Significance
Metastasis accounts for most cancer-associated death. To metastasize, cells can move collectively where cells travel together as cohorts to invade surrounding tissues. However, the mechanisms by which cells move collectively are unclear. Utilizing a combination of in vitro, ex vivo, and in silico approaches, we demonstrated that cancer cell collective invasion is regulated by the energetic states of leader–follower cells. Leader cells require more energy than follower cells, and forward invasion of the leader cell consumes and depletes its available energy. A follower cell then takes over the leader position to sustain invasion. Clinically, there is significant interest in metabolic inhibitors to treat cancer, and our results suggest that metastatic migration might also be inhibited by targeting metabolic pathways.
Keywords: cancer invasion, collective migration, leader cell, cancer metabolism, bioenergetics
Abstract
The ability of primary tumor cells to invade into adjacent tissues, followed by the formation of local or distant metastasis, is a lethal hallmark of cancer. Recently, locomoting clusters of tumor cells have been identified in numerous cancers and associated with increased invasiveness and metastatic potential. However, how the collective behaviors of cancer cells are coordinated and their contribution to cancer invasion remain unclear. Here we show that collective invasion of breast cancer cells is regulated by the energetic statuses of leader and follower cells. Using a combination of in vitro spheroid and ex vivo organoid invasion models, we found that cancer cells dynamically rearrange leader and follower positions during collective invasion. Cancer cells invade cooperatively in denser collagen matrices by accelerating leader–follower switching thus decreasing leader cell lifetime. Leader cells exhibit higher glucose uptake than follower cells. Moreover, their energy levels, as revealed by the intracellular ATP/ADP ratio, must exceed a threshold to invade. Forward invasion of the leader cell gradually depletes its available energy, eventually leading to leader–follower transition. Our computational model based on intracellular energy homeostasis successfully recapitulated the dependence of leader cell lifetime on collagen density. Experiments further supported model predictions that decreasing the cellular energy level by glucose starvation decreases leader cell lifetime whereas increasing the cellular energy level by AMP-activated kinase (AMPK) activation does the opposite. These findings highlight coordinated invasion and its metabolic regulation as potential therapeutic targets of cancer.
Metastasis accounts for the majority of cancer-associated deaths (1). Local invasion of primary tumor cells into surrounding tissues is one of the very early steps in the invasion–metastasis cascade (2, 3). Although cancer invasion has often been envisioned as a single cell process mediated by signaling pathways leading to epithelial-to-mesenchymal transition (4), emerging evidence suggests that the majority of solid tumors also employ a collective strategy to invade as cohesive tumor clusters (5, 6). The collective behaviors of tumor clusters often make them more metastatic than single tumor cells (6–8).
Recent work has shown that many types of cancer cells including breast cancer invade into the surrounding tissues as collective strands (9–12). Those collective strands are often led by cells of a more invasive phenotype termed leader cells, such as highly metastatic cancer cells (9, 12) and cancer-associated fibroblasts (10, 13). Leader cells can remodel the stromal matrix through force-mediated matrix deformation, protease-mediated matrix degradation (9–11, 14), and polarized assembly of fibronectin (15, 16). Microtracks created by leader cells through matrix remodeling can be utilized by less invasive follower cells (9–11). Follower cells communicate with leader cells through adhesion-based mechanical interactions (12, 13), collectively migrate within the microtracks, and further expand the tracks (11).
Cancer invasiveness is also associated with its unique metabolic characteristics (17). Many cancer cells prefer to use the less efficient but more rapid glycolytic pathway (18) instead of oxidative phosphorylation to produce ATP, even with the presence of abundant oxygen, a phenomenon known as the Warburg effect (19). Although the increased biomass generation from aerobic glycolysis can promote cancer cell growth and proliferation (20), the increased lactate generation and excretion, on the other hand, favors protease-mediated matrix remodeling and thus enhancing cancer invasion (17). Glycolytic production of ATP has also been reported to regulate leader–follower rearrangement during vessel branching (21, 22) and promote cancer invasion indirectly (23). On the other hand, oxidative phosphorylation may provide ATP to the most energy-demanding regions, such as the leading edge of invasive cancer cells, causing a localized reversal of the Warburg effect (24). The invasive behavior of cancer cells has been proposed to follow physical laws such that minimization of cellular energy costs in a physically challenging environment favors a collective strategy (25). Consistent with this idea, cancer cells were found to switch from single cell dissemination to collective invasion in denser matrices (26) where more energy is required for individual cancer cells to migrate (27).
Although cancer cell metabolism is closely related to cancer invasion and metastasis, its impact on leader–follower interaction and its contribution to collective invasion is still not clear. In this paper, utilizing a combination of in vitro and ex vivo invasion assays, genetically encoded molecular probes for intracellular ATP/ADP ratio (28), and computational modeling that generates testable predictions, we demonstrated that breast cancer cells collectively invade into the surrounding matrices in a coordinated manner regulated by the energetic statuses of leader and follower cells. When encountering a physically challenging environment, leader and follower cells frequently switch positions to collectively overcome the energy barrier of invasion.
