Significance
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is important for neuronal development. Mechanistically, UCH-L1 has an inhibitory effect on mTOR-containing complexes thereby controlling protein synthesis and degradation. Neurodegeneration arising from UCH-L1 deficiency is the result of an early increased protein turnover (synthesis and decay), which strains neurons by increasing energy requirement and endoplasmic reticulum stress. Rapamycin treatment during a critical developmental period partially rescues motor degeneration in adulthood.
Keywords: UCH-L1, neurodegeneration, development, protein synthesis, mTOR
Abstract
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is one of the most abundant and enigmatic enzymes of the CNS. Based on existing UCH-L1 knockout models, UCH-L1 is thought to be required for the maintenance of axonal integrity, but not for neuronal development despite its high expression in neurons. Several lines of evidence suggest a role for UCH-L1 in mUB homeostasis, although the specific in vivo substrate remains elusive. Since the precise mechanisms underlying UCH-L1–deficient neurodegeneration remain unclear, we generated a transgenic mouse model of UCH-L1 deficiency. By performing biochemical and behavioral analyses we can show that UCH-L1 deficiency causes an acceleration of sensorimotor reflex development in the first postnatal week followed by a degeneration of motor function starting at periadolescence in the setting of normal cerebral mUB levels. In the first postnatal weeks, neuronal protein synthesis and proteasomal protein degradation are enhanced, with endoplasmic reticulum stress, and energy depletion, leading to proteasomal impairment and an accumulation of nondegraded ubiquitinated protein. Increased protein turnover is associated with enhanced mTORC1 activity restricted to the postnatal period in UCH-L1–deficient brains. Inhibition of mTORC1 with rapamycin decreases protein synthesis and ubiquitin accumulation in UCH-L1–deficient neurons. Strikingly, rapamycin treatment in the first 8 postnatal days ameliorates the neurological phenotype of UCH-L1–deficient mice up to 16 weeks, suggesting that early control of protein homeostasis is imperative for long-term neuronal survival. In summary, we identified a critical presymptomatic period during which UCH-L1–dependent enhanced protein synthesis results in neuronal strain and progressive loss of neuronal function.
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is a small 27-kDa protein belonging to the UCH family of deubiquitinating enzymes (DUBs) (1) predominantly expressed in neurons where it can comprise up to 5% of total brain protein (2, 3), but is also in other cell types under specialized conditions, e.g., injured kidney podocytes (4) and cancerous cells (5). At a cellular level, UCH-L1 exhibits strong cytoplasmic staining in neurons throughout the brain. A total of 20–50% of UCH-L1 can be membrane associated, which is thought to occur indirectly via macromolecular complexes in neurons (6). Specific in vivo substrates of UCH-L1 have not been identified yet (6). UCH-L1 has high affinity for ubiquitin, which it effectively proteolyzes from small C-terminal amino acids as well as by cleaving diubiquitins in vitro (7). However, compared with UCH-L3, it is a poor hydrolase of ubiquitinated proteins, due to structurally restricted access to its active site (8, 9). UCH-L1 binds monoubiquitin (mUB) in neurons, thereby elongating ubiquitin half-life (10). Indeed, mUB is reduced in spontaneous UCH-L1 mutant mice (11, 12), suggesting that UCH-L1 regulates ubiquitin homeostasis by maintaining a pool of ready-to-use ubiquitin for various cellular events.
Genetic deletion of UCH-L1 results in gracile axonal dystrophy (gad) and premature death with early sensory ataxia followed by motor ataxia (11–13), revealing a crucial role of UCH-L1 in neuronal health. In humans, five pathogenic mutations of UCH-L1 have been discovered, three of which affect the hydrolysis function of the protease and which are associated with the pathogenesis of neurodegenerative diseases (14). A missense mutation in the UCH-L1 gene leads to an I93M substitution at the protein level, which is strongly associated with the development of Parkinson disease (PD) (15). A homozygous missense mutation within the ubiquitin binding domain of UCH-L1 (E7A) results in early onset progressive neurodegeneration in humans similarly to the effects observed in mice with genetic UCH-L1 deletion (16). Protein aggregation is an important hallmark of many neurodegenerative diseases and can arise directly as a result of dysregulated protein synthesis (17). Indeed, UCH-L1 has been shown to regulate the levels of proteins involved in the formation of Alzheimer disease (AD) or PD aggregates (18–20).
In this study, we explored the effects of UCH-L1 deficiency on protein synthesis and decay in the brain at different developmental stages to identify the potential mechanisms underlying neurodegeneration in UCH-L1–deficient mice. By means of behavioral, biochemical, and rescue experiments in newly generated transgenic mice we demonstrate that ablation of UCH-L1 results in a presymptomatic increase in protein turnover followed by proteasomal impairment causing progressive neurodegeneration and motor deficits.
Results
UCH-L1–Deficient Mice Develop Progressive Motor Degeneration.
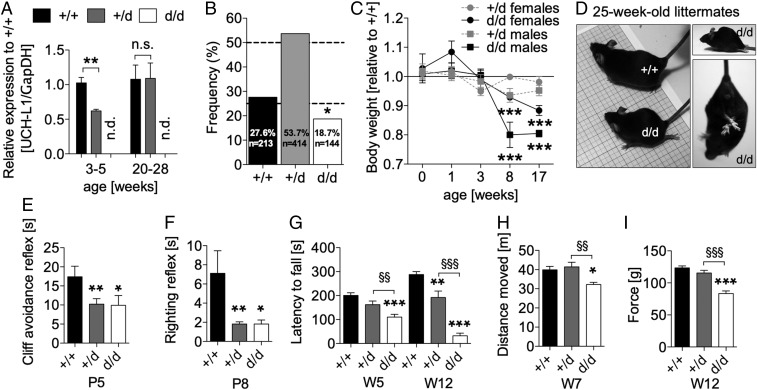
We generated constitutive UCH-L1–deficient mice by flanking exons 1–3 by loxP sites. Crossing UCH-L1 floxed mice with constitutive Cre deleter mice resulted in the generation of UCH-L1 wild-type (Uch-l1+/+) heterozygous (Uch-l1+/d) and knockout (Uch-l1d/d) mice (SI Appendix, Fig. S1). Loss of one UCH-L1 allele led to a 50% reduction of UCH-L1 transcript (Fig. 1A) and protein (SI Appendix, Fig. S2) in juvenile but not adult mice. At postnatal day 0 only 18.7% of newborn pups were Uch-l1d/d, suggesting a developmental perinatal disadvantage due to loss of UCH-L1 expression (Fig. 1B). Uch-l1d/d and not Uch-l1+/d mice were lighter than Uch-l1+/+ mice from postnatal week 8 and displayed dystonic/spastic movements of their hind feet from 10 wk, as well as femoral/gluteal muscle wasting at 20 wk (Fig. 1 C and D). As an indication of neurodegenerative processes we detected spheroid bodies in the cerebellum and enhanced signs of neuronal inflammation in the cerebellum and spinal cord of 25-wk-old Uch-l1d/d mice compared with Uch-l1+/+ littermates (SI Appendix, Fig. S3). Thus, Uch-l1d/d mice develop a similar neurological phenotype to spontaneous UCH-L1–deficient mice, namely the gad mice and nm3419 mice (11, 12). Although Uch-l1+/d and Uch-l1d/d mice exhibited sensorimotor degeneration in adulthood, sensorimotor reflexes at postnatal days 5 and 8 exhibited an unrecognized, paradoxical accelerated execution in comparison with Uch-l1+/+ littermates (Fig. 1 E and F and SI Appendix, Fig. S4). The first signs of motor impairment were detected at 5 wk in the accelerated rotarod test (Fig. 1G and SI Appendix, Fig. S4), which progressed affecting locomotion in the open field test (Fig. 1H and SI Appendix, Fig. S4) and forelimb strength at 12 wk (Fig. 1I and SI Appendix, Fig. S4). Notably, Uch-l1+/d displayed an intermediate postnatal phenotype, highlighting the early importance of UCH-L1 for brain function. Matching the dose-dependent early phenotype of Uch-l1+/d and Uch-l1d/d mice, time course analyses revealed that UCH-L1 transcript, protein, and activity levels were highest in young mice and declined thereafter (SI Appendix, Fig. S5).
Fig. 1.
Following a postnatal period of enhanced reflexes, Uch-l1+/d and Uch-l1d/d mice develop neurologic impairment. (A) qPCR for quantification of total brain UCH-L1 mRNA levels in 3- to 5-wk-old and 20- to 28-wk-old littermates; n = 5–7; **P < 0.01; n.d., not detected; n.s., not significant to +/+ weeks 20–28. (B) Frequency of genotypes resulting from heterozygous breeding; *P < 0.05 to Uch-l1+/+. (C) Development of body weight in Uch-l1+/d and Uch-l1d/d females and males; n = 16–20; ***P < 0.001 to Uch-l1+/+. (D) Micrographs of Uch-l1d/d at 25 wk, demonstrating muscle wasting of the hind legs in comparison with Uch-l1+/+. Postnatal reflexes such as (E) the cliff avoidance reflex postnatal day 5 (P5); n = 21–40 and (F) the righting reflex postnatal day 8 (P8) are enhanced in Uch-l1d/d and Uch-l1+/d mice; n = 21–40. Thereafter, neurological impairment is visible in the (G) accelerated rotarod at weeks 5 and 12 (W5, W12); n = 8–23, (H) open field test at week 7 (W7); n = 18–19, and (I) grip strength test at week 12 (W12); n = 18–19. *P < 0.05; **P < 0.01; ***P < 0.001 to age-matched Uch-l1+/+; §§P < 0.01; §§§P < 0.001 to Uch-l1+/d.
