Abstract
Objective
To systematically review the effect of oral intake of bacterial probiotics on 15 variables related to obesity, diabetes and non-alcoholic fatty liver disease.
Design
Systematic review and meta-analysis.
Data sources
Medline, EMBASE and COCHRANE from 1990 to June 2018.
Eligibility criteria
Randomised controlled trials (≥14 days) excluding hypercholesterolaemia, alcoholic liver disease, polycystic ovary syndrome and children <3 years.
Results
One hundred and five articles met inclusion criteria, representing 6826 subjects. In overweight but not obese subjects, probiotics induced improvements in: body weight (k=25 trials, d=−0.94 kg mean difference, 95% CI −1.17 to −0.70, I²=0.0%), body mass index (k=32, d=−0.55 kg/m², 95% CI −0.86 to −0.23, I²=91.9%), waist circumference (k=13, d=−1.31 cm, 95% CI −1.79 to −0.83, I²=14.5%), body fat mass (k=11, d=−0.96 kg, 95% CI −1.21 to −0.71, I²=0.0%) and visceral adipose tissue mass (k=5, d=−6.30 cm², 95% CI −9.05 to −3.56, I²=0.0%). In type 2 diabetics, probiotics reduced fasting glucose (k=19, d=−0.66 mmol/L, 95% CI −1.00 to −0.31, I²=27.7%), glycated haemoglobin (k=13, d=−0.28 pp, 95% CI −0.46 to −0.11, I²=54.1%), insulin (k=13, d=−1.66 mU/L, 95% CI −2.70 to −0.61, I²=37.8%) and homeostatic model of insulin resistance (k=10, d=−1.05 pp, 95% CI −1.48 to −0.61, I²=18.2%). In subjects with fatty liver diseases, probiotics reduced alanine (k=12, d=−10.2 U/L, 95% CI −14.3 to −6.0, I²=93.50%) and aspartate aminotransferases (k=10, d=−9.9 U/L, 95% CI −14.1 to -5.8, I²=96.1%). These improvements were mostly observed with bifidobacteria (Bifidobacterium breve, B. longum), Streptococcus salivarius subsp. thermophilus and lactobacilli (Lactobacillus acidophilus, L. casei, L. delbrueckii) containing mixtures and influenced by trials conducted in one country.
Conclusions
The intake of probiotics resulted in minor but consistent improvements in several metabolic risk factors in subjects with metabolic diseases.
Trial registration number
CRD42016033273.
Keywords: obesity, diabetes, non-alcoholic fatty liver disease, probiotics, bifidobacterium, lactobacillus
Strengths and limitations of this study.
We underscored food grade bacterial probiotics’ inherent effects, analysed a large panel of variables and performed subgroup explorations analysis to detect a disease stage severity dependence.
We performed, for the first time, subgroup explorations to detect bacterial species contribution.
We integrated both exploratory and secondary outcomes, 43 trials conducted in one country, trials with small sample size, heterogeneous study populations, incomplete information on drug treatment, dietary and physical activity records.
For some trials, parameters necessary for the estimation of effect sizes were unknown and we had to base our calculations on assumptions that could be only in part derived from the data at hand.
Introduction
The high prevalence of obesity, diabetes and non-alcoholic fatty liver disease (NAFLD) is a global health problem resulting in considerable healthcare costs. Lifestyle changes are regarded as cornerstones in the management of these tightly linked disorders that progress over an individual’s lifetime.
The rapid progression of these diseases is linked to changes in a myriad of environmental factors interacting with genetic and epigenetic factors. The gut microbiota is a key player at the interface between environmental changes and host biology. Metabolic traits, such as obesity, diabetes and non-alcoholic steatohepatitis are associated with changes in gut microbiota diversity and composition.1 2 Gut microbiota profile is also associated with specific dietary patterns3 and respond to dietary4 5 and therapeutic interventions.6 However, until recently, causal roles of the gut microbiota in the development and maintenance of chronic metabolic disorders is suggested mainly based on findings in rodents.7
Probiotics are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’.8 In rodents, numerous studies have shown beneficial effects of probiotics on energy, glucose and lipid metabolism,9 although not consistently.10 In humans, the number of studies with probiotics nearly doubled over the last 2 years. Several meta-analyses evaluated the impact of probiotics on metabolic variables11–17 but explorations to detect a disease stage severity dependence are scarce. Furthermore, no meta-analysis evaluated the contribution of bacteria used in foods that are listed as biological agents under European Food Safety Authority qualified presumption of safety list and none examined the specific contribution of bacterial species.18
We thus conducted a systematic review and meta-analysis aiming to investigate the impact of probiotics on 15 variables related to obesity, diabetes and NAFLD. We investigated multiple outcomes, many of which are inter-related so that concomitant effects would corroborate consistent probiotics effects. We aimed at elucidating whether there is an overall effect of probiotics on metabolic impairments and, if so, under which conditions the effect occurs. These goals were pursued by investigating the following questions: (1) What is the effect of probiotics on different outcomes related to obesity, metabolic syndrome, diabetes and NAFLD? (2) Is there effect heterogeneity by gender? (3) Is there effect heterogeneity in the following study populations: normal weight (NW), overweight (OW), obese (OB), metabolic syndrome, impaired fasting glucose (IFG), type 2 diabetes (T2DM), gestational diabetes, NAFLD? (4) Is there effect heterogeneity by total daily dose, food form and probiotics species (or combination of them)?
Methods
The search strategy, eligibility criteria and outcomes were described a priori (PROSPERO CRD42016033273).
Data sources
We searched PUBMED/MEDLINE, EMBASE and the COCHRANE CENTRAL library for eligible articles published in English, French, German, Spanish or Portuguese between January 1990 and June 2018. We also searched the reference list of the identified papers.
Study selection
We included human intervention studies that fulfilled the following inclusion criteria: (1) randomised controlled trials (parallel or cross-over), (2) published since 1990, (3) treatment duration of at least 14 days (at which some metabolic effect may occur, yet the short duration may reduce a chance to detect an effect), (4) use of probiotics (following genera: Bifidobacterium, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Propionibacterium and Streptococcus) as key independent variables and (5) reported data on at least one difference between baseline and end of intervention related to the metabolic impairment using search terms: obese/-ity, diabetic/-es, weight, metabolic syndrome, glucose intolerant, glucose tolerant, glucose tolerance, glucose intolerance, insulin resistance, insulin sensitivity, impaired fasting glucose, waist circumference, abdominal adiposity, abdominal obesity, central obesity, visceral adipose tissue, visceral fat, visceral adiposity, fat, fatty liver, non-alcoholic fatty liver and irrespective of whether the investigators considered the outcome as primary or secondary criteria (see online supplementary file).
bmjopen-2017-017995supp001.pdf (831KB, pdf)
The outcomes were body weight (BW), body mass index (BMI), waist circumference (WC), body fat mass (BFM) (determined using bioelectrical impedance or dual energy X-ray absorptiometry), subcutaneous adipose tissue mass (SAT), visceral adipose tissue mass (VAT), fasting glucose (FG), glycated haemoglobin (HbA1c), insulin (INS), homeostatic model of insulin resistance (HOMA-IR), C-reactive protein (CRP), triglycerides (TG), alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT) and gamma-glutamyl transferase (GGT). We excluded studies that administered probiotics with other functional ingredients with the exception of fermentable fibres at a maximum dose of 1.5 g/day. Fructo-oligosaccharides improve probiotics’ survival in the gastrointestinal system19; however, we assumed that three times a day intake of 0.5 g would be insufficient to exert a significant prebiotic effect. Subjects who had isolated hypercholesterolaemia, alcoholic liver disease, polycystic ovary syndrome and children with an age of <3 years were excluded. Three authors (BG, HK, MMS) independently performed the study selection and any disagreement was resolved on a discussion with a fourth author (JS or JMF).
