Significance
The emergence of uniquely human genes during hominid speciation enabled numerous human-specific adaptations that presumably included changes in resilience to disease but potentially increased susceptibility as well. Here we show that the transgenic expression of one such gene, called CHRFAM7A, changes the mouse reservoir of hematopoietic stem cells in bone marrow and amplifies the mouse inflammatory response in a model of human systemic inflammatory response syndrome (SIRS). Because the CHRFAM7A gene is a dominant-negative inhibitor of ligand binding to α7 nicotinic acetylcholine receptor (α7nAChR), a neurotransmitter receptor implicated in immunity, inflammation, neurodegeneration, and cognitive function, the results underscore the importance of understanding the contribution of species-specific genes to human disease and the impact they may have on the fidelity of animal models for translational medicine.
Keywords: inflammation, injury, hematopoietic stem cells, acetylcholine receptor, α7 nicotinic acetylcholine receptor
Abstract
A subset of genes in the human genome are uniquely human and not found in other species. One example is CHRFAM7A, a dominant-negative inhibitor of the antiinflammatory α7 nicotinic acetylcholine receptor (α7nAChR/CHRNA7) that is also a neurotransmitter receptor linked to cognitive function, mental health, and neurodegenerative disease. Here we show that CHRFAM7A blocks ligand binding to both mouse and human α7nAChR, and hypothesized that CHRFAM7A-transgenic mice would allow us to study its biological significance in a tractable animal model of human inflammatory disease, namely SIRS, the systemic inflammatory response syndrome that accompanies severe injury and sepsis. We found that CHRFAM7A increased the hematopoietic stem cell (HSC) reservoir in bone marrow and biased HSC differentiation to the monocyte lineage in vitro. We also observed that while the HSC reservoir was depleted in SIRS, HSCs were spared in CHRFAM7A-transgenic mice and that these mice also had increased immune cell mobilization, myeloid cell differentiation, and a shift to inflammatory monocytes from granulocytes in their inflamed lungs. Together, the findings point to a pathophysiological inflammatory consequence to the emergence of CHRFAM7A in the human genome. To this end, it is interesting to speculate that human genes like CHRFAM7A can account for discrepancies between the effectiveness of drugs like α7nAChR agonists in animal models and human clinical trials for inflammatory and neurodegenerative disease. The findings also support the hypothesis that uniquely human genes may be contributing to underrecognized human-specific differences in resiliency/susceptibility to complications of injury, infection, and inflammation, not to mention the onset of neurodegenerative disease.
Although it is generally agreed that the emergence of new, species-specific genes helped drive the phenotypic evolution of humans, just as they have done in other species (1, 2), there is a lack of direct evidence that links these genes to normal human biology and/or to the onset of traits that might be uniquely human in disease. While these genes may well have enabled adaptations of the species, contributed to processes that are now considered normal human physiology, and even altered human resilience and susceptibility to disease and infection (3), their direct study is unusually difficult. First and foremost, these genes are either absent in animal models of human disease (4–6), or alternatively present in animals but not humans (7, 8). To date, the study of their significance can only be inferred by human clinical research.
The CHRFAM7A gene is a case in point (9, 10). It originally arose in the hominin genome when five exons of the UL Kinase-4 gene on chromosome 3 translocated to, and then fused with, the Dupa gene (11, 12), a gene that is found in many species that originated with 5 exons partially duplicated from the 10-exon α7 nicotinic acetylcholine receptor (α7nAChR/CHRNA7) gene on the forward strand of chromosome 15 (Fig. 1A). Accordingly, CHRFAM7A is a hybrid gene that is in close physical proximity to CHRNA7 and encodes a protein with structural similarity to α7nAChR. Both genes are independently regulated by unique promoters that drive their differential expression (13, 14). Moreover, several genetic studies have tied the CHRFAM7A/CHRNA7 locus and a 2-bp mutation in CHRFAM7A on chromosome 15 to nicotine dependence, schizophrenia, cognitive dysfunction, and bipolar and neurodegenerative disease (9, 10).
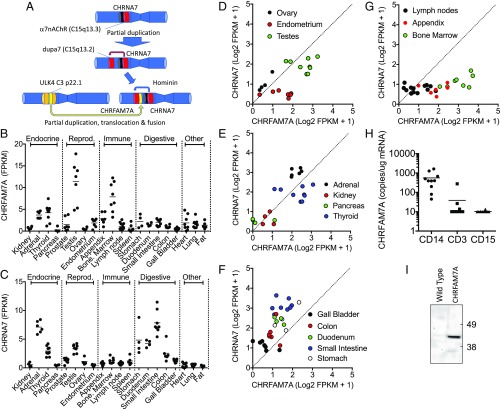
Fig. 1.
CHRFAM7A is widely distributed in human tissues and overrepresented in bone marrow. (A) Schematic representation of the origin of CHRFAM7A in the human genome. The 10-exon α7nAChR (CHRNA7) gene of many species underwent a partial duplication and translocation to create a 5-exon gene in near proximity to CHRNA7. A second partial translocation of 5 exons originating from a ULK-4 (FAM) gene of chromosome 3 then fused with this sequence to create a unique chimeric 10-exon gene called CHRFAM7A that is found in both Homo sapiens (https://www.ensembl.org/Homo_sapiens/Info/Index) and Homo neanderthalis gene databases (neandertal.ensemblgenomes.org), and encodes identical 412-amino acid proteins with a new and unique 27-amino acid sequence in its extracellular ligand-binding domain. (B and C) Mining the distribution of CHRFAM7A and CHRNA7 expression in human tissues. Gene expression data were scraped from the Human Protein Atlas (Methods) as curated on GenTree in July 2018. It shows that CHRFAM7A is widely expressed in humans, particularly in reproductive and immune tissues. These data, along with expression in discrete areas of the brain, were confirmed by qPCR (SI Appendix, Fig. S1). (D–G) Proportional representation of CHRFAM7A and CHRNA7 expression in human tissues. There is bias in CHRFAM7A expression in reproductive tissues like endometrium and testes (D) but expression appears evenly distributed between CHRFAM7A and CHRNA7 in endocrine tissues (E). This is in contrast to digestive tissues like the small intestine, colon, and duodenum, which show a bias toward the parental CHRNA7 gene expression (F). Immune tissues like the lymph nodes, bone marrow, and appendix show a preponderance of CHRFAM7A gene expression (G). (H) Expression of CHRFAM7A expression in circulating immune cells from healthy volunteers. CHRFAM7A expression is readily detected in CD14+ myeloid cells, with the expression of CHRFAM7A in lymphocytes (CD3+ cells) and granulocytes (CD15+ cells) at the detection limit (10 copies per μg mRNA) of the assay. (I) The bone marrow of transgenic mice expresses a CHRFAM7A protein. Immunoblotting of bone marrow lysates with an antibody raised to a unique 27-amino acid sequence of CHRFAM7A not present in CHRNA7 reveals translation of a protein with the predicted 43-kDa molecular size of CHRFAM7A. It is not detected in bone marrow lysates prepared from wild-type C57 mice. FPKM, fragments per kilobase of transcript per million mapped reads.