Monoubiquitin Levels Are Reduced at Post- but Not Presymptomatic Time Points in UCH-L1–Deficient Brains.
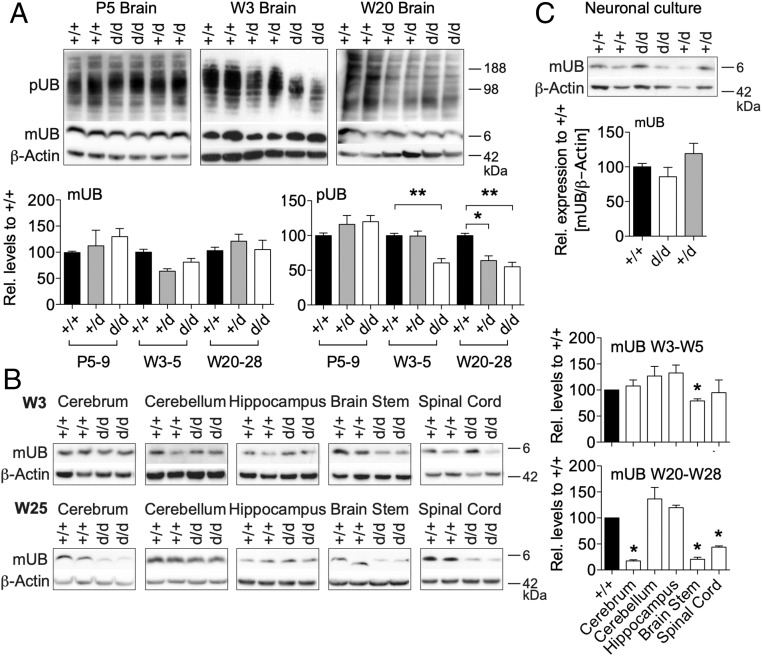
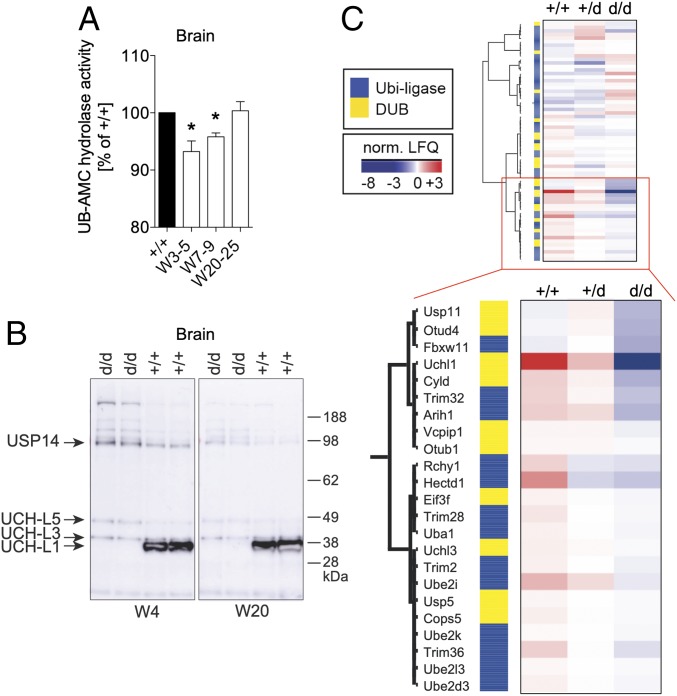
The accelerated execution of postnatal sensorimotor reflexes paired with the high expression levels of UCH-L1 in young mice suggests that UCH-L1 is required for brain development during the first postnatal weeks. UCH-L1 was suggested to be critically important for maintaining free ubiquitin levels and for the function of the ubiquitin proteasome system in neurons (2). We therefore analyzed the levels of mUB and polyubiquitin in total brain lysates and specific brain areas with age. In whole brain lysates of wild-type mice, levels of mono- and polyubiquitin decreased with age (SI Appendix, Fig. S6), suggesting a pronounced need for ubiquitination in brain development. To our surprise, mUB levels in whole brain lysates were unchanged in 5-d-old and 3- and 20-wk-old Uch-l1+/d and Uch-l1d/d mice; however, levels of polyubiquitinated proteins were drastically decreased in 3-wk-old Uch-l1d/d brains compared with Uch-l1+/+ mice and remained lower compared with Uch-l1+/+ mice upon aging (Fig. 2A). Uch-l1+/d brains showed a significant reduction of pUB only at 20–28 wk of age compared with Uch-l1+/+ mice. Investigation of specific brain areas showed that mUB levels were slightly reduced only in the brainstem of 3-wk-old Uch-l1d/d mice in comparison with Uch-l1+/+ littermates (Fig. 2B). With aging, mUB levels additionally decreased in cerebrum and spinal cord of 25-wk-old Uch-l1d/d mice compared with wild-type littermates (Fig. 2B), correlating with the magnitude of UCH-L1 expression in the five brain areas studied (SI Appendix, Fig. S7A). In Uch-l1+/d mice, mUB levels were comparable to Uch-l1+/+ mice at 3–5 wk and decreased at 20–28 wk in the cerebrum compared with Uch-l1+/+ mice (SI Appendix, Fig. S7B). Further, mUB levels did not differ between primary cultured neurons of Uch-l1+/+, Uch-l1+/d, and Uch-l1d/d littermates (Fig. 2C), suggesting that UCH-L1 is not required for the initial maintenance of the mUB pool in young brains. Measurement of overall UCH hydrolase activity exhibited only a 5% reduction of UCH activity in 3-wk-old Uch-l1d/d brains (Fig. 3A). Since other UCH enzymes could be up-regulated in young brains to stabilize the mUB pool in the situation of UCH-L1 loss, we searched for a compensatory up-regulation of other DUBs. However, no increase of the other main UCH family members UCH-L3, UCH-L5, or UCH-X4 transcripts was observed (SI Appendix, Fig. S8), suggesting either functional compensation by other UCH enzymes or that UCH activity is not the predominant function of UCH-L1 in the brain at the analyzed time points. We thus analyzed the activity of other DUBs using the UB-VME activity-based probe. No strong activation of other UCH enzymes was noted in 4- or 20-wk-old Uch-l1d/d brains, except for a slight enhancement of USP14 activity (Fig. 3B). Furthermore, proteomic analyses of postnatal day 5 brains exhibited down-regulation or no change of most deubiquitinating enzymes, as opposed to the strong regulation of UCH-L1 by knockout (Fig. 3C and SI Appendix, Fig. S9). Some E1, E2, and E3 enzymes such as UbE2r2, UBE2h, RNF40, and Smurf1 were up-regulated in Uch-l1d/d mice in comparison with Uch-l1+/+ littermates. Taken together, the data indicate that UCH-L1 loss is not compensated by other DUBs, and that the loss in brain mUB occurs when neurodegeneration is already advanced, suggesting that the reduced mUB level in neurons is one of the effects and not the cause of the neurodegenerative processes in Uch-l1d/d mice. On the other hand, with advanced neurodegeneration, UCH-L1 deficiency resulted in an accumulation of polyubiquitinated proteins in 25-wk-old cerebella predominantly in the white matter, as well as in cultured Uch-l1d/d neurons (SI Appendix, Fig. S10), indicating a complex time- and brain area-dependent perturbation of protein homeostasis in UCH-L1 deficiency.
Fig. 2.
UCH-L1–deficient mice exhibit an age- and brain area-dependent reduction in monoubiquitin. (A) Western blot (WB) for monoubiquitin (mUB) and polyubiquitin (pUB) in Uch-l1+/d, Uch-l1d/d, and Uch-l1+/+ brain lysates of postnatal day 5 (P5), 3-wk-old (W3), and 20-wk-old (W20) mice. Densitometric quantification; 4 exp. n = 8–17; *P < 0.05, **P < 0.01 to Uch-l1+/+. (B) WB for mUB in five distinct brain areas of 3- and 25-wk-old mice. Graphs exhibit densitometric analysis; 3 exp. n = 5–8; *P < 0.05. (C) WB for mUB and UCH-L1 in cultured neurons from Uch-l1+/d, Uch-l1d/d, and Uch-l1+/+ littermates. Densitometric quantification; 4 exp. n = 7–8.
Fig. 3.