Data extraction
Two reviewers independently extracted data from the original publications: [author, year of publication, country of origin, design of the trial, experimental intervention (type of bacterial species and, if given, subspecies), dose, food form and duration of intervention, comparator/control, characteristics of the population (descriptive statistics of age, sex and BMI), medication for T2DM, descriptive statistics for each outcome (mean/median, SD/SE, CI, etc before and after the intervention)]. We extracted data and calculated the mean and SE for each outcome and time point using standardised units (see online supplementary file). If provided, we extracted statistics quantifying the absolute change postintervention versus preintervention and/or the results of a hypothesis test investigating the statistical significance of this change.
Risk of bias (quality) assessment
We calculated a quality score based on 10 factors, according to a standardised procedure (PEDro tool based on the Delphi list) and classified studies as high quality (≥8 points), moderate quality (>6 and <8 points) or low quality (≤6 points).
Calculation of effect measures
As effect measures, we considered the mean difference in absolute change from baseline between the probiotics and control groups. For studies with more than two measurements during the follow-up, we calculated the change between the last preintervention measurement and the measurement at the end of the administration period. Missing SE for the difference between interventions for change-from-baseline parameter were estimated using the formula recommended in the Cochrane handbook based on an assumption of the magnitude of correlation among the repeated outcome measurements (intrasubject correlation). Since studies did not explicitly report coefficients of intrasubject correlation, we assumed for all outcomes an intermediate magnitude of intrasubject coefficient of 0.5. We conducted sensitivity analyses to examine the robustness of the meta-analysis results with respect to this assumption by varying the intrasubject correlation within realistic ranges (details see below).
Data synthesis
We calculated summary meta-analysis estimates for the difference between probiotic and control, including 95% CI and p values, by using the random effects method (DerSimonian-Laird estimator20) that allows for heterogeneity in results between studies. There was within publication and within study dependence structure because some authors reported multiple randomised trial results investigating different outcomes in the same study population within the same paper. However, because we aimed to synthesise outcomes separately and as separate research questions, we did not adjust our estimates for this multiplicity.
Assessment of study heterogeneity
We examined probiotic effect heterogeneity by forest plots visualising the study-specific efficacy estimates and 95% CIs as well as a χ2 heterogeneity test. To identify any dose–response relationship pattern, we plotted the study-specific efficacy estimates ordered by the total daily dose of probiotics administered. To explore whether the heterogeneity pattern is related to study-specific variables, we generated stratified forest plots grouping studies by characteristics of the intervention, food form and specific study populations. To quantify the magnitude of heterogeneity, we calculated the between study variance (τ²) and the I² statistic, that is, the percentage of variation in effect estimates attributable to heterogeneity. Further, we applied a hypothesis test for heterogeneity based on the Q χ2 statistic.21 Forest plots were grouped in different levels of heterogeneity based on the I² value: low (I²: 0%–25%), intermediate (I²: 25%–50%), moderate (I²: 50%–75%) and high (I²≥75%).
To investigate the possible source of heterogeneity, we performed sensitivity analysis restricted to studies without children, pregnant woman, subjects undergoing gastric surgery and without only one country, due to a high preponderance of the conducted studies. If feasible, depending on the heterogeneity pattern identified, we calculated the following efficacy estimates in total and for subgroups for each outcome: (1) gender; (2) study population. The treatment and placebo groups were classified as NW: 18.5≥BMI<25 kg/m², OW: 25≥BMI<30 kg/m², OB: BMI≥30 kg/m², IFG: 5.6–6.9 mmol/L, T2DM: FG ≥7.0 mmol/L or HbA1C≥6.5% and NAFLD (biopsy or ultrasound diagnosed). For study population characteristics undescribed in the original publication, we used the mean of treatment group value to assign the study population. In trials with mixed study populations, the study population would contribute to each study population specific meta-analysis. (3) Total daily dose. (4) Food forms categorised as (capsule or powder or sachet or pill) and (yoghourt or fermented milk). (5) Characteristics of probiotics interventions: species and subspecies.
Assessment of publication bias
To visually identify publication bias or other small study effects, we used funnel plots or simple scatterplots of the SE versus the study-specific effect estimates including 95% pseudo confidence limits. Potential small study effects were visualised showing the Egger’s line in the funnel plot, a line resulting from a linear regression of the effect estimates on their SE, weighing by the inverse of the variance of the intervention effect estimate. Further, we calculated a Galbraith’s radial plot showing the effect estimate divided by its SE against the precision of the effect estimate. In addition, for groups with more than 10 studies, we tested for small study effects using Egger’s test and Begg’s rank correlation test.22 23 In case of significant funnel asymmetry, we further examined the potential reasons by stratified funnel plots grouping studies by study characteristics that might relate to study size (eg, quality score, baseline value, population, etc). For outcomes with evidence of residual small study effects that could not be explained by known study-specific characteristics (eg, due to publication bias), we conducted a sensitivity analysis recalculating the summary estimates by a trim and fill algorithm.24
Sensitivity analysis
First, we conducted a sensitivity analysis aimed to assess robustness of results regarding our assumption of intrasubject correlation (r=0.5) used to impute SD of change measures. We varied intrasubject correlations within specific ranges obtained from confidence limits of correlation coefficients that were back calculated from studies that explicitly reported SDs of changes by using an approximation formula from the Cochrane handbook. Second, to evaluate the robustness of results obtained with respect to study quality, specific probiotic species or study populations, we conducted sensitivity analyses by recalculating the meta-analytic summary efficacy estimates excluding trials with low and intermediate quality score levels, or studies including the Bacillus coagulans (former Lactobacillus sporogenes) strain or studies including children, pregnant women or gastric surgery. Finally, we investigated the sensitivity of our results with respect to estimates from studies conducted in one country where we observed a high number of trials with similar design and methods and we recalculated estimates excluding those studies. All statistical hypothesis testing was conducted two-sided (p<0.05). All calculations were conducted using the software STATA (StataCorp. 2013. Stata Statistical Software: Release 15. College Station, Texas, USA: StataCorp LP).
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
We identified 1934 records, from which we selected 986 individual abstracts and 161 potentially relevant articles for full review (figure 1). We identified 105 articles19 25–128 reporting data from 99 different research studies including 111 different randomised comparisons of probiotics versus control (further called randomised clinical trials [RCTs], see online supplementary table 1-2) with the following outcomes: BW (number of RCTs: k=58, n=3422 individuals, median=77.4 kg), BMI (k=68, n=4015, 28.2 kg/m²), WC (k=26, n=1583, 98.8 cm); BFM (k=27, n=1562, 27.8 kg), SAT and VAT (k=5, n=543, 192.4 cm² and 114.7 cm², respectively), FG (k=83, n=5188, 6.1 mmol), HbA1c (k=28, n=1796, 6.3%), INS (k=63, n=3854, 11.0 mU/L), HOMA-IR (k=52, n=3513, 3.2), CRP (k=41, n=2376, 3.6 mg/L), TG (k=74, n=4461, 145.4 mg/dL), ALAT (k=26, n=1466, 38.6 IU/L), ASAT (k=23, n=1340, 36.1 IU/L) and GGT (k=14, n=816, 41.5 IU/L). The median duration of the follow-up was 8 weeks (range: 2–28 weeks), probiotics dose ranged from 107 to 1012 CFU daily, and 43 trials were conducted in one country (Iran). Using a PEDro tool-based quality score evaluated for each research study, 69 (70%) studies showed high quality, 15 (15%) studies showed moderate quality and 15 (15%) studies showed low quality (see online supplementary table 3).
