Protein homeostasis is tightly regulated, and multiple cellular mechanisms are in place to dispose of misfolded or no-longer-needed proteins. One of the key players is the ubiquitin (Ub)-proteasome system (UPS), in which a variety of specific ligases mark substrate proteins with a Ub “flag” to be recognized by the proteolytic proteasome complex and to be subsequently degraded. Long poly-Ub chains are believed to be essential to effect a proteolytic signal, although the proteasomal Ub receptors do not require long chains to perform their activating function. In PNAS, Sun et al. (1) use a clever approach based on the impressive chemical synthesis of poly-ubiquitinated substrate proteins to address part of this mystery by showing why poly-Ub chains are important for effective proteasomal degradation.
A typical 26S proteasome consists of a cylindrical 20S core particle (20S-CP) responsible for catalytic protein degradation, and a 19S regulatory particle involved in the recognition, unfolding, and translocation of ubiquitinated substrates to the core particle (2). Three intrinsic 19S subunits (Rpn1, Rpn10, and Rpn13), together with three reversibly associated shuttling factors [Rad23 (Rad23A/Rad23B in mammals), Dsk2 (UBQLN1-4 in mammals), and Ddi1], act as Ub receptors that target ubiquitinated substrates for degradation (3–5). In concert, one or more of the proteasome-associated deubiquitinating enzymes (DUBs)—USP14 and UCH37—subsequently trim down the Ub chain (6, 7). The intrinsic 19S subunit Rpn11 serves as final gatekeeper and balances the en bloc removal of the remaining Ub chain and substrate degradation (8–10). Its well-timed actions prevent the substrate protein from escaping degradation while simultaneously warranting that no Ub is degraded by the 20S-CP. Finally, the substrates are unfolded and translocated into the interior of the 20S-CP for degradation (11). The typical signal for proteasome-mediated degradation is believed to be a Lys48-linked tetra-Ub chain; however, this classical selection criterion seems less generally applicable because substrates equipped with different Ub linkage types, shorter poly-Ub chains, and even monoubiquitinated proteins have now been reported to be degraded by the proteasome in specific cases (12–16). The subtle differences and mechanistic intricacies of proteasome-mediated degradation between short- and long-Ub-chain–carrying substrates is not yet fully understood.
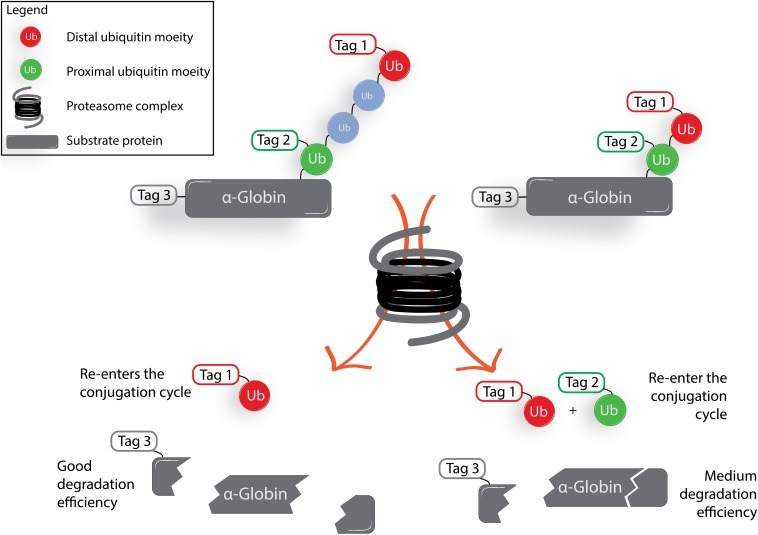
The study by Brik, Ciechanover, and coworkers (1) discloses part of the mystery on what roles Ub chains with different lengths play in proteasomal degradation. To do so, the authors chemically prepared mono-, di-, and tetra-ubiquitinated α-globin using native chemical ligation technology based on the reaction of a δ-thiolysine peptide and a peptide-thioester, followed by radical desulfurization (17, 18). The emergence of technologies to prepare Ub-based reagents, poly-Ub chains, and ubiquitinated proteins carrying native/artificial and stabilized linkages has been very helpful for studying and disclosing the molecular details of many aspects of the complex world of Ub biology (19–21). The impressive chemical modular construction of ubiquitinated α-globin proteins allows for the introduction of “handles” at specific positions at will. Sun et al. placed HA, Myc, and FLAG tags on predetermined positions, allowing them to investigate the fate of the proximal Ub, the distal Ub, and the α-globin protein when subjected to proteasomal degradation in rabbit reticulocyte fraction II, a crude cell extract containing active proteasome, ubiquitination machinery (E1 and most of the E2 and E3 enzymes), and DUBs, but not Ub.
The authors show that when employing tagged tetra-Ub α-globin, the distal Ub moiety (depicted in red in Fig. 1) is released by DUB action and subsequently reenters the Ub conjugation cycle to ubiquitinate other endogenous proteins, whereas the proximal Ub moiety (depicted in green in Fig. 1) is released and reconjugated 10-fold less efficiently. When performing a similar experiment on the shorter, di-Ub–modified protein, both proximal Ub and distal Ub are released from α-globin by DUB action and both reenter the conjugation cycle to a similar extent. This shows that the proximal Ub in the longer tetra-Ub chains is more shielded from DUB activity than the proximal Ub in the shorter di-Ub chains.
Fig. 1.
Simplified schematic representation of results obtained by Sun et al. (1). Synthetic ubiquitinated α-globin was mixed with rabbit fraction II. The di-Ub–modified α-globin induces a different proteolytic outcome compared with the tetra-Ub–modified α-globin.
