Almost all organisms, from insects to mammals, have coevolved with microorganisms, establishing symbiotic interactions. Whereas most such interactions are based on nutrition (1), there are other examples, among which one of the most well studied is symbioses involving light-emitting bacteria. The bobtailed squid, Euprymna scolopes, forms a symbiosis with the bioluminescent Vibrio fischeri, which produces light that puts the nocturnal squid into stealth mode, blending in with moon- and starlight. A central goal for all symbiotic research is to delineate whether, and how, the symbiosis signals to distal organs of the host. In PNAS, by using the binary squid–vibrio model and isogenic bacterial mutants, Moriano-Gutierrez et al. (2) demonstrate that light produced by the bacteria has a dramatic impact on the transcriptome in the light organ as well as the eye, whereas the response in the gills is independent of the bioluminescence produced by V. fischeri.
During the past 20 y, there has been a dramatic revival in the interest of studying the microbiome and symbiotic interactions between microorganisms living inside and on the surface of other living organisms. Such symbiotic interactions occur in all domains of life, from the binary symbiosis of lichen, which arises from algae or cyanobacteria living among fungi filaments, to the complex interactions between trillions of bacteria and the human host. With numerous studies associating altered microbiota with different disease states, the microbiome has become a hot topic. However, due to the complexity of the human microbiome, few, if any, studies have provided causal evidence for the underpinning of the symbiosis among specific strains, their traits, and the host. The interaction between the human microbiome and the host can be induced by several different bacteria that often form networks (or guilds) that, as an aggregate, perform important functions such as metabolism of dietary fibers (3). In contrast, the bobtailed squid specifically selects bioluminescent V. fischeri from the seawater to colonize the light organ, which induces tissue remodeling and light production (4). Because the squid can be reared either with V. fischeri (SYM) or without V. fischeri (APO) and the bacteria are genetically modifiable, it provides a unique system to investigate specific bacterial factors shaping the symbiosis. Twenty years ago, it was demonstrated that mutants in the operon encoding the bioluminescent machinery failed both to induce remodeling of the light organ and to develop persistent colonization (5). However, it has been unclear whether, and how, V. fischeri can signal other organs in the squid. At present, similar questions are being asked in many different symbiotic model systems: Which bacteria and by which pathways can they signal to distant tissues? In mammals, it is clear that the gut microbiota can communicate with distant organs by activating enteroendocrine cells in the gut that signal to distant organs via hormones, through neuronal signaling both within the enteric nervous system and through the vagus nerve, as well as by producing metabolites and products that can act as messengers per se (6). However, few, if any, microbial genes that have pronounced effects on host physiology have been identified in specific bacteria in naturally occurring mammalian ecosystems. In this respect, the squid–vibrio model provides a unique opportunity to investigate a nonnutritional symbiosis and the resulting effects on distant organs using squid exposed to their normal environmental microbiota with or without V. fischeri.
In PNAS, Moriano-Gutierrez et al. (2) address how the symbiotic V. fischeri affects the transcriptome locally in the light organ and distantly in the gills and the eye. This is an interesting selection of organs, as only the light organ is in contact with V. fischeri cells, whereas the gill has immune functions and is in contact with all bacteria in the surrounding water. In contrast, the eye is considered immune privileged and thus should not respond to bacteria. Furthermore, the light organ and the eye have had convergent evolution, wherein the light organ has evolved eyelike optical structures similar to large crystallin lenses to project emitted light, and the epithelial crypt cells can detect light (7).
First, Moriano-Gutierrez et al. (2) compared the transcriptional profiles in the three organs in juvenile (24 h posthatch) and adult animals. The profiles of the gill and the light organ grouped more closely together, likely because these organs are in direct contact with bacteria, which the eye is not, and thus likely have more cells with immune functions present. Similar findings have been obtained by comparing germ-free and colonized mice in which the ileum, which is immunologically active and in direct contact with bacteria, has twice as many microbially regulated genes compared with the colon in which the epithelium is separated from the microbiota by a mucus layer (8). Furthermore, few microbial genes were identified in epididymal and subcutaneous white adipose tissues, which are distant from the bacteria (9), suggesting that the effects in distant organs may be secondary due to host adaptation or circulating microbial metabolites.
