Abstract
Objectives
To estimate self-reported human papillomavirus (HPV) disease-related psychosocial impact among male and female patients in South Korea.
Design
In this multicentre cross-sectional study, psychosocial impacts were estimated using a one-time survey capturing HPV Impact Profile (HIP) results, CuestionarioEspecifico en Condilomas Acuminados (CECA; in Spanish)—‘Specific questionnaire for Condylomata Acuminata’ and the EuroQol-5 Dimension (EQ-5D) surveys. Student’s t-tests or Mann-Whitney U tests were used for continuous comparisons; χ2 or Fisher’s exact tests were applied for categorical comparisons.
Setting
5098 clinics throughout Seoul, Busan, Daegu, Kwangju and Daejeon (South Korea).
Participants
Patients with and without genital warts (GW) (males) and selected HPV diseases (females) visiting primary care physicians, obstetricians/gynaecologists, urologists and dermatologists with 2–30 years experience.
Results
Of 150 male and 250 female patients, HIP scores showed 85.3% of male patients with GW and 32.0% without reported moderate psychological impact (p<0.0001). In categorised total scores, 88.5% of female patients with and 66.0% without selected HPV-related diseases reported moderate or high psychological impacts (p=0.0004). In the CECA questionnaire, male patients had mean (SD) scores of 10.51 (3.79) in ‘emotional health’ and 15.90 (6.13) in ‘sexual activity’. Female patients with GW reported lower scores in both dimensions with mean scores of 7.18 (4.17) in ‘emotional health’ and 10.97 (5.80) in ‘sexual activity’ (p<0.0001), indicating worse health-related quality of life (HRQoL). For the EQ-5D, male patients with GW reported lower mean Visual Analogue Scale (VAS) scores than those without (75.1 vs 81.13, p<0.0135). Mean VAS score and utility values were lower for females with HPV-related diseases than those without (72.18 vs 76.86 and 0.90 vs 0.94, respectively).
Conclusion
In South Korea, GW in men and HPV-related diseases in women negatively impact patient well-being and HRQoL scores. Among women, those with GW suffered a greater psychosocial impact than those with other selected HPV-related diseases.
Keywords: genital warts, psychosocial impact, South Korea
Strengths and limitations of this study.
Cross-sectional Human Papillomavirus (HPV) Impact Profile, Cuestionario Especifico en Condilomas Acuminados and EuroQol-5 dimension surveys completed by patients were logged by multiple physician specialties and in different geographical regions in South Korea.
Patients were stratified into male and female groups and further stratified by genital warts (GW) or HPV-related disease status (with/without).
Patient survey results were used to assess psychosocial burden (general health, sexual activity, cervical cancer screening behaviour, psychosocial impact, GW experience, sociodemographic information).
Selection bias may have occurred due to the convenience sample approach used.
Introduction
Human papillomaviruses (HPVs) are common sexually transmitted viral infections in young people.1–3 Of the 130 HPV types that have been identified and sequenced, approximately 40 have a predilection for the anogenital region. Although they are readily transmitted, and most are transient, they can cause disease that manifests as genital warts (GW) and squamous intraepithelial lesions on cervical Papanicolaou (Pap) screenings and high-risk types are the cause of anogenital cancers. HPV types 6 and 11 alone are estimated to cause most viral sexually transmitted infections (STIs).4 5 GW can be exophytic, confluent cauliflower-like tumours, and their typical morphologies can aid in diagnosis, although they can also be flat or atypical.6–8
To date, few studies have focused on HPV prevalence and related disease among men and women residing in South Korea. The study, conducted among South Korean women, observed a low-risk HPV prevalence of 10.3% among those ages 20–29 years and 3.2% among women ages 50–59 years.9 While another study observed an overall GW prevalence in South Korea of 0.7%.10
Studies have shown that GW infection can have a tremendous psychosocial impact on patients.11–13 Some of the highest rates of GW occur in adolescents and young adults at a time when individuals are particularly impacted by the stigma associated with a visible STI. Several key emotions have been identified in GW patients including anger, disgust, shame, embarrassment, depression, anxiety, worry and a feeling of being less desirable, which all can have an impact on sexual relationships.14 Research by Maw et al found that up to two-thirds of male and female GW patients made lifestyle changes that impacted their relationships.15
Recognising the profound impact that GW and other HPV-related diseases can have on patients has led to the creation of tools to assess the burden of these conditions, including the self-administered HPV Impact Profile (HIP) as developed and validated by Mast et al, the EuroQoL-5 Dimensions (EQ-5D), and the Cuestionario Especifico en Condilomas Acuminados (CECA; in Spanish)—‘Specific questionnaire for Condylomata Acuminata’. These are standardised and commonly used instruments to measure health-related quality of life (HRQoL).16–18 The HIP was used in a study of Taiwanese women, and results showed that an abnormal Pap result (including abnormal results and any grade of cervical cancer) has a significant psychosocial impact, and a greater impact for those diagnosed with GW.19 Pirotta et al 20 also found a significant psychosocial impact on Australian women screened for and diagnosed with an HPV-related disease. These women were found to be more likely to have their social lives disrupted, even more so than those being treated for high-grade cervical dysplasia.20
Literature on the psychosocial impact of GW in South Korea is scarce. Most available research in the country focuses on cervical cancer, HPV knowledge, and attitudes or intention towards HPV vaccination.21–23 The aim of this study was to evaluate the psychosocial burden of HPV-related diseases, including GW, in South Korea among male and female patients ages 20–60 years.
Materials and methods
Study design
A cross-sectional study was conducted from 28 July 2011 to 30 November 2011 in five major cities of South Korea: Seoul, Busan, Daegu, Kwangju and Daejeon (see online supplementary appendix table A1). The study targeted clinics where cervical cytology screenings, including GW screenings, were performed, and where men and women were seen for HPV-related diseases. No confidential patient-level data were collected for this study.
bmjopen-2018-025035supp001.pdf (208.9KB, pdf)
Inclusion and exclusion criteria
Participating physicians
Participating physicians were identified through an Intercontinental Marketing Services database, a database of nationwide clinics published by Health Insurance Review and Assessment. This database includes information pertaining to 5098 clinics in the five targeted cities (see online supplementary appendix table A2). All data collection for this study was conducted in the office or clinic of the participating physicians.
