Abstract
Background & Aims:
Non-alcoholic fatty liver disease is an inflammatory condition that results in progressive liver disease. It is unknown if individuals with hepatic steatosis, but not known to have liver disease, have higher serum concentrations of markers of systemic inflammation and oxidative stress
Methods:
We collected data from 2482 participants from the Framingham Heart Study (mean age, 51±11 years; 51% women) who underwent computed tomography and measurement of 14 serum markers of systemic inflammation. Heavy alcohol users were excluded. Liver:Phantom ratio (LPR, a continuous parameter of liver attenuation relative to a calibration phantom) was used to identify individuals with radiographic evidence of liver fat. Primary covariates included age, sex, smoking, alcohol, aspirin use, hypertension, dyslipidemia, diabetes, and cardiovascular disease. Body mass index and visceral fat were secondary covariates. We used multivariable linear regression models to assess the association between liver fat and systemic inflammatory markers.
Results:
In multivariable-adjusted models, liver fat was associated with the following inflammatory markers: high-sensitivity C-reactive protein (P<.001), urinary isoprostanes (P<.001), interleukin 6 (P<.001), intercellular adhesion molecule 1 (P<.001), and P-selectin (P=.002). Additional adjustment for body mass index or visceral fat attenuated the results slightly, although all associations remained statistically significant (P for all ≤.01).
Conclusion:
In a community-based cohort, individuals with hepatic steatosis without known liver disease had higher mean serum concentrations of systemic markers of inflammation. Studies are needed to determine whether treatment of hepatic steatosis reduces systemic inflammation.
Introduction
Non-alcoholic fatty liver disease (NAFLD), characterized by hepatic steatosis in the absence of secondary causes, is a common and morbid cause of liver disease worldwide.1–3 Most people with NAFLD have simple steatosis that does not progress to fibrosis1, and many consider hepatic steatosis without fibrosis to be an innocuous condition.4
Multiple studies have observed associations between NAFLD and cardiovascular disease, including coronary artery calcium,5,6 microvascular function,7 and diastolic dysfunction.6 Systemic inflammation is a major component of cardiovascular disease8 and is associated with the metabolic syndrome,9 obesity,10,11 and diabetes.12 An increased influx of free fatty acids to the liver may lead to increased transcription of systemic inflammatory mediators, which accelerate cardiovascular disease.13 Since NAFLD is strongly associated with obesity and insulin resistance, it is important to understand the extent to which NAFLD is associated with systemic inflammation after accounting for potential confounding factors. In several small studies, participants with NAFLD and insulin resistance14,15 or the metabolic syndrome16 had higher measures of systemic inflammation compared to those without NAFLD. In hospital-based cohorts, several studies observed an association between those with NAFLD or hepatic fibrosis and measures of systemic inflammation.17,18,27–29,19–26 Prior studies of the association between NAFLD and inflammatory makers have largely been limited by small sample sizes16,17,31–33,18,20,24,25,27–30 or measurement of a single or few inflammatory markers10,14,35,15,19,21,22,24,25,33,34, and have predominantly included those with more advanced NAFLD.23,26,29 The relationship between hepatic steatosis and systemic inflammation among community-dwellers not selected for liver disease has largely been unexplored.
Thus, we evaluated the association between liver fat as measured on non-contrast enhanced computed tomography and multiple biomarkers of systemic inflammation and oxidative stress among community-dwelling participants in the Framingham Heart Study (FHS). We hypothesized that liver fat is associated with multiple markers of systemic inflammation even after accounting for obesity and other covariates.
Participants and Methods
Participants (n=3,394) from the FHS second and third generation cohorts who participated in a multi-detector computed tomography substudy were eligible for inclusion.36 The FHS is a multi-generational community-based epidemiological study of cardiovascular disease in Massachusetts, USA, beginning in 1948, including over 14,000 participants (https://www.framinghamheartstudy.org).37
We excluded participants for: inadequate image capture of the liver (n=265); excess alcohol use (>14 drinks/week for women, >21 drinks/week for men, n=484)2,38; missing information on alcohol use (n=26); and missing covariates (n=137) (Figure 1). The study protocol was approved by the Institutional Review Board at Boston University Medical Center; all participants provided written informed consent.