Moriano-Gutierrez et al. (2) observed that the transcriptional profiles of juvenile and adult animals were relatively similar in the gill and more different in the light organ; the largest separation occurring between samples was observed in the eye. This observation suggests that the eye has significant postnatal developmental regulation, although some caution should be made since the data are based on relatively few replicates.
Next, the authors focused in on the adult animals to investigate how the presence or absence of the symbiont regulates gene expression. Not surprisingly, the light organ demonstrated the most pronounced response to the presence of V. fischeri, which colonizes this organ at low levels but rapidly expands into a significant biomass. V. fischeri induced genes in the light organ that were related to morphological changes upon colonization with the bacterium, including loss of a specific ciliated epithelia and development of crypts. Restructuring of the tissue is also a well-known phenotype upon colonization of germ-free mice, in which the intestinal villi undergo dramatic shortening and widening associated with increased angiogenesis upon colonization with bacteria (10). However, V. fischeri also induced expression of genes associated with the eye functions, such as genes involved in lens formation, phototransduction, and visual perception. However, it remains unclear whether these transcriptional responses are converted into functional proteins (and the physiologic implications) or are associated with maturation of the light organ in preparation for emitting light.
More surprisingly, the eye had twice as many symbiosis-regulated genes compared with the gill. This suggests that the gill, although immunologically active, does not respond to the V. fischeri community populating the light organ, which is likely due to V. fischeri being present at relatively low abundance in the surrounding water, which the gills are directly exposed to. Thus, to observe V. fischeri-induced gene expression in the gills, the squids likely have to be reared under germ-free conditions to reduce the contribution of bacteria in the surrounding water. But why does the eye transcriptionally respond to bacteria in the light organ? First, the data suggest the presence of communication between the light organ and the eye. This connection appears
The study by Moriano-Gutierrez et al. demonstrates the power of the squid–vibrio system in identifying microbial functions and their effect on the host.
to be specific for the squid, as the gut microbiota did not affect transcription in the mouse eye (2), and could potentially be related to the convergent evolution of the two organs. Second, the findings also suggest that regulation of the eye transcriptome could be dependent on bacterial light production.
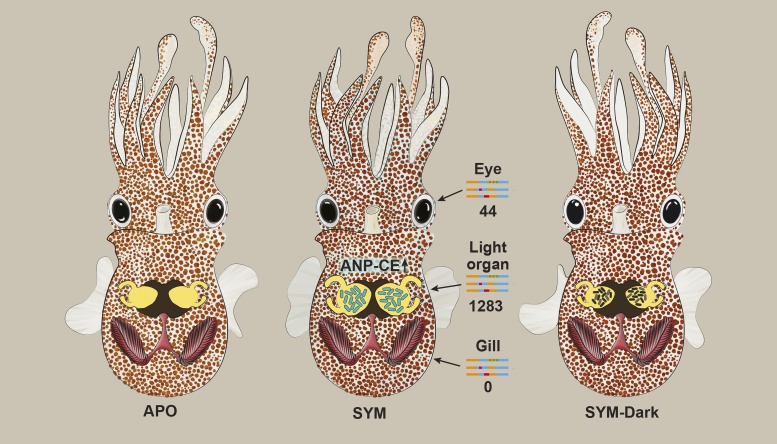
To address this possibility, Moriano-Gutierrez et al. (2) colonized juvenile squids for 24 h with either wild-type bacteria or an isogenic dark mutant lacking the lux operon (SYM-dark; Fig. 1). Impressively, two-thirds of the 1,919 regulated genes in the light organ required light production from the symbiont to be regulated, clearly demonstrating that light production is a key feature of the symbiosis between the squid and V. fischeri. Among these were the genes involved relating to eye functions, suggesting that light production can serve as a cue for maturation of the light organ. Since the gill had limited transcriptional response to the symbiont, light production did not appear to regulate the transcriptome. In contrast, in the eye, 44 genes were specifically regulated by light production from V. fischeri within the light organ. This clearly highlights that light production from V. fischeri is essential for regulation of gene expression not only in the light organ, but also in the eye. However, it is at present unclear why the squid eye would respond to light production in the light organ and how such signals are transmitted to the eye. Are these signals mediated by direct sensing of bioluminescence by the eye, by light receptors in the light organ transmitting nervous signals to the eye, or through indirect effects mediated by differential responses of the wild-type and mutant bacteria in the symbiosis? Furthermore, it is unclear how these transcriptional responses in the eye translate into physiological behavior in the squid. At the present time, these questions are challenging to address in an animal host for which the genome just has become available (11) and because of the lack of genetic tools.