Patient and public involvement
Participant physicians invited their patients for study participation as part of routine practice by asking their patients if they were willing to participate in a one-time survey and giving them a patient informed consent form with a short description of the survey. The physician provided verification on the survey regarding to which group the patient belonged (GW or control group) and administered the survey in the physician’s office. Once the survey was completed, the patient’s survey was placed in a sealed envelope and left at the physician’s office to be sent or picked up by a research coordinator. Patients were not involved in the recruitment to and conduct of the study. Physicians were asked to read the corresponding questions to the patients to avoid any misinterpretation of questions. The results will not be disseminated to study participants. The current study was not a randomised controlled trial.
Female patients
Female patients were included in the study if they were between the ages of 20 and 60 years, experienced an HPV-related event within the past 3 months, were in good self-reported health and belonged to one of the following categories: (1) Abnormal Pap test result with no definitive histology, conforming to the Bethesda Category-2001 category of squamous or glandular cell abnormality (eg, atypical squamous cells of undetermined significance, atypical glandular cells of undetermined significance, low-grade squamous intraepithelial lesion or high-grade squamous intraepithelial lesion) and no previous high-risk HPV test performed; (2) receipt of positive high-risk HPV DNA test results after an abnormal Pap test, as defined in the previous category; (3) diagnosis of external GW or treatment for recurrences; (4) histological diagnosis of HPV-related cervical dysplasia cervical lesion (eg, CIN1, CIN2, CIN 3); (5) normal Pap result with no abnormal Pap test or definitive therapy within the past year or (6) two or more of the above conditions (not including GW patients) were categorised in the upper level of disease. To enable categorising of women into discreet disease groups of CIN versus GW, female patients were excluded from the study if they were diagnosed with GW and had any of the following: precancerous cervical lesions, abnormal Pap and HPV-positive or abnormal Pap test results.
The control group was selected from the same clinic as the case group. Physicians provided verification on the survey regarding patient groups (GW or control group) and gave them the survey to complete in the physician’s office. The physician sample was divided across primary care physicians (general practitioners and internal medicine), obstetrics/gynaecologists, urologists and dermatologists. The control group consisted of patients who have never had GW or received treatment for it or had surgery or therapy in the genital area and included all other patients from a physician’s practice or clinic.
Male patients
Male patients were included in the study if they were between ages 20 and 60 years, in good self-reported physical health and belonged to one of the following categories: (1) newly diagnosed or existing external GW within the past 3 months of study recruitment and (2) patients who had never been diagnosed with GW, prescribed GW treatment, or had surgery or therapy in the genital area.
Male and female patients were excluded from the study if they: (1) had presence of any other concurrent/active STI; (2) were concurrently enrolled in clinical studies of investigational agents; (3) had a history of known prior or recent (within 1 year of the enrolment date) HPV vaccination; (4) had ongoing alcohol or drug abuse; (5) were unable to give informed consent or (6) had presence of any condition, which, in the opinion of the investigator, could interfere with the evaluation of the study objectives.
Survey instruments
To measure the psychosocial burden (general health, sexual activity, cervical cancer screening behaviour, psychosocial impact, GW experience, sociodemographic information), participants completed the three validated questionnaires; HIP, CECA (in Spanish—‘Specific questionnaire for Condylomata Acuminata’) and EQ-5D surveys, which were translated to the Korean language and culturally pretested. Questionnaires were administered by the participating physician after patients were diagnosed with HPV-related disease. A pilot test was conducted using a small sample of physicians representing all four types of study physicians (two per specialty, eight total). This was to ensure that all survey questions and exercises were understood by respondents, and included culturally appropriate information.
HIP is a validated, 29-item self-administered questionnaire, designed to measure the psychosocial impact of HPV-related health conditions in women.15 The response for each item ranges from 0 (lowest impact) to 10 points (highest impact). Items in the HIP survey were linearly transformed to a 0–100 scale, with higher scores indicating better health. To create scale scores, the mean was computed as the sum of the item scores over the number of items answered to account for missing data. If more than 50% of items on the scale were missing, the score was not computed. To create the total scale score, the mean was computed as the sum of all items over the number of items answered on all scales.24 The scale uses visual-spatial, numeric and verbal descriptive anchors to assess subject responses. This survey was adapted for use in male patients in consultation with the original developer and has undergone cognitive testing in the USA. Overall, HIP scores are categorised as: no or little impact (mean HIP score <40), moderate impact (between 40 and 70) and heavy psychological impact (mean HIP score >70).19
The CECA survey includes 10 questions across two domains: emotional and sexual activity.18 25 CECA scores range from 0 (worst HRQoL) to 100 (best HRQoL). The EQ-5D survey is a two-part questionnaire, including descriptive and thermometer or Visual Analogue Scale (VAS), and serves as a generic validated instrument for use as a measure of HRQoL.26 VAS scores range from 0 (death) to 100 (perfect health).
Statistical analysis
All study outcomes were summarised descriptively. A descriptive analysis of the EQ-5D questionnaire was performed and numbers and percentages were provided. The Japanese version of the EQ-5D Instrument was used in this study to estimate the utilities associated with EQ-5D health status.27 Japan was the first Asian country to develop its own preference EQ-5D weights in 2002. The model was chosen to represent Asian preference weights.28 VAS scores and utility values were reported using the mean and SD of the VAS score. VAS scores ranged from 0 (worst HRQoL) to 100 (best HRQoL), and utility values from 0 (death) to 1 (perfect health).
Scores obtained for male and female patients were compared according to GW diagnosis (in men) and HPV-related disease or GW diagnosis (in female patients). For continuous variables, comparisons were performed using the Student’s t-test or Mann-Whitney U test. In addition, the effect size (mean difference between the two means divided by the pooled SD) between groups has been calculated. For categorical variables, differences between the groups were analysed using the χ2 or Fisher’s exact test depending on patient distribution across response categories.
CECA scores were reported using the mean, SD and 95% CI. Student’s t-tests were performed to compare CECA scores according to gender.
Results
Sociodemographic and clinical characteristics
A total of 400 patients participated in the study. Table 1 shows age, marital status, race, highest educational degree and sexual activity according to gender and HPV diagnosis status. Approximately half of the patients included in the study were age 30–44 years (45.3%), 85.9% were in a committed relationship, 51.6% were married, 2.3% earned an education lower than grade 12 (including vocational studies), 75.7% were employed and 22.5% had no health insurance or other healthcare coverage.
