Significance
Bacteria contain peptidoglycan (PG) in their cell envelope to protect them against intracellular osmotic pressure and environmental stress. PG is a large elastic polymer made up of glycan strands interlinked by short peptide chains that form a mesh-like sacculus. In many bacteria, the peptide cross-links are of two types: the predominant d-alanine−meso-diaminopimelic acid (mDAP) and the rare mDAP−mDAP cross-links. Here, we report the importance of mDAP cross-links in PG synthesis during cell growth by identifying a previously unknown hydrolytic enzyme that cleaves such cross-links in the PG sacculi of Escherichia coli. In summary, this study clarifies the role of PG hydrolysis in bacterial cell wall synthesis, thereby rendering it an alternative drug target for development of new antimicrobial agents.
Keywords: bacteria, peptidoglycan, mDAP cross-link, MepK, YcbK
Abstract
Bacteria are surrounded by a protective exoskeleton, peptidoglycan (PG), a cross-linked mesh-like macromolecule consisting of glycan strands interlinked by short peptides. Because PG completely encases the cytoplasmic membrane, cleavage of peptide cross-links is a prerequisite to make space for incorporation of nascent glycan strands for its successful expansion during cell growth. In most bacteria, the peptides consist of l-alanine, d-glutamate, meso-diaminopimelic acid (mDAP) and d-alanine (d-Ala) with cross-links occurring either between d-Ala and mDAP or two mDAP residues. In Escherichia coli, the d-Ala−mDAP cross-links whose cleavage by specialized endopeptidases is crucial for expansion of PG predominate. However, a small proportion of mDAP−mDAP cross-links also exist, yet their role in the context of PG expansion or the hydrolase(s) capable of catalyzing their cleavage is not known. Here, we identified an ORF of unknown function, YcbK (renamed MepK), as an mDAP−mDAP cross-link cleaving endopeptidase working in conjunction with other elongation-specific endopeptidases to make space for efficient incorporation of nascent PG strands into the sacculus. E. coli mutants lacking mepK and another d-Ala−mDAP–specific endopeptidase (mepS) were synthetic sick, and the defects were abrogated by lack of l,d-transpeptidases, enzymes catalyzing the formation of mDAP cross-links. Purified MepK was able to cleave the mDAP cross-links of soluble muropeptides and of intact PG sacculi. Overall, this study describes a PG hydrolytic enzyme with a hitherto unknown substrate specificity that contributes to expansion of the PG sacculus, emphasizing the fundamental importance of cross-link cleavage in bacterial peptidoglycan synthesis.
Cell envelopes of bacteria have a mesh-like exoskeleton called peptidoglycan (PG; also called murein) to protect them against turgor and environmental stress conditions. It also confers mechanical strength and shape to bacterial cells. PG is a single, large, covalently cross-linked macromolecule made up of multiple linear glycan strands that are interconnected by short peptide chains (Fig. 1) (1–4). The glycan strands are polymers of alternating β-1,4-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) disaccharide units in which the d-lactoyl moiety of each MurNAc residue is covalently attached to the first amino acid of the peptide chain. Typically, the peptide chains consist of two to five amino acids, and in Escherichia coli, a pentapeptide consists of l-alanine (l-Ala1)−d-glutamic acid (d-Glu2)−meso-diaminopimelic acid (mDAP3)−d-Ala4−d-Ala5. Nearly 40% of the neighboring peptide chains are cross bridged to each other, either between the d-Ala4 and mDAP3 (d-Ala−mDAP, or 4−3) or between mDAP3 and mDAP3 (mDAP−mDAP, or 3−3) residues (2, 5). Of these, the 4−3 cross-links are more predominant (∼93%) and are catalyzed by d,d-transpeptidase activity of high-molecular-weight penicillin-binding proteins (PBPs) that include PBP1A, PBP1B, PBP2, and PBP3 (6). On the contrary, 3−3 cross-links are much less abundant (about 7%) and result from the activity of l,d-transpeptidases LdtD and LdtE (7).
Fig. 1.
Schematic representation of the PG sacculus of E. coli. The structure and composition of glycan strands and peptide chains are shown. Listed are the known PG hydrolases of E. coli (3, 4, 10). Cleavage sites of the hydrolases are indicated by the scissors symbol. MepK (indicated in red) is identified in this study.
Synthesis of PG is a complex process that occurs in two distinct cellular compartments: the cytosol and the periplasm (8). The precursor UDP-MurNAc-pentapeptide is synthesized in the cytosol by sequential enzymatic reactions catalyzed by MurA, -B, -C, -D, -E, and -F before being transferred to a membrane-bound lipid carrier (C55-bactoprenol phosphate) to generate lipid I. Subsequently, a molecule of UDP-GlcNAc is added to lipid I to yield lipid II, which is then flipped across the inner membrane into the periplasm. Here, the membrane-bound d,d-transpeptidases catalyze the formation of 4−3 cross-links by cleaving the d-Ala4−d-Ala5 peptide bond of the incoming disaccharide pentapeptide (donor) to link the d-Ala4 to the d-center of the mDAP3 residue of an adjacent peptide chain (acceptor) (6). On the contrary, l,d-transpeptidases (LdtD and LdtE) catalyze the formation of 3−3 cross-links by cleaving the mDAP3−d-Ala4 peptide bond of an existing tetrapeptide of the PG sacculus (donor) to link the mDAP3 to the d-center of the mDAP3 residue of an adjacent peptide (acceptor) (9).