Fig. 1.
Light-producing V. fischeri induce gene expression in the light organ and the eye. Juvenile squids colonized with wild-type V. fischeri (SYM), but not those colonized with isogenic Δlux mutants (SYM-dark), induce profound gene expression in the light organ (including genes associated with the function of the eye) (1,283 genes) and, to a lower extent, in the eye (44 genes), but not in the gill (0 genes). One gene, ANP-CE, which is associated with cell swelling, was demonstrated to be expressed in crypt cells that undergo swelling after colonization with wild type, but not Δlux mutants. Adapted from data presented in figure 4 of ref. 2.
One clear advantage of the system used by Moriano-Gutierrez et al. (2) is that they study a naturally occurring symbiosis and can use genetics to clarify pathways in the bacterium that may have an impact on host physiology, which can be difficult to approach in more complex symbioses. Thus, it is tempting to speculate that perhaps some conserved cellular functions, such as epithelial integrity and innate immune responses that may be hard to address in complex microbial ecosystems, could be studied using the squid–vibrio system, in which microbial functions can readily be evaluated by genetic manipulation of the bacterium. Reciprocally, some of the host responses that can be hard to study mechanistically in the squid could potentially be addressed in more genetically tractable organisms such as Drosophila, zebrafish, or even mice. One such example is Moriano-Gutierrez et al.’s (2) study of the atrial natriuretic peptide-converting enzyme (ANP-CE) gene. ANP-CE had significantly higher expression in the light organ of SYM animals compared with SYM-dark animals. This protein is associated with cell swelling (12), which is a hallmark following colonization by wild-type V. fischeri, but not SYM-dark mutants (5). Indeed, SYM animals demonstrated increased expression of ANP-CE in the crypt cells, which was not observed in the SYM-dark mutants (Fig. 1). These results emphasize the importance of also determining the spatial expression pattern in the host tissues, which may provide deeper understanding of the functional implications of transcriptome data. However, to causally link ANP-CE expression to crypt-cell swelling, interference of ANP-CE expression of function needs to be performed. Interestingly, Lactobacillus rhamnosus GR-1 administration to rats exposed to sustained coronary artery occlusion exhibited a significant attenuation of left ventricular hypertrophy based on tissue weight assessment and expression of atrial natriuretic peptide (13). These changes were associated with improved hemodynamic parameters, reflecting both enhanced systolic and diastolic left ventricular function and highlighting that microbial regulation of this gene is associated with tissue remodeling.
The study by Moriano-Gutierrez et al. (2) demonstrates the power of the squid–vibrio system in identifying microbial functions and their effect on the host. This system provides a potential avenue by which to study conserved cellular functions that can explain specific interactions in more complex symbiotic systems such as the mammalian gut.
Acknowledgments
I thank Anna Hallén for creating the artwork. F.B. is funded by the Wenner-Gren Foundations and is a recipient of the Torsten Söderberg Professorship in Medicine and the European Research Council Consolidator Grant 615362-METABASE.
Footnotes
The author declares no conflict of interest.
See companion article on page 7990.
References
- 1.Ley RE, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriano-Gutierrez S, et al. Critical symbiont signals drive both local and systemic changes in diel and developmental host gene expression. Proc Natl Acad Sci USA. 2019;116:7990–7999. doi: 10.1073/pnas.1819897116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 4.McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 5.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol. 2000;182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 7.Pankey MS, Minin VN, Imholte GC, Suchard MA, Oakley TH. 2014. Predictable transcriptome evolution in the convergent and complex bioluminescent organs of squid. 111:E4736–E4742. [DOI] [PMC free article] [PubMed]
- 8.Larsson E, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mardinoglu A, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11:834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belcaid M, et al. Symbiotic organs shaped by distinct modes of genome evolution in cephalopods. Proc Natl Acad Sci USA. 2019;116:3030–3035. doi: 10.1073/pnas.1817322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takei Y. Does the natriuretic peptide system exist throughout the animal and plant kingdom? Comp Biochem Physiol B Biochem Mol Biol. 2001;129:559–573. doi: 10.1016/s1096-4959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 13.Gan XT, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]