UCH-L1 deficiency is not significantly compensated by other DUBs. (A) UCH-based activity assay in brain lysates of 3- to 5-, 7- to 9-, and 20- to 25-wk-old Uch-l1d/d measures the hydrolysis of AMC from the synthetic substrate Ub-AMC by UCH deubiquitinating enzymes; n = 4–10; *P > 0.05 to Uch-l1+/+. (B) Ubiquitin-derived activity-based assay in brain lysates of 4- or 20-wk-old Uch-l1d/d or Uch-l1+/+ using the ubiquitin-vinylmethylester probe (HA-Ub-VME), which covalently binds to the active site (Cys) of DUBs. WB for the HA-tag exhibits active UCH-L1 with bound HA-Ub-VME probe at 38 kDa, which is absent in the Uch-l1d/d lysates. The other bands correspond to other active deubiquitinating enzymes as published (57); n = 3–5. (C) Proteome analyses of postnatal day 5 Uch-l1+/+, Uch-l1+/d, and Uch-l1d/d brains. Heat map shows extracted normalized label-free quantification (LFQ) intensities of all proteins assigned with deubiquitinase (DUB) activity or protein-ubiquitin ligase activity. The mean of each genotype is presented; n = 3. Significant alterations (FDR < 0.05, after correction for multiple testing) were UCH-L1 and HECTD1. The majority of DUB showed a decreased trend with knockout.
Proteasomal Activity Is Enhanced in Young UCH-L1–Deficient Mice Followed by an Age-Dependent Decline.
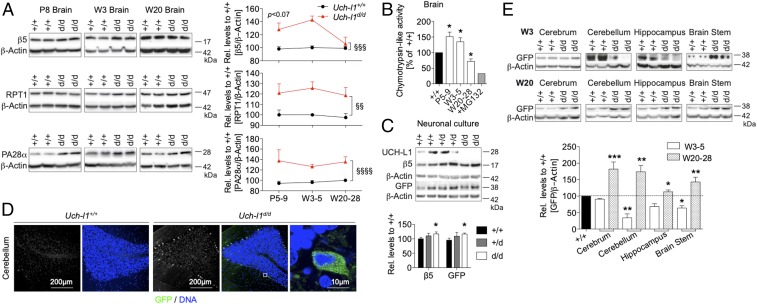
UCH-L1 deficiency resulted in accelerated postnatal reflexes with decreased levels of polyubiquitinated proteins in juvenile mice, followed by motor degeneration and an abnormal accumulation of polyubiquitinated proteins in old adult mice. The proteasome is integral to protein homeostasis since it degrades misfolded, damaged, or aggregated proteins. Also, proteasome function declines with age and neurodegeneration (15). We therefore hypothesized that the abnormal levels of polyubiquitinated proteins in UCH-L1–deficient brains might indicate altered proteasome activity, with decreased levels of polyubiquitin relating to enhanced proteasomal degradation of polyubiquitinated proteins. To examine whether this was the case, we quantified levels, composition, and overall activity of the 26S proteasome to evaluate how the proteasome is affected by loss of UCH-L1 with age. Protein levels of the 20S core component β5, which harbors the chymotrypsin-like activity, were elevated up to 3 wk of age in Uch-l1d/d mice (Fig. 4A). Levels of the 19S regulatory cap protein RPT1 and PA28α, a constituent of the alternative PA28 regulatory cap, were also up-regulated in Uch-l1d/d brains (Fig. 4A). These data suggest that UCH-L1 deficiency results in an up-regulation of proteasomal levels. Chymotrypsin-like activity, representing the main proteolytic activity of the proteasomal 20S core unit was increased in 5- to 8-d-old and 3- to 5-wk-old Uch-l1d/d brains compared with Uch-l1+/+ littermates (Fig. 4B). In contrast, chymotrypsin-like activity was significantly reduced in 20- to 28-wk-old Uch-l1d/d brains, suggesting that the primary effect of early increases in proteasome levels and activity is followed by secondary late onset decline of proteasome function with age under UCH-L1 deficiency. To assess whether the observed decrease in chymotrypsin-like activity of the proteasome in old Uch-l1d/d brains reflected a reduction in protein degradation by the ubiquitin/proteasome system (UPS), we used UBG76V-green fluorescent protein (GFP) transgenic mice (21) in which GFP is tagged to a constitutively active proteasomal degradation signal (UBG76V), leading to polyubiquitination and proteasomal degradation of GFP (21). We crossed this line to the Uch-l1d/d or Uch-l1+/+ background to assess the UPS status in Uch-l1d/d brains. Our results revealed an accumulation of UBG76V-GFP protein in isolated primary culture neurons of Uch-l1d/d mice, despite an increase in proteasome levels as indicated by β5 levels (Fig. 4C), and in the cerebrum, cerebellum, hippocampus, and brainstem of 20- to 28-wk-old UCH-L1–deficient mice (Fig. 4 D and E). Immunofluorescence for GFP showed that UBG76V-GFP accumulation was confined to neurons in respective brain areas (Fig. 4D and SI Appendix, Fig. S11). Of note, UBG76V-GFP accumulation was not apparent in 3- to 5-wk-old brain areas of Uch-l1d/d mice (Fig. 4E). At 3 wk of age, UBG76V-GFP protein levels were comparable (cerebrum and hippocampus) or decreased (cerebellum and brainstem) in comparison with Uch-l1+/+ littermates, possibly reflecting an enhanced removal of UBG76V-GFP through the elevated proteasomal activity. Taken together, these results indicate that proteasomal removal of target proteins is impaired in aged UCH-L1–deficient mice, following a phase of enhanced removal in juvenile mice.
Fig. 4.
Proteasomal activity is enhanced in young UCH-L1–deficient mice followed by an age-dependent decline. (A) WB for the 20S core proteasomal subunit β5, which carries the chymotrypsin-like activity of the proteasome, of the regulatory 19S cap protein RPT1 and of the PA28α regulatory cap protein exhibits a postnatal and persistent up-regulation of proteasomal capacity in brain lysates of postnatal day 8 (P8), week 3 (W3), and week 20 (W20) Uch-l1d/d and Uch-l1+/+. Right graphs exhibit densitometric quantifications; 3 exp. n = 5–9; §§P < 0.01, §§§P < 0.001, §§§§P < 0.0001 (effect of genotype by two-way ANOVA). (B) Chymotrypsin-like activity of the proteasome; n = 5; *P > 0.05 to Uch-l1+/+. 10 µM MG132 was added to the brain lysate as an assay control. To assess proteasome function, UCH-L1–deficient mice were crossed to UBG78V-UB transgenic mice. (C) WB for UCH-L1, β5, and UBG76V-GFP in primary neurons from Uch-l1+/+, Uch-l1+/d, and Uch-l1d/d. Uch-l1d/d UBG76V-GFP neurons exhibit GFP accumulation despite increased proteasome levels; 3 exp. n = 7–10; *P < 0.05 to Uch-l1+/+. (D) Confocal images of UBG78V-GFP (green; DNA = blue) in the cerebellum of 20-wk-old Uch-l1d/d and Uch-l1+/+ UBG76V-GFP transgenic littermates exhibit GFP accumulation in neurons of Uch-l1d/d mice. (E) WB for UBG78V-GFP accumulation in four distinct brain areas of 3-wk- and 20-wk-old Uch-l1d/d versus Uch-l1+/+ UBG76V-GFP littermates, graph demonstrates densitometric quantification; 2 exp. n = 8–10; *P < 0.05; **P < 0.01; ***P < 0.001 to Uch-l1+/+.
Protein Synthesis Is Enhanced in UCH-L1–Deficient Neurons.