Because the PG sacculus is a continuous molecular network that completely encircles the cytoplasmic membrane, the growth of a cell is tightly coupled to expansion of PG, requiring the coordinated activity of hydrolases that cleave the cross-links and synthases that form the cross-links (1). Given that the PG is interconnected by two types of cross-bridges (4–3 and 3–3), it is expected that the cleavage of both of these cross-links is a prerequisite to make space for the incorporation of incoming murein strands for its successful expansion. We previously showed cleavage of 4−3 cross-links is crucial for PG enlargement as an E. coli mutant lacking three d,d-endopeptidases, MepS, MepM, and MepH (specific to d-Ala−mDAP cross bridges), is unable to incorporate new murein and undergoes rapid lysis (10). However, it is not clear how the 3−3 cross-linkages affect PG enlargement because they are also expected to hinder opening of the mesh for the incorporation of new PG material.
In this study, we show that 3−3 cross-link cleavage contributes to PG enlargement by identifying an enzyme of previously unknown specificity, YcbK (renamed murein endopeptidase K, MepK), as a murein hydrolase that cleaves 3−3 cross-links in E. coli. Extensive genetic and molecular analyses indicate that mepK functions in conjunction with other elongation-specific d,d-endopeptidases to contribute to synthesis of PG. Biochemical studies demonstrate MepK is a PG hydrolase with the ability to cleave the 3−3 cross-links of soluble muropeptides and of intact PG sacculi. MepK orthologs from other gram-negative bacteria also exhibit similar substrate specificity. To the best of our knowledge, MepK is the only enzyme reported so far in any bacterial genera to exhibit 3−3 cross-link–specific catalytic activity.
Results
MepK Functions in Conjunction with Other Elongation-Specific Endopeptidases.
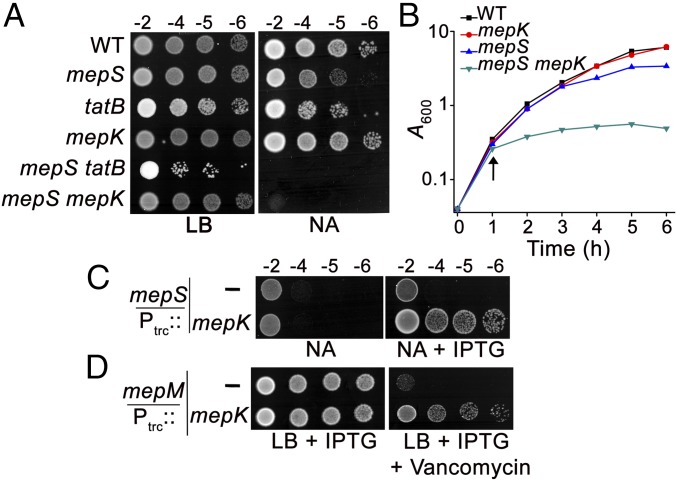
To understand the mechanism of cross-link cleavage during PG enlargement, we made an attempt to identify mutations that confer additive sickness to an E. coli strain lacking a major elongation-specific endopeptidase, MepS (10, 11). We observed that deletion of tatB, a gene encoding a component of the twin-arginine translocase (TatABC) secretion pathway, resulted in synthetic sickness in a strain deleted for MepS on media of low osmolarity such as LB without NaCl and nutrient agar (NA) (Fig. 2A). Deletions in tatA or tatC also behaved similarly to that of tatB, indicating the importance of a functional Tat system in the growth of the mepS deletion mutant. TatABC is a secretion system that facilitates transport of folded proteins from the cytosol into the periplasm across the inner membrane. In E. coli, ∼27 proteins are known to be transported by the TatABC pathway (12), and we tested the genes which are crucial for growth of the mepS mutant by examining the phenotypes of several double-deletion mutants lacking each of the TAT substrates. Interestingly, a double mutant lacking MepS and YcbK, an ORF of unknown function (hereafter designated MepK), behaved exactly like that of the ∆mepS ∆tatB strain, suggesting the deficiency of MepK in the periplasm as the basis of synthetic sickness of the ∆mepS ∆tatB double mutant (Fig. 2A). Consistent with the viability assay, the cells of the double mutant (∆mepS ∆mepK) bulge and lyse in low-osmolarity conditions (Fig. 2B and SI Appendix, Fig. S1A). However, the ∆mepK single mutant did not exhibit any discernible phenotype except a moderate increase in sensitivity to certain β-lactam antibiotics such as ampicillin and cephalexin (ref. 13, SI Appendix, Fig. S1B).
Fig. 2.
