (e) low calcification susceptibility; and- specifically for TAVR application (f) crimping stability. In recent years, new polymer technologies have emerged and subsequently novel polymeric aortic valve have been developed, showing promising early in vitro results.4 Examples for such devices include the Polynova xSIBS valve that was design-optimized based on the device thrombogenic emulation (DTE) methodology for reduction of stress accumulation in the leaflets, improved hemodynamics, and minimization of platelet activation and thrombosis.9 In-vitro hemodynamic testing with the xSIBS valve demonstrated comparable performance to a clinically-used tissue valve, with reduced regurgitation fraction.9 A Hyaluronan-Polyethylene SAVR valve showed good potential in terms of material properties, valve hydrodynamics, hydrophilicity, blood clotting, and platelet and leukocyte adhesion.26,38 The Triskele (UCL TAV™, University College London, London, UK) TAVR valve utilizes urethane (POSS-PCU) leaflets and skirt, demonstrated in vitro testing comparable hydrodynamic performance to other, clinically-used, tissue TAVR valves.27 Foldax Inc. (Salt Lake City, UT, USA) utilizes an elastomer in their prosthetic heart valve products,10,11 which include aortic and mitral surgical valves, and a transcatheter valve. Strait Access Technologies (SAT, Cape Town, South Africa) develops a heparinized polyurethane TAVR valve for rheumatic heart disease (RHD) patients and recently passed over 600 M cycles in vitro accelerated durability testing.31 From the checkered past with surgical polymeric heart valves, it is clear that in order to develop a successful polymeric TAVR valve the polymer technology alone may not be enough, and a new dedicated approaches might be needed. While the early in vitro results of these new polymeric valve technologies seem promising, some important hemodynamic testing data is not available yet to truly evaluate their performance under conditions that TAVR valves operate under.
In the current study, we present a novel polymeric aortic valve for TAVR application that was developed in collaboration between Stony Brook University (Stony Brook, NY, USA) and Polynova Cardiovascular Inc. (Stony Brook, NY, USA). The new polymeric TAVR valve is a second generation valve that was derived from the Polynova SAVR valve that was previously described (Fig. 1a).8,9 This unique polymeric valve combines a novel polymer, xSIBS, and a unique design optimization methodology of the leaflets. xSIBS- a thermoset polymer, is the cross-linked version of the clinically-used SIBS (poly-(styrene-block-isobutylene-block-styrene)) and was shown to have excellent hemocompatibility and resistance to calcific deposition.25,33 It retains SIBS high hemocompatibility and biostability characteristics, yet with improved strength and mechanical stability.9,33The leaflets of the previous generation SAVR polymeric valve were designed using our DTE methodology for optimizing the valve hydrodynamic, thrombogenic and durability performance.9 In the new TAVR valve we kept the optimized leaflets design, yet adjusted the leaflets nominal position (zero-stress configuration) to be semi-open during their high-precision fabrication process that utilize electric discharge machining (EDM) to fabricate the molds. This was intended for further reduction of the flexural stress accumulated over the cardiac cycle (Fig. 1a). In addition, we have improved the manufacturing process to yield higher quality polymer, further enhancing the valve durability in terms of both design and manufacturing.
FIGURE 1.
(a) Illustration of the first generation Polynova polymeric SAVR valve with normally-closed leaflets conformation (left), and the second generation polymeric TAVR valve with semi-open leaflets (right). (b) Illustration of the compression mold used to prototype the polymeric TAVR valve.
The goal of the present study was to evaluate the hemodynamic performance of the novel polymeric TAVR valve. Our approach for in vitro hemodynamic testing comprises of three distinct tests that cover various aspects of the hemodynamic performance—including such that are unique to TAVR valves and are essentially not tested by others: (i) baseline hydrodynamic performance as described by ISO 5840–3, (ii) complementary hydrodynamic testing in a patient-specific CAVD anatomy, and (iii) flow-induced thrombogenicity. This hemodynamics testing approach allows us to predict the valve performance more realistically already in the early stage of bench testing, and modify the design if needed accordingly. Evaluation of the valve stability (durability, calcification susceptibility, and crimping stability) that is being performed as well will be described in a following paper.
MATERIALS AND METHODS
The Polynova TAVR valve was prototyped at size 20-mm (lumen diameter 18.4 mm, Outer diameter 20.0 mm). This size was chosen to fit the valve for future animal studies. The hemodynamic testing was performed in comparison to two clinically-used tissue valves of equivalent size: (i) Carpentier-Edwards PERIMOUNT Magna Ease SVAR valve size 19-mm (Edwards, Irvine, CA, USA), which is commonly considered a gold standard reference for valve testing (lumen diameter 18 mm); (ii) Inovare TAVR valve (Braile Biomédica, Brazil) size 20-mm (lumen diameter 19 mm).
Valve Prototyping
The polymeric valve prototypes were manufactured in-house using vacuum compression molding. A compression mold was fabricated at micron-precision using Electric Discharge Machining (EDM) (Fig. 1b). The molding process included placing raw xSIBS pellets in inside the mold, and then closing the mold under heat (220 °C) and pressure (4 Tons) for 1 h. The mold was designed to be connected to a vacuum line and hold vacuum throughout the molding process, therefore enhancing the density and quality of the molded polymer. The cured valves were then sutured to laser-cut nitinol stents (6–0 Silk black braided, Ethicon Inc., NJ, USA).