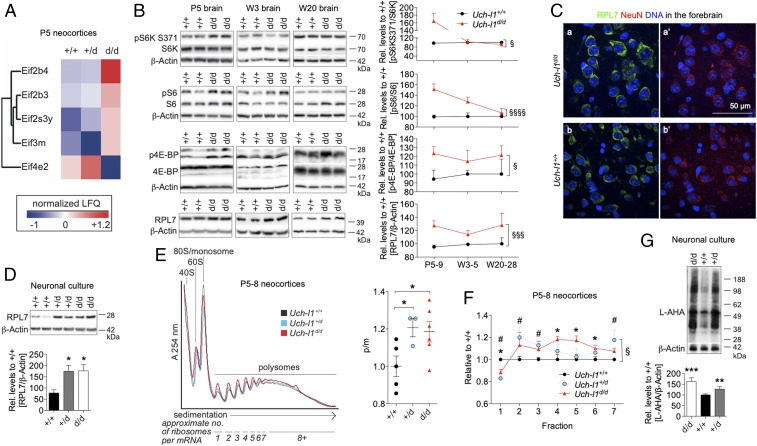
Protein homeostasis regulates the concentration, localization, and interactions of proteins from synthesis to degradation. We observed that proteasomal degradation was enhanced in young mutant brains and wondered whether this was necessary to balance for enhanced protein synthesis. We established that S6 ribosomal protein phosphorylation (as a measure of global protein synthesis activity) is highest in postnatal brains of wild-type mice, significantly decreasing with age (SI Appendix, Fig. S12A) as expected (22, 23). To assess whether protein synthesis was enhanced in the postnatal period of Uch-l1d/d mice, we interrogated the newly generated proteomics dataset for significantly changed proteins defined as log2 fold change >1 (+/+ versus d/d) and a false discovery rate (FDR) of 0.2 after correction for multiple testing. We found an increase of several activating eIF members, of Eif2 and -3, a key complex in activation of translation. In addition, Eif4e2, a described repressor of translational activation (24), was most strongly decreased (Fig. 5A). Time course analyses of the kinases involved in transcriptional activation such as the p70 ribosomal S6 protein kinases 1 and 2 (S6K1/2), ribosomal protein S6 (S6), which is phosphorylated by S6K1/2 (25), and the eukaryotic initiation factor 4E-binding proteins (4E-BPs) demonstrated enhanced phosphorylation levels of all three, especially in postnatal Uch-l1d/d brains in comparison with wild-type littermates (Fig. 5B). Of note, phosphorylated levels of S6K and S6 were significantly elevated in both Uch-l1+/d and Uch-l1d/d postnatal brains compared with Uch-l1+/+ mice (SI Appendix, Fig. S12B). However, 20- to 25-wk-old UCH-L1–deficient brains did not show increased pS6K/S6K, pS6/S6, or p4E-BP/4E-BP levels in comparison with Uch-l1+/+ littermates (Fig. 5B), suggesting that UCH-L1 deficiency profoundly affects protein synthesis in the postnatal period when pS6/S6 expression is at its highest. Corroborating the finding of enhanced activation of proteins regulating protein translation, the ribosomal protein RPL7 associated with enhanced ribogenesis was increased in Uch-l1d/d brains (Fig. 5B), especially in neurons, a finding confirmed by Western blotting for RPL7 in UCH-L1–deficient cultured primary neurons (Fig. 5D) and by staining for RPL7 in forebrain neurons of 3-wk-old Uch-l1d/d mice (Fig. 5C) compared with wild-type littermates. To examine the impact of UCH-L1 deficiency on translation in the brain in vivo, we performed polysome profiling of neocortices from Uch-l1+/d, Uch-l1d/d mice and littermate controls (Fig. 5 E and F). Quantitative analysis of the polysome to monosome ratio (P/M) revealed a mild but significant increase in Uch-l1+/d and Uch-l1d/d (Fig. 5E). Moreover, we also detected a significant shift of ribosomes from lighter to heavier polysomal regions in Uch-l1+/d and Uch-l1d/d brains (Fig. 5F). Thus, more ribosomes are engaged with mRNAs in Uch-l1+/d and Uch-l1d/d neocortices, consistent with increased mRNA translation in brains deficient for UCH-L1 function. To further assess the rate of protein synthesis in neurons, we monitored incorporation of the methionine analog 4-azido-l-homoalanine HCl (l-AHA) in a Click-iT-SPAAC (strain-promoted azide alkyne click chemistry) reaction with primary neurons. Following a pulse of 1 h with l-AHA, both Uch-l1d/d and Uch-l1+/d neurons incorporated significantly more l-AHA than neurons derived from Uch-l1+/+ littermates, showing that the absence of UCH-L1 promoted increased protein synthesis (Fig. 5G).
Fig. 5.
Protein synthesis is enhanced in UCH-L1–deficient neurons. (A) Heat map of significantly changed eIF (eukaryotic translation initiation) subunit protein expression determined by proteomic analysis. Proteins of the eIF complex quantified with fold change of log2(d/d/control) or log2(d/+/control) larger 1 and FDR < 0.2, means per genotype; n = 3. (B and D) WB for the activation [phosphorylation (p)] of kinases involved in transcriptional activation such as the p70 ribosomal S6 protein kinases (S6K), ribosomal protein S6 (S6), the eukaryotic initiation factor 4E-binding proteins (4E-BPs), and for the large ribosomal subunit RPL7 in (B) postnatal day 5 (P5), 3-wk-old (W3), and 20-wk-old (W20) brain lysates of Uch-l1d/d and Uch-l1+/+ and in (D) cultured neurons. Right graphs demonstrate densitometric quantification in B 3 exp. n = 5–9; §P < 0.05, §§§P < 0.001, §§§§P < 0.0001 (effect of genotype by two-way ANOVA). In D 3 exp. n = 5–9; *P < 0.05 (Bonferroni test after one-way ANOVA). (C) Confocal images for ribosomal RPL7 (a and b), which localizes to NeuN positive neurons (a′ and b′) in frontal cortical neurons of 3-wk-old Uch-l1d/d and Uch-l1+/+. (E) Overlay of polysome profiles from neocortices of Uch-l1+/+, Uch-l1+/d, and Uch-l1d/d postnatal day 5. Graph: normalized polysome-to-monosome (p/m) ratio for Uch-l1+/d and Uch-l1d/d vs. Uch-l1+/+ neocortices; 3 exp. n = 3–6; *P < 0.05. (F) Bin analysis of changes in polysome fractions; 3 exp. n = 3–6; #P < 0.05 Uch-l1+/+ versus Uch-l1+/d and *P < 0.05 Uch-l1+/+ versus Uch-l1d/d at the indicated fraction, §P < 0.05 (effect of genotype x fraction, mixed two-way ANOVA). Note the significant shift of ribosomes from lighter to heavier polysomes in Uch-l1+/d and Uch-l1d/d. (G) Measurement of new protein synthesis rate in primary neurons by incorporation of the methionine analog l-AHA HCl during a 1-h pulse. Densitometric quantification of l-AHA incorporation; 3 exp. n = 8–14; **P < 0.01; ***P < 0.01 to Uch-l1+/+.
Alteration of Protein Homeostasis Strains UCH-L1–Deficient Neurons.
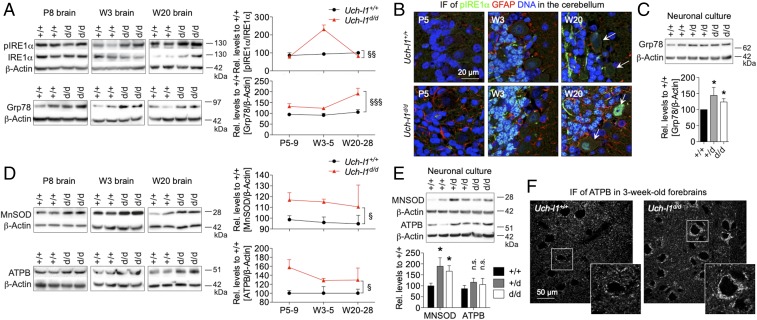
Accumulation of misfolded proteins in the endoplasmic reticulum (ER) as in the setting of increased protein synthesis, leads to a condition referred to as ER stress. To counteract the accumulation of misfolded proteins, cells activate the serine/threonine kinase IRE1α to up-regulate molecular chaperones such as Grp78 via activation of the transcription factor X-box binding protein (26). Grp78 is situated in the ER lumen (27) where it is involved in the folding of newly synthesized proteins (28) and in the retrograde transport of aberrant proteins across the ER membrane for degradation by the proteasome (29). Western blot analyses exhibited a significant activation of IRE1α in brain lysates (Fig. 6A) of 3- to 5-wk-old Uch-l1d/d mice versus Uch-l1+/+, whereby immunofluorescence analyses confirmed enhanced pIRE1α expression in neurons, as shown exemplarily for the cerebellum (Fig. 6B). In line with these observations, Western blot analyses exhibited an early and persistent induction of Grp78 in brains and cultured neurons of Uch-l1d/d mice, compared with wild-type littermates (Fig. 6 A and C). Together, these findings support the occurrence of ER stress and the involvement of UCH-L1 in regulation of protein synthesis in neurons at the age of 3 wk, shortly before the first symptoms are observed in 5-wk-old UCH-L1–deficient mice. Since processes such as protein synthesis and proteasomal degradation highly depend on sufficient ATP levels, we searched for further signs of neuronal stress due to energy depletion or reactive oxidative species. Whereas the mitochondrial membrane ATP synthase subunit beta (ATPB) is involved in ATP production from ADP, the manganese superoxide dismutase (MnSOD) scavenges reactive oxygen species (ROS) in stress-induced diseases. Time course analyses exhibited elevated levels of both ATPB and of MnSOD, which were most pronounced in postnatal days 5–9 and 3- to 5-wk-old Uch-l1d/d brains (Fig. 6D). This finding was corroborated in primary culture Uch-l1d/d neurons, in which MnSOD levels were significantly enhanced and ATPB levels slightly up-regulated in comparison with primary culture Uch-l1+/+ neurons (Fig. 6E). Immunofluorescent analyses exhibited an enhanced signal for ATPB in neurons, as demonstrated in neurons of the forebrain (Fig. 6F). Taken together these results indicate enhanced neuronal strain in the form of ATP depletion and ER stress in UCH-L1–deficient mice secondary to accelerated protein synthesis and degradation.
Fig. 6.