Genetic interactions between mepS, mepK, and mepM. (A) WT (MG1655) and its mutant derivatives were grown in LB and serially diluted, and 5 µL of each dilution were spotted on indicated plates and incubated overnight at 30 °C. (B) Cultures of WT and its mutant derivatives grown overnight were diluted 1:100 into fresh LB and grown until A600 of 0.3 at 30 °C. Cultures were pelleted, washed, and resuspended in nutrient broth (NB) and grown at 30 °C. Growth was monitored every 1 h. The arrow indicates the time of the shift to NB. (C) WT and ∆mepS strains carrying either vector (pTrc99a; Ptrc::) or pPK2 (Ptrc::mepK) were grown, and viability was tested on NA plates at 37 °C with or without Isopropyl thiogalactopyranoside (IPTG) (0.5 mM) as described above. (D) WT and ∆mepM strains carrying vector or pPK2 (Ptrc::mepK) were grown in LB and tested for viability at 37 °C on LB plates supplemented with vancomycin (250 µg/mL) and IPTG (0.5 mM).
MepK belongs to the M15 family of peptidases that also comprises DdpX, a peptidase that cleaves d-Ala−d-Ala dipeptide in the cytosol of E. coli (14, 15). In addition, a mepK homolog of Klebsiella pneumoniae is predicted to be a PG hydrolase (16). Hence, we tested whether increased expression of mepK is able to compensate the loss of MepS and/or MepM, the elongation-specific PG hydrolases (10). The absence of MepS results in sensitivity on NA at 42 °C (17), and this growth defect was significantly suppressed when mepK (Ptrc::mepK) was introduced in multiple copies (Fig. 2C). In addition, mepK overexpression suppressed the vancomycin sensitivity of a ΔmepM deletion mutant, indicating that MepK compensates for the loss of either mepS or mepM (Fig. 2D). A double mutant lacking mepS and mepM does not grow on rich media such as LB but grows well on minimal media (10). Additional copies of mepK could also support, albeit partially, the growth of a ΔmepS ΔmepM double mutant on LB (SI Appendix, Fig. S2A). Moreover, deletion of mepK conferred significant additive sickness to the ΔmepS ΔmepM strain growing on minimal media, with the triple-deletion strain growing very poorly with extensive lysis and cell death (SI Appendix, Fig. S2 B and C). These observations collectively indicated contribution of MepK to the functions of elongation-specific d,d-endopeptidases, MepS and MepM.
MepK Modulates mDAP−mDAP Cross-Links of Peptidoglycan.
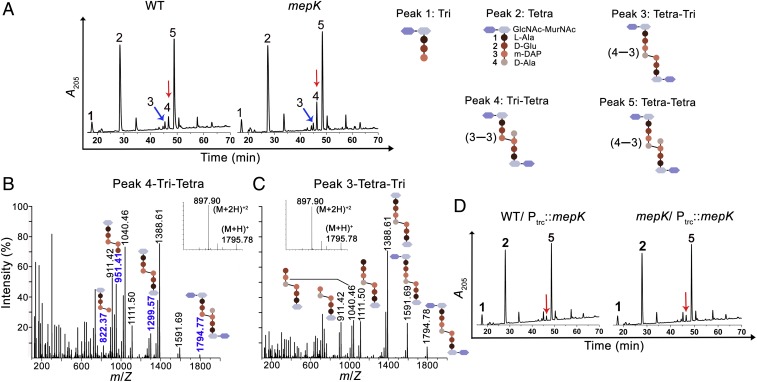
As the above observations suggest that MepK is a PG peptidase, we examined the composition of PG of a mutant lacking MepK. The PG sacculi from WT and the ΔmepK mutant were prepared and digested with a muramidase (mutanolysin), and the resulting muropeptides were separated by reverse-phase high pressure liquid chromatography (RP-HPLC). Analysis of the chromatograms revealed a single significant alteration in the PG of the ΔmepK strain compared with the WT. A muropeptide peak eluted at 47 min (denoted as 4 in Fig. 3A) was found to be increased 1.75-fold in the ΔmepK mutant (with peak 4 constituting about 4% of the total soluble muropeptides in the WT and 7% in the ΔmepK mutant) (Fig. 3A and SI Appendix, Table S3). Mass spectrometry (MS) data indicated the molecular mass of peak 4 is 1,794 Da, suggesting it is a cross-linked dimer of a disaccharide tetrapeptide and a disaccharide tripeptide (Fig. 3B). Further analysis by tandem mass spectrometry revealed these dimers are cross-linked by the rare mDAP−mDAP linkages (3−3; tri-tetra; Fig. 3B). On the other hand, peak 3, which also had an identical molecular mass of 1,794 Da, was a dimer cross-linked by d-Ala−mDAP linkages (4−3; tetra-tri; Fig. 3C). In addition, multiple copies of plasmid-borne mepK (Ptrc::mepK) were able to decrease the tri-tetra peak to 2% of total soluble muropeptides in both the WT and ΔmepK strain (Fig. 3D and SI Appendix, Table S3), suggesting that MepK directly or indirectly modulates the level of 3−3 cross-links in PG sacculi.
Fig. 3.