Baseline Hydrodynamic Performance
Baseline hydrodynamic performance of the valves was performed according to the test conditions described in ISO 5840–3 for prosthetic heart valves. Testing was performed in a Vivitro Pulse Duplicator (Vivitro Labs, Victoria, BC) at average Cardiac Outputs (CO) of 4, 5, 6, and 7 L/min, Heart Rate (HR) of 70 bpm, systole-diastole ratio of 0.35–0.65, aortic pressure of 120/80 mmHg, and mean arterial pressure (MAP) of about 100 mmHg. Silicone fitting that resembles the aortic root was fabricated to match the valves anchoring requirements as follows: The polymeric valves (n = 3) and Inovare valves (n = 2) were deployed in a 19 mm diameter annulus (5% oversizing), and the Perimount Magna Ease SAVR valve (n = 1) was placed in a 20.5 mm annulus with an undercut to fit and hold its suturing ring. The Inovare valves were balloon inflated to 3.5 atm inside the silicone fitting according to the manufacturer instructions. Blood-analog fluid was used as the working fluid (50.3% Glycerol, 48.8% ddH2O, 0.9% NaCl, by weight; yielding dynamic viscosity μ = 3.5 mPa s, and density ρ = 1121 kg/m3, at 37 °C). Pressure (aortic and ventricular) and flow recordings were filtered using a 30 Hz analog low pass filter (LPF). In each test condition the system was allowed to stabilize and then 10 consecutive cycles of pressure and flow were recorded. Hydrodynamic performance was evaluated by calculating the valves Effective Orifice Area (EOA, Eq. (1) per ISO 5840–3), average systolic trans-valvular pressure drop (ΔP) and regurgitation flow. In addition, high frame rate video images (402 fps) of the valves were taken for qualitative assessment of the valves’ leaflet kinematics (uEye 3140CP2, IDS, Germany).
| (1) |
where the EOA is calculated in cm2, QRMS is the root mean square forward flow (mL/s) during the positive differential pressure period (ΔP > 0), ΔP (mmHg) is the mean pressure difference (measured during the positive differential pressure period), and ρ (g/cm3) is the density of the test fluid.
Patient-Specific Hydrodynamic Performance
While ISO-compliant pulse duplicators (PD) provide baseline hydrodynamic performance of prosthetic valves, it tends to overestimate TAVR valve performance due to the simplified anatomical modeling of the aortic annulus and aorta,30 and has been shown to have a significant effect on turbulence as well as on the sinus flow.13,14 We therefore complemented the hydrodynamic assessment by testing the valves under more realistic deployed TAVR conditions in a patient-specific anatomical model of an AS patient with an advanced stage CAVD, using the Replicator pulse duplicator system (Vascular Simulations LLC, Stony Brook, NY, USA).30 Importantly, the setup and testing conditions performed in this section were also compliant with ISO guidelines. CT scans of the patient were provided anonymously by Stony Brook University Hospital after obtaining approval from the institutional review board. The scans were then processed for obtaining a CAD model that comprised of the lumen of the aortic valve to the ascending aorta segment, and aortic valve calcifications patterns, as previously described.5 The CAD model was then scaled down to reach an aortic annulus size of 19 mm, to match the Vivitro silicone fitting size (Fig. 2). 3D printing and molding of the aortic arch model was performed as previously described.30 Several calcified aortic valve replicas were manufactured. Each of the test valves was then deployed in them: the 20-mm Polynova valves (n = 3) with the nitinol stent were deployed manually, while the 20-mm Inovare valves (n = 2) were balloon inflated to 3.5 atm according to the manufacturer guidelines. For testing the Perimount Magna Ease SAVR valve (n = 1), an aortic annulus model was prepared without the leaflets; the SAVR valve was mounted onto the aortic annulus in a supraannular position, with a thin plastic ring holding the suturing ring tight in place.
FIGURE 2.
(a) The CAVD ascending aorta and valve model used for the patient-specific testing in this study, and (b) the matching molded valve. The valve and calcifications were colored for visualization purposes. (c) Example of the pressure and flow waveforms recorded in the Replicator pulse duplicator with a Polynova polymeric TAVR valve at CO of 4 L/min.
Test conditions were similar to the baseline hydrodynamic experiments, except that the maximum CO simulated was 5 L/min. Similar blood analog fluid was used as in the baseline hydrodynamic testing at 37 °C. Similar to the baseline hydrodynamic testing, in each test condition the system was allowed to stabilize and then 10 consecutive cycles of pressure and flow were recorded. An electromagnetic flow meter (Carolina Medical Electronics, NC, USA) was placed in the left ventricle outflow tract for direct measurement of the aortic flow and regurgitation (Same flowmeter used in the Vivitro PD). Ventricular and aortic pressures were measured using catheter-tip transducers (Micro Cath 825–0101, Millar Inc., TX, USA). Flow and pressure recordings (PowerLab 8/35, ADInstruments, Australia) were filtered using a digital 30 Hz LPF. Endoscopic camera was used to record the valves from the ascending aorta position. Angiography videos (Artis Zeego, Siemens Healthcare GmbH, Germany) of contrast agent injection (Omnipaque® 240, Iohexol, GE Healthcare Inc, NJ, USA), 25 mL at 14 mL/s,24 were taken for CO of 5 L/min by placing a 5F pigtail catheter (Cook Medical Inc., IN, USA) at the aortic root location, and used for visualizing regurgitation. Exemplary flow and pressure waveforms from the Replicator are depicted in Fig. 2b.