Results
Cancer Cells Dynamically Reorganize Their Relative Positions Within Collectively Invading Strands.
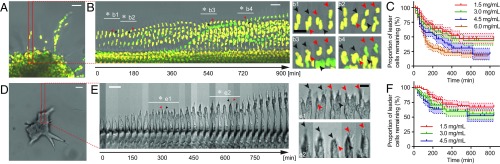
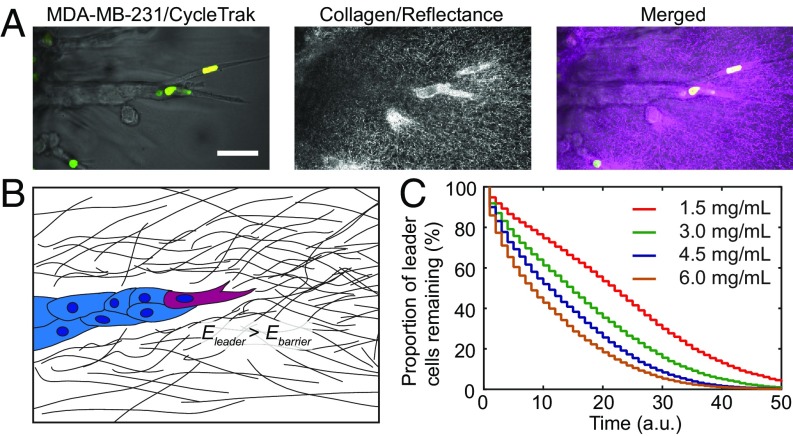
To facilitate the study of leader–follower interaction during cancer invasion, MDA-MB-231 breast cancer cells expressing the CycleTrak nuclear sensor (29) were formed into multicellular spheroids (9). The spheroids were then embedded in 1.5–6.0-mg/mL type I collagen gels. The cells invaded into the collagen matrix as collective strands (Fig. 1A), consistent with a previous report (9). Leader cells are often described as more migratory but less proliferative than follower cells during collective migration (SI Appendix, Fig. S1A) (30). Interestingly, we found that not all cells within the strands migrate outward. The cells frequently switched their relative positions even when the strands themselves were continuously invading (Fig. 1B and Movie S1). Specifically, each leader cell remained in the leader position of the invading strand for a limited time before it was replaced as a leader by a follower cell (Fig. 1B). A brief period of pause or retraction of the original leader cell, and thus the strand, was often observed before the emergence of a new leader (Fig. 1B), leading to temporary invasion interruptions and saltatory motions (SI Appendix, Fig. S1B). Consistently, forward invasion of the strand was interrupted when the original leader cell was laser ablated. The invasion recovered 12 h post ablation when a new leader emerged (SI Appendix, Fig. S1C).
Fig. 1.
Dynamic reorganization of collectively invading breast cancer cells in vitro and ex vivo. (A) A representative MDA-MB-231 spheroid expressing the CycleTrak nuclear marker (green/yellow, different colors indicate different cell cycle phases) is invading upward into 4.5-mg/mL collagen with collective strands. (B) Cells dynamically reorganize their relative positions within a typical strand (boxed region from A) during invasion, resulting in leader cell turnovers (asterisks; frames under white lines are enlarged on the Right to highlight leader turnover). Note that the invasion is interrupted when the original leader cells (black arrowheads) pause or move backward before the emergence of the new leaders (red arrowheads). (C) Leader–follower switching during spheroid invasion is more frequent in high-density collagen than in lower-density collagen (n > 50 for each condition; P < 0.0001 from a logarithmic-rank test for trend; shades represent standard error (s.e.); complete leader cell lifetime data are available in Dataset S1). (D) A representative mouse mammary tumor virus–polyomavirus middle T-antigen (MMTV-PyMT) mouse tumor organoid invading into 4.5-mg/mL collagen matrix. (E) A representative strand (boxed region from D) exhibits intermittent short-period pauses or retractions during forward invasion, many of them correlate with leader cell turnover events (as indicated with asterisks; black arrowheads, old leader; red arrowheads, new leader; frames under the white lines are enlarged on the Right to highlight leader turnover). (F) Leader cell lifetime during organoid invasion decreases with increasing collagen density (n > 45 for each condition; P = 0.05 from a logarithmic-rank test for trend; shades represent s.e.). [Scale bar, 50 µm (A and B, Left and D and E, Left), 25 µm (B, Right and E, Right).]
Additionally, using MDA-MB-231 cells expressing photoactivatable mCherry (31), we found the percentage of activated leader cells decreased gradually following photoactivation (SI Appendix, Fig. S1 D and E). Moreover, isolated mCherry-positive leader cells did not always assume a leader position when cocultured with isolated follower cells (SI Appendix, Fig. S1F). These data confirm the plasticity of breast cancer cells in collective invasion.