Several recent studies have suggested that CHRFAM7A encodes a dominant-negative inhibitor of ligand binding to α7nAChR in vitro (15–18), which can also be observed at the neuromuscular junction of transgenic mice (19). As such, CHRFAM7A has been implicated in the regulation of numerous α7nAChR-dependent antiinflammatory processes as well as in mental health, cognition, and neurodegenerative disease (20). In peripheral tissues, there has been significant interest in the role of α7nAChR in the neural control of inflammation (21), where it has been implicated in gauging both local and systemic inflammatory responses after burn, hemorrhage, sepsis, arthritis, and wound healing, to name a few (22, 23). With no cognate CHRFAM7A hybrid gene in other species, however, the physiological and pathophysiological consequences of its expression in humans are not known and not manifested in standard models of human disease (3, 10, 13). Accordingly, because α7nAChR agonists have been very effective in mouse models of human disease but nevertheless disappointing in human clinical studies (24), we deemed it necessary to assess the significance of CHRFAM7A to cholinergic signaling in an animal model of inflammation. To this end, we engineered transgenic mice to express CHRFAM7A in both central nervous system and peripheral tissues, as it is normally observed in humans. We then explored the consequences of its expression for the inflammatory response to injury, namely the phenomena of bone marrow exhaustion and emergency myelopoiesis that characterize the systemic inflammatory response syndrome (SIRS) that leads to organ failure and death in humans after severe injury.
Results
CHRFAM7A Is Widely Distributed in Human Tissues.
Like many new genes that emerge during speciation (25–29), we found that the expression of CHRFAM7A is high in reproductive tissues (Fig. 1B). In addition, we noted overrepresentation of CHRFAM7A relative to CHRNA7 in human bone marrow (Fig. 1C). This observation supports our earlier hypothesis that the emergence of CHRFAM7A could have contributed changes to immunity and human resilience/sensitivity to injury, infection, and/or inflammatory responsiveness (3, 9, 30). Because the product of CHRFAM7A regulates ligand binding to α7nAChR (15–18) encoded by CHRNA7, we compared the relative expression of CHRNA7 and CHRFAM7A in human tissues (Fig. 1 D–H). Reproductive organs showed bias toward increased CHRFAM7A expression (Fig. 1D), while endocrine tissues displayed no difference (Fig. 1E). Digestive tissues showed increased CHRNA7 expression over CHRFAM7A (Fig. 1F), possibly reflecting the protective role of CHRNA7 in the neural control of gut inflammation (31–33). Bone marrow, however, like in the appendix, was particularly elevated in relative CHRFAM7A expression (Fig. 1G). In blood, circulating CD14+ monocytes, but not CD3+ lymphocytes or CD16+ granulocytes, express CHRFAM7A (Fig. 1H). Additional surveys of human peripheral and central nervous system tissues confirmed this wide tissue distribution of CHRFAM7A (SI Appendix, Fig. S1).
CHRFAM7A Expression Increases Differentiation of Hematopoietic Stem Cells to Monocytes over Granulocytes in Vitro.
To develop a tractable experimental model capable of evaluating the functional consequences of CHRFAM7A, we first showed that the CHRFAM7A gene encodes a functional protein in mouse cells (SI Appendix, Fig. S2) that inhibits ligand binding to both human α7nAChR transfected in BALB/c 3T3 fibroblasts (SI Appendix, Fig. S2 A–E) and endogenous mouse α7nAChR on RAW264-7 macrophages (SI Appendix, Fig. S2 F–L). Most notably, we observed the same increase in cell–cell adhesion in mouse RAW264-7 macrophages (SI Appendix, Fig. S2H) as we previously reported in CHRFAM7A-transfected human THP1 monocytes (13). Because these findings establish that CHRFAM7A is active in a mouse genetic background, we then generated two independent lines of transgenic CHRFAM7A mice (SI Appendix, Figs. S3 and S4) that produce a 43-kDa CHRFAM7A protein in tissues under control of the EF1α promoter, in tissues such as bone marrow (Fig. 1I). These naive mice were indistinguishable from their wild-type siblings and showed no morphological or gross hematological phenotype (SI Appendix, Fig. S5).
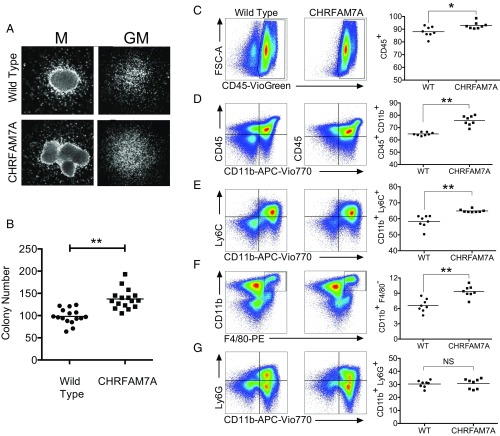
Based on the elevated expression of CHRFAM7A in human bone marrow (Fig. 1 B and G) and monocytes (Fig. 1H), we focused on the effects of CHRFAM7A expression on myelopoiesis, and specifically on whether CHRFAM7A would affect colony formation of bone marrow cells differentiated with GM-CSF in vitro (Fig. 2). To this end, bone marrow from both transgenic and sibling mice was placed into MethoCult for 7 d and colony-forming potential was measured. Viability (SI Appendix, Fig. S6) and morphology (Fig. 2A) of the colonies that emerged were indistinguishable. Unexpectedly, significantly more colonies from CHRFAM7A-transgenic mouse bone marrow expanded from equal numbers of starting cells in differentiation media (Fig. 2B). Analyses of these colonies using flow cytometry revealed a significant increase in the percentage of some, but not all, lineage-committed CD45+ and CD11b+ cells (Fig. 2 C–G). While there was increased differentiation of cells to CD11b+Ly6C+ monocytes (Fig. 2E) and CD11b+F4/80+ macrophages (Fig. 2F), compared with cells from wild-type controls, there was no difference in the percentage of CD11b+Ly6G+granulocytes (Fig. 2G). These data suggest that CHRFAM7A promoted expansion of monocytes over granulocytes.