Figure 1.
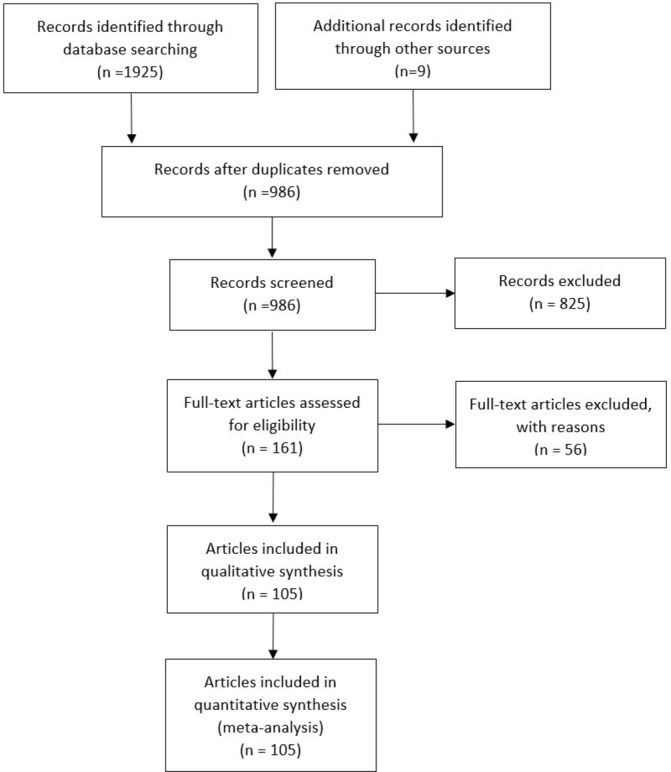
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Anthropometric variables and BMI
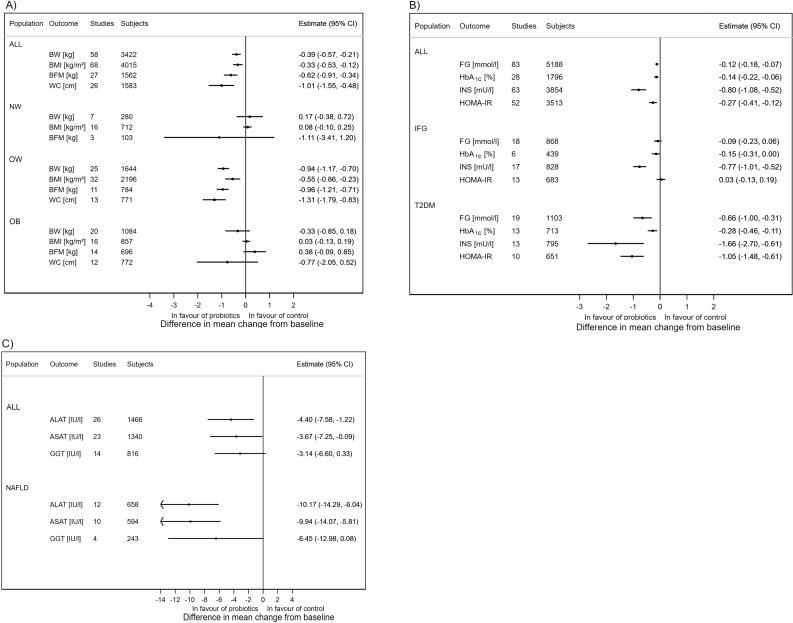
We first examined probiotic effects in all study populations. We observed a minor but significant mean difference in absolute changes for anthropometric parameters, BW: k=58, d=−0.39 kg (95% CI −0.57 to −0.21; I²=22.6%, median duration of the follow-up 10 weeks), BMI: k=68, d=−0.33 kg/m² (95% CI −0.53 to −0.12; I²=86.3%), WC: k=26, d=−1.01 cm (95% CI −1.55 to −0.48; I²=35.6%), BFM: k=27, d=−0.62 kg (95% CI −0.91 to −0.34; I²=16.3%), VAT: k=5, d=−6.30 cm² (95% CI −9.05 to −3.56; I²=0.0%), and SAT: k=5, d=−4.99 cm² (95% CI −7.55 to −2.44; I²=0.0%) with probiotic administration (figure 2A, see online supplementary figures 1–18). Sensitivity analysis excluding different types of trials showed that the effect estimates were robust with respect to study quality, specific study populations (children, pregnancy, gastric surgery) and conserved even when studies from Iran were excluded (see online supplementary table 4–6, supplementary figures 19–24).
Figure 2.
Results of meta-analyses: overall estimates and estimates obtained in specific study populations: (A) anthropometric variables and BMI, (B) glucose homeostasis, (C) liver enzymes. (X-axis) Absolute difference in mean change from baseline (probiotics—control groups): random effects estimate and 95% CI; outcomes (measurement unit): ALAT, alanine aminotransferase; ALL, all studies pooled; ASAT, aspartate aminotransferase; BFM, body fat mass; BMI, body mass index; BW, body weight; FG, fasting glucose; GGT, gamma-glutamyl transferase; HbA1c, glycated haemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; IFG, impaired fasting glucose; INS, fasting insulin; NAFLD, non-alcoholic fatty liver disease; NW, normal weight; OB, obese; OW, overweight, T2DM, type 2 diabetes mellitus, WC, waist circumference.
Glucose homeostasis and systemic inflammation
We also found a significant mean difference in absolute changes for parameters related to glucose homeostasis and systemic inflammation: FG: k=83, d=−0.12 mmol (95% CI −0.18 to −0.07; I²=55.4%), HbA1c: k=28, d=−0.14 pp (95% CI −0.22 to −0.06; I²=81.3%), INS: k=63, d=−0.80 mU/L (95% CI −1.08 to −0.52, I²=72.8%), HOMA-IR: k=52, d=−0.27 pp (95% CI −0.41 to −0.12; I²=74.9%) and CRP: k=41, d=−0.48 mg/L (95% CI −0.76 to −0.21; I²=67.1%) with probiotics administration (figure 2B, see online supplementary figures 25-39). Sensitivity analysis showed that effect estimates were robust with respect to study quality and specific study populations (children, pregnancy, gastric surgery). Sensitivity analysis recalculating the meta-analysis estimates excluding all studies conducted in Iran revealed non-significant effect estimates for all these parameters (see online supplementary table 7–9, supplementary figures 40–44).
TG and liver function
We observed a significant change in TG: k=74, d=−5.40 mg/dL (95% CI −9.17 to −1.63; I²=35.9%), ALAT: k=26, d=−4.40 U/L (95% CI −7.58 to −1.22; I²=92.9%) and ASAT: k=23, d=−3.67 U/L (95% CI −7.25 to −0.09; I²=96.8%) as well as a borderline significant trend in GGT: k=14, d=−3.14 U/L (95% CI −6.60 to 0.33; I²=86.9%) with high heterogeneity (figure 2C, see online supplementary figures 45–56). Sensitivity analysis showed that effect estimates were robust with respect to study quality and specific study populations (children, pregnancy, gastric surgery). After excluding the trials from Iran, the effect estimates became non-significant (see online supplementary table 9–10, supplementary figures 57–60).
Subgroup analysis by gender
Only a small fraction of studies reported gender-specific estimates. Thus, the power to identify effect heterogeneity by gender was limited for most outcomes. The only evidence for heterogeneity of treatment effect by gender we observed was for FG, INS and HOMA-IR (effect only observed in women, not shown).