Table 1.
Sociodemographic characteristics and sexual activity of survey participants in South Korea by gender and GW diagnosis (men) or HPV-related diseases (women)
| Men (n=150) | P value | Women (n=250) | P value | Overall | |||
| With GW (n=75) |
No GW (n=75) |
HPV disease (n=200) |
No HPV disease (n=50) |
||||
| Age | |||||||
| Mean | 34.07 | 37.32 | 0.0422 | 34.90 | 35.56 | 0.6695 | 35.28 |
| SD | 9.48 | 9.96 | 9.63 | 10.32 | 9.77 | ||
| Age group | |||||||
| 20–29 years | 26 (34.7%) | 19 (25.3%) | 0.0194 | 70 (35.0%) | 18 (36.0%) | 0.9538 | 133 (33.3%) |
| 30–44 years | 40 (53.3%) | 33 (44.0%) | 86 (43.0%) | 22 (44.0%) | 181 (45.3%) | ||
| 45–60 years | 9 (12.0%) | 23 (30.7%) | 44 (22.0%) | 10 (20.0%) | 86 (21.5%) | ||
| Valid, n | 75 | 75 | 200 | 50 | 400 | ||
| Committed relationship | |||||||
| Yes | 66 (88.0%) | 62 (83.8%) | 0.4596 | 170 (85.4%) | 44 (88.0%) | 0.6398 | 342 (85.9%) |
| No | 9 (12.0%) | 12 (16.2%) | 29 (14.6%) | 6 (12.0%) | 56 (14.1%) | ||
| Valid, n | 75 | 74 | 199 | 50 | 398 | ||
| Marital status | |||||||
| Married | 32 (42.7%) | 45 (60.8%) | 0.0976 | 98 (49.0%) | 31 (62.0%) | 0.4302 | 206 (51.6%) |
| Widowed/divorced | 1 (1.3%) | 1 (1.4%) | 9 (4.5%) | 2 (4.0%) | 13 (3.3%) | ||
| Separated | 2 (2.7%) | 6 (3.0%) | 1 (2.0%) | 9 (2.3%) | |||
| Never married | 40 (53.3%) | 28 (37.8%) | 87 (43.5%) | 16 (32.0%) | 171 (42.9%) | ||
| Valid, n | 75 | 74 | 200 | 50 | 399 | ||
| Highest degree | |||||||
| Less than grade 12 including vocational education | 1 (1.3%) | 1 (1.3%) | 0.1752 | 4 (2.0%) | 3 (6.0%) | 0.1159 | 9 (2.3%) |
| High school graduate/GED | 18 (24.0%) | 11 (14.7%) | 67 (33.8%) | 21 (42.0%) | 117 (29.4%) | ||
| Some college/technical school including associate’s degree | 12 (16.0%) | 6 (8.0%) | 54 (27.3%) | 7 (14.0%) | 79 (19.8%) | ||
| Baccalaureate degree | 40 (53.3%) | 48 (64.0%) | 68 (34.3%) | 16 (32.0%) | 172 (43.2%) | ||
| Ever had sexual intercourse | |||||||
| Yes | 75 (100.0%) | 70 (93.3%) | 0.0229 | 198 (99.0%) | 46 (92.0%) | 0.0038 | 389 (97.3%) |
| No | 5 (6.7%) | 2 (1.0%) | 4 (8.0%) | 11 (2.8%) | |||
| Valid, n | 75 | 75 | 200 | 50 | 400 | ||
| Age at first sexual intercourse | |||||||
| Mean | 20.63 | 21.93 | 0.0470 | 21.92 | 22.84 | 0.1847 | 21.78 |
| SD | 3.95 | 3.79 | 4.19 | 4.18 | 4.11 | ||
| Valid, n patients | 75 | 68 | 196 | 45 | 384 | ||
| No of sex partners in the last 5 years | |||||||
| No partners | 2 (2.9%) | 0.0014 | 6 (3.0%) | 2 (4.3%) | 0.6072 | 10 (2.6%) | |
| 1 partner | 9 (12.0%) | 30 (42.9%) | 103 (52.3%) | 30 (65.2%) | 172 (44.3%) | ||
| 2–5 partners | 47 (62.7%) | 25 (35.7%) | 65 (33.0%) | 13 (28.3%) | 150 (38.7%) | ||
| 6–10 partners | 10 (13.3%) | 9 (12.9%) | 9 (4.6%) | 28 (7.2%) | |||
| 11–15 partners | 3 (4.0%) | 1 (1.4%) | 3 (1.5%) | 1 (2.2%) | 8 (2.1%) | ||
| 16–20 partners | 1 (1.3%) | 2 (2.9%) | 4 (2.0%) | 7 (1.8%) | |||
| 21–25 partners | 3 (4.0%) | 2 (1.0%) | 5 (1.3%) | ||||
| 26–50 partners | 1 (1.3%) | 2 (1.0%) | 3 (0.8%) | ||||
| More than 50 partners | 1 (1.3%) | 1 (1.4%) | 3 (1.5%) | 5 (1.3%) | |||
| Valid, n | 75 | 70 | 197 | 46 | 388 | ||
| Frequency of condom use | |||||||
| Never | 16 (21.3%) | 26 (37.7%) | 0.0614 | 86 (43.7%) | 24 (52.2%) | 0.2860 | 152 (39.3%) |
| Less than half the time | 28 (37.3%) | 13 (18.8%) | 30 (15.2%) | 10 (21.7%) | 81 (20.9%) | ||
| About half the time | 10 (13.3%) | 12 (17.4%) | 31 (15.7%) | 3 (6.5%) | 56 (14.5%) | ||
| Not always but more than half the time | 16 (21.3%) | 10 (14.5%) | 26 (13.2%) | 3 (6.5%) | 55 (14.2%) | ||
| Always | 5 (6.7%) | 7 (10.1%) | 15 (7.6%) | 5 (10.9%) | 32 (8.3%) | ||
| I have not had sexual intercourse in the last 12 months | 1 (1.4%) | 9 (4.6%) | 1 (2.2%) | 11 (2.8%) | |||
| Valid, n | 75 | 69 | 197 | 46 | 387 | ||
| Sexual partners in lifetime | |||||||
| Heterosexual partners | 74 (100.0%) | 70 (100.0%) | 198 (100.0%) | 46 (100.0%) | 388 (100.0%) | ||
| Valid, n | 74 | 70 | 198 | 46 | 388 | ||
| Sexual partners in the last 12 months | |||||||
| Heterosexual partners | 74 (100.0%) | 69 (100.0%) | 194 (100.0%) | 46 (100.0%) | 383 (100.0%) | ||
| Mean | 2.57 | 1.80 | 0.0829 | 2.06 | 1.20 | 0.2046 | 2.01 |
| SD | 2.81 | 2.49 | 4.50 | 0.63 | 3.62 | ||
| Valid, n | 74 | 69 | 194 | 46 | 383 | ||
GED, general educational development; GW, genital warts; HPV, human papillomavirus.