MepK modulates mDAP cross-links of PG sacculi. (A) HPLC chromatograms depicting the soluble muropeptides of WT and ∆mepK mutants. Muropeptide peaks (1–5) were collected and analyzed by MS, and structures are depicted. The red arrow represents peak 4; the blue arrow represents peak 3. (B and C) Mass spectra of peaks 3 and 4 show identical molecular mass (M+H)+ of 1,795.78 Da (Inset). Tandem MS indicated peak 4 is a 3−3 cross-linked dimer (tri-tetra) and peak 3 is a 4−3 cross-linked dimer (tetra-tri). Fragments specific to the 3−3 dimer are shown in blue. (D) HPLC chromatograms of WT and ∆mepK mutant strains carrying pPK2 (Ptrc::mepK). Cultures were grown to an A600 of ∼1 in LB containing 0.5 mM IPTG followed by isolation and analysis of PG sacculi. The red arrow represents peak 4.
In light of an earlier report that MepA, a member of the LAS metallopeptidase family (18) and a known 4−3 endopeptidase, is also able to cleave 3−3 cross-links in vitro (5), we examined PG composition of a double-deletion mutant lacking mepA and mepK and found it to be comparable to that of the single mepK deletion mutant (SI Appendix, Fig. S3). Moreover, mepA deletion did not confer any additive sickness to the mepS mepK mutant, suggesting MepA may not have a role in the turnover of 3−3 cross-linkages under normal laboratory growth conditions.
ldtDE Is Epistatic to mepK.
l,d-transpeptidases, LdtD and LdtE, catalyze the formation of 3−3 cross-links in the PG sacculi of E. coli (7); hence, we examined the effect of LdtDE activity on the phenotypes of mepK. The tri-tetra peak (peak 4) was completely absent in strains lacking ldtDE (irrespective of the presence or absence of mepK), indicating that l,d-transpeptidase activity leads to the accumulation of 3−3 cross-links in the mepK mutant (Fig. 4A). Consistent with this, overexpression of LdtD (PT5-lac::ldtD) was extremely toxic in a ΔmepK mutant, with its PG sacculi accumulating significantly higher levels of 3−3 cross-links compared with the WT (Fig. 4 B and C).
Fig. 4.
Genetic interactions between mepK, mepS, and ldtDE. (A) HPLC chromatograms of PG sacculi isolated from ldtDE and ldtDE mepK mutants. The absence of peak 4 is indicated by a red arrow. (B) HPLC chromatograms of WT and ∆mepK mutant carrying vector alone (pCA24N) or vector carrying ldtD (PT5-lac::ldtD). Strains were grown to an A600 of ∼1 in LB containing IPTG (20 µM for WT and 6 µM for ∆mepK mutant) followed by isolation and analysis of PG sacculi. MS analysis indicated peaks a, b, and c are tetra (Gly4), tri-tetra (Gly4), and tri-tri, respectively (SI Appendix, Fig. S6). (C) WT and ∆mepK mutant carrying vector (pCA24N) or its derivative (PT5-lac::ldtD) were grown, and their viability was tested on indicated plates at 37 °C with or without IPTG (20 µM). (D) Growth of WT and its mutant derivatives was tested on indicated plates at 37 °C as described above. (E) ∆mepS and ldtDE ∆mepS strains carrying either vector or pPK2 (Ptrc::mepK) were grown in LB, and viability was examined on NA plates with or without IPTG (0.5 mM) at 37 °C.
Similarly, introduction of LdtDE deletion abrogated the growth defects of the mepS mepK double mutant (Fig. 4D). LdtDE deletion was also able to confer partial growth advantage to a ΔmepS single mutant, indicating that the 3−3 cross-links are deleterious in the absence of MepS (Fig. 4 D and E). The above results clearly indicate that ldtDE is epistatic to mepK; however, we observed that overexpression of mepK was able to confer further growth to a mepS ldtDE double mutant, suggesting MepK may have an additional function which is independent of LdtDE (Fig. 4E). These observations are discussed below.
MepK Contributes to PG Synthesis Along with MepS and MepM.
Our results thus far indicated MepK is a putative 3−3 cross-link–specific PG hydrolase functioning in conjunction with MepS and MepM, the elongation-specific 4−3 endopeptidases. Hence, we reasoned that cleavage of 3−3 cross-links may also contribute to PG expansion and therefore measured PG synthesis in various mutants deleted for MepK/MepS/MepM/LdtDE using a 3H-mDAP incorporation assay (ref. 10, Fig. 5). Lack of MepK alone resulted in a somewhat lower rate of PG synthesis; however, its absence in ΔmepS or ΔmepM strains led to a further decrease in 3H-mDAP incorporation compared with the WT, clearly indicating that the combined activity of these peptidases contributes to optimal PG synthesis. Deletion of ldtDE in the ΔmepS ΔmepK mutant was able to restore PG synthesis to that of a single ΔmepS mutant, validating the earlier findings of l,d-transpeptidase activity being epistatic to MepK (Fig. 4D).
Fig. 5.
MepK contributes to optimal PG synthesis. Incorporation of new murein using 3H-mDAP was measured in WT and its various mutant derivatives. Strains were grown in minimal A media supplemented with 0.5% casamino acids (CAA) and 0.2% glucose at 37 °C. In this medium, all strains grew equally well, and their growth rates were comparable. At an A600 of ∼0.7, a 0.5-mL aliquot was pulsed with 3H-mDAP and processed as described in Materials and Methods. All strains carried lysA deletion to prevent conversion of mDAP into lysine. Values are expressed in percentages, and WT is normalized to 100. Data shown represent three independent experiments.