Similar dynamic reorganization of cancer cells was also observed during the invasion of tumor organoids generated from MMTV-PyMT mice (Fig. 1 D and E, SI Appendix, Fig. S1G, and Movie S2), which were characterized by keratin-14 positive leader cells (12). We found that keratin-14 was expressed not only in the leader cell at the very front, but also in cells immediately following it (SI Appendix, Fig. S1H), suggesting that those keratin-14 positive cells may collectively serve as a leader cohort and may switch positions with each other.
We next quantified the lifetime of each leader cell during invasion and found that it decreased as the density of the collagen matrix increased (Fig. 1 C and F). Given that individual cell migration is more difficult in denser collagen matrices (27), the more frequent leader–follower switching in denser collagen matrices implies that cancer cells invade into a physically challenging environment via a relaylike manner. Together, these observations suggest that timely leader–follower turnover may be a collective strategy cancer cells use to sustain continuous invasion where migratory cells take turns in leading the invasion.
Leader Cells Increase Glucose Uptake When Invasion Becomes Difficult.
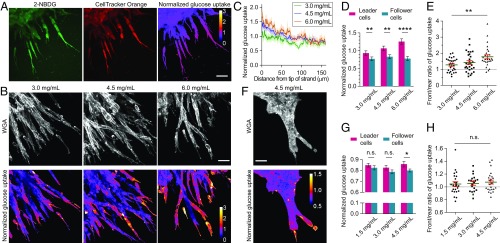
It was previously shown that breast cancer cells invaded cooperatively toward a glucose gradient to minimize thermodynamic costs (25). As glucose is the main source of cellular energy production and increased glucose uptake has often been associated with cancer invasiveness (32), we next investigated if the leader–follower behavior can be explained by a difference in glucose uptake and thereby a difference in cellular energy states. To measure glucose uptake along invading strands in vitro, we incubated MDA-MB-231 spheroids with the fluorescent glucose analog (2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose) (2-NBDG). Its intensity was then normalized against the CellTracker Orange CMRA dye through ratio imaging to remove artifacts due to light scattering and attenuation in 3D (Fig. 2A). Leader cells exhibited higher glucose uptake than follower cells (Fig. 2 B–E) with the absence of an external glucose gradient (SI Appendix, Fig. S2), and this difference increased with the density of collagen matrices (Fig. 2 D and E). A similar difference between the glucose uptake of leader and follower cells was also observed for ex vivo organoids invading in high-density collagen (Fig. 2 F–H). These results indicate that leader cells respond to an increasingly challenging extracellular environment by increasing glucose uptake and energy production. This increased energy metabolism of leader cells may be used to compensate for the increased energy consumption due to high cytoskeletal contractility (SI Appendix, Fig. S3 A and B) and tensile forces (33), high strain energy transmitted to the extracellular matrix (SI Appendix, Fig. S3C), as well as high matrix metalloproteinase activities at the invasion front (11).
Fig. 2.
Glucose uptake along collectively invading strand in vitro and ex vivo. (A) Glucose uptake along invading strands in a representative spheroid is measured by the glucose analog 2-NBDG (green) and normalized to the CellTracker Orange CMRA dye (red) to obtain a ratiometric normalized glucose uptake value (heat map). (B) MDA-MB-231 leader cells exhibit higher normalized glucose uptake than follower cells as shown by the ratio heat maps alongside with wheat germ agglutinin (WGA) labeling of cell boundaries. (C) Normalized glucose uptake decreases along invading strands from tip to rear in vitro (averaged from n > 10 strands for each condition; shades represent s.e.). (D and E) The difference between leader and follower cells in normalized glucose uptake increases with collagen density in vitro (n = 29, 29, and 27, respectively; P = 0.0059, 0.0037, and <0.0001 from the paired t tests in D; P = 0.0035 from the ANOVA test in E). (F–H) Normalized glucose uptake is higher in leader cells than in follower cells during organoid invasion and exhibits a similar trend as collagen density increases (n = 24, 20, and 24, respectively; P = 0.32, 0.094, and 0.029 from the paired t tests in G; P = 0.78 from the ANOVA test in H). Strand tips orient toward the lower-right corner of the images. *P < 0.05, **P < 0.01, and ****P < 0.0001; n.s., not significant. (Scale bar, 50 µm.)
Cellular ATP/ADP Ratio Correlates with Leader Cell Invasion.