Fig. 2.
MethoCult identifies an increase in colony-forming activity and bias to monocyte lineage. (A) Morphology of colonies from wild-type and CHRFAM7A-transgenic mice. The appearance of macrophage (M) and granulocyte-monocyte (GM) colonies expanded from 3 × 103 bone marrow cells from wild-type and CHRFAM7A-transgenic mice in GM differentiating MethoCult is morphologically indistinguishable. Cells were examined under phase-contrast microscopy and photographed. (B) Colony numbers in GM differentiating MethoCult. After 7 d in MethoCult culture, the numbers of colonies that arose after plating 3 × 103 wild-type or CHRFAM7A-transgenic bone marrow cells per dish were determined. Differences in 16 replicates performed from 8 donor mice were analyzed by unpaired, two-tailed t test (P < 0.01). The horizontal line shows the mean. (C–G) Flow cytometry of bone marrow cells differentiated in vitro. After the exclusion of debris, doublets, and nonviable cells (SI Appendix, Fig. S10), flow cytometry was used to identify and measure changes in myeloid differentiation including CD45+ (C) and CD45+CD11b+ (D) leukocytes, and CD11b+Ly6C+ monocytes (E) and F4/80+CD11b+ macrophages (F). There was no difference in CD11b+Ly6G+ granulocytes (G). Differences in eight mice were analyzed by unpaired two-tailed t test (*P < 0.05, **P < 0.01; NS, not significantly different). The horizontal line shows the mean.
CHRFAM7A Expression Increases the Hematopoietic Stem Cell Reservoir.
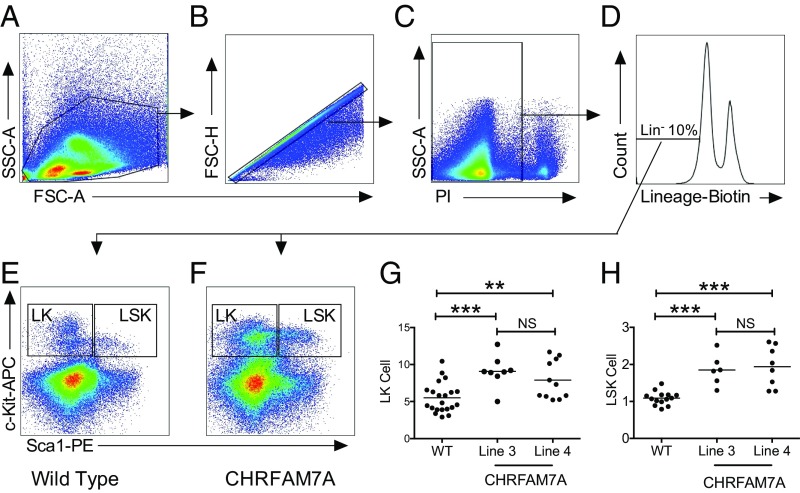
To assess whether CHRFAM7A also affects myeloid cell expansion in vivo, we analyzed the bone marrow of CHRFAM7A-transgenic mice for hematopoietic stem cells (HSCs) and compared the findings with sibling controls and wild-type C57BL/6J mice (Fig. 3 and SI Appendix, Fig. S7). To detect HSCs, we used c-Kit and Sca1 antibodies on the subpopulation of lineage-negative (Lin−) cells and quantified the Lin−Sca1+c-Kit+ (LSK) and Lin−c-Kit+ (LK) cells in bone marrow of wild-type and CHRFAM7A mice (Fig. 3 A–F). As shown in Fig. 3 G and H, there were significantly more LSK and LK cells in the bone marrow of CHRFAM7A mice compared with wild-type controls. This increase was also observed in two independent lines of CHRFAM7A-transgenic mice. Because the percentages of cells recovered from sibling or commercial C57BL/6J mice were indistinguishable (SI Appendix, Fig. S7 A and B), we concluded that the increased colony formation in vitro (Fig. 2) was attributable to an increase in the HSC reservoir in the bone marrow of CHRFAM7A-transgenic mice.
Fig. 3.
Hematopoietic stem cells are increased in CHRFAM7A-transgenic bone marrow. (A–F) Flow cytometry of mouse lineage− Sca1+ c-Kit+ (LSK) hematopoietic stem cells. After the exclusion of debris (A), doublets (B), and nonviable (C) cells from the gate shown in A, ∼10% of cells were lineage (Lin−) marker-negative (D) and used to gate for c-Kit and Sca1 expression in Lin− bone marrow cells from wild-type (E) or CHRFAM7A (F) mice. These analyses revealed two subsets of cells enriched for Lin−, c-Sca1+, and Kit+ (LSK) and Lin− c-Kit+ (LK) cells. (G and H) Quantification of mouse LSK and LK hematopoietic stem cells. Each of the gates shown in B and C (Far Right) was applied to bone marrow cells of either wild-type and sibling nontransgenic mice or CHRFAM7A-transgenic mice harvested from line 3 or line 4. Having demonstrated that the numbers were not different between siblings and wild-type C57/Bl6 mice (SI Appendix, Figs. S6 and S7), data were combined. One-way ANOVA was used to compare groups (**P < 0.01, ***P < 0.001). The line shows the mean.
CHRFAM7A Protects Burn-Induced HSC Exhaustion and Redirects Myelopoiesis to Monocyte Lineage.