MepK Is an mDAP−mDAP Cross-Link–Specific l,d-Endopeptidase.
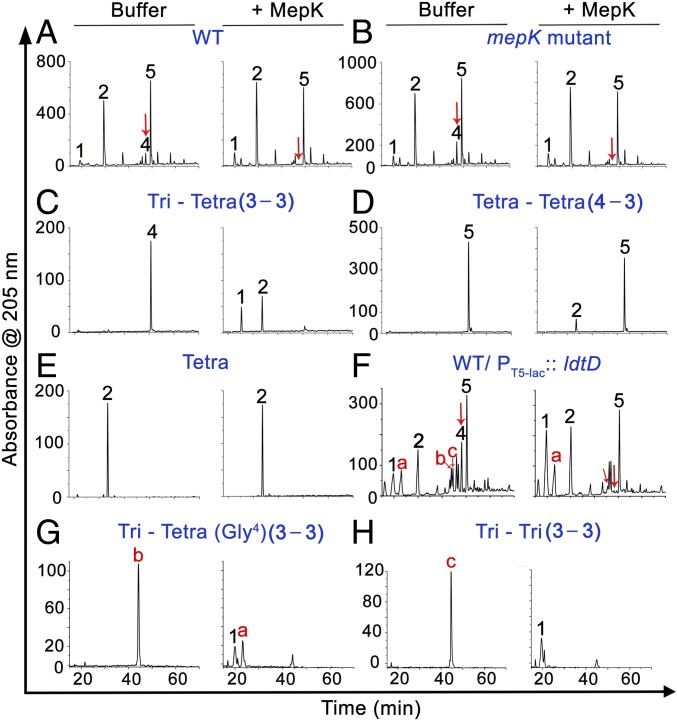
To examine the in vitro activity of MepK, we overexpressed and purified a histidine-tagged signal-less MepK derivative (MepK31-182-His6). Purified MepK was able to show a zone of clearance in a zymogram assay, indicating either PG binding or hydrolysis (SI Appendix, Fig. S4). Incubation of total soluble muropeptides (obtained by mutanolysin digestion of PG sacculi from WT or the mepK deletion mutant) with MepK resulted in complete loss of tri-tetra (peak 4) with a concomitant increase in tripeptide disaccharide (tri, peak 1) and tetrapeptide disaccharide (tetra, peak 2) (Fig. 6 A and B). Cleavage of tri-tetra into tri and tetra was also confirmed using purified tri-tetra as a substrate (Fig. 6C). MepK was also able to weakly cleave the tetra-tetra dimer (peak 5) (Fig. 6 A, B, and D) but had no activity on the tetra monomer (Fig. 6E). MepM, a d,d-endopeptidase specific to 4−3 cross-links (10), was not able to cleave the 3−3 cross-links of peak 4 but was able to cleave the 4−3 cross-linked muropeptides (peaks 3 and 5) (SI Appendix, Fig. S5).
Fig. 6.
HPLC chromatograms showing the biochemical activity of MepK. Soluble muropeptides of WT (A), soluble muropeptides of mepK mutant (B), purified tri-tetra (C), purified tetra-tetra (D), purified tetra (E), soluble muropeptides of WT/PT5-lac::ldtD (F), purified tri-tetra (Gly4) (G), and purified tri-tri (H) were incubated either with buffer or with MepK (5 µM) for 20 h and separated by RP-HPLC. Note that MepK cleaved peaks 4, b, and c completely (red arrows) and partially cleaved peak 5 (F). All peaks were identified by MS and tandem MS (SI Appendix, Fig. S6).
To further confirm the catalytic specificity of MepK, we used soluble muropeptides of a strain carrying more copies of LdtD (WT/PT5-lac::ldtD) as its substrates. As expected, LdtD overexpression resulted in accumulation of a large number of 3−3 cross-linked muropeptides and also tetrapeptides with glycine at position 4 (Fig. 4B and SI Appendix, Fig. S6). Consistent with earlier results, MepK completely hydrolyzed all of the 3−3 cross-linked muropeptides (peaks b, c, and 4) and also partially cleaved the tetra-tetra dimer (peak 5) (Fig. 6F), clearly indicating that MepK is predominantly a 3−3 cross-link–specific PG hydrolase with a minor activity on 4−3 cross-linked muropeptides. Further, we purified two types of 3−3 cross-linked muropeptides designated as b (tri-tetra with Gly4) and c (tri-tri). Incubation of MepK with b and c yielded tri and tetra (Gly4) and monomers of tri, respectively, confirming the ability of MepK to effectively cleave 3−3 cross-linked muropeptides (Fig. 6 G and H). In addition, MepK was able to cleave the 3−3 cross-links of intact PG sacculi as well as those of the soluble muropeptides (SI Appendix, Fig. S7). Taken together, these results demonstrate that MepK is primarily a 3−3 cross-link–specific l,d-endopeptidase and also a weak d,d-endopeptidase.