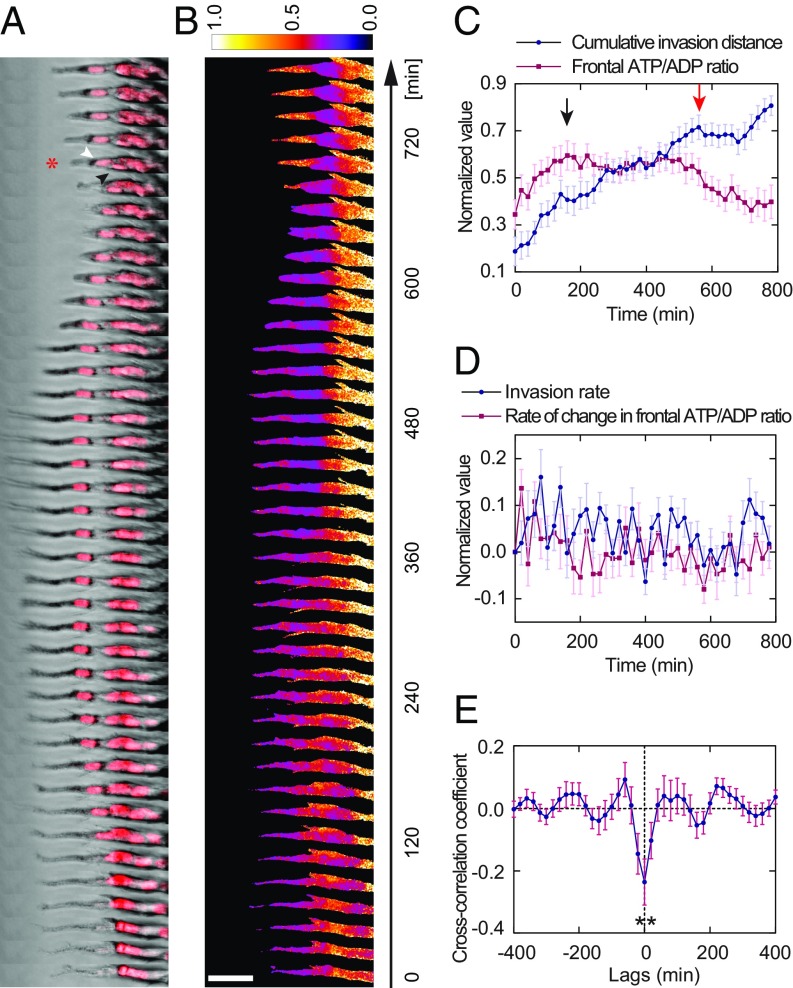
To further elucidate how the cellular energy state affects leader–follower behavior during cancer collective invasion, we employed the previously developed molecular probe for the intracellular ATP/ADP ratio, PercevalHR (28). MDA-MB-231 cells expressing the PercevalHR probe were used to generate spheroids, and the spheroids were embedded in 4.5-mg/mL collagen. The dynamic behavior of the cellular energy state during invasion was then recorded by time-lapse imaging (Movie S3). We found that, although the ATP/ADP ratio of the leader cell was not always the highest among all cells within the same strand, if it drops too low, the leader cell position will be replaced by a more energetic follower cell (Fig. 3 A and B). Quantitative analysis revealed that a peak in the leader ATP/ADP ratio was reached before a major retraction step in the cumulative invasion distance (Fig. 3C), suggesting that a rise in cellular energy level is the driving force of forward invasion. In addition, the rate of change in the leader ATP/ADP ratio and the rate of invasion frequently exhibited opposite signs (Fig. 3D) and correlated negatively with each other (Fig. 3E), implying that forward invasion of the leader consumes and depletes its available energy. These data highlight a connection between the cellular energy state and the leader–follower state.
Fig. 3.
Dynamics of cellular ATP/ADP ratio correlates with leader cell invasion. (A) Time-series images of an leftward invading MDA-MB-231 strand with the PercevalHR ATP/ADP ratio sensor and DRAQ5 nuclear labeling (red) show a follower cell taking over the leader position (as indicated by the asterisk at 700 min; black arrowhead, old leader; white arrowhead, new leader). (B) Heat map of the same strand shows that the normalized ATP/ADP ratio correlates with leader cell invasion. The leader cell invades forward when the ATP/ADP ratio is high (0–160 min). As it invades, the ATP/ADP ratio drops because energy is being consumed (180–320 min). The original leader cell stops invading (340–480 min) and retracts (500–660 min) as the ATP/ADP ratio drops below a certain threshold. Leader–follower switching eventually takes place to sustain continued invasion (680–780 min). (C) Comparison of cumulative invasion distance and frontal ATP/ADP ratio (n = 21 strands; normalized to the range of [0, 1] for each strand) suggests that forward invasion of the leader cell depletes its ATP/ADP ratio (black arrow indicates the ATP/ADP ratio peak), causing invasion to stall (red arrow indicates a major retraction). (D) Rate of change in frontal ATP/ADP ratio and invasion rate of the strands frequently display opposite signs (n = 21 strands; normalized to the range of [0, 1] for each strand, which is then shifted to retain the signs of the variable before normalization). (E) Forward invasion depletes cellular energy as indicated by a negative correlation between the rates (n = 21 strands; P = 0.0043 from the one-sample t test compared with a value = 0). The ATP/ADP ratio in the frontal 25-µm segment of each strand is used to represent the leader cell ATP/ADP ratio. (Scale bar, 50 µm.)