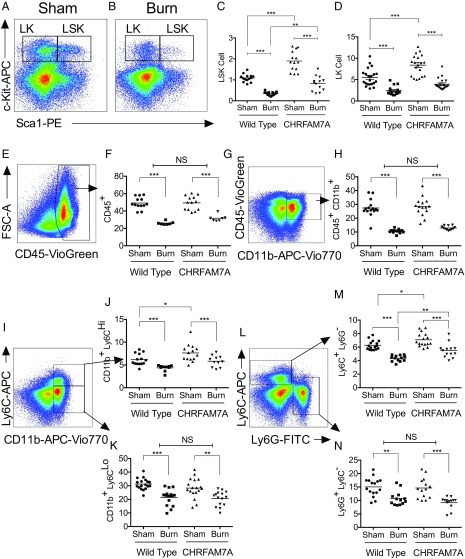
In humans (SI Appendix, Fig. S8), the percentage of CD34+ circulating progenitor cells decreases after severe burn injury, presumably as cells are mobilized into affected tissues. To explore whether the transgenic expression of CHRFAM7A would affect the fate of LSK cells in vivo, we used an experimental model of burn injury that induces emergency myelopoiesis and the depletion of HSCs in bone marrow like SIRS to cause significant morbidity and mortality in humans (34, 35). In this model, a cutaneous burn mobilizes myeloid cells from the bone marrow and stimulates immune cell trafficking to peripheral tissues like lung (36–39) to cause long-term depletion of HSCs and bone marrow exhaustion (37). Accordingly, it is possible to monitor changes in bone marrow by flow cytometry (Fig. 4 A and B) and quantify the decrease of LK and LSK cells as they differentiate and mobilize after burn injury to wild-type and CHRFAM7A-transgenic mice (Fig. 4 C and D). Unlike wild-type mice, there was no near depletion of LK or LSK cells in the bone marrow of CHRFAM7A mice after burn injury. Instead, the percentage of LSK cells most notably decreased to levels that resembled those of uninjured wild-type mice. These findings suggest that CHRFAM7A spares the bone marrow HSC reservoir from depletion following severe injury.
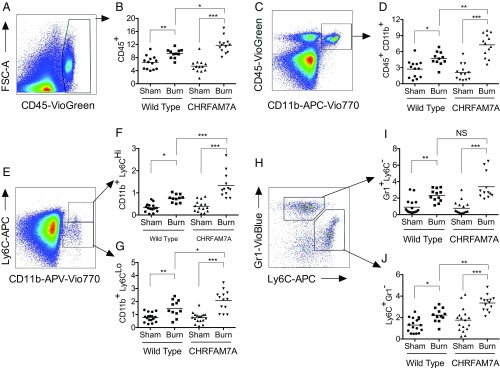
Fig. 4.
CHRFAM7A spares bone marrow HSCs after burn with bias for monocyte lineage. (A and B) Flow cytometry analysis of mouse c-Kit+ Sca1+ lineage− hematopoietic stem cells after thermal injury. The gating strategy that separates LSK and LK hematopoietic progenitor populations in mouse bone marrow without (A) or after (B) a 4-h burn injury enables quantification of the LK (c-Kit+Sca1−) and LSK (c-Kit+Sca1+) percentage of cells gated out of the ∼10% lineage-negative staining cells. (C and D) Quantification of mouse hematopoietic LSK and LK stem cells in bone marrow of wild-type and CHRFAM7-transgenic mice. Quantification of LSK (C) and LK (D) hematopoietic progenitor populations from WT and CHRFAM7A mouse bone marrow after sham or a 4-h thermal injury. With no difference between C57BL/6 or siblings and transgenic line 3 or 4, data were combined for analyses by one-way ANOVA (**P < 0.01, ***P < 0.001). (E–N) Quantification of mouse myeloid cells in bone marrow after burn injury. After the exclusion of debris, doublets, and nonviable cells (SI Appendix, Fig. S10), resident myeloid cell subset lineages were assessed by flow cytometry in bone marrow without (sham) or with thermal injury (burn) in wild-type and CHRFAM7A-transgenic mice. Differences in immune cells including CD45+ leukocytes (E and F), CD45+CD11b+ monocytes (G and H), CD11b+Ly6CHigh/Low (I–K), and Ly6CHighLy6GLow and Ly6GHighLy6CLow (L and M) inflammatory, resident, and patrolling macrophages and granulocytes were calculated. With no observable differences between wild-type C57BL/6 siblings and commercial mice or between CHRFAM7A-transgenic lines 3 and 4, respectively (SI Appendix, Fig. S7), data were combined and differences were analyzed by one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001).
We next determined whether the sparing of the HSC reservoir in CHRFAM7A mice was accompanied by changes in commitment of myeloid cells to monocyte lineage, like that observed in vitro. In both wild-type and CHRFAM7A mice, these analyses (Fig. 4 E–N) identified a burn-induced reduction in all cell types evaluated, including resident CD45+ and CD45+CD11b+ leukocytes (Fig. 4 E–H), CD11b+Ly6CHigh inflammatory monocytes (Fig. 4 I and J), and Ly6G+Ly6C− granulocytes (Fig. 4 K and N), presumably as cells are mobilized to peripheral tissues. The monocyte response of both wild-type and CHRFAM7A-transgenic mice to burn injury was qualitatively similar but quantitatively distinct. This was because CHRFAM7A mice started with a higher number of basal CD11b+Ly6CHigh inflammatory monocytes in their bone marrow (Fig. 4 I, J, L, and M). In contrast, we noted that the burn-induced decreases in CD11b+Ly6CLow monocytes (Fig. 4 I and K) and Ly6G+Ly6C− granulocytes (Fig. 4 L and N) were not different, reflecting a shift in the mobilization of CD11b+Ly6CHigh (Fig. 4J) and Ly6G−Ly6C+ (Fig. 4M) inflammatory monocytes over CD11b+Ly6CLow (Fig. 4K) or Ly6G+Ly6C− (Fig. 4N) cells. These data establish that the CHRFAM7A-dependent increase in monocyte lineage shown in vitro is also detected in vivo. The phenotype appears to be bone marrow-dependent, because the splenic myeloid cell response to burn was not affected by CHRFAM7A expression (SI Appendix, Fig. S9). We concluded that the main consequence of CHRFAM7A expression is likely tied to its ability to increase myelopoiesis, and with it spare the HSC reservoir in the bone marrow niche following injury.
CHRFAM7A Increases and Redirects Differentiation of Myeloid Cells Mobilized to Injured Lung.
Despite CHRFAM7A sparing the HSC reservoir after injury, the CHRFAM7A-transgenic mice also show an increase in myeloid cell mobilization to affected lungs (Fig. 5). This myeloid cell mobilization appears injury-dependent. The percentages of CD45+ leukocytes were similar in uninjured wild-type and CHRFAM7A mouse lungs. In contrast, the percentages of CD45+ (Fig. 5 A and B) and CD45+CD11b+ (Fig. 5 C and D) leukocytes mobilized to lungs post injury were significantly increased in CHRFAM7A mice. This is consistent with an early and rapid mobilization of inflammatory cells that would characterize a SIRS-like response (37, 40).