Comparison between the valves was performed pairwise using the student T test between the polymeric valve and the SAVR reference valve, and between the polymeric valve and the TAVR valve. All data sets were confirmed to have a normal distribution. The statistical analysis was performed per CO (intra-CO), per pulse duplicator (Vivitro, Replicator), on the EOA, average transvalvular pressure drop, and total regurgitation fraction. p value < 0.05 was considered significant.
Flow-Induced Thrombogenicity
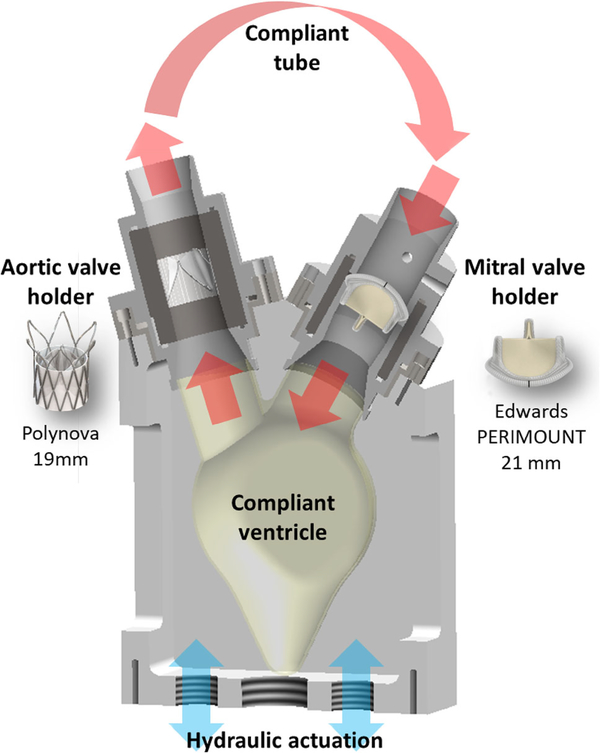
Thrombogenicity of the valves was measured by means of bulk-flow platelet activation as previously described.9Briefly, 120 mL of blood was obtained from healthy volunteers (n = 10) of both sexes, screened for antiplatelet medication and a history of smoking according to institutional IRB protocols. The blood was processed to produce Gel-Filtered Platelets (GFP) buffer with platelets concentration of 15,000/μL. The test valves (n = 1 per valve group) were mounted inside a closed flow-loop, at the outflow tract of a mock silicone left ventricle (Fig. 3). A 21-mm Carpentier-Edwards Perimount Magna Ease SAVR valve was used in the mitral valve position for all the test cases. In order to be able to differentiate thrombogenicity of the test valves, it is important that all the setup components (in this case it includes the mitral valve) will have minimum thrombogenicity, and preferably less than the test valves. The inflow and outflow ports were connected with a Penrose tubing (CR Bard, Covington, GA, USA), which served as a compliance reservoir. A pulsatile reciprocating pump (Model 1423 blood pump, Harvard Apparatus, Holliston, MA, USA), was used to compress/relax the ventricle model and drive flow through the flow loop. 200 mL of GFP buffer were used per test. For consistency with our previous valve thrombogenicity measurements performed in a pulsatile left ventricle recirculation flow loop, the tests were performed at pump rate of 90 bpm, Stroke Volume (SV) of 65 mL (corresponding to a CO of 5.85 L/min), and systole/diastole ratio of 0.375.6,7 Each test was run for a duration of 30 min, with 50 μL samples duplicates taken every 10 min. Platelet activation state (PAS) was measured as platelet thrombin generation in the flow loop.15,32 For each valve, the slope of the PAS, aka platelet activation rate (PAR), was calculated from the linear best-fit curve per experiment, and was accounted for the valve flow-induced thrombogenicity. Wilcoxon signed-rank test was performed on valve pairs to compare the PAR. The null hypothesis was assumed with α = 0.05. Between tests, the bioprosthetic valves were cleaned thoroughly by immersing them in 10% Tween 80 solution (Sigma Aldrich, St. Louis, MO) for 5 min, followed by three rinsing cycles in saline, 5 min each.37 All the other setup components, including the polymeric valve, were carefully cleaned with soap solution (0.5% SDS + 50 mM NaOH), and then washed with copious amounts of distilled water.
FIGURE 3.
Illustration of the experimental flow loop used for the flow-induced thrombogenicity testing. The test valves were mounted at the mock left ventricle outflow tract. A reference 21-mm Carpentier-Edwards Perimount Magna Ease SAVR valve was used in the mitral position.