Cellular Energy Level Determines Leader Cell Fate as Explained by a Mathematical Model.
Our experimental results suggest a link between the cellular energy state and the cancer invasion. Based on these observations, we hypothesized that the fate of a leader cell is determined by its energy level during cancer collective invasion: Leader cells respond to a physically challenging environment by increasing their energy level, which is consumed gradually during forward invasion; when the energy level drops below a threshold, the original leader stops forward invasion; a more energetic follower cell may take over the leader position, or the original leader cell may rest to rebuild its energy level before it can invade again.
To test this hypothesis, we developed a simple mathematical model based on intracellular energy homeostasis (SI Appendix, Supplementary Materials and Methods) and the interaction between the leader cell and the surrounding matrix (Fig. 4A). In the model, a cell’s energy level is determined by a balance between energy production and energy consumption. To invade forward, the leader cell’s energy level needs to be above a threshold to overcome the energy barrier imposed by the surrounding matrix (Fig. 4B). By assuming a positive correlation between the energy barrier and the density of the collagen matrix (27), computer simulations (SI Appendix, Table S1) of the model successfully recapitulated the dependence of leader cell lifetime on collagen density (Fig. 4C). These results suggest that increased leader–follower switching in high-density collagen is the preferred strategy cancer cells use to overcome an increased energy barrier of invasion.
Fig. 4.
Intracellular energy state determines leader cell fate as explained by modeling. (A) An invading strand of MDA-MB-231 cells expressing the CycleTrak sensor in 1.5-mg/mL collagen shows the interaction between the leader cells and the collagen matrix. (B) Illustration of the model shows the leader cell overcoming the energy barrier imposed by the collagen matrix to invade. (C) Model simulation of leader cell lifetime recapitulates its dependence on the collagen density/energy barrier. a.u., arbitrary unit. (Scale bar, 50 µm.)
Manipulating Cellular Energy Level Modulates Leader Cell Lifetime.
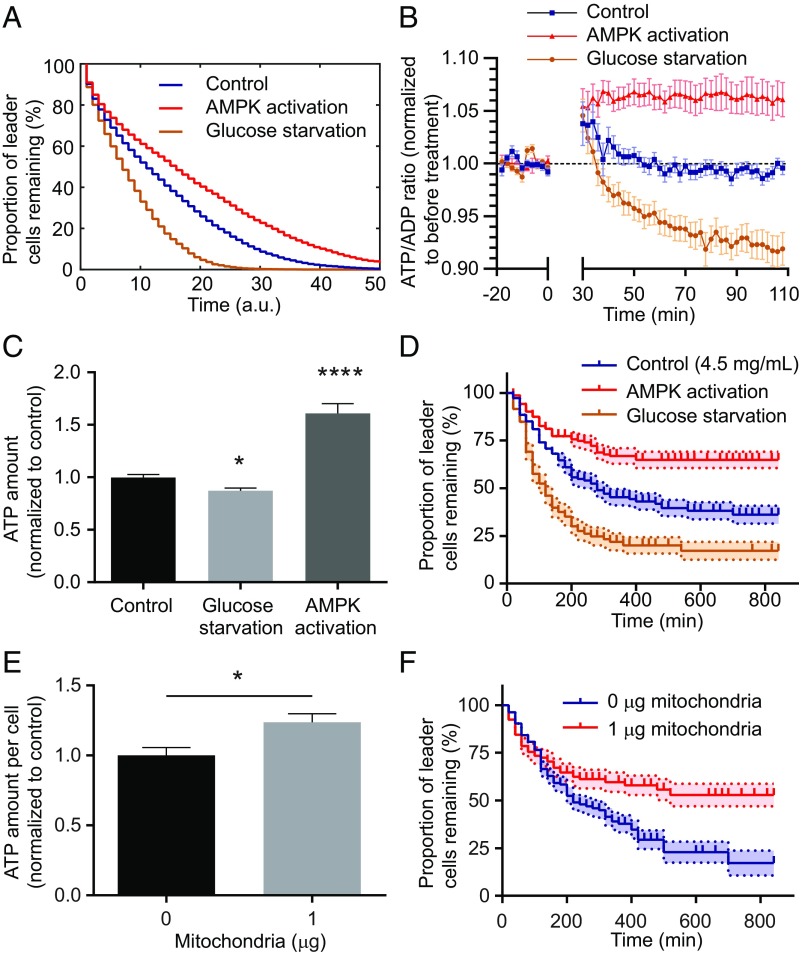
Our computational model further predicted that globally increasing cellular energy levels would prolong the duration before the energy level of the leader cell drops below the threshold, thus increasing leader cell lifetime, whereas decreasing the cellular energy levels would do the opposite (Fig. 5A). To test these predictions, 200-µM AICAR was used to activate AMPK and increase cellular energy levels (24), and media containing 0-mM glucose was used to decrease cellular energy levels. AMPK is an intracellular energy sensor that increases ATP production and decreases ATP consumption when activated (34). AICAR treatment increased the cellular ATP/ADP ratio within 30 min (Fig. 5B), and the increased energy level was maintained 20 h after treatment (Fig. 5C). Consistent with previous reports (27, 28), glucose starvation decreased the ATP/ADP ratio gradually 30 min after treatment (Fig. 5B), and the decreased energy level was maintained for 20 h following treatment (Fig. 5C).