Fig. 5.
CHRFAM7A increases myeloid migration to lung after burn injury with a bias toward monocyte lineage. Flow cytometric analyses of enzymatically digested lung cells were obtained from mice 4 h after a 7-s thermal injury to show that, after the exclusion of debris, doublets, and nonviable cells (SI Appendix, Fig. S10), different myeloid immunophenotypes can be distinguished, identified, and quantified. As such, total CD45+ staining leukocytes (A and B), CD45+CD11b+ monocytes (C and D), and CD11b+Ly6CHigh/Low inflammatory/patrolling monocytes (E–G) are all significantly increased in lung after burn injury in both wild-type and CHRFAM7A-transgenic mice. Whereas basal percentages are not qualitatively different, there is a significantly increased quantitative response in lungs of CHRFAM7A mice to the burn injury in CD45+ staining leukocytes (B, P < 0.05), CD45+CD11b+ monocytes (D, P < 0.05), and CD11b+Ly6CHigh/Low inflammatory/patrolling monocytes (F, P < 0.001; G, P < 0.05). In contrast, the percentage of Gr1+Ly6C− granulocytes (H and I) increased in response to burn injury (P < 0.05) but there was no effect of CHRFAM7A. The Ly6C+Gr1− monocyte population, however (H and J), is similarly increased by burn (P < 0.05) but further increased (P < 0.01) in lungs of CHRFAM7A-transgenic mice. Differences between the groups were analyzed using one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001).
There was also a significant difference in the appearance of more differentiated myeloid cells in the CHRFAM7A-transgenic mice compared with their wild-type counterparts (Fig. 5 E–J). Basal percentages of lung immune cells measured in sham mice were the same in all groups, but there were burn-induced differences and CHRFAM7A-dependent differences in monocytic but not granulocytic lineage. These included increased CD11b+Ly6CHigh inflammatory and CD11b+Ly6CLow patrolling monocytes (Fig. 5 E–G and J) in the injured lung. In contrast, while the percentages of granulocytes (i.e., Gr1+Ly6C−; Fig. 5 H and I) were increased in injured lungs, there was no difference between wild-type and CHRFAM7A mice. Like bone marrow in vitro and in vivo, the expression of CHRFAM7A redirects myeloid differentiation to monocytes, presumably to alter the cellular response in the inflamed target tissue.
Discussion
We observed an increased HSC reservoir in the bone marrow of two independent lines of CHRFAM7A-transgenic mice. These findings imply that the emergence of CHRFAM7A expression in humans is relevant to physiological and pathophysiological processes in which the HSC reservoir is thought to be critically important. Examples include the HSC response to injury as shown here, but likely extend to other inflammatory responses, to infection and changes observed during lifespan, healthspan, and normal aging (34, 41–48). Because α7nAChR has been detected in a subset of bone marrow stem cells and cholinergic signaling in bone marrow tied to HSC and leukocyte migration (49, 50), the findings are also compatible with, and add to, predictions that the interference of agonist binding to α7nAChR by CHRFAM7A expression will impact α7nAChR-dependent activities. That being said, they do not preclude the possibility that there exist CHRFAM7A activities that are independent of α7nAChR or couple with other nAChRs. These findings are compatible with the immunophenotyping of bone marrow, spleen, and lung myeloid cells showing a CHRFAM7A-dependent bias toward monocyte differentiation after induction of SIRS and an exacerbation of myeloid cell mobilization to lung in the experimental mouse model of emergency myelopoiesis. Accordingly, the data all support a function for CHRFAM7A in human inflammation, in the development, maintenance, and mobilization of bone marrow HSCs, and, by inference, human-specific resilience and susceptibility to disease. With this in mind, we speculate that the wide distribution of CHRFAM7A in humans could be regulating α7nAChR-dependent inflammatory responses in a species-specific manner and gauging α7nAChR activity in disease processes where it has already been implicated: arthritis, cancer, cardiovascular disease, injury repair, sepsis, tissue regeneration, and impaired wound healing (51–55).
There are a large number of studies that have characterized the inflammatory consequences of severe burn, trauma, and infection for both the depletion and mobilization of cells in tissues like lung (37, 38, 45, 56). Acute injury, including severe cutaneous burn, results in a rapid response by the immune system. While these immune cells respond and traffic to sites of tissue injury, the bone marrow must also recover from the injury in a process called emergency myelopoiesis (34, 41–48). This myelopoiesis occurs through differentiation of HSCs in bone marrow and provides a renewed source of immune cells capable of responding to subsequent insults (57, 58). Myelopoiesis, however, also occurs in extramedullary tissues such as the spleen (59). Here, we find that CHRFAM7A expression increased the bone marrow reservoir of HSCs, but there was no effect on myelopoiesis in the spleen. Interestingly, expansion of the HSC reservoir and increased myelopoiesis have been shown to improve animal survival to challenges, for example with LPS (58). It is therefore interesting to speculate that this retention of HSCs in CHRFAM7A-transgenic mice could even be protective. If so, the inflammatory responses that accompany severe injury could have helped drive the emergence of CHRFAM7A, its selection, and its subsequent retention in the hominid genome. To this end, we have noted that some injuries, such as burn, track to species-specific behaviors that are both unique to, and ubiquitous within, human societies.
Surprisingly, we noted that in the SIRS response after severe burn injury at 4 h, there is an increase in immune cell trafficking to the lung in the CHRFAM7A-transgenic mice, while these same animals retain more HSCs in their bone marrow than their wild-type counterparts. These observations raise the possibility that in addition to altering the acute injury response, the retention of an HSC reservoir could increase the availability of HSCs to better respond to subsequent disruptions, called a “second hit” (42, 45, 60). This clinical phenomenon is critical to survival after injury and polytrauma because it has the potential to limit the SIRS response that leads to acute lung injury and the onset of multiorgan dysfunction syndrome that accompanies severe injury and leads to high mortality (34, 42, 43, 60).