Population subgroup analysis
Subgroup analysis in different populations revealed specific effects for anthropometric variables (see online supplementary table 4–6, supplementary figures 61–71). In OW subjects, we obtained a significant mean difference in probiotics administration compared with placebo for: BW (k=25, d=−0.94 kg, 95% CI −1.17 to −0.70, I²=0.0%), BMI (k=32, d=−0.55 kg/m², 95% CI −0.86 to −0.23, I²=91.9%), WC (k=13, d=−1.31 cm, 95% CI −1.79 to −0.83, I²=26.2%), BFM (k=11, d=−0.96 kg, 95% CI −1.21 to −0.71, I²=0.0%), VAT (k=5, d=−6.30 cm², 95% CI −9.05 to −3.56, I²=0.0%) and SAT (k=5, d=−4.99 cm², 95% CI −7.55 to −2.44, I²=0.0%). In contrast, the effect estimates were smaller and non-significant in OB subjects (figure 2A).
Subgroup analysis in different populations revealed an interesting pattern for glucose homeostasis variables (see online supplementary table 7–8, supplementary figures 72–82). In subjects with impaired fasting glucose, we observed significant effects on HbA1c (k=6 including no trial from Iran, d=−0.15 pp, 95% CI −0.31 to 0.00, I²=89.7%) and INS (k=17 including five trials from Iran, d=−0.77 mU/L, 95% CI −1.01 to −0.52, I²=0.0%) (median duration of the follow-up 10 weeks). In type 2 diabetic subjects, probiotics reduced FG (k=19 including nine trials from Iran, d=−0.66 mmol/L, 95% CI −1.00 to −0.31, I²=27.7%), HbA1c (k=13 including seven trials from Iran, d=−0.28 pp, 95% CI −0.46 to −0.11, I²=54.1%), INS (k=13 including eight trials from Iran, d=−1.66 mU/L, 95% CI −2.70 to −0.61, I²=37.8%) and HOMA-IR (k=10 including six trials from Iran, d=−1.05 pp, 95% CI −1.48 to −0.61, I²=18.2%) (figure 2B). Probiotics induced improvements in INS (k=6 including four trials from Iran, d=−3.17 mU/L, 95% CI −4.88 to −1.46, I²=73 .8%) and HOMA-IR (k=6 including four trials from Iran, d=−0.71 pp, 95% CI −1.05 to −0.36, I²=66.4%) in women with gestational diabetes.
In subjects with fatty liver diseases, probiotics reduced ALAT (k=12 including eight trials from Iran, d=−10.2 U/L, 95% CI −14.3 to −6.0, I²=93.50%), ASAT (k=10 including seven trials from Iran, d=−9.9 U/L, 95% CI −14.1 to −5.8, I²=96.1%) (figure 2C, see online supplementary figures 83–84). Also, BW (k=5, d=−1.83 kg, 95% CI −3.49 to −0.17; I²=0.0%), BMI (k=11, d=−1.21 kg/m², 95% CI −2.18 to −0.24; I²=88.5%) and components of metabolic syndrome WC (k=4, d=−1.81 cm, 95% CI −3.20 to −0.43; I²=0.0%), FG (k=12 including nine trials from Iran, d=−0.30 mmol/L, 95% CI −0.52 to −0.08, I²=68.5%) and TG (k=11 including seven trials from Iran, d=−12.89 mg/dL, 95% CI −21.82 to −3.97; I²=33.2%) were reduced in subjects with fatty liver diseases (see online supplementary table 4,5,7,9). Finally, only four trials reported results in subjects with metabolic syndrome (data not shown).
Dose, food form and species subgroup analysis
Except for BMI, subgroup analysis did not reveal a dose-dependent effect. Subgroup analysis revealed no common or unique food form effect (see online supplementary table 4–10).
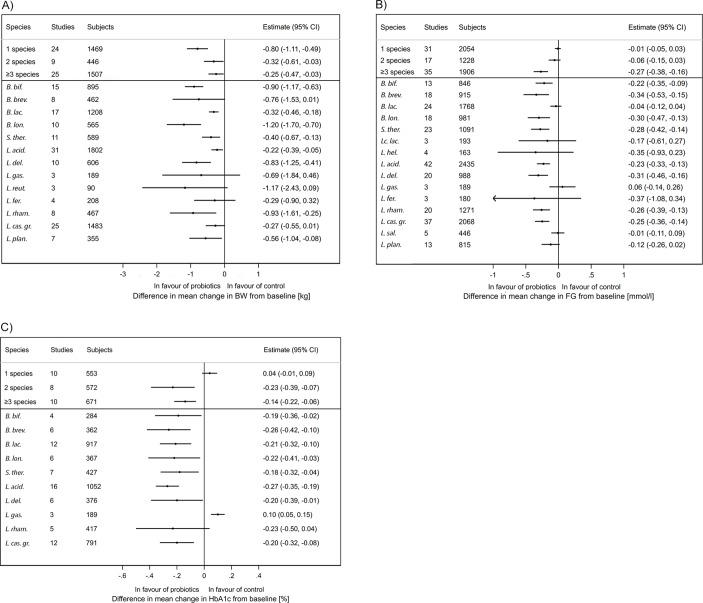
We observed a significant effect with a single bacterial species on all anthropometric variables. In contrast, a subgroup analysis showed that the use of three or more species has significant effects on an increased number of outcomes: BW, BMI, FG, HbA1c, INS, HOMA-IR, TG, ALAT and ASAT (figure 3A-C, see online supplementary table 4–10). Multiple variables BW, BMI, FG, HbA1C, INS, TG, ALAT and ASAT were significantly reduced in interventions with Bifidobacterium breve (trend for BW), B. longum, Streptococcus salivarius subsp. thermophilus, Lactobacillus acidophilus, L. casei group (trend for BW) and L. delbrueckii containing mixtures.
Figure 3.
Results of subgroup meta-analyses stratified by probiotics species and total number of different species administered (data shown for at least three trials): (A) body weight (kg), (b) fasting glucose (mmol/L), (C) glycated haemoglobin (%). (X-axis) Absolute difference in mean change from baseline (probiotics group—control group): random effects estimate and 95% CI. B. bif, Bifidobacterium bifidum; B. brev, Bifidobacterium breve; B. lac., Bifidobacterium animalis subsp. lactis; B. lon., Bifidobacterium longum; S. therm., Streptococcus salivarius subsp. thermophilus; Lc. lact, Lactococcus lactis; L. hel., Lactobacillus helveticus; L. acid., Lactobacillus acidophilus; L. del., Lactobacillus delbrueckii; L. gas., Lactobacillus gasseri; L. reut., Lactobacillus reuteri; L. fer., Lactobacillus fermentum; L. rham., Lactobacillus rhamnosus; L. cas. gr., Lactobacillus casei or paracasei; L. sal., Lactobacillus salivarius; L. plan., Lactobacillus plantarum.
Robustness of results regarding assumptions of intra-subject correlation
With the exception of ALAT, ASAT and TG, estimation of intrasubject correlation showed lower 90% CI bounds that were larger than 0.5. Therefore, for the majority of outcomes, the assumption of a correlation coefficient of 0.5 was found to be conservative since an assumed higher correlation coefficient would result in larger and more significant treatment effect values. For ALAT, ASAT and TG, the lower bounds of the 90% CI for the within subject correlation were below 0.5. Sensitivity analysis using these lower bound CI values as estimates for within subject correlation did not alter the conclusions reached for these outcomes.