The valves were tested without oversizing (fully deployed diameter) inside a valve-specific fitting, made of ultra-high molecular weight (UHMW) Polyethylene. The valves were mounted in the outflow tract such that the fitting ensured smooth flow into and out of the valve lumen (i.e., without their stents), and with a potential paravalvular region that may otherwise exist in a patient specific CAVD anatomy excluded. In order to ensure no material-related bias, and that platelet activation results were attributed to the valves’ hydrodynamics, an additional semi-static test was performed (see Online Appendix 1).
Plots of the pressure and flow waveforms for the three test valves in the three flow setups can be seen in Online Appendix 2.
RESULTS
Hydrodynamic Performance
Baseline hydrodynamic performance testing of the four test valves was performed in an ISO-compliant pulse duplicator (Vivitro PD) (Online Video 1). Snapshots of the valves at peak diastole and systole are depicted in Fig. 4, allowing a qualitative comparison of the valves’ effective orifice area (EOA) at peak systole, as well as evaluating the shape of the orifice. The polymeric TAVR valve had the largest orifice area. The Perimount Magna Ease had slightly lower orifice area, and the 20 mm Inovare the lowest. Same trend was seen for the shape of fully open orifice; the polymeric valve had the most circular opening, and the Inovare valve (both sizes) the least.
FIGURE 4.
Top—the prosthetic aortic valves tested in this study. Middle—front view of the test valves at peak diastole as recorded in the Vivitro PD system at CO of 5 L/min. Bottom—front view of the test valves at peak systole as recorded in the Vivitro PD system.
Besides testing the hydrodynamic performance in an ISO-compliant pulse duplicator (‘baseline’ performance), we complemented the hydrodynamic assessment in a patient-specific CAVD anatomy. This is necessary for predicting more realistically the performance of the valve by taking into account the anatomical variability of CAVD patients, as well as the effect of the diseased native valve leaflets with embedded calcifications, which are essentially absent in the ISO-compliant PDs.30 Endoscopic video from the Replicator is available (Online Video 2). Figure 5 depicts the valves deployed in the patient-specific CAVD valve model before it was mounted inside the matching aorta in the Replicator system for testing. It also shows examples of subtracted X-ray angiography images taken with each of the valves that allowed us to qualitatively assess the aortic regurgitation (Online Videos 3–5).
FIGURE 5.
a The test valves deployed in the CAVD aortic valve model, side and top views, and b the deployed valves mounted in the matching CAVD aorta in the Replicator, visualized using subtracted X-ray angiograms (see Online Videos 2–4).
Quantitative forward flow measurements and analysis that were performed both in the standard Vivitro PD and in the patient specific Replicator PD are depicted side by side in Fig. 6. In both systems the EOA trend was in agreement with the qualitative analysis of Fig. 4. In the ISO standard PD, the polymeric TAVR valve had the largest EOA for all CO tested (1.65–1.83 cm2), trailing slightly behind was the Perimount Magna Ease SAVR valve (1.57–1.76 cm2), and the Inovare with the lowest (1.32–1.55 cm2). The transvalvular pressure drop trend was different: the SAVR valve had lower pressure drop (11.18–21.85 mmHg) than the polymeric valve (13.85–24.68 mmHg). The Inovare valve had the highest pressure drop (17.00–29.65 mmHg). This trend was consistent for all the CO tested, with significant difference between the polymeric valve and the reference valves intra-CO (p < 0.001).
FIGURE 6.
Results of hemodynamics testing in the Vivitro PD and in the dedicated patient specific Replicator PD: forward flow hydrodynamic analysis by means of (a) EOA and (b) transvalvular pressure drop (ΔP), as calculated from the Vivitro PD system for CO of 4.0–7.0 L/min (left) and the Replicator (right) for CO of 4.0–5.0 L/min. Mean EOA values of the CAVD aortic valves models pre-TAVR is plotted in green at the top-right graph. Values represent mean 6 standard deviation. p < 0.001 for all cases (Polynova vs. reference valves, intra-CO, intra-PD; and same valve inter-PD for a given CO). PD pulse duplicator, EOA effective orifice areal, ΔP mean transvalvular pressure drop, CO cardiac output.
In the Replicator, the CAVD patient-based aortic valves had EOA of 0.58 ± 0.005 cm2 (Fig. 6) and pressure drop of 63.11 ± 1.32 mmHg for CO of 4 L/min, which corresponds to the classification of severe aortic stenosis.2,35 Following TAVR deployment, the EOA increased in the polymeric valve to the largest degree (to 1.27–1.32 cm2). With the Inovare valve deployed, the EOA increased to a lesser degree of 1.08–1.11 cm2 (Fig. 6). The SAVR valve had values comparable to the polymeric TAVR valve, ranging 1.37–1.38 cm2. The SAVR valve had the lowest transvalvular pressure drop, (17.90–24.11 mmHg), followed by the polymeric valve (27.46–33.80 mmHg), and the Inovare valve (30.91–38.33 mmHg). In the Replicator, the difference between the polymeric valve and reference valves intra-CO was also found to be significant (p < 0.001).
Regurgitation flow analysis in the standard PD indicated that while the polymeric valve had better performance in systole, it had higher values of overall regurgitation fraction (11.87–21.28% for CO of 7–4 L/min, respectively) (Fig. 7). The Inovare valve had overall regurgitation fraction of 7.26–9.39%. The SAVR valve had the lowest regurgitation ranging 1.97–2.48%.