Nearly 20 y ago, the possibility that taxonomically restricted genes specify species-specific responsiveness garnered attention with the discovery of cmah (7), a gene whose product is absent in humans but nevertheless regulates resilience, susceptibility, and the response to infection and injury in other species (8, 61, 62). The present study with CHRFAM7A in transgenic mice was made possible because we were first able to show that CHRFAM7A recognizes and regulates the mouse form of α7nAChR (19). We posit that it is this interaction that most likely generates the HSC phenotype reported here and predict that CHRNA7 knockout mice could have the same phenotype. That being said, it is noteworthy that this transgenic mouse approach cannot address the possibility that CHRFAM7A, perhaps as homopentamers or heteropentamers with other cholinergic subunits, has distinct activities or regulates other uniquely human genes. To this end, we note that gene expression analyses after stable transfection of the monocyte THP1 cell line (13) suggested that CHRFAM7A regulates TPTEP1 (63), a uniquely human endogenized retroviral sequence that encodes an AT-hook protein reminiscent of transcription factors (64). These findings suggest the existence of human-specific signaling that would be absent in the transgenic background. This possibility makes it critically important to determine the consequences of uniquely human gene expression to human physiology and, even more importantly, the development of better methods to study their effects in animal models of human disease.
Finally, it is noteworthy that the findings presented here point to how the emergence of a species-specific gene might gauge myeloid cell mobilization, and with it, inflammatory responsiveness. It is a mechanism that might be contributing to the observation that systemic inflammatory responses to severe injury and infection in humans and mice are genetically distinct (65–67). Qualitatively, different species share numerous inflammatory responses (56, 68) that can be ascribed to common cellular mechanisms. These include immune cell mobilization to the sites of tissue injury and infection (40) for pro- and antiinflammatory functions in pathogen phagocytosis, infection control, and tissue repair (69). CHRFAM7A, however, presents a unique molecular variation to an antiinflammatory pathway that is otherwise shared by numerous species. This variation could be critical in targeting human therapeutics. For example, it is interesting to speculate that the translational gap that characterizes the efficacy of drugs targeting α7nAChR in animal models and humans (24) might be attributable to the presence of CHRFAM7A in humans but not other species. If so, regulating CHRFAM7A expression in humans could confer a newfound efficacy in humans and utility of a wide class of drugs originally developed to target, and proven effective to treat, α7nAChR-mediated inflammatory and neurodegenerative disease in animal models.
Methods
Details for all materials and methods are described in SI Appendix.
Gene Expression in Normal Human Tissues.
Two strategies were used to assess gene expression in normal tissues: (i) mining (August 2018) expression data in the Human Protein Atlas (https://www.proteinatlas.org) as reported in GenTree (gentree.ioz.ac.cn), a database comparing species-specific gene assembly (29), and (ii) qRT-PCR of cDNAs in OriGene Tissue Scan Arrays prepared of normal human peripheral tissues and human brain.
Recruitment of Human Volunteers for Blood Donation.
Blood was collected from normal and burn patients after informed consent. The University of California, San Diego, Institutional Review Board approved the consent forms, specimen collection protocols, and guidelines for study procedures.
Isolation of CD14+, CD15+, and CD3+ Cells from Blood, HSC Colony Formation, and in Vitro Studies.
An autoMACS Pro Separator (Miltenyi Biotec) was used to isolate CD3+, CD14+, and CD15+ cells from human blood according to the manufacturer’s recommendations, and cells were processed for RNA isolation, cDNA synthesis, and PCR as described below. In vitro colony formation of bone marrow cells from sibling (nontransgenic) and transgenic mice was assessed in MethoCult colony formation assays according to the manufacturer’s (Stem Cell Technologies) recommendations. Cells were diluted in granulocyte-macrophage (GM) MethoCult media, incubated 7 d, photographed, and assessed by flow cytometry. Mouse BalbC 3t3 fibroblasts and RAW264.7 macrophages were cultured, transfected, and analyzed for CHRFAM7A expression and bungarotoxin binding by immunoblotting, fluorescence microscopy, and flow cytometry, as described in the methods of SI Appendix. The effects of CHRFAM7A on RAW cell morphology was assessed by microscopy (SI Appendix, Fig. S2). To purify leukocytes from human blood and process for extraction, the μMACS mRNA Isolation Kit (Miltenyi Biotec; 130-075-201) was used per the manufacturer’s protocol with mechanical shearing and cDNA synthesis as described in further detail in SI Appendix.
Transgenic Mice and PCR.
Transgenic mice were generated by contract with Cyagen with CHRFAM7A under control of the elongation factor 1A (EF1A) promoter (SI Appendix, Fig. S1). Of the four mouse lines obtained (SI Appendix, Fig. S2), lines 3 and 4 were used in all of the experiments to ensure that differences could be attributed to CHRFAM7A, and nontransgenic siblings were used as controls as indicated. Primers for PCR included CHRFAM7A [forward (F)] 5′-ATAGCTGCAAACTGCGATA-3′ and [reverse (R)] 5′-CAGCGTACATCGATGTAGCAG-3′; human CHRNA7 (F) 5′-ACATGCGCTGCTCGCCGGGA-3′ and (R) 5′-GATTGTAGTTCTTGACCAGCT-3′; mouse CHRNA7 (F) 5′-CGTGGGCCTCTCTGTAGTGG-3 and (R) 5′-CTGGAGTTGGGGCACAGTGC-3′; and rat/mouse GAPDH QuantiTect QT01658692 and human GAPDH (F) 5′-CATGAGAAGTATGACAACAGCCT-3′ and (R) 5′-AGTCCTTCCACGATACCAAAGT-3′. Relative gene expression was calculated by either comparing the thermal cycle number (Ct) with that of GAPDH or with a standard curve generated with plasmid, and expressed as either a reciprocal of dCt, plasmid copy number, or fold increase using the ∆∆Ct method.
Animal Model of Cutaneous Thermal Injury.
All animal studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (70) and preapproved by the University of California, San Diego, Animal Subjects (Institutional Animal Care and Use) Committee. CHRFAM7A-transgenic lines were propagated as heterozygotes, and sibling nontransgenic and CHRFAM7A-transgenic mice were obtained through the University of California, San Diego, breeding core once identified as transgenic by tail clip analyses (Transnetyx). Only male mice were used in the studies reported here, because female mice have differential sensitivity to thermal injury. Age-matched C57BL/6 mice were also purchased from Jackson Laboratories for cross-over studies. Animals received buprenorphine for pain control, and 8 cm2 exposed to steam for 7 s. Animals then received an s.c. injection of 1 mL normal saline and sacrificed 4 h later.
Flow Cytometry.