Discussion
This meta-analysis revealed that probiotics consumption improves anthropometric parameters and BMI with small effect sizes. The effects on glucose homeostasis, systemic inflammation, TG and liver function were as well of small size and influenced by Iranian studies. These effect estimates became non-significant when excluding Iranian trials. This could be due to multiple factors like design, clinical practices, genetics, diets, lifestyle and/or environmental factors the strains tested could favour a response to probiotics in Iran. Finally, this meta-analysis did not explore the contribution of the Iranian studies in different study populations and care should be taken when generalising those findings.
A weight loss of 3% to 5% results in clinically relevant reductions in cardiovascular risk factors and weight loss of 2% to 5% results in modest lowering of HbA1c in OW and OB adults with T2DM.129 We found that probiotics consumption resulted in BW loss in OW individuals (0.94 kg, 1.2%), which is below the clinically meaningful threshold, suggesting that probiotics might be a complement to standard weight loss approaches. In addition to its effects on BW, probiotics consumption decreased BFM and WC, indicating improved body composition and fat distribution. This was corroborated in studies using computer tomography76–79 124 and probiotic interventions reduced both visceral and subcutaneous adipose tissue. In OB subjects, however, the effects of probiotics were non-significant. Obesity is a chronic disease characterised by severe gut microbial dysbiosis and OB subjects may be resistant to probiotics or require long-term administration. The median intervention duration with probiotics was 8 weeks in OW subjects and it remains to be explored whether probiotics could achieve or contribute to a clinically meaningful weight loss as part of a long-term lifestyle intervention.
Probiotics intake improved glucose control in subjects with impaired fasting glucose and T2DM. The HbA1c effect size was doubled in diabetics and INS was proportionally reduced, indicating improved insulin resistance. Probiotics intake reduced BW in subjects with T2DM indicating that BW loss contributes to the better glycaemic control. Overall, the metabolic status of subjects with T2DM improved as indicated by reduced TG and CRP, although additional research in these population is warranted. Finally, in subjects with T2DM, the mean difference in HbA1c with probiotics was −0.28, which is promisingly close to the HbA1c reductions of 0.5–2 pp associated with medical nutrition therapy.130
Probiotic intake reduced liver enzyme activity in subjects with fatty liver disease. These changes occurred with reduced BW and components of metabolic syndrome (WC, FG, TG) yet surprisingly, the surrogate of insulin resistance remained unchanged. This is promising although the number of trials is small (12), the trials were of short duration (≤12 weeks except98 119) and the majority of them were performed in Iran. Additional evaluation of liver pathology using non-invasive assessments and liver biopsy would be needed to support a clinical recommendation.
Previous meta-analyses suggested benefits with multistrain probiotic mixes compared with a single strain. We corroborated that probiotic mixes composed of three or more species resulted in improvements and identified core species: B. breve, B. longum, Streptococcus salivarius subsp. thermophilus, L. acidophilus, L. casei group, L. delbrueckii. Interestingly, yoghourt starter bacteria include L. delbrueckii subsp. bulgaricus and S. thermophilus and this finding is of interest in respect to an inverse association between yoghourt and BW change and the incidence of T2DM.131 132 In diabetics, treatment with metformin and acarbose changes faecal microbiota with an increased abundance of lactobacilli (which belongs to the Firmicutes phyla) and bifidobacteria (Actinobacteria) and these species could potentially contribute to the antidiabetic effect.133–135 The intake of probiotics on top of medication may induce even more pronounced changes in microbiota of the small intestine and this raises the question whether probiotics could become a part of the nutritional strategy in diabetes management.
Different probiotic species and strains may have multiple modes of actions affecting sugar digestion and absorption,136 fat absorption,106 gut barrier function,112 115 137 low-grade inflammation, 39 71 81 99 110 111 116 138 bile acid metabolism,97 139 incretin secretion120 and gut microbiota ecosystems19 44 87 115 119 and, in particular, short chain fatty acid production.121 It is reasonable to anticipate that these multiple effects may combine to induce significant effects, particularly in the case of multispecies mixes. However, the exact mechanism behind probiotics’ efficacy is unknown and currently limited to hypotheses. The majority of studies examined in the current analysis poorly described the rationale of the probiotics selected, dosing remained arbitrary and, overall, the approaches were rather empiric. Therefore, further research is warranted to select the probiotics strains not only for their capacity to comply with food grade status, but also for their capacity to survive through gastrointestinal tract (assuming this is required) and affect relevant biological target(s).
Limitations of the study
We integrated both exploratory and secondary outcomes, trials with small sample size, heterogeneous study populations, no or incomplete information on concomitant hypoglycaemic drug treatment and no or incomplete dietary and physical activity records. The heterogeneity of probiotic strains implies a limitation that we aimed to reduce by the subgroup meta-analysis at the species level. Thirty-eight per cent of trials were conducted in Iran and published within the last 6 years. These trials reported very similar designs, sample sizes, methodology, endpoints and results. Exclusion of these trials render the effect of probiotics non-significant for FG, HbA1c, INS, HOMA-IR, CRP, TG, ALAT and ASAT. The influence of these trials on the overall meta-analysis is clear though the precise reason why is difficult to know with certainty. Care is required in interpreting these data in generalising them to subjects outside of Iran. Furthermore, non-published results from studies that did not show significant results could introduce a potential bias. While our diagnostic publication bias analyses indicated evidence for publication bias (both asymmetric funnel plots and Egger’s test) for outcomes FG, HbA1c, INS, ALAT, ASAT and GGT, correction of estimates by imputing non-published studies demonstrated no effect of small study size. While including studies with low methodological quality may influence our analyses, recalculating summary estimates with filtering study quality demonstrated that our results were robust despite potential bias. For some trials, important parameters necessary for the estimation of effect sizes were unknown and we had to base our calculations on assumptions that could be only in part derived from the data at hand. For example, if SD of changes were not explicitly reported, we had to impute these by assuming a value for the intrasubject correlation. A sensitivity analysis varying the correlation assumptions within realistic ranges of correlation showed robustness of results. Even though for some outcomes the true correlation between subjects might be higher, we chose a conservative estimate (r=0.5) aimed at avoiding any overestimation bias of effect sizes and type I error inflation. Finally, we did not adjust our estimates for multiplicity tests.
Significance of the study
This meta-analysis showed that the intake of probiotics resulted in minor improvements of BW and body composition in OW subjects. Probiotics administration may provide improved glucose control and insulin function in type 2 diabetic subjects, and liver enzymes in those with fatty liver disease. These improvements were observed with B. breve, B. longum, Streptococcus salivarius subsp. thermophilus, L. acidophilus, L. casei and L. delbrueckii containing mixtures.
Unanswered questions and future research
This review and meta-analysis brought forth several questions: First, what are the underlying mechanisms? Second, how can multispecies mixes be optimised to induce synergistic effects? Third, can probiotics become part of standard dietary recommendations for obesity, diabetes and non-alcoholic disease management? Finally, with these questions, this study paves the way for clinical studies examining the potential of probiotic mixes as part of a long-term dietary intervention.
Supplementary Material
Acknowledgments
We thank Murielle Gagneau, Danone Nutricia Research, for her guidance and review of the systematic review and meta-analysis protocol, Agnès Meunier, Danone Nutricia Research, for her initial bibliography screen, Marion Genser, BGStats Consulting for her systematic literature review and data extraction, Quentin Dornic, Danone Nutricia Research and Kevin J Carroll, KJC Statistics Ltd for their statistical review and Timothy Swartz, Institute of Cardiometabolism and Nutrition, for his English editing.
Footnotes
HK and BG contributed equally.