UCH-L1 defiency results in neuronal strain. Uch-l1d/d brains were analyzed for the occurrence of ER stress and for signs of energy depletion. (A) WB for the activation [phosphorylation (p)] of the sensor of unfolded proteins in the lumen of the ER, IRE1α, and of the molecular chaperone Grp78 in brain lysates of postnatal day 8 (P8), 3-wk-old (W3), and 20-wk-old (W20) Uch-l1d/d in comparison with Uch-l1+/+. Right graphs exhibit densitometric quantifications; 3 exp. n = 5–9; §§P < 0.01, §§§P < 0.001 (effect of genotype by two-way ANOVA). (B) Confocal images of pIRE1α (green), glial fibrillary acidic protein (GFAP, red), and DNA (blue) in cerebella of Uch-l1d/d in comparison with Uch-l1+/+ mice. Arrows: Purkinje cells. Note the enhanced signal for pIRE1α in the stratum granulosum in 3- and 20-wk-old Uch-l1d/d cerebella. (C) WB for Grp78 levels in primary neurons of Uch-l1d/d in comparison with Uch-l1+/+. Lower graph exhibits densitometric quantifications; 3 exp. n = 5–9; *P < 0.05 to Uch-l1+/+. WB for the mitochondrial membrane ATP synthase (ATPB) and the detoxifying enzyme manganese superoxide dismutase (MnSOD) in (D) brain lysates of postnatal day 8 (P8), 3-wk-old (W3), and 20-wk-old (W20) Uch-l1d/d; 3 exp. n = 5–15; and (E) in primary neurons of Uch-l1d/d in comparison with Uch-l1+/+. Graphs (D and E) exhibit densitometric quantifications; 3 exp. n = 8; §P < 0.05; *P < 0.05 to Uch-l1+/+, n.s., not significant. (F) Confocal images for ATBP in forebrain neurons of 3-wk-old Uch-l1d/d mice in comparison with Uch-l1+/+ mice. Arrows: Purkinje cells, note the enhanced signal for ATBP in Uch-l1d/d neurons.
mTORC1 Inhibition Restricts Protein Synthesis and Ubiquitin Accumulation in UCH-L1–Deficient Neuronal Cultures.
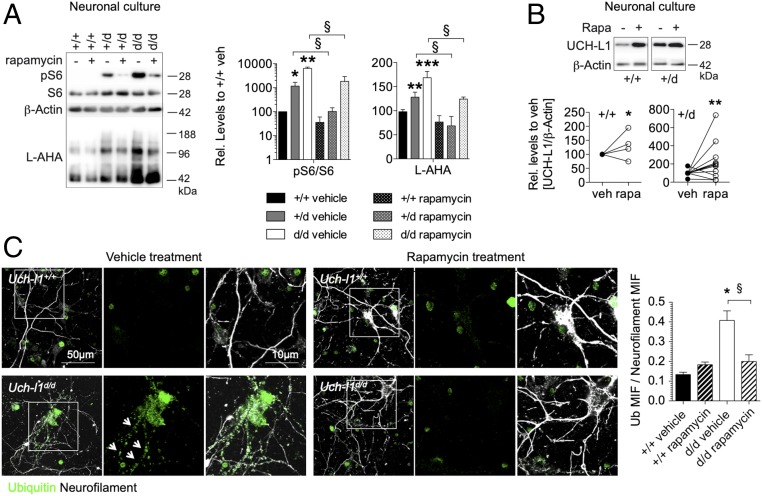
To address how UCH-L1 deficiency might affect protein synthesis, and whether suppression of enhanced protein synthesis could ameliorate the phenotype of UCH-L1–deficient mice, we analyzed activity levels of mammalian target of rapamycin (mTOR). mTOR complexes with raptor to form the mTOR complex 1 (mTORC1) or with rictor to form the mTOR complex 2 (mTORC2). During growth factor and nutrient sufficiency, mTORC1 phosphorylates S6K, S6, and 4E-BP to coordinately up-regulate protein translation and biosynthesis (25). In comparison with Uch-l1+/+ littermates, Uch-l1d/d brains exhibited enhanced phosphorylation and thereby activation of mTOR exclusively during the postnatal period (SI Appendix, Fig. S13A). Furthermore, levels of raptor but not rictor were enhanced in postnatal day 5 and 3- to 5-wk-old Uch-l1d/d brains (SI Appendix, Fig. S13B). This result suggests an enhanced activity of mTOR through mTORC1 rather than mTORC2 and corroborates our finding of enhanced phosphorylation of mTORC1 downstream targets S6K, S6, and 4E-BP phosphorylation demonstrated in Fig. 5A. Given the findings of enhanced mTORC1 activity in the postnatal period, we hypothesized that inhibition of mTORC1 activity could be an effective measure to dampen the postnatally enhanced protein synthesis and resultant neuronal strain and neurodegeneration in Uch-l1d/d brains. First, rapamycin, a mTORC1 inhibitor which acutely inhibits mTORC1 signaling activity (30), was used to treat primary cultured neurons. Indeed, as in vivo, pS6/S6 levels were significantly elevated both in Uch-l1+/d and Uch-l1d/d primary culture neurons with a concomitant increase in protein synthesis as shown by l-AHA incorporation (Fig. 7A). Rapamycin treatment reduced levels of pS6 in Uch-l1+/d and Uch-l1d/d primary culture neurons with a concomitant reduction in protein synthesis (Fig. 7A). Notably, rapamycin treatment increased UCH-L1 protein in Uch-l1+/+ and Uch-l1+/d neurons (Fig. 7B), suggesting that mTORC1 inhibition by rapamycin can also induce protein synthesis of particular proteins such as UCH-L1 in the regulation of protein synthesis. Analysis of ubiquitin staining on neurons showed strong ubiquitin accumulation in Uch-l1d/d neurons, which was reduced following rapamycin treatment. Also, neurofilament staining was ameliorated (Fig. 7C). Together these findings indicate that reduction of unbalanced protein synthesis in UCH-L1 deficiency restores protein homeostasis and prevents accumulation of polyubiquitinated proteins in primary culture neurons.
Fig. 7.
mTORC1 inhibition by rapamycin reduces protein synthesis and ubiquitin accumulation in Uch-l1d/d cultured neurons. Primary neurons were treated for 1 h with rapamycin (rapa) or vehicle (veh). (A) Measurement of new protein synthesis rate by incorporation of the methionine analog l-AHA during a 1-h pulse and WB for biotin; pS6 to S6 ratio to control for mTORC1 inhibition. Right graph exhibits densitometric quantification of l-AHA incorporation; 4 exp. n = 4–22; *P < 0.05; **P < 0.01; ***P < 0.001 to Uch-l1+/+ vehicle, §P < 0.05 to Uch-l1+/d or to Uch-l1d/d vehicle. (B) WB for UCH-L1. Densitometric analyses exhibit the change of UCH-L1 protein levels with rapamycin treatment in individual (paired) neuronal cultures from Uch-l1+/+ or Uch-l1+/d littermate embryos; 2 exp. n = 4–9; *P < 0.05; **P < 0.01. (C) Confocal images for ubiquitin (green) and neurofilament (white) in primary neurons of Uch-l1d/d and Uch-l1+/+ with or without treatment with rapamycin or vehicle for 24 h. Arrows point toward ubiquitin aggregates in neurofilament positive processes. Graph exhibits quantification of mean intensity of fluorescence (MIF) of ubiquitin to neurofilament; 2 exp. n = 6; *P < 0.05 to Uch-l1+/+ vehicle; §P < 0.05 to Uch-l1d/d rapamycin.
Postnatal Inhibition of mTORC1 Ameliorates the Neurodegenerative Phenotype of UCH-L1 Deficiency.
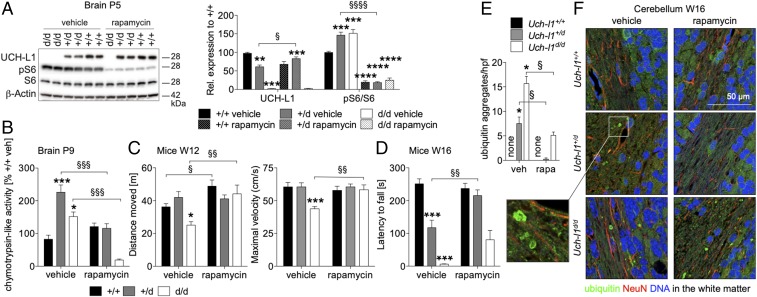
Finally, we addressed whether dampening protein synthesis by rapamycin treatment could also block UCH-L1–dependent motor degeneration in vivo. Postnatal days 1–8 treatment of litters from heterozygous breeding pairs with rapamycin reduced pS6/S6 levels of all three genotypes below untreated Uch-l1+/+ levels (Fig. 8A), demonstrating successful mTORC1 inhibition. As in cultured neurons, rapamycin treatment significantly elevated UCH-L1 protein levels in Uch-l1+/d mice to almost Uch-l1+/+ protein levels (Fig. 8A). Postnatal reflexes were normalized in Uch-l1+/d and Uch-l1d/d mice following treatment with rapamycin (SI Appendix, Fig. S14). We next evaluated whether rapamycin treatment ameliorated the molecular phenotype of Uch-l1+/d and Uch-l1d/d mice. Chymotrypsin-like activity of the proteasome was significantly reduced in brains of Uch-l1+/d and Uch-l1d/d mice at the termination of rapamycin treatment on postnatal day 9 (Fig. 8B), suggesting that rapamycin treatment reduced the need for enhanced proteasomal activity caused by UCH-L1 deficiency, as the burden of protein synthesis was reduced. Finally, we analyzed the effects of postnatal rapamycin treatment on motor function in adult mice using the open field and accelerated rotarod tests in 12- and 16-wk-old mice, respectively. Rapamycin treatment rescued motor deficits of Uch-l1d/d mice in the open field (Fig. 8C) and ameliorated the performance of Uch-l1d/d and Uch-l1+/d mice on the accelerated rotarod (Fig. 8D). Morphologically, postnatal rapamycin treatment significantly ameliorated the extent of ubiquitin aggregates in the white matter of the cerebellum by immunofluorescence when analyzed in 16-wk-old Uch-l1+/d and Uch-l1d/d mice (Fig. 8 E and F). Taken together, these data show that postnatal dampening of mTOR activity ameliorates the neurological phenotype of UCH-L1 deficiency.