Bone marrow cells from wild-type, sibling (nontransgenic), or transgenic mice were analyzed after 7 d of differentiation in GM MethoCult in vitro using a myeloid cell antibody panel capable of detecting monocytes, macrophages, granulocytes, and dendritic cells with anti-Gr1, anti-CD45, anti-Ly6G, anti-F4/80, anti-CD11c, anti-Ly6C, and anti-CD11b, and propidium iodide (PI) to cells to assess cell viability (SI Appendix, Table S1). Compensation settings were established using MACS Comp Bead Kits. To assess bone marrow directly, cells were collected from tibia and femur and stained for analyses in a multipanel flow cytometer (MACS; Miltenyi Biotec) using an antibody panel capable of detecting c-kit+ and Sca1+ cells in a lineage-negative (Lin−) HSC population called LSK cells (SI Appendix, Table S2). In contrast, lung or spleen tissues were dissociated into single-cell suspensions using an enzymatic digestion and a mechanical gentleMACS dissociator according to the manufacturer’s instructions. Splenic single-cell suspensions were then submitted to myeloid cell immunostaining using the antibody panel of SI Appendix, Table S1 (above), while another panel (SI Appendix, Table S3) was adapted for the detection of lung alveolar macrophages and dendritic cells). A myeloid subset of cells in lung was measured using a panel composed of anti-Gr1, anti-MHCII, anti-F4/80, anti-CD11c, anti-Ly6C, and anti-CD11b (SI Appendix, Table S4). Compensation settings were set by MACS Comp Bead Kits. With no difference between C57BL/6 or siblings and transgenic lines 3 or 4 (SI Appendix, Figs. S6 and S7), data from both mouse lines were combined for analyses unless indicated otherwise.
Statistical Analysis.
All data and calculations were performed using GraphPad Prism software (version 6.0) including ANOVA and t tests where appropriate as indicated in the figure legends.
Data Availability.
The authors confirm that all relevant data are included in the paper and/or its SI Appendix and available on request from the authors. Source data for Fig. 1 are provided from the Human Protein Atlas (https://www.proteinatlas.org) as scraped from GenTree (gentree.ioz.ac.cn) and described in Methods.
Supplementary Material
Acknowledgments
We thank Ms. Emelie Amburn and Ms. Annemarie Hageny for expert technical assistance, Professor Raul Coimbra for discussions regarding the cholinergic control of inflammation, and the Center for Academic Research and Training in Anthropogeny (CARTA) at University of California, San Diego (https://carta.anthropogeny.org), for insight into the implications of anthropogeny for medicine and health and the cellular and molecular explorations of anthropogeny. Research was supported by operating grants from the Department of Defense (W81XWH-10-1-0527), National Institutes of Health (1R01GM121530-02), and University of California, San Diego, Department of Surgery Reinvestment Fund; and Fellowship Training grants from the National Institutes of Health (T32-DC000024), Shock Society (T. W. Chan), and Surgical Infection Society (E.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821853116/-/DCSupplemental.
References
- 1.Chen S, Krinsky BH, Long M. New genes as drivers of phenotypic evolution. Nat Rev Genet. 2013;14:645–660. doi: 10.1038/nrg3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long M, VanKuren NW, Chen S, Vibranovski MD. New gene evolution: Little did we know. Annu Rev Genet. 2013;47:307–333. doi: 10.1146/annurev-genet-111212-133301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird A, Costantini T, Coimbra R, Eliceiri BP. Injury, inflammation and the emergence of human-specific genes. Wound Repair Regen. 2016;24:602–606. doi: 10.1111/wrr.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florio M, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- 5.Stahl PD, Wainszelbaum MJ. Human-specific genes may offer a unique window into human cell signaling. Sci Signal. 2009;2:pe59. doi: 10.1126/scisignal.289pe59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki IK, et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through delta/notch regulation. Cell. 2018;173:1370–1384.e16. doi: 10.1016/j.cell.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou HH, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okerblom JJ, et al. Loss of CMAH during human evolution primed the monocyte-macrophage lineage toward a more inflammatory and phagocytic state. J Immunol. 2017;198:2366–2373. doi: 10.4049/jimmunol.1601471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantini TW, Dang X, Coimbra R, Eliceiri BP, Baird A. CHRFAM7A, a human-specific and partially duplicated α7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J Leukoc Biol. 2015;97:247–257. doi: 10.1189/jlb.4RU0814-381R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinkus ML, et al. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology. 2015;96:274–288. doi: 10.1016/j.neuropharm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gault J, et al. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7) Genomics. 1998;52:173–185. doi: 10.1006/geno.1998.5363. [DOI] [PubMed] [Google Scholar]
- 12.Riley B, Williamson M, Collier D, Wilkie H, Makoff A. A 3-Mb map of a large segmental duplication overlapping the alpha7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics. 2002;79:197–209. doi: 10.1006/geno.2002.6694. [DOI] [PubMed] [Google Scholar]
- 13.Costantini TW, et al. A human-specific α7-nicotinic acetylcholine receptor gene in human leukocytes: Identification, regulation and the consequences of CHRFAM7A expression. Mol Med. 2015;21:323–336. doi: 10.2119/molmed.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang X, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A: A human-specific α7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 2015;29:2292–2302. doi: 10.1096/fj.14-268037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lucas-Cerrillo AM, et al. Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: Potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606. doi: 10.1074/jbc.M110.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasala M, Corradi J, Bruzzone A, Esandi MDC, Bouzat C. A human-specific, truncated α7 nicotinic receptor subunit assembles with full-length α7 and forms functional receptors with different stoichiometries. J Biol Chem. 2018;293:10707–10717. doi: 10.1074/jbc.RA117.001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldifassi MC, et al. Interaction of the α7-nicotinic subunit with its human-specific duplicated dupα7 isoform in mammalian cells: Relevance in human inflammatory responses. J Biol Chem. 2018;293:13874–13888. doi: 10.1074/jbc.RA118.003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, et al. The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J Biol Chem. 2014;289:26451–26463. doi: 10.1074/jbc.M114.582858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan T, et al. CHRFAM7A alters binding to the neuronal alpha-7 nicotinic acetylcholine receptor. Neurosci Lett. 2019;690:126–131. doi: 10.1016/j.neulet.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lendvai B, Kassai F, Szájli A, Némethy Z. α7 nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull. 2013;93:86–96. doi: 10.1016/j.brainresbull.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Fujii T, et al. Physiological functions of the cholinergic system in immune cells. J Pharmacol Sci. 2017;134:1–21. doi: 10.1016/j.jphs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis AS, van Schalkwyk GI, Bloch MH. Alpha-7 nicotinic agonists for cognitive deficits in neuropsychiatric disorders: A translational meta-analysis of rodent and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:45–53. doi: 10.1016/j.pnpbp.