Contributors: Study concept and design: HK, BG, JS, SR, KC. Systematic literature review: BG, HK, JS, MMS, JMF. Data extraction: BG, HK, JS, MMS, JMF. Concept and conduct of statistical analysis: BG. Interpretation of data: HK, BG, MMS, SR, JS, KC. Drafting of the manuscript: HK, BG. Critical revision HK, BG, MMS, JMF, SR, JS, KC. All authors approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: Danone Nutricia Research, Palaiseau, France. The work of BGStats Consulting was funded by Danone Research, Palaiseau, France. Danone Research supported collaborative work of the Institute of Cardiometabolism and Nutrition. JS received consultancy fee from Danone Research.
Competing interests: HK and JMF are employees of Danone Nutricia Research; BG received funding from Danone Research; JS received consultancy fees from Danone Research, is presently member of the Scientific Advisory Board of Actial SRL and acted as expert for Actial in a court hearing; KC, MMS and SR have a collaborative agreement with Danone Research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Authors have included all available data in the research through supplementary figures and tables.
Patient consent for publication: Not required.
References
- 1. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 2. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60. 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 3. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8. 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 5. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016;352:560–4. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 7. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Tang H, Zhang C, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. Isme J 2015;9:1–15. 10.1038/ismej.2014.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cani PD, Van Hul M. Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotechnol 2015;32:21–7. 10.1016/j.copbio.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 11. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2013;19:6911–8. 10.3748/wjg.v19.i40.6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan Y, Sun J, He J, et al. Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PLoS One 2015;10:e0132121 10.1371/journal.pone.0132121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res 2015;35:566–75. 10.1016/j.nutres.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 14. Sun J, Buys NJ. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomised placebo-controlled trials. Br J Nutr 2016;115:1167–77. 10.1017/S0007114516000076 [DOI] [PubMed] [Google Scholar]
- 15. Borgeraas H, Johnson LK, Skattebu J, et al. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2018;19 10.1111/obr.12626 [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Juan QF, He YW, et al. Multiple effects of probiotics on different types of diabetes: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Pediatr Endocrinol Metab 2017;30:611–22. 10.1515/jpem-2016-0230 [DOI] [PubMed] [Google Scholar]
- 17. Yao K, Zeng L, He Q, et al. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit 2017;23:3044–53. 10.12659/MSM.902600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. EFSA Journal 2018;16:e05315. [Google Scholar]
- 19. Sanchez M, Darimont C, Drapeau V, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 2014;111:1507–19. 10.1017/S0007114513003875 [DOI] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- 25. Agerholm-Larsen L, Raben A, Haulrik N, et al. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr 2000;54:288–97. 10.1038/sj.ejcn.1600937 [DOI] [PubMed] [Google Scholar]
- 26. Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, et al. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr 2016;116:8:1394–401. 10.1017/S0007114516003457 [DOI] [PubMed] [Google Scholar]
- 27. Ahn HY, Kim M, Ahn YT, et al. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr Metab Cardiovasc Dis 2015;25:724–33. 10.1016/j.numecd.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 28. Aihara K, Kajimoto O, Hirata H, et al. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr 2005;24:257–65. 10.1080/07315724.2005.10719473 [DOI] [PubMed] [Google Scholar]
- 29. Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci 2016;8:256 10.3389/fnagi.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016;32:315–20. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 31. Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014;39:1276–85. 10.1111/apt.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011;15:1090–5. [PubMed] [Google Scholar]
- 33. Andreasen AS, Larsen N, Pedersen-Skovsgaard T, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 2010;104:1831–8. 10.1017/S0007114510002874 [DOI] [PubMed] [Google Scholar]
- 34. Asemi Z, Khorrami-Rad A, Alizadeh SA, et al. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 2014;33:198–203. 10.1016/j.clnu.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 35. Asemi Z, Samimi M, Tabassi Z, et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr 2013;67:71–4. 10.1038/ejcn.2012.189 [DOI] [PubMed] [Google Scholar]
- 36. Asemi Z, Zare Z, Shakeri H, et al. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 2013;63(1-2):1–9. 10.1159/000349922 [DOI] [PubMed] [Google Scholar]
- 37. Asgharian A, Askari G, Esmailzade A, et al. The effect of symbiotic supplementation on liver enzymes, c-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: a clinical trial. Int J Prev Med 2016;7:59 10.4103/2008-7802.178533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asgharian A, Mohammadi V, Gholi Z, et al. The effect of synbiotic supplementation on body composition and lipid profile in patients with nafld: a randomized, double blind, placebo-controlled clinical trial study. Iranian Red Crescent Medical Journal 2017;19 10.5812/ircmj.42902 [DOI] [Google Scholar]
- 39. Badehnoosh B, Karamali M, Zarrati M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. The Journal of Maternal-Fetal & Neonatal Medicine 2018;31:1128–36. 10.1080/14767058.2017.1310193 [DOI] [PubMed] [Google Scholar]
- 40. Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, et al. The consumption of synbiotic bread containing lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J Am Coll Nutr 2016;35:506–13. 10.1080/07315724.2015.1032443 [DOI] [PubMed] [Google Scholar]
- 41. Barreto FM, Colado Simão AN, Morimoto HK, et al. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014;30:939–42. 10.1016/j.nut.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 42. Behrouz V, Jazayeri S, Aryaeian N, et al. Effects of probiotic and prebiotic supplementation on leptin, adiponectin, and glycemic parameters in non-alcoholic fatty liver disease: a randomized clinical trial. Middle East J Dig Dis 2017;9:150–7. 10.15171/mejdd.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernini LJ, Simão AN, Alfieri DF, et al. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2016;32:716–9. 10.1016/j.nut.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 44. Brahe LK, Le Chatelier E, Prifti E, et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br J Nutr 2015;114:406–17. 10.1017/S0007114515001786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burton KJ, Rosikiewicz M, Pimentel G, et al. Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. Br J Nutr 2017;117:1312–22. 10.1017/S0007114517000885 [DOI] [PubMed] [Google Scholar]
- 46. Chung HJ, Yu JG, Lee IA, et al. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio 2016;6:64–76. 10.1002/2211-5463.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Roos NM, Schouten G, Katan MB. Yoghurt enriched with Lactobacillus acidophilus does not lower blood lipids in healthy men and women with normal to borderline high serum cholesterol levels. Eur J Clin Nutr 1999;53:277–80. 10.1038/sj.ejcn.1600722 [DOI] [PubMed] [Google Scholar]
- 48. Dolatkhah N, Hajifaraji M, Abbasalizadeh F, et al. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J Health Popul Nutr 2015;33:25 10.1186/s41043-015-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ebrahimi ZS, Nasli-Esfahani E, Nadjarzade A, et al. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord 2017;16:23 10.1186/s40200-017-0304-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012;28:539–43. 10.1016/j.nut.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 51. Ekhlasi G, Kolahdouz Mohammadi R, Agah S, et al. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci 2016;21:106 10.4103/1735-1995.193178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eslamparast T, Poustchi H, Zamani F, et al. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 2014;99:535–42. 10.3945/ajcn.113.068890 [DOI] [PubMed] [Google Scholar]