FIGURE 7.
Regurgitation flow analysis during diastole. (a) Total regurgitation fraction (%SV); (b) Closing regurgitation fraction (%SV); (c) Leakage regurgitation fraction (%SV). Left—results obtained from the Vivitro PD for baseline hydrodynamic analysis. Right—results obtained from the patient-specific model in the Replicator. Values in (a) equal (b) + (c). Values are presented as mean 6 standard deviation. p < 0.001 for all intra-CO, intra-PD cases (Polynova vs. reference valves). Inter-PD analysis (Vivitro vs. replicator for same valve at similar CO): *p = 0.02, †p > 0.05. PD pulse duplicator, CO cardiac output.
Regurgitation values in the patient-based model were overall very similar to those measured in the ISO-compliant pulse duplicator, both for total regurgitation fraction, as well as after the breakdown to closing and leakage fractions (Fig. 7). Slight decrease in regurgitation was evident with the TAVR valves in the Replicator. In contrast, the SAVR valve had slight increase in overall regurgitation. In both pulse duplicators, regurgitation fraction was found to be significant between the valves intra-CO and intra-PD (p < 0.001). When comparing the same valve inter-PD at similar CO, differences in some cases were only marginally significant (p = 0.02), and others not significant (p > 0.05) (Fig. 7). This again indicates the overall similarity between the two pulse duplicators in terms of regurgitation, despite the presence of calcific deposits of this specific patient case.
Flow-Induced Thrombogenicity
Platelet activation was measured as function of the valves’ hydrodynamics in the pulsatile LV using the PAS assay and given by the PAR (platelet activation rate- the slope of the PAS over circulation time). Comparing the thrombogenic performance of the polymeric valve with the two equivalent-size reference valves, the thrombogenicity trend followed that of the baseline forward-flow hydrodynamic performance (Fig. 8). The polymeric valve had the least thrombogenic potential, followed by the SAVR valve (with non-significant difference), and lastly the Inovare valve (p < 0.005) with increase by a factor of 6.5 in PAR as compared to the polymer valve thrombogenic performance.
FIGURE 8.
Platelet activation state (PAS) and rate (PAR) of the test valves. n = 10 for all valves. Values represent average 6 standard error. *p < 0.005.
DISCUSSION
In the present study we introduce a novel polymeric aortic valve for transcatheter applications, and demonstrate its hemodynamic performance profile experimentally in three different systems. Polymeric valves have great potential to overcome many of the limitations associated with tissue-based valves, both in terms of clinical complications, as well as in engineering design freedom and automation of reproducible and precise production. The polymeric valve presented herein has demonstrated the potential to be a viable aortic valve that may effectively substitute tissue-based prosthetic aortic valves in the near future; having already met the ISO standard for durability (> 200 M cycles per ISO 5840–3), calcification resistance, crimping stability, and efficient hemodynamics. The latter is presented here and discussed in details. The former criteria, which are related to the valve stability, will be described in detail in a following paper.
Our combined comprehensive approach for evaluating the valves hemodynamics with three types of specific tests that complement each other, provides a more realistic in vitro prediction of the valve performance in its intended clinical environment (deployment in a CAVD anatomy). The three tests include a thorough evaluation of the valve hemodynamics: baseline standard PD hydrodynamic testing according to ISO 5840–3, patient-based testing that allows evaluation of the hydrodynamic performance change that is both patient- and procedural-dependent (was also compatible with the ISO guidelines), and a dedicated test that evaluates the valve’s thrombogenic potential by measuring the flow induced platelet activation under semi-physiological pulsatile LV flow conditions. This testing approach allowed us to predict our innovative TAVR polymeric valve performance in realistic scenarios at an early R&D stage in order to examine whether further design modifications may be needed in order to optimize its design. Traditionally this is performed only in pre-clinical studies, with major drawbacks (i) there is no large animal CAVD model that represent a CAVD anatomy, (ii) at this advanced R&D stage device companies would rather not retreat to the drawing board in order to redesign and achieve better pre-clinical performance, i.e., either the TAVR valve is somewhat successful, or the design is abandoned.