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luis Villanueva-Cañas J, et al. New genes and functional innovation in mammals. Genome Biol Evol. 2017;9:1886–1900. doi: 10.1093/gbe/evx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 27.Toll-Riera M, et al. Origin of primate orphan genes: A comparative genomics approach. Mol Biol Evol. 2009;26:603–612. doi: 10.1093/molbev/msn281. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YE, Long M. New genes contribute to genetic and phenotypic novelties in human evolution. Curr Opin Genet Dev. 2014;29:90–96. doi: 10.1016/j.gde.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YE, Vibranovski MD, Landback P, Marais GA, Long M. Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol. 2010;8:e1000494. doi: 10.1371/journal.pbio.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baird A, Coimbra R, Dang X, Eliceiri BP, Costantini TW. Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 2016;5:66–71. doi: 10.1016/j.bbacli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheadle GA, Costantini TW, Bansal V, Eliceiri BP, Coimbra R. Cholinergic signaling in the gut: A novel mechanism of barrier protection through activation of enteric glia cells. Surg Infect (Larchmt) 2014;15:387–393. doi: 10.1089/sur.2013.103. [DOI] [PubMed] [Google Scholar]
- 32.Costantini TW, et al. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: Modulation of intestinal occludin expression. J Trauma. 2010;68:1349–1354, discussion 1354–1356. doi: 10.1097/TA.0b013e3181dccea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini TW, et al. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: A cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181:478–486. doi: 10.1016/j.ajpath.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Loftus TJ, Mohr AM, Moldawer LL. Dysregulated myelopoiesis and hematopoietic function following acute physiologic insult. Curr Opin Hematol. 2018;25:37–43. doi: 10.1097/MOH.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ipaktchi K, et al. Attenuating burn wound inflammatory signaling reduces systemic inflammation and acute lung injury. J Immunol. 2006;177:8065–8071. doi: 10.4049/jimmunol.177.11.8065. [DOI] [PubMed] [Google Scholar]
- 36.Muthu K, et al. Perturbed bone marrow monocyte development following burn injury and sepsis promote hyporesponsive monocytes. J Burn Care Res. 2008;29:12–21. doi: 10.1097/BCR.0b013e31815fa499. [DOI] [PubMed] [Google Scholar]
- 37.Posluszny JA, Jr, et al. Burn injury dampens erythroid cell production through reprioritizing bone marrow hematopoietic response. J Trauma. 2011;71:1288–1296. doi: 10.1097/TA.0b013e31822e2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rea S, et al. Bone marrow-derived cells in the healing burn wound—More than just inflammation. Burns. 2009;35:356–364. doi: 10.1016/j.burns.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med (Berl) 2011;89:985–995. doi: 10.1007/s00109-011-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brakenridge SC, et al. The impact of age on the innate immune response and outcomes after severe sepsis/septic shock in trauma and surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;85:247–255. doi: 10.1097/TA.0000000000001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Efron PA, et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery. 2018;164:178–184. doi: 10.1016/j.surg.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horiguchi H, et al. Sepsis and Critical Illness Research Center Investigators Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loftus TJ, et al. Sepsis and Critical Illness Research Center investigators: Protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7:e015136. doi: 10.1136/bmjopen-2016-015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 46.Nacionales DC, et al. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol. 2015;195:2396–2407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stortz JA, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84:342–349. doi: 10.1097/TA.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stortz JA, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49:249–258. doi: 10.1097/SHK.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gahring LC, et al. Nicotinic receptor alpha7 expression identifies a novel hematopoietic progenitor lineage. PLoS One. 2013;8:e57481. doi: 10.1371/journal.pone.0057481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-García A, et al. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood. 2019;133:224–236. doi: 10.1182/blood-2018-08-867648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126:2443–2451. doi: 10.1182/blood-2015-07-533588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colmegna I, Weyand CM. Haematopoietic stem and progenitor cells in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:252–260. doi: 10.1093/rheumatology/keq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giles AJ, et al. The functional interplay between systemic cancer and the hematopoietic stem cell niche. Pharmacol Ther. 2016;168:53–60. doi: 10.1016/j.pharmthera.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rennert RC, Sorkin M, Garg RK, Gurtner GC. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen Med. 2012;7:833–850. doi: 10.2217/rme.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinhoff G, et al. Stem cells and heart disease—Brake or accelerator? Adv Drug Deliv Rev. 2017;120:2–24. doi: 10.1016/j.addr.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitroulis I, Kalafati L, Hajishengallis G, Chavakis T. Myelopoiesis in the context of innate immunity. J Innate Immun. 2018;10:365–372. doi: 10.1159/000489406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitroulis I, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172:147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutta P, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212:497–512. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lasanianos NG, Kanakaris NK, Dimitriou R, Pape HC, Giannoudis PV. Second hit phenomenon: Existing evidence of clinical implications. Injury. 2011;42:617–629. doi: 10.1016/j.injury.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Chandrasekharan K, et al. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med. 2010;2:42ra54. doi: 10.1126/scitranslmed.3000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okerblom J, et al. Human-like Cmah inactivation in mice increases running endurance and decreases muscle fatigability: Implications for human evolution. Proc Biol Sci. 2018;285:20181656. doi: 10.1098/rspb.2018.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang Q, Ding J, Xu R, Xu Z, Zheng S. The novel human endogenous retrovirus-related gene, psiTPTE22-HERV, is silenced by DNA methylation in cancers. Int J Cancer. 2010;127:1833–1843. doi: 10.1002/ijc.25213. [DOI] [PubMed] [Google Scholar]
- 64.Aravind L, Landsman D. AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 1998;26:4413–4421. doi: 10.1093/nar/26.19.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seok J, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg. 2015;78:671–686. doi: 10.1097/TA.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao W, et al. Inflammation and Host Response to Injury Large-Scale Collaborative Research Program A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lowry DM, et al. The vagus nerve alters the pulmonary dendritic cell response to injury. J Surg Res. 2014;192:12–18. doi: 10.1016/j.jss.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 70.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed Natl Acad Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all relevant data are included in the paper and/or its SI Appendix and available on request from the authors. Source data for Fig. 1 are provided from the Human Protein Atlas (https://www.proteinatlas.org) as scraped from GenTree (gentree.ioz.ac.cn) and described in Methods.