The sexual activity of surveyed patients according to gender and GW or selected HPV-related disease is shown in table 1. Male GW patients reported a younger age at first intercourse compared with female patients (20.6 (4.0) vs 21.9 (4.2)), and had a greater number of sexual partners (p=0.0014) than those without GW. A higher percentage of female patients with HPV-related diseases reported having had sexual intercourse compared with those without HPV-related diseases (99.0% vs 92.0%, p=0.0038). No statistically significant differences were observed for any of the remaining sexual activity questions, as reported in table 1.
Psychosocial impact
HIP scores for male and female patients are summarised in table 2. Significantly higher HIP scores were observed among men with GW compared with those without GW for all domains of the score except for ‘control-life impact’. Eighty-five per cent of men with GW and 32.0% of men without GW reported a moderate psychological impact (p<0.0001).
Table 2.
HIP questionnaire scores of participating patients by GW and HPV-related diagnosis in South Korea
| Men (n=150) | ES | P value | Women (n=250)* | ES | P value | |||
| With GW (n=75) |
No GW (n=75) |
HPV disease (n=200) |
No HPV disease (n=50) |
|||||
| HIP total score | ||||||||
| Mean | 50.90 | 36.13 | 1.69 | <0.0001 | 53.37 | 44.98 | 0.68 | <0.0001 |
| 95% CI | (48.8 to 53.0) | (34.3 to 38.0) | (51.8 to 55.0) | (41.4 to 48.6) | ||||
| Valid, n | 75 | 75 | 199 | 50 | ||||
| Worries and concerns | ||||||||
| Mean | 49.65 | 24.25 | 1.51 | <0.0001 | 57.19 | 41.94 | 0.63 | <0.0001 |
| 95% CI | (45.5 to 53.8) | (20.8 to 27.7) | (54.2 to 60.2) | (35.5 to 48.4) | ||||
| Valid, n | 75 | 75 | 199 | 49 | ||||
| Emotional impact | ||||||||
| Mean | 49.10 | 33.98 | 1.19 | <0.0001 | 56.08 | 42.32 | 0.84 | <0.0001 |
| 95% CI | (46.0 to 52.2) | (31.3 to 36.6) | (53.8 to 58.4) | (37.8 to 46.8) | ||||
| Valid, n | 75 | 75 | 199 | 50 | ||||
| Sexual impact | ||||||||
| Mean | 47.53 | 41.20 | 0.51 | 0.0019 | 50.81 | 49.80 | 0.07 | 0.6550 |
| 95% CI | (45.1 to 50.0) | (38.1 to 44.3) | (48.9 to 52.8) | (45.3 to 54.3) | ||||
| Valid, n | 75 | 75 | 197 | 49 | ||||
| Self-Image | ||||||||
| Mean | 49.00 | 41.63 | 0.76 | <0.0001 | 47.66 | 45.17 | 0.19 | 0.2226 |
| 95% CI | (46.5 to 51.5) | (39.8 to 43.5) | (45.9 to 49.5) | (41.4 to 48.9) | ||||
| Valid, n | 75 | 75 | 199 | 50 | ||||
| Partner issues and transmission | ||||||||
| Mean | 62.16 | 42.12 | 1.40 | <0.0001 | 58.86 | 47.23 | 0.62 | 0.0001 |
| 95% CI | (59.1 to 65.2) | (38.5 to 45.8) | (56.2 to 61.5) | (41.8 to 52.6) | ||||
| Valid, n | 74 | 66 | 185 | 47 | ||||
| Interactions with doctors | ||||||||
| Mean | 51.31 | 33.28 | 0.90 | <0.0001 | 46.75 | 45.73 | 0.20 | 0.6611 |
| 95% CI | (47.4 to 55.3) | (25.2 to 41.4) | (44.8 to 48.7) | (40.8 to 50.6) | ||||
| Valid, n | 71 | 30 | 199 | 50 | ||||
| Control-life impact | ||||||||
| Mean | 49.69 | 52.13 | 0.23 | 0.1643 | 48.48 | 52.37 | 0.31 | 0.0641 |
| 95% CI | (47.3 to 52.0) | (49.6 to 54.7) | (46.6 to 50.4) | (49.2 to 55.5) | ||||
| Valid, n | 75 | 75 | 199 | 50 | ||||
| HIP total score categorised | ||||||||
| No or little impact | 11 (14.7%) | 51 (68.0%) | <0.0001 | 23 (11.5%) | 17 (34.0%) | 0.0004 | ||
| Moderate impact | 64 (85.3%) | 24 (32.0%) | 168 (84.0%) | 30 (60.0%) | ||||
| Heavy psychological impact | 9 (4.5%) | 3 (6.0%) | ||||||
| Valid, n | 75 | 75 | 200 | 50 | ||||
HIP items range from 0 (lowest impact) to 10 points (highest impact).
CECA scores range from 0 (worst HRQoL) to 100 (the best HRQoL).
EQ-5D range from 0 (worst imaginable health state) to 100 (best imaginable health state).
ES >0.01 is considered significant.
*HPV, human papillomavirus is included in this table.
CECA, Cuestionario Especifico en Condilomas Acuminados; EQ-5D, EuroQol-5 Dimension; ES, effective size; GW, genital warts; HIP, Human Papillomavirus Impact Profile; HRQoL, health-related quality of life.
When comparing women diagnosed with HPV-related disease to those without disease, significant differences were observed for the ‘worries and concerns’, ‘emotional impact’ and ‘partner’s issues and transmission’ domains. In all domains, female patients with HPV-related disease had higher scores, reflecting a higher psychological impact (88.5% of female patients with selected HPV-related diseases vs 66.0% without reported a moderate or heavy psychological impact (p=0.0004)).