Evaluation of the valves’ forward flow performance of the polymeric valve demonstrated that it has excellent orifice area, both in terms of the EOA and in the shape of the orifice that was the most circular as compared to the reference valves. The EOA of the polymeric valve was significantly higher than that of the equivalent-size Inovare valve for all flow rates tested (Fig. 6), with mean difference of 0.32 ± 0.01 cm2 in the baseline hydrodynamic testing, and 0.20 ± 0.01 cm2 in the patient-based hydrodynamic testing. Comparison to the equivalent-size Perimount Magna Ease SAVR valve, which is considered a gold-standard reference for valve testing, showed slight advantage in EOA for the polymeric valve in the baseline hydrodynamic testing. In the patient-based testing however the SAVR valve EOA was slightly higher. Higher drop in hydrodynamic performance of the TAVR valves relative to the SAVR valve was expected when moving from baseline to patient-based testing; mainly because the SAVR lumen size and shape are less affected by the native anatomy, while the lumen diameter of the deployed TAVR valves is impaired by oversizing and presence of calcific deposits. Such restriction imposed by the calcified patient-specific anatomy affects the level of expansion of the TAVR valve, shape of expansion (Fig. 5a), and importantly may also affect the leaflet kinematics of the under-expanded valve.30The differences in performance per valve when comparing the Vivitro to the Replicator are as follows: the SAVR valve had the least drop in terms of EOA and transvalvular pressure drop, with average decline of 0.22 cm2 and 7.92 mmHg, respectively. These values in the Replicator are consistent with previously reported clinical measurements,16,29 and demonstrate again the importance of testing prosthetic heart valves in challenging anatomical replica as complementary to baseline hydrodynamic testing. The Inovare valve had slightly higher drop in performance, on average 0.26 cm2 and 15.19 mmHg for EOA and pressure drop, respectively. The polymeric valve had higher drop in performance, on average 0.38 cm2 in EOA, and 15.07 mmHg in pressure drop. In terms of transvalvular pressure drop, the polymeric valve had consistently better values (lower) compared to the 20-mm Inovare valve: 3.87 ± 1.02 mmHg in the baseline tester, and 3.99 ± 0.77 mmHg in the patient-based tester. Compared to the SAVR reference valve, pressure drop was higher in the polymeric valve by average in 2.48 ± 0.27 mmHg in the baseline tester. This difference was increased to 9.63 ± 0.08 mmHg in the Replicator, due to the increased restriction in opening of the deployed self-expanding stented TAVR polymeric valve. The Inovare valve, being balloon-expandable, was less affected by size of the mock annulus- following the balloon expansion to 3.5 atm the deployed diameter was measured about 1 mm larger at the ventricular and aortic sides (larger than the nominal valve size of 20 mm). It was not possible to measure the actual deployed diameter in the center of the valve.
While differences in hydrodynamic performance between the baseline and patient-based testing were notable for the forward flow analysis, the regurgitation flow values were overall quite similar with the patient based studies. Nevertheless, slight decrease in regurgitation fraction was noticed for both TAVR valves. In the patient-specific mock aortic annulus the regurgitation was dominated mostly by the pattern of the calcific deposits. These lead to suboptimal fit between the deployed valve and the mock annulus, creating small gaps where PVL occurs.30 The slightly higher regurgitation values that were measured in the simplified aortic root of the ISO-compliant PD was rather unexpected, and might indicate an overestimation. Rahmani et al. reported on baseline hydrodynamic performance with the Triskele polymeric TAVR valve, with total regurgitation fraction in the range of about 10–20% for equivalent CO and stent oversizing as tested here. The reference TAVR valves in that study with clinically-used valves, also reached relatively high regurgitation values of up to 70% of SV.27 Although the regurgitation values of our polymeric valve were higher than those of the reference TAVR valves, they are still marginally within the accepted values per ISO 5840–3 (EOA ≥ 0.85 cm2, transvalvular regurgitation fraction ≤ 10%, total regurgitation fraction ≤ 17.5%, at deployed valve diameter of 20 mm, CO 5 L/min, MAP 100 mmHg, HR 70 bpm, systolic duration 35%). Comparing the current polymeric TAVR design to its predecessor polymeric SAVR valve, the latter had leaflets molded in a normally-closed position (Fig. 1a) with very low regurgitation fraction of ~ 2 to 4%.9 One of the design changes between the SAVR and the TAVR polymeric valves was the addition of a sleeve for attaching the stent. Apparently, the addition of the sleeve in the leaflet region resulted in much smaller neo-sinuses (Fig. 1a), not allowing the formation of strong enough recirculation zone in the sinuses to assist a faster closing of the leaflets.23 This design feature will be addressed accordingly in the next design iteration.
The flow-induced platelet activation thrombogenicity testing followed the trend of the forward flow hydrodynamic results, with the polymeric valve being the least thrombogenic, followed by the SAVR valve (no statistical significance), and with the 20-mm Inovare being the most thrombogenic. In this regard, despite the higher regurgitation values found for the TAVR polymeric valve, it still exhibited the lowest thrombogenic potential of all the valves tested.
In this study the TAVR valves were tested under 5% oversizing. At this level of oversizing, the polymeric valves has started to show pinwheeling at the free edge of the leaflets during diastole (Fig. 4 bottom). In comparison, When we tested the valves at full expansion (no oversizing), no pinwheeling was observed (see Online Appendix 3). Since the eventual intended use of the polymeric TAV is under oversizing of 5% and more, and since pinwheeling might have an effect on the valve durability,21 we will likely make the free edges of the polymeric leaflet shorter in the next design iteration.
One of the limitations of this study was that the hydrodynamic analysis did not include spatial analysis of flow patterns, e.g., using numerical simulations or quantitative visualization such as PIV. We are currently addressing this both by performing matching computational Fluid–Structure Interaction (FSI) simulations, and by incorporating means of visualization to the models (both baseline and patient-specific). Other limitation of the study was that the patient-specific hydrodynamics included here a single patient-specific case. However, as there is no generic patient based CAVD model, this far exceeds what was performed before in vitro. To the best of our knowledge this is the first study to investigate prosthetic valves-either SAVR or TAVR using such more clinically relevant approach. In that, we are already expanding our CAVD anatomy models to include a bigger range of challenging anatomies for valve testing, including bileaflet aortic valves (BAV) anatomies.