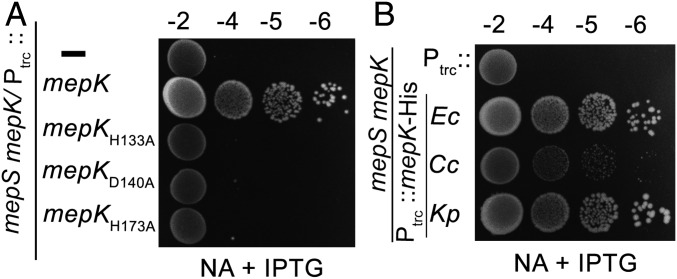
Based on homology modeling, an earlier study predicted an MepK ortholog of K. pneumoniae to be a metal-binding PG hydrolase (ref. 16, SI Appendix, Fig. S8A). To validate the function of MepK, we created site-directed mutations in the predicted metal-binding site by modifying each of the amino acid residues H133, D140, and H173 into an l-alanine in a Ptrc::mepK plasmid system. Complementation assays (Fig. 7A) and the analysis of PG composition (SI Appendix, Fig. S8B) showed that these mutant plasmids are not functional, although they are able to express MepK to a similar extent (SI Appendix, Fig. S8C), indicating the importance of these active-site amino acid residues.
Fig. 7.
Functionality of mepK variants and orthologs. (A) ∆mepS ∆mepK strain carrying either vector (Ptrc), pPK17 (Ptrc::mepK), pPK19 (Ptrc::mepKH133A), pPK20 (Ptrc::mepKD140A), or pPK21 (Ptrc::mepKH173A) was grown in LB, and viability was tested on NA plates with IPTG (0.5 mM) at 30 °C. (B) ∆mepS ∆mepK strain carrying vector (Ptrc), pPK17 (Ptrc::mepK-HisE. coli;Ec), pPK23 (Ptrc::mepK-HisC. crescentus;Cc), or pPK25 (Ptrc::mepK-HisK. pneumoniae;Kp) was grown in LB and processed as described above.
MepK is conserved in most classes of gram-negative bacteria but not in gram-positive organisms. To examine the function of mepK, we cloned its orthologs from two model organisms, Caulobacter crescentus and Klebsiella pneumoniae. Viability assays indicated that both of the homologs complement ΔmepK ΔmepS growth defects (Fig. 7B). However, we found weak complementation with mepK of C. crescentus, most likely due to its poor expression (SI Appendix, Fig. S8D). In addition, the recombinant MepKKp-His protein was able to cleave the 3−3 cross-links of soluble muropeptides of E. coli (SI Appendix, Fig. S8E), suggesting a conserved function of MepK in these bacteria.
Discussion
In this study, we report identification of a previously unknown peptidoglycan hydrolase, MepK, which cleaves the 3−3 cross-linked muropeptides of PG sacculi in E. coli. To the best of our knowledge, MepK is the only enzyme known so far across the bacterial genera to exhibit such catalytic specificity. We also show that cross-link cleavage mediated by the combined activity of both 3−3-specific (MepK) and 4−3-specific (MepS/MepM) endopeptidases is required to make optimal space for the incorporation of new murein material, thereby confirming the earlier observations of cross-link cleavage being an important determinant of PG expansion during growth of a bacterium.
Role of MepK in PG Enlargement.
PG hydrolysis is an indispensable step of cell wall synthesis and cell growth in several gram-negative and gram-positive bacterial genera (10, 19–22). In E. coli and other gram-negative bacteria, cleavage of d-Ala−mDAP cross-links mediated by d,d-endopeptidases is crucial for PG enlargement (10, 20, 22). In this context, our results show that cleavage of mDAP cross-links also plays a role in PG enlargement. The synthetic and additive phenotypes of the mepS mepK double mutant (Figs. 4D and 5) indicate that in the absence of MepS, a major 4−3 endopeptidase, activity of MepK is required. Loss of these synthetic phenotypes in the absence of l,d-transpeptidases suggests that the 3−3 cross-links are deleterious and are capable of hindering the progress of nascent PG incorporation during growth of the PG sacculus (Fig. 4D). Consistent with this, the absence of 3−3 cross-links partially relieves the growth defects of the mepS mutant (Fig. 4 D and E).
As shown in Fig. 4E, overexpression of mepK was able to further confer growth to an ldtDE mepS double mutant. This LdtDE-independent activity of MepK is most likely due to its weak 4−3 cross-link cleaving ability (Fig. 6 D and F). However, this weak activity may not significantly contribute to PG enlargement under normal physiological conditions. In a recent study, a mutant of E. coli was constructed which contains exclusively 3−3 cross-links in its PG sacculus (9). As this strain is completely dependent on LdtD for the formation of cross-links, MepK may become indispensable in this strain to open up the 3−3 cross-links for PG expansion and hence for its growth and viability.
How Is 3−3 Cross-Linkage Regulated?