HIP scores by specific HPV-related disease were also conducted. In all domains except for ‘control-life impact’ and ‘emotional impact’, significant differences were identified. Higher scores and thereby higher psychological impact were reported by patients with external GW. All GW patients had either moderate or heavy psychological impact (90.0% and 10.0%, respectively). In all domains, female patients with selected HPV-related diseases had higher scores, reflecting a higher psychological impact (see online supplementary appendix table A3).
CECA scores stratified by gender are shown in figure 1. Women with GW reported significantly lower scores on the ‘emotional health’ (mean (SD), 7.2 (4.2)) and ‘sexual activity’ dimensions (11.0 (5.8)) compared with men with GW—‘emotional health’ dimension (10.5 (3.8)) and ‘sexual activity’ dimension (15.9 (6.1))—indicating worse HRQoL among women.
Figure 1.
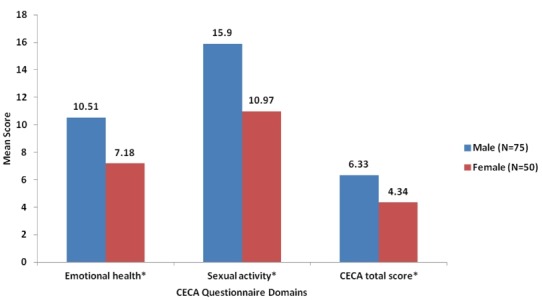
CECA questionnaire scores by male and female patients with GW in South Korea. CECA, Cuestionario Especifico en Condilomas Acuminados (in Spanish)—‘Specific questionnaire for Condylomata Acuminata’; GW, genital warts. *p-value <0.05.
No significant differences were observed for problems reported by male patients in the EQ-5D descriptive system by GW diagnosis, as most male patients reported no problems. Among those who reported problems, the most frequent were ‘pain–discomfort’ (10.7%) and ‘anxiety–depression’ (12.7%, table 3). Female patients with selected HPV-related diseases reported more problems related to the EQ-5D ‘anxiety–depression’ dimension than those without. Thirty-one per cent of those with selected HPV-related diseases reported feeling moderately or extremely anxious or depressed, compared with 10.0% of female patients without HPV-related diseases. There were no statistically significant differences in the remaining EQ-5D items between female patients with and without HPV-related diseases (table 3).
Table 3.
EQ-5D descriptive system results by male and female patients with and without GW and selected HPV-related diseases in South Korea
| Men (n=150) | P value | Women (n=250) | P value | |||
| With GW (n=75) |
No GW (n=75) |
HPV disease (n=200) |
No HPV disease (n=50) |
|||
| Mobility | ||||||
| I have no problems walking about | 73 (97.3%) | 75 (100.0%) | 0.1545 | 193 (97.0%) | 49 (98.0%) | 0.6979 |
| I have some problems walking about | 2 (2.7%) | 6 (3.0%) | 1 (2.0%) | |||
| Valid, n | 75 | 75 | 199 | 50 | ||
| Self-care | ||||||
| I have no problems with self-care | 75 (100.0%) | 75 (100.0%) | – | 197 (99.0%) | 50 (100.0%) | 0.7762 |
| I have some problems washing or dressing myself | 1 (0.5%) | |||||
| I am unable to wash or dress myself | 1 (0.5%) | |||||
| Valid, n | 75 | 75 | 199 | 50 | ||
| Usual activities | ||||||
| I have no problems with performing my usual activities | 72 (96.0%) | 75 (100.0%) | 0.0802 | 196 (98.5%) | 49 (98.0%) | 0.8044 |
| I have some problems with performing my usual activities | 3 (4.0%) | 3 (1.5%) | 1 (2.0%) | |||
| Valid, n | 75 | 75 | 199 | 50 | ||
| Pain–discomfort | ||||||
| I have no pain or discomfort | 65 (86.7%) | 69 (92.0%) | 0.2900 | 165 (82.9%) | 40 (80.0%) | 0.6291 |
| I have moderate pain or discomfort | 10 (13.3%) | 6 (8.0%) | 34 (17.1%) | 10 (20.0%) | ||
| Valid, n | 75 | 75 | 199 | 50 | ||
| Anxiety–depression | ||||||
| I am not anxious or depressed | 66 (88.0%) | 65 (86.7%) | 0.8061 | 136 (68.3%) | 45 (90.0%) | 0.0078 |
| I am moderately anxious or depressed | 9 (12.0%) | 10 (13.3%) | 56 (28.1%) | 5 (10.0%) | ||
| I am extremely anxious or depressed | 7 (3.5%) | |||||
| Valid, n | 75 | 75 | 199 | 50 | ||
HIP items range from 0 (lowest impact) to 10 points (highest impact).
CECA scores range from 0 (worst HRQoL) to 100 (the best HRQoL).
EQ-5D range from 0 (worst imaginable health state) to 100 (best imaginable health state).
CECA, Cuestionario Especifico en Condilomas Acuminados; EQ-5D, EuroQol-5 Dimension; GW, genital warts; HIP, Human Papillomavirus Impact Profile; HPV, human papillomavirus; HRQoL, health-related quality of life.
EQ-5D descriptive system responses were also compared among female patients by HPV-related disease (see online supplementary appendix table A4). The only two dimensions with significant differences were ‘pain/discomfort’ (p=0.0146) and ‘anxiety/depression’ (p=0.0387). A higher percentage of female GW patients reported being ‘moderately anxious or depressed’ and ‘extremely anxious or depressed’ (48.0%), followed by those with precancerous lesions (34.7%) and those presenting abnormal Pap test and HPV positive results (24.0%) (see online supplementary appendix table A4).
Table 4 shows VAS scores and utility values obtained from male participants according to GW diagnosis. Those patients with GW reported significantly lower mean VAS scores (75.3) than those without (81.1, p=0.0135). No significant differences in utility values according to GW diagnosis were identified.
Table 4.