The hemodynamic results presented here allowed us to get a comprehensive evaluation on our polymeric TAVR valve performance, which is our 1st generation TAVR design. The complementary results allows us to determine what specific design features should be optimized in the next design iteration: This may include, e.g., (a) enlarging the neo-sinuses for increasing closing speed of the leaflets, (b) molding the valve together with the stent (sutureless valve) for minimizing regurgitation, and (c) shortening the leaflets’ free edge for minimizing pinwheeling during diastole.
CONCLUSIONS
In the current study we presented a novel polymeric TAVR valve device and focused on a comparative and comprehensive evaluation of the valve hemodynamic performance. The performance was very promising, with excellent EOA, pressure drop, and thrombogenicity level compared to clinically-used reference TAVR and SAVR valves. While regurgitation values were higher than the reference valves those still complied with the ISO standard. Hemodynamic-wise, the results strongly indicate that the Polynova polymeric TAVR valve can outperform clinically-used tissue-valves.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Braile Biomédica (Brazil), for providing us with the Inovare valve samples. This project was supported by NIH-NIBIB Quantum Award Phase II-U01EB012487 (DB), NHLBI STTR R41-HL134418 (DB), and the Center for Biotechnology: a New York State Center for Advanced Technology, New York State Department of Economic Development; and corporate support.
ABBREVIATIONS
- AS
Aortic stenosis
- CAVD
Calcific aortic valve disease
- CO
Cardiac output
- DTE
Device thrombogenic emulation
- EOA
Effective orifice area
- GFP
Gel-filtered platelets
- HR
Heart rate
- MAP
Mean arterial pressure
- PD
Pulse duplicator
- PVL
Paravalvular leak
- SAVR
Surgical aortic valve replacement
- SV
Stroke volume
- TAVR
Transcatheter aortic valve replacement
Footnotes
CONFLICT OF INTEREST
Author OMR is a consultant for Polynova Cardiovascular Inc. Authors MJS and DB has stock ownership in Polynova Cardiovascular Inc. Authors BK, WCC, MB and GM declare that they have no conflicts of interest.
ELECTRONIC SUPPLEMENTARY MATERIAL
The online version of this article (https://doi.org/10.1007/s10439-018-02119-7) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Alavi SH, Groves EM, and Kheradvar A. The effects of transcatheter valve crimping on pericardial leaflets. Ann. Thorac. Surg 97:1260–1266, 2014. [DOI] [PubMed] [Google Scholar]
- 2.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and The Society of Cardiovascular, and The Society for Cardiovascular, Interventions, S. Society of Thoracic, Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, and Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114:e84–e231, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Arsalan M, and Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nat. Rev. Cardiol 13:360–367, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Bezuidenhout D, Williams DF, and Zilla P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 36:6–25, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M, Marom G, Ghosh RP, Fernandez HA, Taylor JR, Slepian MJ Jr, and Bluestein D. Effect of balloon-expandable transcatheter aortic valve replacement positioning: a patient-specific numerical model. Artif. Organs 40(12):E292–E302, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claiborne TE, Girdhar G, Gallocher-Lowe S, Sheriff J, Kato YP, Pinchuk L, Schoephoerster RT, Jesty J, and Bluestein D. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna aortic bioprosthetic valves. ASAIO J. 57:26–31, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Claiborne TE, Sheriff J, Kuetting M, Steinseifer U, Slepian MJ, and Bluestein D. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. J. Biomech. Eng 135:021021, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claiborne TE, Slepian MJ, Hossainy S, and Bluestein D. Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert Rev. Med. Devices 9:577–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claiborne TE, Xenos M, Sheriff J, Chiu W-C, Soares JS, Alemu Y, Gupta S, Judex S, Slepian MJ, and Bluestein D. Towards optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO J. 59:275–283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandeniyage LS, Adhikari R, Bown M, Shanks R, Adhikari B, Easton CD, Gengenbach TR, Cookson D, and Gunatillake PA. Morphology and surface properties of high strength siloxane poly(urethane-urea)s developed for heart valve application. J. Biomed. Mater. Res. B 2018. 10.1002/jbm.b.34101. [DOI] [PubMed] [Google Scholar]
- 11.Dandeniyage LS, Gunatillake PA, Adhikari R, Bown M, Shanks R, and Adhikari B. Development of high strength siloxane poly(urethane-urea) elastomers based on linked macrodiols for heart valve application. J. Biomed. Mater. Res. B 106(5):1712–1720, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Dasi LP, Hatoum H, Kheradvar A, Zareian R, Alavi SH, Sun W, Martin C, Pham T, Wang Q, Midha PA, Raghav V, and Yoganathan AP. On the mechanics of transcatheter aortic valve replacement. Ann. Biomed. Eng 45:310–331, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatoum H, Dollery J, Lilly SM, Crestanello J, and Dasi LP. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: an in vitro study. J. Thorac. Cardiovasc. Surg 2018. 10.1016/j.jtcvs.2018.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatoum H, Yousefi A, Lilly S, Maureira P, Crestanello J, and Dasi LP. An in vitro evaluation of turbulence after transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg 2018. 