Fig. 5.
Leader cell lifetime can be modulated by increasing or decreasing cellular energy level. (A) Model predicts that a drop in intracellular energy level by glucose starvation decreases leader cell lifetime and that an elevated energy level due to AMPK activation increases leader cell lifetime. a.u., arbitrary unit. (B) Glucose deprivation decreases the cellular ATP/ADP ratio over time for MDA-MB-231 cells on a glass substrate, whereas AMPK activation by 200-µM 5-aminoimidazole-4-carboxamide riboside (AICAR) increases the ATP/ADP ratio (n > 15 cells for each condition, treatment added at t = 0 min). (C) Glucose starvation decreases total ATP concentration, whereas AMPK activation increases total ATP concentration 20 h after treatment (n = 24, 14, and 16, respectively; P = 0.0052, and <0.0001 from unpaired t tests compared with the control). (D) Experiments verify that leader cell lifetime in spheroids is decreased (P < 0.0001 from the logarithmic-rank test) in glucose-free medium and increased (P < 0.0001 from logarithmic-rank test) when treated with 200-µM AICAR to activate AMPK (n > 100 for each condition; shades represent s.e.). (E) Artificially transferred mitochondria (1 µg/100,000 recipient cells) increases cellular ATP concentration compared with the control (0 µg) 4 d after transfer (n = 5 for each group; P = 0.023 from unpaired t test). (F) Leader cells with artificially transferred mitochondria have increased lifetime compared with control ones within coculture spheroids in 4.5-mg/mL collagen (n > 100 for each condition; P = 0.0051 from the logarithmic-rank test). *P < 0.05 and ****P < 0.0001.
We then treated invading spheroids of MDA-MB-231 cells expressing the CycleTrak sensor with 200-µM AICAR to increase cellular energy levels or with glucose starvation to decrease cellular energy levels. As predicted by our model, leader cell lifetime in 4.5-mg/mL collagen was indeed increased by AMPK activation and decreased by glucose starvation (Fig. 5D). Cell migration speed within the invading strands was not affected by the treatments (SI Appendix, Fig. S4), suggesting that the prolonged or shortened leader cell lifetime is not caused by a global modulation of cell migration.
To further test the hypothesis that leader cell lifetime can be modulated by cellular energy metabolism, we artificially transferred exogenous mitochondria from cancer associated fibroblasts to MDA-MB-231 cells (35). We found that the transferred mitochondria increased ATP production in cancer cells (Fig. 5E), consistent with previous reports (35, 36). Cells with exogenous mitochondria were then mixed with control cells to generate coculture spheroids. We found that leader cells with exogenous mitochondria had significantly increased lifetimes compared with control cells within the same spheroids (Fig. 5F), likely due to their elevated energy levels. Together, these data validated the hypothesis that cancer cell invasion can be controlled by its energetic state.
Discussion
Using a combination of in vitro and ex vivo invasion experiments and computational modeling, we demonstrated that breast cancer cells collectively invade into surrounding tissues in a coordinated relaylike manner, which is regulated by the energy states of leader and follower cells.
By tracking the movement of individual breast cancer cells within invading strands of in vitro MDA-MB-231 spheroids and ex vivo MMTV-PyMT organoids, we observed dynamic reorganization of the relative positions both between leader and follower cells and among follower cells (Movie S1). Similar leader cell rearrangement or leader position competition has also been observed in other cancer types (37) and during angiogenic sprouting (22, 38, 39). The extent of leader–follower turnover may be regulated by the extracellular matrices and cell types. We found that the leader–follower turnover frequency increases with collagen density and that leader cells in organoids are more stable than in spheroids. In a more heterogeneous cell population, leader cells and follower cells may have more distinct phenotypes and thus less likely to switch positions with each other (37). An extreme example is when invasive cells are mixed with noninvasive cells to make heterotypic tumor spheroids where invasive cells exclusively become the leader (9). Nevertheless, leader–follower switching may still occur as long as there are more than one leader-capable cells within the same strand. Collective invasion of MMTV-PyMT organoids are often led by keratin-14 positive cells (12). We found that those keratin-14 positive cells occupy not only the leader positions, but also some follower positions (SI Appendix, Fig. S1H). Leader–follower switching may then take place among those keratin-14 positive leader-capable cells.