EQ-5D VAS scores and utility values by male patients with and without GW and female patients with and without selected HPV-related disease in South Korea
| Men (n=150) | ES | P value | Overall | Women (n=250) | ES | P value | Overall | |||
| With GW (n=75) |
No GW (n=75) |
HPV disease (n=200) |
No HPV disease (n=50) |
|||||||
| VAS (EQ-5D) | ||||||||||
| Mean | 75.31 | 81.13 | 0.41 | 0.0135 | 78.16 | 72.18 | 76.86 | 0.30 | 0.0606 | 73.14 |
| 95% CI | (71.6 to 79.0) | (78.4 to 83.9) | (75.8 to 80.5) | (69.9 to 74.4) | (72.6 to 81.1) | (71.2 to 75.1) | ||||
| Valid, n | 74 | 71 | 145 | 190 | 49 | 239 | ||||
| Utility values | ||||||||||
| Mean | 0.95 | 0.95 | <0.01 | 0.7527 | 0.95 | 0.90 | 0.94 | 0.27 | 0.0773 | 0.91 |
| 95% CI | (0.9 to 1.0) | (0.9 to 1.0) | (0.9 to 1.0) | (0.9 to 0.9) | (0.9 to 1.0) | (0.9 to 0.9) | ||||
| Valid, n | 75 | 75 | 150 | 199 | 50 | 249 | ||||
CECA scores range from 0 (worst HRQoL) to 100 (the best HRQoL).
EQ-5D range from 0 (worst imaginable health state) to 100 (best imaginable health state).
HIP items range from 0 (lowest impact) to 10 points (highest impact).
ES >0.01 is considered significant.
CECA, Cuestionario Especifico en Condilomas Acuminados; EQ-5D, EuroQol-5 Dimension; ES, effect size; GW, genital warts; HIP, Human Papillomavirus Impact Profile; HPV, human papillomavirus; HRQoL, health-related quality of life; VAS, Visual Analogue Scale.
Female patients with selected HPV-related diseases showed numerically lower mean VAS scores (72.2) and utility values (0.90) than those without selected HPV-related diseases (76.86 and 0.94, respectively), but the differences were not significant. When comparing selected HPV-related diseases, the lowest VAS and utility scores (worst HRQoL) were observed in GW patients (p<0.0001, see online supplementary appendix table A5).
Discussion
This cross-sectional study estimated the psychosocial burden of GW and HPV-related diseases in South Korea by obtaining self-reported HPV disease-related information among male and female patients age 20–60 years presenting to clinics where cervical cytology screenings, including GW screenings, were performed, and where men and women were seen for HPV-related diseases. To our knowledge, this is the first study that has looked at the psychosocial burden of GW and HPV-related disease on patients’ QoL in South Korea. Higher HIP score values, reflecting a greater psychosocial impact of the disease, were recorded for men with GW than for those without GW (50.90 vs 36.13) and in women diagnosed with HPV-related diseases than for those without (53.37 vs 44.98).
Overall, female patients had a greater psychosocial impact compared with male patients (HIP scores: 51.69 vs 43.51). Similarly, male patients had better HRQoL indicating lower psychosocial impact compared with female patients, as assessed by CECA scores (6.33 vs 4.34). VAS scores ranged from 0 (worst imaginable health status) to 100 (best imaginable health status), and female patients reported worse health status (73.14) compared with male patients (78.16), particularly female patients with HPV-related diseases (72.18). In addition, GW patients reported worse HRQoL scores compared with those without GW in the disease-specific HIP questionnaire. Furthermore, women reported poorer health status following a GW diagnosis than a CIN diagnosis. These results are consistent with a Chinese study by Wang et al 29 which reported that female GW patients had the highest mean HIP scores (52.2), showing a significant psychological impact, followed by patients with precancerous cervical lesions (48.6), HPV after abnormal Pap test results (45.8), abnormal Pap test results without HPV test (44.1), and those who were HPV negative after abnormal Pap test results (43.1).
In the current study, HRQoL results suggest that GW in males and HPV-related disease (high-grade dysplasia requiring ablation treatment) in female patients had a negative impact on patient well-being and HRQoL scores. This study also observed that female GW patients suffered a major impact compared with those with other selected HPV-related diseases. Previous studies have shown that patients with GW had significantly lower QoL, and substantial psychosocial burden with higher social stigma—especially when GW infection is symptomatic, visible to the naked eye, and found in the genital region.30–32 In addition, a study that compared GW patients with asymptomatic genitourinary internal medicine patients observed that patients with GW had a significantly higher psychological burden because of the GW infection compared with the other patients. The study also observed that infection with GW influences the patient’s physical well-being and has a potentially detrimental effect on the patient’s emotions.33
This could explain the observed poorer health status in GW patients evaluated in this study. Furthermore, the highest score was in the ‘partner issue and transmission’ category, followed by ‘worries and concerns’ and ‘emotional impact’, with a HIP mean score >60. The lowest scores were in the ‘control-life impact’ category (mean HIP score 45.20). A similar study using the HIP survey instrument in Australia found that the largest impact of GW on QoL was in the domains of ‘sexual impact’, ‘self-image’ and ‘partner and transmission’.24
Based on EQ-5D survey results, GW and selected HPV-related disease patients reported more problems related to ‘anxiety–depression’ than those without these conditions. The current study detected a lower impact of GW as assessed by EQ-5D than in the previous Canadian study.34 HRQoL scores in each of the questionnaires reported by female study patients were descriptively compared among the study subgroups (abnormal Pap result, abnormal Pap and HPV positive results, precancerous lesions, and external GW). While GW has an impact on HRQoL in the current study, the precise impact is difficult to assess due to scarcity of data and the heterogeneity of the instruments used to compare scores of GW patients with those of the general population.35
Shi et al conducted a similar study in mainland China in 2012 and found that 56.4% of patients reported some problems in the ‘anxiety and depression’ dimension (highest), followed by ‘pain and discomfort’ (24.7%) and ‘mobility’ (3.5%).17 In a study from the UK, Woodhall et al 36 found that female GW patients had lower VAS and EQ-5D index scores than control patients, even after adjusting for age and gender. The difference was particularly notable in young women. Consistent with the current study results, Woodhall et al also reported that the ‘pain and discomfort’ and ‘depression and anxiety’ dimensions were the two most affected domains.
This study also observed that 60% of women with no GW reported a moderate impact in the HIP scoring. Reasons for this impact level among these patients were not evaluated. However, there is the possibility that these patients may have had other conditions during presentation at the clinic that may have impacted their HIP score.