- 53. Eslamparast T, Zamani F, Hekmatdoost A, et al. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br J Nutr 2014;112:438–45. 10.1017/S0007114514000919 [DOI] [PubMed] [Google Scholar]
- 54. Famouri F, Shariat Z, Hashemipour M, et al. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr 2017;64:413–7. 10.1097/MPG.0000000000001422 [DOI] [PubMed] [Google Scholar]
- 55. Feizollahzadeh S, Ghiasvand R, Rezaei A, et al. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type ii diabetes mellitus. Probiotics Antimicrob Proteins 2017;9:41-47 10.1007/s12602-016-9233-y [DOI] [PubMed] [Google Scholar]
- 56. Firouzi S, Majid HA, Ismail A, et al. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr 2017;56 10.1007/s00394-016-1199-8 [DOI] [PubMed] [Google Scholar]
- 57. Firouzi S, Mohd-Yusof BN, Majid HA, et al. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: a randomized controlled trial. BMC Complement Altern Med 2015;15:433 10.1186/s12906-015-0952-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gøbel RJ, Larsen N, Jakobsen M, et al. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr 2012;55:673–8. 10.1097/MPG.0b013e318263066c [DOI] [PubMed] [Google Scholar]
- 59. Gomes AC, de Sousa RG, Botelho PB, et al. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity 2017;25:30–8. 10.1002/oby.21671 [DOI] [PubMed] [Google Scholar]
- 60. Hajifaraji M, Jahanjou F, Abbasalizadeh F, et al. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J Clin Nutr 2018;27:581–91. 10.6133/apjcn.082017.03 [DOI] [PubMed] [Google Scholar]
- 61. Hariri M, Salehi R, Feizi A, et al. The effect of probiotic soy milk and soy milk on anthropometric measures and blood pressure in patients with type II diabetes mellitus: A randomized double-blind clinical trial. ARYA Atheroscler 2015;11(Suppl 1):74–80. [PMC free article] [PubMed] [Google Scholar]
- 62. Higashikawa F, Noda M, Awaya T, et al. Improvement of constipation and liver function by plant-derived lactic acid bacteria: a double-blind, randomized trial. Nutrition 2010;26:367–74. 10.1016/j.nut.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 63. Higashikawa F, Noda M, Awaya T, et al. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr 2016;70:582–7. 10.1038/ejcn.2016.17 [DOI] [PubMed] [Google Scholar]
- 64. Hove KD, Brøns C, Færch K, et al. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur J Endocrinol 2015;172:11–20. 10.1530/EJE-14-0554 [DOI] [PubMed] [Google Scholar]
- 65. Hulston CJ, Churnside AA, Venables MC. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br J Nutr 2015;113:596–602. 10.1017/S0007114514004097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hütt P, Songisepp E, Rätsep M, et al. Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef Microbes 2015;6:233–43. 10.3920/BM2014.0035 [DOI] [PubMed] [Google Scholar]
- 67. Ibrahim NS, Muhamad AS, Ooi FK, et al. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl Physiol Nutr Metab 2018;43:180–6. 10.1139/apnm-2017-0464 [DOI] [PubMed] [Google Scholar]
- 68. Ilmonen J, Isolauri E, Poussa T, et al. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr 2011;30:156–64. 10.1016/j.clnu.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 69. Inoue K, Shirai T, Ochiai H, et al. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 2003;57:490–5. 10.1038/sj.ejcn.1601555 [DOI] [PubMed] [Google Scholar]
- 70. Ivey KL, Hodgson JM, Kerr DA, et al. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr 2014;68:447–52. 10.1038/ejcn.2013.294 [DOI] [PubMed] [Google Scholar]
- 71. Jafarnejad S, Saremi S, Jafarnejad F, et al. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: a randomized controlled clinical trial. J Nutr Metab 2016;2016:1–8. 10.1155/2016/5190846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jamilian M, Bahmani F, Vahedpoor Z, et al. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double-blind, placebo-controlled trial. Arch Iran Med 2016;19:687–82. doi:0161910/AIM.004 [PubMed] [Google Scholar]
- 73. Javadi L, Ghavami M, Khoshbaten M, et al. The Effect of Probiotic and/or Prebiotic on Liver Function Tests in Patients with Nonalcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Iran Red Crescent Med J 2017;19 10.5812/ircmj.46017 [DOI] [Google Scholar]
- 74. Javadi LGM, Khoshbaten M, Safaiyan A, et al. The potential role of probiotics or/and prebiotic on serum lipid profile and insulin resistance in alcoholic fatty liver disease: A double blind randomized clinical trial. Crescent Journal of Medical and Biological Sciences 2017;4:131–8. [Google Scholar]
- 75. Jones RB, Alderete TL, Martin AA, et al. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatr Obes 2018;13:705–14. 10.1111/ijpo.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jung SP, Lee KM, Kang JH, et al. Effect of lactobacillus gasseri bnr17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med 2013;34:80–9. 10.4082/kjfm.2013.34.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jung S, Lee YJ, Kim M, et al. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J Funct Foods 2015;19:744–52. 10.1016/j.jff.2015.10.006 [DOI] [Google Scholar]
- 78. Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010;64:636–43. 10.1038/ejcn.2010.19 [DOI] [PubMed] [Google Scholar]
- 79. Kadooka Y, Sato M, Ogawa A, et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr 2013;110:1696–703. 10.1017/S0007114513001037 [DOI] [PubMed] [Google Scholar]
- 80. Karamali M, Dadkhah F, Sadrkhanlou M, et al. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab 2016;42:234–41. 10.1016/j.diabet.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 81. Karbaschian Z, Mokhtari Z, Pazouki A, et al. Probiotic supplementation in morbid obese patients undergoing One Anastomosis Gastric Bypass-Mini Gastric Bypass (OAGB-MGB) surgery: a randomized, double-blind, placebo-controlled, clinical trial. Obes Surg 2018;28:2874–85. 10.1007/s11695-018-3280-2 [DOI] [PubMed] [Google Scholar]
- 82. Khalili L, Alipour B, Asghari Jafar-Abadi M, et al. The effects of lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J 2019;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kijmanawat A, Panburana P, Reutrakul S, et al. The effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. J Diabetes Investig 2019;10 10.1111/jdi.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim M, Kim M, Kang M, et al. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct 2017;8:250–61. 10.1039/C6FO00993J [DOI] [PubMed] [Google Scholar]
- 85. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr 2017;36:1245–9. 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 86. Laitinen K, Poussa T, Isolauri E, et al. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009;101:1679–87. 10.1017/S0007114508111461 [DOI] [PubMed] [Google Scholar]
- 87. Lee SJ, Bose S, Seo JG, et al. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: a randomized double-blind controlled clinical trial. Clin Nutr 2014;33:973–81. 10.1016/j.clnu.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 88. Lee Y, Ba Z, Roberts RF, et al. Effects of Bifidobacterium animalis subsp. lactis BB-12® on the lipid/lipoprotein profile and short chain fatty acids in healthy young adults: a randomized controlled trial. Nutr J 2017;16:39 10.1186/s12937-017-0261-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lindsay KL, Kennelly M, Culliton M, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr 2014;99:1432–9. 10.3945/ajcn.113.079723 [DOI] [PubMed] [Google Scholar]
- 90. Lindsay KL, Brennan L, Kennelly MA, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am J Obstet Gynecol 2015;212:496.e1–496.e11. 10.1016/j.ajog.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 91. Madjd A, Taylor MA, Mousavi N, et al. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: a randomized controlled trial. Am J Clin Nutr 2016;103:323–9. 10.3945/ajcn.115.120170 [DOI] [PubMed] [Google Scholar]
- 92. Mahboobi S, Iraj B, Maghsoudi Z, et al. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: a randomized clinical trial. Int J Prev Med 2014;5:1239–46. [PMC free article] [PubMed] [Google Scholar]