Leader–follower dynamics in angiogenesis is often regulated by a gradient of VEGF (30, 40). Although cancer cell invasion is rarely regulated by VEGF gradients, it has been recently reported that a glucose gradient can induce MDA-MB-231 cells to penetrate into a collagen matrix with multiple leaders at different places competing for the frontmost position (25). Our spheroid and organoid experiments suggest that such dynamic coordination and competition occur naturally during collective invasion of breast cancer cells without the requirement of a gradient in growth factors or nutrients. However, in the absence of a glucose gradient, the leader cell was much shorter lived with a median lifetime ranging from 120 to 480 min (Fig. 1C), compared with 70 h with the presence of a glucose gradient (25), suggesting that cancer invasion could be enhanced by guided signals within the tumor microenvironment. Even though an external glucose gradient was not applied, we still observed higher glucose uptake in leader cells than in follower cells. In addition, the difference between leader and follower cells in glucose uptake increased with collagen density. Combined with the findings that glucose uptake in isolated MDA-MB-231 cells also increases with collagen density (27) and that leader cells create microtracks for less invasive follower cells (9–11), it is likely that the leader position is responsible for mediating the most significant cell-matrix interactions, and the extracellular matrix architecture and nutrient availability predominantly affect the behavior of leader cells compared with follower cells. Indeed, we found that leader cells have higher cytoskeletal contractility than follower cells and transmit more strain energy to the surrounding matrix (SI Appendix, Fig. S3), and the leader position was also reported to have high protease activities (11). This is consistent with the idea that leader cells bear the majority of the energetic costs associated with matrix deformation and degradation during invasion (25).
With the PercevalHR probe to measure the intracellular ATP/ADP ratio, we observed a positive correlation between invasion rate and energy consumption rate (Fig. 3 D and E), suggesting that faster invasion leads to faster energy depletion. The high-energy consuming rate of leader cells eventually lead to a decrease in ATP/ADP ratio and leader–follower transition and may require mitochondria accumulation and AMPK activation to compensate for the energy deficit, similar to that which happens in the leading edge of individually invading cancer cells (24). A peak of the ATP/ADP ratio in the leader cell typically occurred before it reached its maximum invasion distance (Fig. 3 A–C), implying that a rise in leader energy level drives the invasion of the strands. In addition, we previously reported that the ATP/ADP ratio in isolated breast cancer cells increases with collagen density (27), consistent with the idea that the threshold energy level of leader cells needed to overcome the invasion barrier increases with collagen density. We then built all these concepts into a computational model to help understand the energetic regulation of collective cancer invasion. By simulating the intracellular energy homeostasis of the leader cell and defining the lifetime of leader cells in the model as the duration of time when the leader cell energy level is above the invasion threshold, the model successfully reproduced the dependence of leader cell lifetime on collagen density. In addition, the model predictions that decreasing cellular energy levels by glucose starvation decrease leader cell lifetime and that increasing cellular energy levels by AMPK activation or delivery of exogenous mitochondria increases leader cell lifetime were verified by experiments.
Overall, our data suggest that cancer cells employ relaylike collective migration as a strategy to invade into a physically challenging and energy demanding environment. The leader cell requires more energy compared with follower cells to break the invasion energy barrier, whereas its forward invasion depletes the available energy until cellular energy levels drop below the threshold for invading. Following that, the original leader cell may rest to return to a high-energy state, or a more energetic follower cell may take over the leader position. In some cases, we have observed a delay before the new leader cell is able to invade, resulting in intermittent interruptions of forward invasion of the strands (Figs. 1 B and E and 3A), suggesting that a timely leader–follower switching is required for sustained invasion. Similar saltatory motion has also been observed during collective migration along 2D strips (41, 42) and 3D microtubes (43), although leader cells in those systems do not encounter resistance from the matrix in front of them. As a result, leader cells usually have a higher speed than follower cells when migrating into an open space (41, 42), whereas leader cell migration is usually slower than follower cells when invading into a 3D matrix (SI Appendix, Fig. S1B). When 3D invasion becomes difficult, as in the case of a high-density collagen matrix, cancer cells were found to switch from a single cell dissemination mode to a collective mode (26). This is consistent with the idea that collective migration is more energy efficient than single cell migration, and energy efficiency may be critical for cancer dissemination in a challenging environment. Moreover, we found cancer cells employ a relaylike strategy by promptly replacing low-energy old leaders with high-energy new leaders, so as to collectively overcome the increased energy barrier. In addition, leader and follower cancer cells are able to communicate and cooperate through signaling to promote collective invasion (37). The cooperative behaviors of cell clusters has also been reported to increase the migration speed of border cells in the Drosophila ovary (44) and enhance the abilities of mammary epithelial cells and lymphocytes to sense weak guidance signals (45, 46), which may also apply to and contribute to cancer invasion. This cooperative behavior of cancer invasion may become a new target for cancer therapeutics. Treatments that aim to disrupt the communication and cooperation between leader and follower cells and that aim to disrupt the metabolic regulation of leader–follower behaviors may turn intermittent interruption of invasion into long-term interruptions and prevent collective invasion.