Overall, the results of the current study suggest that a GW diagnosis has a great psychosocial impact on female patients. Other studies have provided evidence that the psychosocial impact of sexually transmitted disease diagnoses may be greater for women than for men. The origin of these differences is not clear, but they may be due to sexual infectivity and reproductive health.36 Furthermore, research among women who received abnormal cervical smear test results have indicated that they often experienced psychosocial consequences including anxiety, fears about cancer, sexual difficulties, changes in body image and concerns regarding loss of reproductive function.13 37 Shi et al 17 also indicated in their study that culture plays an important role, as conservative cultures (such as South Korea) view a diagnosis of a sexually transmitted disease such as GW as disgraceful. Consequently, patients would not seek support, even from their own families.16 Additionally, continued study of HPV natural history among men from different geographical regions is necessary to elucidate the underlying HPV-related diseases occurring in these populations.
Limitations
The current study is limited, as selection bias may have occurred due to the convenience sample approach used. The data were collected in participating physician offices and clinics through questionnaires and interview-based surveys. Patients may have given expected rather than truthful answers, which may not give the true psychosocial impact. Moreover, only patients who sought professional GW treatment were included in the study, which may not be generalisable to the entire South Korean GW population. As the study was cross-sectional in design, it can only report the impact of GW on the patients at the time the survey was taken, rather than longer term impact. However, in a longitudinal study conducted to determine the impact of HPV status on QoL in oral cavity and oropharyngeal squamous cell carcinoma, results showed that QoL scores were lower in HPV positive patients.38 The study design is a simple descriptive comparison of outcomes, so potential factors that might mediate or moderate the psychosocial effects of GW were not evaluated. We recommend that for future studies on GW in South Korea, multivariate analysis is carried out to address these factors.
Conclusion
The diagnosis of GW, a common sexually transmitted disease, has significant associated morbidity—largely due to the psychosocial impact GW have on patients. Prevention of all HPV-related diseases, cancers, and non-cancerous lesions is important. Vaccines that have broad protection against multiple HPV types should be considered. In addition, the results of this study can help direct guidelines for patient counselling and health education and emphasise the need to include HPV vaccine programmes as a part of national vaccine programmes. The purpose of this study was to determine the psychosocial impact of GW among male and female patients in South Korea using various validated tools, given that literature related to the psychosocial impact of GW is scarce in this country. The current study results, using HRQoL, suggest that GW in males and high-grade dysplasia requiring ablation treatment in female patients have a negative impact on patient well-being and HRQoL. The psychosocial burden was particularly greater among female GW patients compared with those with other selected HPV-related disease.
Although recent studies have looked at the psychosocial impact of GW on HRQoL in other places like China,11 Singapore,12 and the UK,13 this study highlights the psychosocial impact of GW on HRQoL for infected patients in South Korea. Previously published studies used for comparison to the results of this study vary substantially in methodology and are different in nature due to the dissimilarities of GW across regions and cultures. However, the current study offers baseline data, and further research is encouraged to measure the psychosocial burden of GW in South Korea. Despite its limitations, the current study offers groundwork for measurement of the psychosocial impact of GW in South Korea that was previously unavailable.
Supplementary Material
Acknowledgments
The authors acknowledge Furaha Kariburyo of STATinMED Research, for medical writing assistance.
Footnotes
Contributors: TSL, SK-T, PKS, KY, AK, ARG, SMG, WJ, NL and MR conceived and designed the experiments for this manuscript. NL, MR, SK-T, PKS and AK performed the experiments for this manuscript. NL and MR analysed the data for this manuscript. NL and MR contributed reagents/materials/analysis tools for this manuscript. All authors contributed to the writing of this manuscript.
Funding: This study was funded by Merck & Co.
Competing interests: TSL has no conflicts to declare. KY was a paid contractor for Merck & Co at the time of the study and was an employee of Cubist Pharmaceuticals December 2014–July 2015, which was acquired by Merck & Co in January 2015. AK, SK-T and PKS are employees of Merck & Co. SMG received grants to her institution from the Commonwealth Department of Health for HPV genoprevalance surveillance postvaccination, Merck & Co, and Glaxo Smith Kline to perform phase 3 clinical vaccine trials: Merck to evaluate HPV in RRP postvaccination programme; CSL for HPV in cervical cancer study, and VCA for a study on the effectiveness of a public health HPV vaccine study, and a study on the associations of early-onset cancers. SMG also received speaking fees from MSD and SPMSD for work performed in her personal time. Merck & Co also paid for travel and accommodation to present at HPV Advisory board meetings. ARG is a member of Merck & Co advisory boards. Her institution has received grants and contracts to support HPV-related research. NL and MR are employees of IQVIA, Barcelona, Spain, which is a paid consultant to Merck & Co. WJ has no conflicts to declare.
Ethics approval: The study was approved by the National Evidence-based Healthcare Collaborating Agency (NECA), Borame University Hospital, the SMG-SNU University Medical Center and the Ewha University Mokdong Hospital ethics committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data collected is the property of Merck & Co, and can be accessed with permission from Merck & Co.
Patient consent for publication: Not required.