Fig. 8.
Postnatal rapamycin treatment ameliorates neurodegenerative phenotype of UCH-L1–deficient mice. Litters from Uch-l1+/d females crossed with Uch-l1+/d males were treated on a daily basis with rapamycin (0.1 mg/kg) or vehicle (NaCl/ethanol) from postnatal day 1–8. Thereafter, treatment was stopped. (A) WB for the phosphorylation (p) status of mTORC1 substrate S6 ribosomal protein (S6) in brains from one litter. Graph demonstrates densitometric quantification; 4 litters n = 6–22; **P < 0.01, ***P < 0.001, ****P < 0.0001 to Uch-l1+/+; §P < 0.05, §§§§P < 0.0001 to Uch-l1+/d vehicle. (B) Chymotrypsin-like activity of the proteasome in brain lysates postnatal day 9 (P9), 24 h after termination of short-term treatment with rapamycin or vehicle; 3 litters n = 3–11; *P < 0.05 and ***P < 0.001 to Uch-l1+/+ vehicle; §§§P < 0.001 genotype comparison. (C) Measurement of the distance moved in the open field test and the maximal velocity performed at 12 wk of age, demonstrating partially rescued phenotype in Uch-l1d/d mice; 5 litters n = 5–17; *P < 0.05, ***P < 0.001 to Uch-l1+/+ vehicle; §P < 0.05; §§P < 0.01 genotype comparison. (D) Accelerated rotarod tests performed at 16 wk of age, demonstrating rescued neurological phenotype in Uch-l1+/d mice, 5 litters n = 2–15; ***P < 0.001 to Uch-l1+/+ vehicle, §§P < 0.01 to Uch-l1+/d vehicle. (E and F) Ubiquitin aggregates within the cerebellar white matter were quantified (E) and visualized (F) by immunofluorescent staining for ubiquitin (green), glial fibrillary acidic protein (GFAP, red), and DNA (blue). Graph exhibits quantification of five high power fields (hpf) of n = 3 mice per genotype and treatment; *P < 0.05 to vehicle Uch-l1+/+; §P < 0.05 to respective vehicle-treated genotype.
Discussion
UCH-L1 is one of the most abundant and enigmatic enzymes of the CNS. As a synopsis of findings based on existing spontaneous UCH-L1 knockout models, UCH-L1 is thought to be required for the maintenance of axonal integrity, but not for neuronal development, despite its high expression in neurons (6). We generated a new transgenic mouse model to investigate the mechanisms underlying neurodegeneration due to UCH-L1 deficiency. Based on our data we propose that UCH-L1 has a previously unrecognized role in neuronal development. By performing biochemical and behavioral analyses at pre- and postsymptomatic time points, we can show that UCH-L1 deficiency results in enhanced neuronal protein synthesis during the first postnatal weeks, leading to ER stress, energy depletion, proteasomal impairment with accumulation of nondegraded ubiquitinated protein, and neurodegeneration starting around the fifth postnatal week. Strikingly, we found that treatment of mice lacking UCH-L1 with the protein synthesis modulator rapamycin directly after birth could rescue neurological phenotypes later in life.
Our Uch-l1d/d mice show a phenotype of sensory and motor ataxia consistent with that described in gad and nm3419 spontaneous UCH-L1 mutants (11, 12). In contrast to the proposal that neurodegeneration is caused by reduced levels of mUB (10, 12), we found that mUB levels are grossly normal in postnatal, and 3- to 5-wk-old Uch-l1d/d mice and in primary neurons, suggesting that UCH-L1 is not primarily required to maintain the mUB pool in young neurons. UCH-L1’s proposed role in maintaining a pool of ubiquitin in the cytosol is based on its hydrolytic activity to cleave short peptides from the C terminus of ubiquitin in vitro (6), which was minimally reduced in Uch-l1d/d mice. Loss of UCH-L1 did not result in a compensatory up-regulation of other known DUBs. Rather, levels of other DUBs were found to be down-regulated by proteomic analyses. Thus, UCH-L1 is so unique from its structural (and thereby potentially functional) characteristics (31) (quoted as “the most complicated eukaryotic protein structure discovered to date”) (6), that a compensation is not possible by other DUBs. Since reduced levels of mUB could not explain the early behavioral phenotype of the Uch-l1d/d mice, we focused on UCH-L1’s role in other processes of the UPS system.
Protein turnover was increased in total brains of postnatal and 3-wk-old UCH-L1–deficient mice and more specifically in neurons. Support for UCH-L1’s function in controlling protein synthesis comes from recent work examining the role of UCH-L1 in synaptic transmission at neuromuscular junctions (13). In UCH-L1–deficient mice, synaptic activity is impaired, with loss of synaptic vesicles and accumulation of branched tubulovesicular structures at presynaptic nerve terminals (13). Indeed, presynaptic protein synthesis is required for synapse formation and components of the mTOR pathway have been shown to control synaptic bouton size, active zone number, and synaptic function (32, 33). Therefore, it is conceivable that the accumulation of tubulovesicular structures and loss of synaptic vesicles in UCH-L1 deficiency could result from UCH-L1–dependent dysregulation of protein synthesis that may involve both up- and down-regulation of protein synthesis of particular proteins. Intriguingly, we observed enhanced postnatal reflexes in UCH-L1–deficient animals reflecting an UCH-L1–dependent acceleration of neonatal development of sensory and motor capabilities. Currently we do not know how UCH-L1 mechanistically modulates neuronal protein synthesis. The enhanced postnatal activity of mTORC1 in UCH-L1–deficient mice suggests that UCH-L1 modulates protein synthesis through the mTOR pathway. mTORC1 activation can increase protein synthesis of some, yet repress translation of other specific proteins. Thereby deviations to an optimal range of protein synthesis that adapts to demand results in the behavioral impairments that develop with age (34–37). Notably, our data showing that rapamycin treatment increases UCH-L1 protein in Uch-l1+/+ and Uch-l1+/d neurons demonstrates that mTORC1 inhibition can also induce protein synthesis of particular homeostatic proteins, such as UCH-L1, important in the regulation of protein synthesis. Previously it was shown that UCH-L1 regulates mTORC1 stability but not levels (38). In our mice, however, amounts of phosphorylated mTOR and raptor were postnatally elevated, suggesting a different level of mTORC1 regulation. UCH-L1 was further ascribed to affect protein synthesis in B cell malignancy by bypassing mTORC1 through association with the translation initiation complex eIF4F, the downstream target of 4E-BP1 (39). In our mice, however, postnatal amounts of phosphorylated 4E-BP1 were significantly enhanced, and dampening of protein synthesis by postnatal rapamycin treatment attenuated the development of neurodegeneration, pointing toward a regulatory effect of UCH-L1 upstream of mTOR.
The GFP degron mouse reporter was designed to give information about the functional status of the ubiquitin/proteasome system as a whole (21). Using this reporter system, we observed increased protein degradation through the proteasome in 3- to 5-wk-old UCH-L1–deficient animals followed by impaired degradation in week 20 mice. However, it needs to be considered that the observed changes in GFP could be due to changes in synthesis, degradation, or both. Nonetheless, chymotrypsin-like activity measurements of the proteasome supported the finding that GFP levels measured in the degron mouse reflected proteasomal activity. Interestingly, in gad or nm3419 spontaneous UCH-L1 mutants proteasomal activity was not obviously impaired (11, 12). It has however been proposed that UCH-L1 is crucial for maintaining proper proteasomal function (10, 40) and is involved in the determination of proteasomal subtype abundance (41), the underlying mechanism(s) however being completely unknown. Nonetheless, enhanced proteasomal abundance and activity in our Uch-l1d/d mice is in accordance with the function of the UPS system to maintain homeostasis in the face of stress by synthesizing and degrading proteins in a synchronized manner (42–44). Of note, we found UCH-L1–deficient mice to increase their proteasomal capacity by up-regulation of the 26S standard proteasome and of the PA28 proteasome. This is of interest, as the 26S proteasome results in an ATP- and ubiquitin-dependent proteolysis, whereas the PA28 proteasome enables cells to perform ATP- and ubiquitin-independent proteolysis (45), a process shown to be of great importance for cellular stress compensation (46). Of note, also the differential distribution of the proteasome forms in specific brain areas is thought to predispose brain areas to stress susceptibility as a predisposition to neurodegeneration (47, 48). Therefore, the initial up-regulation of proteasomal capacity could reflect the need of UCH-L1–deficient neurons to remove flawed newly synthesized proteins and to deal with cellular stress through energy depletion in face of high ATP-consuming cellular processes.