Sun et al. (1) employ the tagged substrates not only to study what happens to the individually tagged proximal and distal Ub moieties, but also to study the rate of degradation of α-globin. The authors find the efficiency of proteasomal degradation of the tetra-Ub-chain–modified protein to be higher than for the di-Ub–modified substrate, and neither mono-Ub, nor free α-globin, is degraded at all. To study the balance between deubiquitination and proteasome-mediated degradation, epoxomicin is introduced to inhibit proteasome activity while DUBs remain active to efficiently remove Ub moieties from the model α-globin protein. Conversely, inhibition of all cysteine DUBs using the inhibitor Ub aldehyde leads to more effective proteasome-mediated degradation of α-globin in the case of tetra-Ub– and di-Ub–modified α-globin compared with the control situation in which DUBs are not inhibited.
Together, these data imply that proteins carrying a tetra-Ub chain, although arguably not absolutely necessary to trigger proteasomal degradation, are degraded more efficiently due to their ability to remain ubiquitinated long enough to effect destruction of the substrates in a cell lysate compared with proteins carrying a shorter Ub chain. The data also show that DUB activity is involved in the regulation of the extent of proteasome-mediated protein degradation.
The work presented by Sun et al. (1) shows how a systematic approach with the right tools allows the study of a very complex degradation system. The use of chemically prepared ubiquitinated substrates is a helpful strategy to decipher, in detail, the roles that some of the proteasomal subunits play; such tools will perhaps unveil even more of the still-remaining mysteries surrounding the UPS in the future.
Acknowledgments
G.J.v.d.H.v.N. and H.O.’s research is supported by the Netherlands Organization for Scientific Research.
Footnotes
Conflict of interest statement: H.O. is a shareholder of the reagent company UbiQ.
See companion article on page 7805.
References
- 1.Sun H, et al. Diverse fate of ubiquitin chain moieties: The proximal is degraded with the target, and the distal protects the proximal from removal and recycles. Proc Natl Acad Sci USA. 2019;116:7805–7812. doi: 10.1073/pnas.1822148116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard JAM, et al. Structure and function of the 26S proteasome. Annu Rev Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science. 2016;351:aad9421. doi: 10.1126/science.aad9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chojnacki M, et al. Polyubiquitin-photoactivatable crosslinking reagents for mapping ubiquitin interactome identify Rpn1 as a proteasome ubiquitin-associating subunit. Cell Chem Biol. 2017;24:443–457.e6. doi: 10.1016/j.chembiol.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins GA, Goldberg AL. The logic of the 26S proteasome. Cell. 2017;169:792–806. doi: 10.1016/j.cell.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B-H, et al. USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature. 2016;532:398–401. doi: 10.1038/nature17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Linden RT, et al. Structural basis for the activation and inhibition of the UCH37 deubiquitylase. Mol Cell. 2015;57:901–911. doi: 10.1016/j.molcel.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worden EJ, Dong KC, Martin A. An AAA motor-driven mechanical switch in Rpn11 controls deubiquitination at the 26S proteasome. Mol Cell. 2017;67:799–811.e8. doi: 10.1016/j.molcel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, et al. Base-CP proteasome can serve as a platform for stepwise lid formation. Biosci Rep. 2015;35:e00194. doi: 10.1042/BSR20140173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma R, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Matouschek A. Recognition of client proteins by the proteasome. Annu Rev Biophys. 2017;46:149–173. doi: 10.1146/annurev-biophys-070816-033719. [DOI] [PubMed] [Google Scholar]
- 12.Shabek N, et al. The size of the proteasomal substrate determines whether its degradation will be mediated by mono- or polyubiquitylation. Mol Cell. 2012;48:87–97. doi: 10.1016/j.molcel.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Kravtsova-Ivantsiv Y, Cohen S, Ciechanover A. Modification by single ubiquitin moieties rather than polyubiquitination is sufficient for proteasomal processing of the p105 NF-kappaB precursor. Mol Cell. 2009;33:496–504. doi: 10.1016/j.molcel.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Lee BH, King RW, Finley D, Kirschner MW. Substrate degradation by the proteasome: A single-molecule kinetic analysis. Science. 2015;348:1250834. doi: 10.1126/science.1250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim W, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajish Kumar KS, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Highly efficient and chemoselective peptide ubiquitylation. Angew Chem Int Ed Engl. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- 18.El Oualid F, et al. Chemical synthesis of ubiquitin, ubiquitin-based probes, and diubiquitin. Angew Chem Int Ed Engl. 2010;49:10149–10153. doi: 10.1002/anie.201005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SK, et al. Synthetic uncleavable ubiquitinated proteins dissect proteasome deubiquitination and degradation, and highlight distinctive fate of tetraubiquitin. J Am Chem Soc. 2016;138:16004–16015. doi: 10.1021/jacs.6b09611. [DOI] [PubMed] [Google Scholar]
- 20.Rösner D, Schneider T, Schneider D, Scheffner M, Marx A. Click chemistry for targeted protein ubiquitylation and ubiquitin chain formation. Nat Protoc. 2015;10:1594–1611. doi: 10.1038/nprot.2015.106. [DOI] [PubMed] [Google Scholar]
- 21.Hewings DS, Flygare JA, Bogyo M, Wertz IE. Activity-based probes for the ubiquitin conjugation-deconjugation machinery: New chemistries, new tools, and new insights. FEBS J. 2017;284:1555–1576. doi: 10.1111/febs.14039. [DOI] [PMC free article] [PubMed] [Google Scholar]