References
- 1. Arima Y, Winer RL, Feng Q, et al. Development of genital warts after incident detection of human papillomavirus infection in young men. J Infect Dis 2010;202:1181–4. 10.1086/656368 [DOI] [PubMed] [Google Scholar]
- 2. Patel H, Wagner M, Singhal P, et al. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis 2013;13:39 10.1186/1471-2334-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pirotta MV, Stein AN, Fairley CK, et al. Patterns of treatment of external genital warts in Australian sexual health clinics. Sex Transm Dis 2009;36:375–9. 10.1097/OLQ.0b013e3181971e4e [DOI] [PubMed] [Google Scholar]
- 4. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218–26. 10.1093/aje/kwf180 [DOI] [PubMed] [Google Scholar]
- 5. Flores-Díaz E, Sereday KA, Ferreira S, et al. HPV-6 molecular variants association with the development of genital warts in men: The HIM Study. J Infect Dis 2016;215:559–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gall SA. Female genital warts: global trends and treatments. Infect Dis Obstet Gynecol 2001;9:149–54. 10.1155/S1064744901000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009;199:805–14. 10.1086/597071 [DOI] [PubMed] [Google Scholar]
- 8. Stamm AW, Kobashi KC, Stefanovic KB. Urologic Dermatology: a Review. Curr Urol Rep 2017;18:6 10.1007/s11934-017-0712-9 [DOI] [PubMed] [Google Scholar]
- 9. Kim MA, Oh JK, Kim BW, et al. Prevalence and seroprevalence of low-risk human papillomavirus in Korean women. J Korean Med Sci 2012;27:922–8. 10.3346/jkms.2012.27.8.922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee TS, Kothari-Talwar S, Singhal PK, et al. A cross-sectional study estimating the burden of illness related to genital warts in South Korea. BMJ Open 2017;7:e01421 10.1136/bmjopen-2016-014217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan LS, Chio MT, Sen P, et al. Assessment of psychosocial impact of genital warts among patients in Singapore. Sex Health 2014;11:313–8. 10.1071/SH13189 [DOI] [PubMed] [Google Scholar]
- 12. Sz Q, Wang SM, Shi JF, et al. Human papillomavirus-related psychosocial impact of patients with genital warts in China: a hospital-based cross-sectional study. BMC Public Health 2014;14:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominiak-Felden G, Cohet C, Atrux-Tallau S, et al. Impact of human papillomavirus-related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health-related quality of life study in the UK. BMC Public Health 2013;13:1065 10.1186/1471-2458-13-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeynes C, Chung MC, Challenor R. ‘Shame on you’ – the psychosocial impact of genital warts. Int J STD AIDS 2009;20:557–60. 10.1258/ijsa.2008.008412 [DOI] [PubMed] [Google Scholar]
- 15. Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998;9:571–8. 10.1258/0956462981921143 [DOI] [PubMed] [Google Scholar]
- 16. Mast TC, Zhu X, Demuro-Mercon C, et al. Development and psychometric properties of the HPV Impact Profile (HIP) to assess the psychosocial burden of HPV. Curr Med Res Opin 2009;25:2609–19. 10.1185/03007990903238786 [DOI] [PubMed] [Google Scholar]
- 17. Shi JF, Kang DJ, Qi SZ, et al. Impact of genital warts on health related quality of life in men and women in mainland China: a multicenter hospital-based cross-sectional study. BMC Public Health 2012;12:153 10.1186/1471-2458-12-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vilata JJ, Varela JA, Olmos L, et al. Validation and clinical use of the CECA, a disease-specific quality of life questionnaire for patients with anogenital condylomata acuminata. Acta Derm Venereol 2008;88:257–62. 10.2340/00015555-0422 [DOI] [PubMed] [Google Scholar]
- 19. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychosom Obstet Gynaecol 2010;31:16–23. 10.3109/01674820903564440 [DOI] [PubMed] [Google Scholar]
- 20. Pirotta M, Stein AN, Conway EL, et al. Genital warts incidence and healthcare resource utilisation in Australia. Sex Transm Infect 2010;86:181–6. 10.1136/sti.2009.040188 [DOI] [PubMed] [Google Scholar]
- 21. Kang HS, Moneyham L. Attitudes toward and intention to receive the human papilloma virus (HPV) vaccination and intention to use condoms among female Korean college students. Vaccine 2010;28:811–6. 10.1016/j.vaccine.2009.10.052 [DOI] [PubMed] [Google Scholar]
- 22. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol 2009;20:1–7. 10.3802/jgo.2009.20.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HW. Knowledge about human papillomavirus (HPV), and health beliefs and intention to recommend HPV vaccination for girls and boys among Korean health teachers. Vaccine 2012;30:5327–34. 10.1016/j.vaccine.2012.06.040 [DOI] [PubMed] [Google Scholar]
- 24. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect 2009;85:508–13. 10.1136/sti.2009.037028 [DOI] [PubMed] [Google Scholar]
- 25. Badia X, Colombo JA, Lara N, et al. Combination of qualitative and quantitative methods for developing a new health related quality of life measure for patients with anogenital warts. Health Qual Life Outcomes 2005;3:24 10.1186/1477-7525-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 27. Tsuchiya A, Ikeda S, Ikegami N, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ 2002;11:341–53. 10.1002/hec.673 [DOI] [PubMed] [Google Scholar]
- 28. Sakthong P, Charoenvisuthiwongs R, Shabunthom R. A comparison of EQ-5D index scores using the UK, US, and Japan preference weights in a Thai sample with type 2 diabetes. Health Qual Life Outcomes 2008;6:71 10.1186/1477-7525-6-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang SM, Shi JF, Kang DJ, et al. Impact of human papillomavirus-related lesions on quality of life: a multicenter hospital-based study of women in Mainland China. Int J Gynecol Cancer 2011;21:182–8. 10.1097/IGC.0b013e3181ffbed8 [DOI] [PubMed] [Google Scholar]
- 30. McCaffery K, Forrest S, Waller J, et al. Attitudes towards HPV testing: a qualitative study of beliefs among Indian, Pakistani, African-Caribbean and white British women in the UK. Br J Cancer 2003;88:42–6. 10.1038/sj.bjc.6600686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health 2010;10:113 10.1186/1471-2458-10-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qi SZ, Wang SM, Shi JF, et al. Human papillomavirus-related psychosocial impact of patients with genital warts in China: a hospital-based cross-sectional study. BMC Public Health 2014;14:739 10.1186/1471-2458-14-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeynes C, Chung MC, Challenor R. ‘Shame on you’–the psychosocial impact of genital warts. Int J STD AIDS 2009;20:557–60. 10.1258/ijsa.2008.008412 [DOI] [PubMed] [Google Scholar]
- 34. Sénécal M, Brisson M, Maunsell E, et al. Loss of quality of life associated with genital warts: baseline analyses from a prospective study. Sex Transm Infect 2011;87:209–15. 10.1136/sti.2009.039982 [DOI] [PubMed] [Google Scholar]
- 35. Scarbrough Lefebvre CD, Van Kriekinge G, Gonçalves MA, et al. Appraisal of the burden of genital warts from a healthcare and individual patient perspective. Pub Health 2011;125:464–75. 10.1016/j.puhe.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 36. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect 2008;84:161–6. 10.1136/sti.2007.029512 [DOI] [PubMed] [Google Scholar]
- 37. Anhang R, Goodman A, Goldie SJ. HPV communication: review of existing research and recommendations for patient education. CA Cancer J Clin 2004;54:248–9. 10.3322/canjclin.54.5.248 [DOI] [PubMed] [Google Scholar]
- 38. Sharma A, Méndez E, Yueh B, et al. Human papillomavirus–positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg 2012;146:739–45. 10.1177/0194599811434707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-025035supp001.pdf (208.9KB, pdf)