Consistent with the observation of ER stress and oxidative stress in juvenile mutant brains and cultured primary neurons of our Uch-l1d/d mice, Tan et al. (49) demonstrated in an in vitro cell culture model that pharmacological UCH-L1 inhibition induces ER stress and cell death. Further, nm3419 mice were described to have ER stress at the presymptomatic stages in corticospinal motor neurons, correlating with disintegration of the apical dendrite and spine loss (50). Interestingly, UCH-L1 is proposed to have an antioxidant function in neurons (17, 51), which could explain the up-regulation of the ROS-detoxifying enzyme MnSOD in neurons of our mice lacking UCH-L1 expression. Consistent with this, down-regulation of UCH-L1 in embryonic carcinoma cells resulted in an increased susceptibility to oxygen and glucose deprivation-induced cell death (52) and gad mice showed increased vulnerability to lipid peroxidation (53, 54). Thus, altered protein synthesis caused by UCH-L1 deficiency at very early developmental time points has acute importance for long-term neuronal health, as suggested by the finding of early onset progressive neurodegeneration in humans with missense mutations in the UCH-L1 gene (16). With age, we saw a significant reduction of proteasome activity with concomitant accumulation of polyubiquitinated proteins, suggesting that prolonged dysregulated protein synthesis with accompanying proteolysis, ER stress, and energy depletion are toxic for neurons. In line with this hypothesis, motor neurons could be particularly susceptible to UCH-L1 loss, as the high energy and protein turnover burdens required to maintain extensive axonal projections imply that motor neurons operate very close to their maximum capacity rendering them vulnerable to defects that other neuronal types might withstand longer. This idea is supported by our finding that reducing protein synthesis by rapamycin treatment during the first postnatal week ameliorates the neurological phenotype and ubiquitin accumulations in Uch-l1+/d and Uch-l1d/d mice up to 16 wk of age.
In conclusion, our study describes the temporal sequence of effects caused by UCH-L1 deficiency yielding insights into the mechanisms underlying neurodegeneration caused by UCH-L1 loss of function. Moreover, we show that a 50% reduction in UCH-L1 can have significant effects at the neuronal and behavioral levels, establishing the importance of UCH-L1 levels in neuronal development and neurodegeneration. Finally, our study reveals that UCH-L1 deficiency affects the ability to regulate translational activity during early synapse development and that this can be pharmacologically addressed, a finding of major importance for patients with juvenile onset neurodegeneration due to mutations in UCH-L1 (16). This result has potential implications beyond UCH-L1 deficiency: timing prophylactic interventions to specific windows may be generally useful for the prevention of neurodegenerative processes.
Materials and Methods
Detailed materials and methods are within SI Appendix.
Generation of UCH-L1 Knockout Mice.
UCH-L1 floxed mice were generated by Genoway (SI Appendix, Fig. S1A), bred to constitutive Cre deleter mice, and backcrossed for 12 generations to the C57BL/6 background. Littermates born from heterozygous parents were used. For rapamycin treatment, pups were treated daily with rapamycin (0.1 mg/kg) or NaCl/ethanol on postnatal days 1–8 between 9 AM and 10 AM. Animal experiments were carried out in accordance with the European Community Council Directive (86/609/EEC), and the procedures used were approved by the State of Hamburg (approval 75/14).
Behavioral Studies.
Offspring were examined for postnatal reflexes, and in adolescence and adulthood for motor coordination (open field and accelerated rotarod test) and strength of the forelimbs (grip strength test). All tests were performed during the dark cycle of the mice under red light.
Primary Neuronal Culture.
Brains from E15.5–E16.5 embryos, arising from heterozygous breeding pairs were isolated and the cerebellum, brainstem, and meninges were removed. The remaining cortex was digested and mechanically dissected. A total of 750,000 cells were plated to grow and differentiate for 7 d. For mTORC1 inhibition, neurons were treated for 1 h with 20 nM rapamycin or dH2O as vehicle.
Ubiquitin-Derived Activity-Based DUB Activity Assay.
The ubiquitin-derived activity-based ubiquitin-vinylmethylester probe (HA-UB-VME), which is an active site-directed probe with broad reactivity toward DUBs, with the exception of few metalloproteases, binds covalently to the active-site (Cys) of most DUBs, which is only accessible in active enzymes. The N-terminal epitope tag hemagglutinin (HA tag) allows detecting the modified DUBs after the separation of a protein mixture by SDS/PAGE, followed by immunoblotting.
Chymotrypsin-Like Activity Assay.
A total of 10 µg total protein was diluted in incubation buffer. After preincubation, the substrate for chymotrypsin-like activity Suc-LLVY-7-amino-4-methylcoumarin (AMC) (Calbiochem, EMD Chemicals, Inc, division of Merck KGaA) was added to the samples at a final concentration of 60 µmol/L. Proteasomal activity was measured at 355 and 460 nm after incubation at 37 °C for 1 h in the dark. MG132 at a concentration of 10 µmol was added to brain lysate as an assay control.
UCH Activity Assay.
Briefly, 10 μg of total brain extract was diluted into UCH reaction buffer. To start the reaction, ubiquitin-AMC was added to a final concentration of 400 nM. Fluorescence intensity was measured at 380 nm and 460 nm at 37 °C and recorded at 1-min intervals for 30 min.
Proteomics.
Snap-frozen, unperfused neocortex from postnatal day 5 Uch-l1+/+, Uch-l1d/+, and Uch-l1d/d mice was homogenized in urea buffer followed by tryptic digestion. Peptides were cleaned up by Stagetips, separated by nLC MS-MS on a 50-cm self-packed C18 fused silica column, and analyzed using a Quadrupole-Orbitrap hybrid mass spectrometer. The raw data and processed mass spectrometry data of this study have been be uploaded to the PRIDE/ProteomExchange database (55, 56) with the dataset identifier PXD010449.
Polysome Profiling and Analysis.
Neocortices were lysed and centrifuged, the resulting supernatants were supplemented with Nonidet P-40 and Triton X-100 (both to 1%), and incubated on ice for 5 min. After centrifugation, sucrose gradients were generated using the Gradient Master 108 programmable gradient pourer. Gradients were fractionated and measured for RNA content using a Piston Gradient Fractionator attached to a UV monitor.
Measurement of Protein Synthesis by Click-iT SPAAC Reaction.
l-AHA HCl (2 mM) was added for 1 h in methionine-free culture DMEM before harvesting. The conjugation reaction was performed by adding DBCO-PEG12-biotin (200 µM final) to the cell extract (10 µL) and incubated for 1 h at 37 °C and analyzed by SDS/PAGE.
Immunohistochemistry and Immunofluorescence.
Antigen retrieval was performed by microwave boiling (10 mM citrate buffer, pH 6.1) or by protease digestion [protease XXIV (Sigma) 5 µg/mL]. Unspecific binding was blocked [5% horse serum (Vector), 30 min at room temperature (RT)]. Antibody incubations of primary antibodies were followed by incubation with biotinylated or AF488 or Cy3-coupled secondary antibodies (1:400, 30 min, RT). Color development was performed with the ZytoChem-Plus AP Polymer Kit (Zytomed) according to the manufacturer’s instructions with neufuchsin. For immunofluorescence, cultured neurons were fixed with 4% PFA for 8 min at RT. Unspecific binding was blocked and 1° antibody incubations (blocking buffer overnight, 4 °C) were followed by incubation with biotinylated or AF488 or Cy3-coupled 2° antibodies.
Electron Microscopy.
Mice were transcardially perfused (4% PFA, 1% glutaraldehyde in 0.1 M PB buffer, pH 7.4). Brains were dissected and postfixed. Infiltration of the embedding medium was performed by immersing the pieces in a 1:1 mixture of propylene oxide and epon and finally in neat epon and hardened at 60 °C. Ultrathin sections were examined in an EM902.
qPCR and RT-PCR Analysis.
Total mRNA was extracted from whole brain using TRIzol and 1 μg was reverse transcribed with random hexamer primer and Moloney Murine Leukemia Virus reverse transcriptase. mRNA expression was quantified with an AbiPrism NN8860 using SYBR green. For RT-PCR, amplification was performed in a Biometra thermocycler.
Immunoblot.
Samples were lysed in T-PER (containing 1 mM sodium fluoride, 1 mM sodium vanadate, 100 nM calyculin A, complete) and denatured with 4× lithium dodecyl sulfate. A total of 10 µg total brain or brain area lysates or 3- to 5-µg neuronal cell lysate were separated on a 4–12% or 16% Bis-Tris NuPage gel. Protein transfer was performed onto PVDF membranes which were blocked before incubation with primary antibodies. Binding was detected by incubation with HRP-coupled secondary antibodies (1:10,000, 3% nonfat milk) and visualized with ECL SuperSignal with Amersham Imager 600.