Several studies reported alterations in the frequency of 3−3 linkages in E. coli growing under different physiological conditions, the stationary phase, and stress (5, 23, 24). It is likely that MepK activity may also be regulated under these conditions. In a transcriptomic study, mepK levels were found to be slightly up-regulated during σE stress response (25). However, preliminary studies done using a mepK::lacZ transcriptional fusion did not reveal any noticeable alterations during envelope stress or the stationary phase. It would be interesting to examine how 3−3 cross-link formation is modulated by the combined activities of LdtDE and MepK. In this context, it is noteworthy that the bicistronic mepK-ycbL operon is located immediately downstream to ldtD in the E. coli genome (and also in many gram-negative bacteria), raising an interesting possibility of their coevolution.
Conservation of MepK Across Bacteria.
MepK belongs to the M15 family of peptidases which includes the VanX type of peptidases as well as DdpX (14, 15). MepK is conserved across most families of gram-negative but not gram-positive bacteria. It has been observed that the proportion of 3−3 and 4−3 cross-links is extremely variable across bacteria (2, 26). For example, 3−3 cross-links account for about 1.2% in Pseudomonas putida, 7% in E. coli, and 40% in Aeromonas sp. and Agrobacterium tumefaciens (26). In members of the Mycobacteriaceae family, PG has extremely high levels of 3−3 cross-links, accounting for almost 60% of the total cross-linkages (27). In these organisms, the 3−3 cross-link hydrolases may have a higher significance.
Materials and Methods
Detailed strain and plasmid constructions, additional materials, methods, Tables S1–S3, and Figs. S1–S8 are listed in SI Appendix.
Media, Bacterial Strains, and Plasmids.
Bacteria were grown either in LB media (0.5% yeast extract, 1% tryptone, and 1% NaCl) or minimal A media supplemented with 0.2% glucose (28). NA has 0.5% peptone and 0.3% beef extract. Antibiotics were used at the following concentrations (µg/mL): ampicillin, 50; chloramphenicol, 30; vancomycin, 250; and kanamycin (Kan), 50. Growth temperature was 37 °C unless otherwise indicated. The bacterial strains and plasmids are listed in SI Appendix.
Molecular and Genetic Techniques.
Recombinant DNA techniques and P1 phage-mediated transductions and transformations were performed using standard methods (28). Deletion mutants are from the Keio mutant collection (29), and wherever necessary, the KanR gene was flipped out using pCP20 plasmid encoding Flp recombinase (30).
Preparation of PG Sacculi.
Isolation of PG was done as described earlier (5). Briefly, cells are grown to an A600 of 1.0, harvested, resuspended in water, and boiled with 8% SDS to solubilize membranes. PG sacculi were collected by high-speed centrifugation and washed with water to remove SDS, followed by treatment with α-amylase and pronase to remove bound glycogen and proteins. Sacculi were again boiled with 8% SDS, collected by ultracentrifugation, and washed with water to remove SDS. Pellet was resuspended and stored at −30 °C. See SI Appendix.
Analysis of PG Sacculi.
Analysis of PG sacculi was done as previously described (5). Intact PG sacculi were digested with mutanolysin and centrifuged to remove the insoluble material. Soluble fraction was reduced with sodium borohydride; samples were loaded onto a reverse-phase C18 column, and fractions were collected. Absorbance was detected at 205 nm. See SI Appendix.
Determination of Enzymatic Activity.
Soluble muropeptides were incubated with either buffer or MepK (5 µM) at 30 °C for 20 h with shaking. Samples were heat inactivated, reduced with sodium borohydride, and separated by RP-HPLC.
Measurement of 3H-mDAP Incorporation into PG Sacculi.
Incorporation of 3H-mDAP into murein sacculi was done as described (10, 31). Strains were grown overnight in LB, washed, and diluted 1:100 into prewarmed minimal A medium supplemented with 0.2% glucose and 0.5% casamino acids. Cells were grown to an A600 of 0.7, and 0.5-mL aliquots were incubated with 5 µCi/mL of 3H-mDAP (14.6 Ci/mmol; Moravek Biochemicals) for 10 min at 37 °C. Reaction was stopped by boiling cells with SDS and then cooling and filtering them. Filters were washed and dried, and the radioactivity was measured using a liquid scintillation counter.
Supplementary Material
Acknowledgments
We thank Sujata Kumari, Vaishnavi Kunteepuram, Aisha Hamid, Nilanjan Som, and Raj Bahadur for plasmids and strains; V. Krishna Kumari and C. Subbalakshmi for HPLC; B. Raman and Y. Kameshwari for mass spectrometry; N. Madhusudhana Rao for advice; and G. Shambhavi for help with the manuscript. We thank National BioResource Project:: E. coli for the Keio mutant collection and the ASKA plasmid library. This work is supported by funds from the Department of Biotechnology (Centre of Excellence on Microbial Biology) and the Council of Scientific and Industrial Research, government of India (to M.R.). P.K.C. acknowledges financial support from the University Grants Commission of India.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816893116/-/DCSupplemental.