- 93. Manzhalii E, Virchenko O, Falalyeyeva T, et al. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J Dig Dis 2017;18:698–703. 10.1111/1751-2980.12561 [DOI] [PubMed] [Google Scholar]
- 94. Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci 2013;38:38–43. [PMC free article] [PubMed] [Google Scholar]
- 95. Minami J, Kondo S, Yanagisawa N, et al. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 2015;4:e17 10.1017/jns.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Miraghajani M, Zaghian N, Dehkohneh A, et al. Probiotic soy milk consumption and renal function among type 2 diabetic patients with nephropathy: a randomized controlled clinical trial. Probiotics Antimicrob Proteins 2017. 10.1007/s12602-017-9325-3 [DOI] [PubMed] [Google Scholar]
- 97. Mobini R, Tremaroli V, Ståhlman M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2017;19 10.1111/dom.12861 [DOI] [PubMed] [Google Scholar]
- 98. Mofidi F, Poustchi H, Yari Z, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr 2017;117:662–8. 10.1017/S0007114517000204 [DOI] [PubMed] [Google Scholar]
- 99. Mohamadshahi M, Veissi M, Haidari F, et al. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014;4:83–8. 10.5681/bi.2014.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mohseni S, Bayani M, Bahmani F, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev 2018;34:e2970 10.1002/dmrr.2970 [DOI] [PubMed] [Google Scholar]
- 101. Nabavi S, Rafraf M, Somi MH, et al. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 2014;97:7386–93. 10.3168/jds.2014-8500 [DOI] [PubMed] [Google Scholar]
- 102. Nabavi S, Rafraf M, Somi M-hossein, et al. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD). J Funct Foods 2015;18:684–91. 10.1016/j.jff.2015.08.031 [DOI] [Google Scholar]
- 103. Naito E, Yoshida Y, Kunihiro S, et al. Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: a randomised, double-blind, placebo-controlled trial. Biosci Microbiota Food Health 2018;37:9–18. 10.12938/bmfh.17-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Naruszewicz M, Johansson ML, Zapolska-Downar D, et al. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr 2002;76:1249–55. 10.1093/ajcn/76.6.1249 [DOI] [PubMed] [Google Scholar]
- 105. Odamaki T, Kato K, Sugahara H, et al. Effect of probiotic yoghurt on animal-based diet-induced change in gut microbiota: an open, randomised, parallel-group study. Benef Microbes 2016;7:473–84. 10.3920/BM2015.0173 [DOI] [PubMed] [Google Scholar]
- 106. Ogawa A, Kadooka Y, Kato K, et al. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis 2014;13:36 10.1186/1476-511X-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Omar JM, Chan Y-M, Jones ML, et al. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J Funct Foods 2013;5:116–23. 10.1016/j.jff.2012.09.001 [DOI] [Google Scholar]
- 108. Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 2015;44:228–37. [PMC free article] [PubMed] [Google Scholar]
- 109. Osterberg KL, Boutagy NE, McMillan RP, et al. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity 2015;23:2364–70. 10.1002/oby.21230 [DOI] [PubMed] [Google Scholar]
- 110. Rajkumar H, Mahmood N, Kumar M, et al. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm 2014;2014:1–8. 10.1155/2014/348959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rajkumar H, Kumar M, Das N, et al. Effect of Probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers. J Cardiovasc Pharmacol Ther 2015;20:289–98. 10.1177/1074248414555004 [DOI] [PubMed] [Google Scholar]
- 112. Sabico S, Al-Mashharawi A, Al-Daghri NM, et al. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. J Transl Med 2017;15:249 10.1186/s12967-017-1354-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, et al. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr 2010;103:1778–83. 10.1017/S0007114509993801 [DOI] [PubMed] [Google Scholar]
- 114. Safavi M, Farajian S, Kelishadi R, et al. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: a randomized triple-masked controlled trial. Int J Food Sci Nutr 2013;64:687–93. 10.3109/09637486.2013.775224 [DOI] [PubMed] [Google Scholar]
- 115. Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci Rep 2017;7:12115 10.1038/s41598-017-12535-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sepideh A, Karim P, Hossein A, et al. Effects of Multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr 2016;35:500–5. 10.1080/07315724.2015.1031355 [DOI] [PubMed] [Google Scholar]
- 117. Sharafedtinov KK, Plotnikova OA, Alexeeva RI, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients--a randomized double-blind placebo-controlled pilot study. Nutr J 2013;12:138 10.1186/1475-2891-12-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shavakhi A, Minakari M, Firouzian H, et al. Effect of a probiotic and metformin on liver aminotransferases in non-alcoholic steatohepatitis: a double blind randomized clinical trial. Int J Prev Med 2013;4:531–7. [PMC free article] [PubMed] [Google Scholar]
- 119. Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes 2018;42 10.1038/ijo.2017.210 [DOI] [PubMed] [Google Scholar]
- 120. Simon MC, Strassburger K, Nowotny B, et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care 2015;38:1827–34. 10.2337/dc14-2690 [DOI] [PubMed] [Google Scholar]
- 121. Stenman LK, Lehtinen MJ, Meland N, et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine 2016;13:190–200. 10.1016/j.ebiom.2016.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab 2014;65:34–41. 10.1159/000365153 [DOI] [PubMed] [Google Scholar]
- 123. Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 2017;125:21–7. 10.1055/s-0042-105441 [DOI] [PubMed] [Google Scholar]
- 124. Takahashi S, Anzawa D, Takami K, et al. Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: a randomized controlled trial. Biosci Microbiota Food Health 2016;35:163–71. 10.12938/bmfh.2016-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, et al. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr 2017;36:85–92. 10.1016/j.clnu.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 126. Tripolt NJ, Leber B, Blattl D, et al. Short communication: effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, β-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome--a pilot study. J Dairy Sci 2013;96:89–95. 10.3168/jds.2012-5863 [DOI] [PubMed] [Google Scholar]
- 127. Vajro P, Mandato C, Licenziati MR, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr 2011;52:740–3. 10.1097/MPG.0b013e31821f9b85 [DOI] [PubMed] [Google Scholar]
- 128. Zarrati M, Salehi E, Nourijelyani K, et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J Am Coll Nutr 2014;33:417–25. 10.1080/07315724.2013.874937 [DOI] [PubMed] [Google Scholar]
- 129. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 130. American Diabetes Association. 5. Prevention or delay of type 2 diabetes. Diabetes Care 2017;40(Suppl 1):S44–S47. 10.2337/dc17-S008 [DOI] [PubMed] [Google Scholar]
- 131. Gijsbers L, Ding EL, Malik VS, et al. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–24. 10.3945/ajcn.115.123216 [DOI] [PubMed] [Google Scholar]
- 132. Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med Overseas Ed 2011;364:2392–404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017;23:850–8. 10.1038/nm.4345 [DOI] [PubMed] [Google Scholar]
- 134. Zhang X, Fang Z, Zhang C, et al. Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Ther 2017;8:293–307. 10.1007/s13300-017-0226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Su B, Liu H, Li J, et al. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes 2015;7:729–39. 10.1111/1753-0407.12232 [DOI] [PubMed] [Google Scholar]
- 136. Panwar H, Calderwood D, Grant IR, et al. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur J Nutr 2014;53:1465–74. 10.1007/s00394-013-0649-9 [DOI] [PubMed] [Google Scholar]
- 137. Kawano M, Miyoshi M, Ogawa A, et al. Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high-fat diet. J Nutr Sci 2016;5:e23 10.1017/jns.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rajkumar H, Kumar M, Das N, et al. Effect of probiotic lactobacillus salivarius ubl s22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther 2015;20:289–98. 10.1177/1074248414555004 [DOI] [PubMed] [Google Scholar]
- 139. Hamad EM, Sato M, Uzu K, et al. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr 2009;101:716–24. 10.1017/S0007114508043808 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017995supp001.pdf (831KB, pdf)