Statistical Analysis.
Values are expressed as mean ± SEM. Effects of genotype were analyzed with t test or one-way ANOVA followed by Bonferroni tests when appropriate. If data did not reach the criteria for parametric statistics, nonparametric statistics (Mann–Whitney’s u test or Kruskal–Wallis’s test followed by Dunn’s tests) were used. Within groups, designs were analyzed with mixed multifactorial ANOVA with Bonferroni post hoc tests when appropriate. Tests were two tailed and significance was set at P < 0.05.
Supplementary Material
Acknowledgments
A.T.R. and J.R. are supported by the Else Kröner Fresenius Stiftung; W.S., M. Sachs, and C.M.-S. by the DFG (Grant SFB1192); and M.M.R. by the DFG [Grants RI2811/1 and RI2811/2 (FOR2743)].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.K. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited with the ProteomeXchange Consortium via the PRIDE partner repository (dataset identifier PXD010449).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812413116/-/DCSupplemental.
References
- 1.Komander D, Clague MJ, Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 2.Day IN, Thompson RJ. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson KD, et al. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 4.Meyer-Schwesinger C, et al. A new role for the neuronal ubiquitin C-terminal hydrolase-L1 (UCH-L1) in podocyte process formation and podocyte injury in human glomerulopathies. J Pathol. 2009;217:452–464. doi: 10.1002/path.2446. [DOI] [PubMed] [Google Scholar]
- 5.Miyoshi Y, et al. High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Sci. 2006;97:523–529. doi: 10.1111/j.1349-7006.2006.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop P, Rocca D, Henley JM. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem J. 2016;473:2453–2462. doi: 10.1042/BCJ20160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: Ubiquitin C-terminal hydrolases. Biochemistry. 1998;37:3358–3368. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- 8.Kurihara LJ, Semenova E, Levorse JM, Tilghman SM. Expression and functional analysis of Uch-L3 during mouse development. Mol Cell Biol. 2000;20:2498–2504. doi: 10.1128/mcb.20.7.2498-2504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das C, et al. Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci USA. 2006;103:4675–4680. doi: 10.1073/pnas.0510403103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osaka H, et al. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet. 2003;12:1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- 11.Saigoh K, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- 12.Walters BJ, et al. Differential effects of Usp14 and Uch-L1 on the ubiquitin proteasome system and synaptic activity. Mol Cell Neurosci. 2008;39:539–548. doi: 10.1016/j.mcn.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Sugiura Y, Myers KG, Liu Y, Lin W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc Natl Acad Sci USA. 2010;107:1636–1641. doi: 10.1073/pnas.0911516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem Int. 2007;51:105–111. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hegde AN, Upadhya SC. The ubiquitin-proteasome pathway in health and disease of the nervous system. Trends Neurosci. 2007;30:587–595. doi: 10.1016/j.tins.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Bilguvar K, et al. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc Natl Acad Sci USA. 2013;110:3489–3494. doi: 10.1073/pnas.1222732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, et al. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem. 2012;120:1129–1138. doi: 10.1111/j.1471-4159.2011.07644.x. [DOI] [PubMed] [Google Scholar]
- 19.Cartier AE, et al. Differential effects of UCHL1 modulation on alpha-synuclein in PD-like models of alpha-synucleinopathy. PLoS One. 2012;7:e34713. doi: 10.1371/journal.pone.0034713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YL, et al. Accumulation of beta- and gamma-synucleins in the ubiquitin carboxyl-terminal hydrolase L1-deficient gad mouse. Brain Res. 2004;1019:1–9. doi: 10.1016/j.brainres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Lindsten K, Menéndez-Benito V, Masucci MG, Dantuma NP. A transgenic mouse model of the ubiquitin/proteasome system. Nat Biotechnol. 2003;21:897–902. doi: 10.1038/nbt851. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 24.Morita M, et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol Cell Biol. 2012;32:3585–3593. doi: 10.1128/MCB.00455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhang C, Wang A. Divergence and conservation of the major UPR branch IRE1-bZIP signaling pathway across eukaryotes. Sci Rep. 2016;6:27362. doi: 10.1038/srep27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN. Localization of the gene encoding human BiP/GRP78, the endoplasmic reticulum cognate of the HSP70 family, to chromosome 9q34. Genomics. 1994;20:281–284. doi: 10.1006/geno.1994.1166. [DOI] [PubMed] [Google Scholar]
- 28.Simons JF, Ferro-Novick S, Rose MD, Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolz A, Wolf DH. Endoplasmic reticulum associated protein degradation: A chaperone assisted journey to hell. Biochim Biophys Acta. 2010;1803:694–705. doi: 10.1016/j.bbamcr.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 31.Sułkowska JI, Rawdon EJ, Millett KC, Onuchic JN, Stasiak A. Conservation of complex knotting and slipknotting patterns in proteins. Proc Natl Acad Sci USA. 2012;109:E1715–E1723. doi: 10.1073/pnas.1205918109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng L, Locke C, Davis GW. S6 kinase localizes to the presynaptic active zone and functions with PDK1 to control synapse development. J Cell Biol. 2011;194:921–935. doi: 10.1083/jcb.201101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol. 2003;13:899–910. doi: 10.1016/s0960-9822(03)00338-5. [DOI] [PubMed] [Google Scholar]
- 34.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niere F, et al. Analysis of proteins that rapidly change upon mechanistic/mammalian target of rapamycin complex 1 (mTORC1) repression identifies Parkinson protein 7 (PARK7) as a novel protein aberrantly expressed in tuberous sclerosis complex (TSC) Mol Cell Proteomics. 2016;15:426–444. doi: 10.1074/mcp.M115.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 37.Niere F, Raab-Graham KF. mTORC1 is a local, postsynaptic voltage sensor regulated by positive and negative feedback pathways. Front Cell Neurosci. 2017;11:152. doi: 10.3389/fncel.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain S, et al. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol. 2013;33:1188–1197. doi: 10.1128/MCB.01389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain S, et al. UCH-L1 bypasses mTOR to promote protein biosynthesis and is required for MYC-driven lymphomagenesis in mice. Blood. 2018;132:2564–2574. doi: 10.1182/blood-2018-05-848515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss A, et al. A role of the ubiquitin-proteasome system in neuropathic pain. J Neurosci. 2002;22:1363–1372. doi: 10.1523/JNEUROSCI.22-04-01363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radón V, et al. Ubiquitin C-terminal hydrolase L1 is required for regulated protein degradation through the ubiquitin proteasome system in kidney. Kidney Int. 2018;93:110–127. doi: 10.1016/j.kint.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 43.Ding Q, Cecarini V, Keller JN. Interplay between protein synthesis and degradation in the CNS: Physiological and pathological implications. Trends Neurosci. 2007;30:31–36. doi: 10.1016/j.tins.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature. 2014;513:440–443. doi: 10.1038/nature13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cascio P. PA28αβ: The enigmatic magic ring of the proteasome? Biomolecules. 2014;4:566–584. doi: 10.3390/biom4020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, et al. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121:3689–3700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson’s disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 48.McNaught KS, Jnobaptiste R, Jackson T, Jengelley TA. The pattern of neuronal loss and survival may reflect differential expression of proteasome activators in Parkinson’s disease. Synapse. 2010;64:241–250. doi: 10.1002/syn.20719. [DOI] [PubMed] [Google Scholar]
- 49.Tan YY, Zhou HY, Wang ZQ, Chen SD. Endoplasmic reticulum stress contributes to the cell death induced by UCH-L1 inhibitor. Mol Cell Biochem. 2008;318:109–115. doi: 10.1007/s11010-008-9862-x. [DOI] [PubMed] [Google Scholar]
- 50.Jara JH, et al. Corticospinal motor neurons are susceptible to increased ER stress and display profound degeneration in the absence of UCHL1 function. Cereb Cortex. 2015;25:4259–4272. doi: 10.1093/cercor/bhu318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spillantini MG, et al. Familial multiple system tauopathy with presenile dementia: A disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA. 1997;94:4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen H, Sikorska M, Leblanc J, Walker PR, Liu QY. Oxidative stress regulated expression of ubiquitin carboxyl-terminal hydrolase-L1: Role in cell survival. Apoptosis. 2006;11:1049–1059. doi: 10.1007/s10495-006-6303-8. [DOI] [PubMed] [Google Scholar]
- 53.Kikuchi T, Mukoyama M, Yamazaki K, Moriya H. Axonal degeneration of ascending sensory neurons in gracile axonal dystrophy mutant mouse. Acta Neuropathol. 1990;80:145–151. doi: 10.1007/BF00308917. [DOI] [PubMed] [Google Scholar]
- 54.Yokota T, et al. Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci USA. 2001;98:15185–15190. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vizcaíno JA, et al. 2016 Update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44:D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deutsch EW, et al. The ProteomeXchange Consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017;45:D1100–D1106. doi: 10.1093/nar/gkw936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borodovsky A, et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9:1149–1159. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.