References
- 1.Höltje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 3.van Heijenoort J. Peptidoglycan hydrolases of Escherichia coli. Microbiol Mol Biol Rev. 2011;75:636–663. doi: 10.1128/MMBR.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 5.Glauner B, Höltje JV, Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 6.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Magnet S, Dubost L, Marie A, Arthur M, Gutmann L. Identification of the L,D-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J Bacteriol. 2008;190:4782–4785. doi: 10.1128/JB.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev. 2008;32:208–233. doi: 10.1111/j.1574-6976.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 9.Hugonnet JE, et al. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. eLife. 2016;5:e19469. doi: 10.7554/eLife.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, SaiSree L, Amrutha RN, Reddy M. Three redundant murein endopeptidases catalyse an essential cleavage step in peptidoglycan synthesis of Escherichia coli K12. Mol Microbiol. 2012;86:1036–1051. doi: 10.1111/mmi.12058. [DOI] [PubMed] [Google Scholar]
- 11.Singh SK, Parveen S, SaiSree L, Reddy M. Regulated proteolysis of a cross-link–specific peptidoglycan hydrolase contributes to bacterial morphogenesis. Proc Natl Acad Sci USA. 2015;112:10956–10961. doi: 10.1073/pnas.1507760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee PA, Tullman-Ercek D, Georgiou G. The bacterial twin-arginine translocation pathway. Annu Rev Microbiol. 2006;60:373–395. doi: 10.1146/annurev.micro.60.080805.142212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu A, et al. Antibiotic sensitivity profiles determined with an Escherichia coli gene knockout collection: Generating an antibiotic bar code. Antimicrob Agents Chemother. 2010;54:1393–1403. doi: 10.1128/AAC.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawlings ND, Barrett AJ. MEROPS: The peptidase database. Nucleic Acids Res. 1999;27:325–331. doi: 10.1093/nar/27.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessard IA, Walsh CT. Mutational analysis of active-site residues of the enterococcal D-ala-D-Ala dipeptidase VanX and comparison with Escherichia coli D-ala-D-Ala ligase and D-ala-D-Ala carboxypeptidase VanY. Chem Biol. 1999;6:177–187. doi: 10.1016/S1074-5521(99)89009-7. [DOI] [PubMed] [Google Scholar]
- 16.Teh BA, et al. Structure to function prediction of hypothetical protein KPN_00953 (Ycbk) from Klebsiella pneumoniae MGH 78578 highlights possible role in cell wall metabolism. BMC Struct Biol. 2014;14:7. doi: 10.1186/1472-6807-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara H, Abe N, Nakakouji M, Nishimura Y, Horiuchi K. Overproduction of penicillin-binding protein 7 suppresses thermosensitive growth defect at low osmolarity due to an spr mutation of Escherichia coli. Microb Drug Resist. 1996;2:63–72. doi: 10.1089/mdr.1996.2.63. [DOI] [PubMed] [Google Scholar]
- 18.Marcyjaniak M, Odintsov SG, Sabala I, Bochtler M. Peptidoglycan amidase MepA is a LAS metallopeptidase. J Biol Chem. 2004;279:43982–43989. doi: 10.1074/jbc.M406735200. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, Ooiwa S, Sekiguchi J. Synthetic lethality of the lytE cwlO genotype in Bacillus subtilis is caused by lack of D,L-endopeptidase activity at the lateral cell wall. J Bacteriol. 2012;194:796–803. doi: 10.1128/JB.05569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dörr T, Cava F, Lam H, Davis BM, Waldor MK. Substrate specificity of an elongation-specific peptidoglycan endopeptidase and its implications for cell wall architecture and growth of Vibrio cholerae. Mol Microbiol. 2013;89:949–962. doi: 10.1111/mmi.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng WL, Kazmierczak KM, Winkler ME. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol Microbiol. 2004;53:1161–1175. doi: 10.1111/j.1365-2958.2004.04196.x. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava D, et al. A proteolytic complex targets multiple cell wall hydrolases in Pseudomonas aeruginosa. MBio. 2018;9:e00972-18. doi: 10.1128/mBio.00972-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernal-Cabas M, Ayala JA, Raivio TL. The Cpx envelope stress response modifies peptidoglycan cross-linking via the L,D-transpeptidase LdtD and the novel protein YgaU. J Bacteriol. 2015;197:603–614. doi: 10.1128/JB.02449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delhaye A, Collet JF, Laloux G. Fine-tuning of the Cpx envelope stress response is required for cell wall homeostasis in Escherichia coli. MBio. 2016;7:e00047-16. doi: 10.1128/mBio.00047-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintela JC, Caparrós M, de Pedro MA. Variability of peptidoglycan structural parameters in gram-negative bacteria. FEMS Microbiol Lett. 1995;125:95–100. doi: 10.1111/j.1574-6968.1995.tb07341.x. [DOI] [PubMed] [Google Scholar]
- 27.Lavollay M, et al. The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by L,D-transpeptidases. J Bacteriol. 2011;193:778–782. doi: 10.1128/JB.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1992. [Google Scholar]
- 29.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wientjes FB, Pas E, Taschner PEM, Woldringh CL. Kinetics of uptake and incorporation of meso-diaminopimelic acid in different Escherichia coli strains. J Bacteriol. 1985;164:331–337. doi: 10.1128/jb.164.1.331-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.