10.1016/j.jtcvs.2018.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesty J, and Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal. Biochem 272:64–70, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Kallis P, Sneddon JF, Simpson IA, Fung A, Pepper JR, and Smith EE. Clinical and hemodynamic evaluation of the 19-mm Carpentier-Edwards supraannular aortic valve. Ann. Thorac. Surg 54:1182–1185, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Kamioka N, Wells J, Keegan P, Lerakis S, Binongo J, Corrigan F, Condado J, Patel A, Forcillo J, Ogburn L, Dong A, Caughron H, Simone A, Leshnower B, Devireddy C, Mavromatis K, Guyton R, Stewart J, Thourani V, Block PC, and Babaliaros V. Predictors and clinical outcomes of next-day discharge after minimalist transfemoral transcatheter aortic valve replacement. JACC Cardiovasc. Interv 11:107–115, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Kheradvar A, Groves EM, Dasi LP, Alavi SH, Tranquillo R, Grande-Allen KJ, Simmons CA, Griffith B, Falahatpisheh A, Goergen CJ, Mofrad MR, Baaijens F, Little SH, and Canic S. Emerging trends in heart valve engineering: Part I. Solutions for future. Ann. Biomed. Eng 43:833–843, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Khoffi F, and Heim F. Mechanical degradation of biological heart valve tissue induced by low diameter crimping: an early assessment. J. Mech. Behav. Biomed. Mater 44:71–75, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Luscher TF Cutting edge research on transcatheter aortic valve implantation: moving indications, complications, and current outcomes. Eur. Heart J 39:633–636, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Martin C, and Sun W. Transcatheter valve underexpansion limits leaflet durability: implications for valve-in-valve procedures. Ann. Biomed. Eng 45:394–404, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marwan M, Mekkhala N, Goller M, Rother J, Bittner D, Schuhbaeck A, Hell M, Muschiol G, Kolwelter J, Feyrer R, Schlundt C, Achenbach S, and Arnold M. Leaflet thrombosis following transcatheter aortic valve implantation. J. Cardiovasc. Comput. Tomogr 12:8–13, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Midha PA, Raghav V, Sharma R, Condado JF, Okafor IU, Rami T, Kumar G, Thourani VH, Jilaihawi H, Babaliaros V, Makkar RR, and Yoganathan AP. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 136:1598–1609, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Min JK, Berman DS, and Leipsic J. Multimodality Imaging for Transcatheter Aortic Valve Replacement. New York: Springer Science & Business Media, 2013. [Google Scholar]
- 25.Pinchuk L, and Zhou Y. Crosslinked polyolefins for biomedical applicatios and method of making same In: USPTO, edited by USPTO. Miami: Innovia LLC, 2009. [Google Scholar]
- 26.Prawel DA, Dean H, Forleo M, Lewis N, Gangwish J, Popat KC, Dasi LP, and James SP. Hemocompatibility and Hemodynamics of Novel Hyaluronan-Polyethylene Materials for Flexible Heart Valve Leaflets. Cardiovasc. Eng. Technol 5:70–81, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahmani B, Tzamtzis S, Sheridan R, Mullen MJ, Yap J, Seifalian AM, and Burriesci G. In vitro hydrodynamic assessment of a new transcatheter heart valve concept (the TRISKELE). J. Cardiovasc. Transl. Res 10:104–115, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Gabella T, Voisine P, Puri R, Pibarot P, and Rodes-Cabau J. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J. Am. Coll. Cardiol 70:1013–1028, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Rosenhek R, Binder T, Maurer G, and Baumgartner H. Normal values for Doppler echocardiographic assessment of heart valve prostheses. J. Am. Soc. Echocardiogr 16:1116–1127, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Rotman OM, Kovarovic B, Sadasivan C, Gruberg L, Lieber BB, and Bluestein D. Realistic vascular replicator for TAVR procedures. Cardiovasc. Eng. Technol 2018. 10.1007/s13239-018-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherman J, Bezuidenhout D, Ofoegbu C, Williams DF, and Zilla P. Tavi for low to middle income countries. Eur. Heart J 38:1182–1184, 2017. [Google Scholar]
- 32.Sheriff J, Bluestein D, Girdhar G, and Jesty J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann. Biomed. Eng 38:1442–1450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheriff J, Claiborne TE, Tran PL, Kothadia R, George S, Kato YP, Pinchuk L, Slepian MJ, and Bluestein D. Physical characterization and platelet interactions under shear flows of a novel thermoset polyisobutylene-based co-polymer. ACS Appl. Mater. Interfaces 7:22058–22066, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, and Kapadia S. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 387:2218–2225, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A, and Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology and E. S. C. C. F. P. Guidelines. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J 28:230–268, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Furnary AP, Li HF, and Grunkemeier GL. Bioprosthetic aortic valve durability: a meta-regression of published studies. Ann. Thorac. Surg 104:1080–1087, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Yin W, Alemu Y, Affeld K, Jesty J, and Bluestein D. Flow-induced platelet activation in bileaflet and monoleaflet mechanical heart valves. Ann. Biomed. Eng 32:1058–1066, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Yousefi A, Bark DL, and Dasi LP. Effect of arched leaflets and stent profile on the hemodynamics of tri-leaflet flexible polymeric heart valves. Ann. Biomed. Eng 45(2):464–475, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.