Materials and Methods
Cell culture, plasmids, reagents, and microscopy are described in SI Appendix, Supplementary Materials and Methods. Manual or automated image analysis was carried out using ImageJ and MATLAB. Computer simulations of the energy-based model were implemented using custom MATLAB scripts with details provided in SI Appendix, Supplementary Materials and Methods and Table S1. All statistical analyses were performed using GraphPad Prism 6. Statistical significance was identified if the tested P value was smaller than 0.05(*), 0.01(**), 0.001(***), or 0.0001(****). When multiple pairwise comparisons were performed, the Bonferroni correction was used to adjust the significance level.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Award 1740900, National Institutes of Health Grant HL127499 (to C.A.R.-K.), and the Scholarship for the Next Generation of Scientists from the Cancer Research Society and NIH Grant K99-CA212270 (to F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809964116/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Talmadge JE, Fidler IJ. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 5.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KJ, Ewald AJ. A collective route to metastasis: Seeding by tumor cell clusters. Science. 2016;352:167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duda DG, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey SP, Starchenko A, McGregor AL, Reinhart-King CA. Leading malignant cells initiate collective epithelial cell invasion in a three-dimensional heterotypic tumor spheroid model. Clin Exp Metastasis. 2013;30:615–630. doi: 10.1007/s10585-013-9565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 11.Wolf K, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 12.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labernadie A, et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19:224–237. doi: 10.1038/ncb3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glentis A, et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat Commun. 2017;8:924. doi: 10.1038/s41467-017-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdogan B, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attieh Y, et al. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly. J Cell Biol. 2017;216:3509–3520. doi: 10.1083/jcb.201702033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han T, et al. How does cancer cell metabolism affect tumor migration and invasion? Cell Adhes Migr. 2013;7:395–403. doi: 10.4161/cam.26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 19.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Cruys B, et al. Glycolytic regulation of cell rearrangement in angiogenesis. Nat Commun. 2016;7:12240. doi: 10.1038/ncomms12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantelmo AR, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunniff B, McKenzie AJ, Heintz NH, Howe AK. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol Biol Cell. 2016;27:2662–2674. doi: 10.1091/mbc.E16-05-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, et al. Minimization of thermodynamic costs in cancer cell invasion. Proc Natl Acad Sci USA. 2013;110:1686–1691. doi: 10.1073/pnas.1221147110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haeger A, Krause M, Wolf K, Friedl P. Cell jamming: Collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim Biophys Acta. 2014;1840:2386–2395. doi: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Zanotelli MR, et al. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Mol Biol Cell. 2018;29:1–9. doi: 10.1091/mbc.E17-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tantama M, Martínez-François JR, Mongeon R, Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 2013;4:2550. doi: 10.1038/ncomms3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridenour DA, McKinney MC, Bailey CM, Kulesa PM. CycleTrak: A novel system for the semi-automated analysis of cell cycle dynamics. Dev Biol. 2012;365:189–195. doi: 10.1016/j.ydbio.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat Rev Clin Oncol. 2009;6:315–326. doi: 10.1038/nrclinonc.2009.64. [DOI] [PubMed] [Google Scholar]
- 31.Subach FV, et al. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat Methods. 2009;6:153–159. doi: 10.1038/nmeth.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 33.Gjorevski N, Piotrowski AS, Varner VD, Nelson CM. Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep. 2015;5:11458. doi: 10.1038/srep11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MJ, Hwang JW, Yun C-K, Lee Y, Choi Y-S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018;8:3330. doi: 10.1038/s41598-018-21539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caicedo A, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. doi: 10.1038/srep09073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konen J, et al. Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nat Commun. 2017;8:15078. doi: 10.1038/ncomms15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 39.Arima S, et al. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 2011;138:4763–4776. doi: 10.1242/dev.068023. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarle V, et al. Modeling collective cell migration in geometric confinement. Phys Biol. 2017;14:035001. doi: 10.1088/1478-3975/aa6591. [DOI] [PubMed] [Google Scholar]
- 42.Vedula SRK, et al. Emerging modes of collective cell migration induced by geometrical constraints. Proc Natl Acad Sci USA. 2012;109:12974–12979. doi: 10.1073/pnas.1119313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xi W, Sonam S, Beng Saw T, Ladoux B, Teck Lim C. Emergent patterns of collective cell migration under tubular confinement. Nat Commun. 2017;8:1517. doi: 10.1038/s41467-017-01390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai D, et al. Modeling and analysis of collective cell migration in an in vivo three-dimensional environment. Proc Natl Acad Sci USA. 2016;113:E2134–E2141. doi: 10.1073/pnas.1522656113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellison D, et al. Cell-cell communication enhances the capacity of cell ensembles to sense shallow gradients during morphogenesis. Proc Natl Acad Sci USA. 2016;113:E679–E688. doi: 10.1073/pnas.1516503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malet-Engra G, et al. Collective cell motility promotes chemotactic prowess and resistance to chemorepulsion. Curr Biol. 2015;25:242–250. doi: 10.1016/j.cub.2014.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.