Figure 1:
Study sample and participant exclusions
Measuring hepatic steatosis
Assessment of hepatic steatosis was standardized relative to a radiographic phantom during eight-slice multi-detector computed tomography (LightSpeed Ultra, General Electric, Milwaukee, WI).36 Full details of the acquisition protocol have been previously published.36 In brief, the mean Hounsfield units from three areas of the liver of at least 100 mm2 (intentionally avoiding blood vessels) was compared to a commercially available calibration control or “phantom,” which was present on all images. Lower values of the Liver:Phantom Ratio (LPR) correspond to more radiographic hepatic steatosis and have previously been shown to be associated with cardiometabolic risk factors.39 Hepatic steatosis was defined as LPR ≤ 0.33 based on prior studies using the liver to spleen ratio.36,39 All image processing was overseen and reviewed by a single radiologist (UH) and has previously been demonstrated to have good inter- and intra-rater reliability.36
Covariates
Covariates were chosen a priori based on their relevance for risk of liver disease or potential influence on a systemic inflammatory state. Covariates included body mass index (BMI, defined by weight (kg) divided by height squared (m2)), hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or treatment with an antihypertensive medication), cardiovascular disease (history of coronary heart disease, stroke, heart failure, or intermittent claudication adjudicated by review of medical records40), or diabetes mellitus (fasting serum glucose ≥ 126 mg/dL or treatment with an anti-hyperglycemic medication). Regular aspirin use was defined as self-reported chronic use of three or more aspirins per week. Lipid-lowering therapy was defined as participant-reported use of medications for “high cholesterol or high triglycerides.” Average alcohol use was participant-reported in drinks per week or drinks per month. Current smoking was defined as one or more cigarette(s) per day over the past year.
Multiple laboratory values also were measured on fasting morning samples, including blood glucose (mg/dL), total cholesterol (mg/dL), high density lipoprotein (mg/dL), cholesterol (mg/dL), triglycerides (mg/dL), and alanine aminotransferase (ALT, U/L). Quantification of visceral adipose tissue (VAT) was done using a semi-automated method previously described which yields excellent inter-reader reproducibility.41 For a secondary analysis, elevated ALT was defined as an ALT > 19 U/L for women and > 30 U/L for men.
Markers of systemic inflammation and oxidative stress
Inflammatory biomarkers were measured at the same time as covariates and include 14 measurements as listed in Table 1. Technical details for these measurements have been previously described42,43 and are summarized in the Supplemental information.
Table 1:
Markers of inflammation and oxidative stress measured
| Marker of inflammation | Biological relevance |
|---|---|
| CD40 ligand (ng/mL) | Marker and mediator of inflammation in atherosclerosis60 |
| Fibrinogen (mg/dL) | Marker of thrombosis and inflammation8 |
| High-sensitivity C-reactive protein (hs-CRP, mg/L) | Marker of systemic inflammation, strong association with cardiovascular disease8 |
| Intercellular adhesion molecule 1 (ICAM1, ng/mL) | Marker associated with progressive atherosclerosis61 |
| Interleukin 6 (IL6, pg/mL) | Pro-inflammatory marker62 |
| Lipoprotein-associated phospholipase A2 activity (Lp-PLA2 activity, nmol/mL/min) | Low density lipoprotein associated with atherosclerosis63 |
| Lipoprotein-associated phospholipase A2 mass (Lp-PLA2 mass, ng/mL) | Low density lipoprotein associated with atherosclerosis63 |
| Monocyte chemoattractant protein 1 (MCP1, pg/mL) | Chemokine associated with endothelial damage and atherosclerosis64 |
| Myeloperoxidase (MPO, ng/mL) | Marker associated with atherosclerosis and cardiovascular disease65 |
| Osteoprotegerin (pmol/L) | Marker of bone metabolism and vascular inflammation66 |
| P-selectin (ng/mL) | Marker associated with inflammatory cell adhesion and atherosclerosis53 |
| Tumor necrosis factor receptor (TNF-α, pg/mL) | Regulator of the inflammatory response67 |
| Tumor necrosis factor receptor 2 (TNFR2, pg/mL) | Marker of inflammation associated with atherosclerosis68 |
| Urinary isoprostanes (pg/mL) Marker of oxidative stress | Marker of oxidative stress69 |
Statistical analyses
Values of inflammatory markers were transformed using the natural logarithm prior to all analyses to normalize skewed distributions. Pearson correlation coefficients were calculated to measure the association between LPR and each inflammatory marker. The primary analysis assessed the relationship between LPR and inflammatory markers using multivariable linear regression models of increasing complexity. Model 1 adjusted for age, sex, average alcohol use, regular aspirin use, and smoking status. Model 2 added adjustment for hypertension, total cholesterol:high density lipoprotein ratio, lipid-lowering therapy, triglycerides, diabetes mellitus, and cardiovascular disease. Model 3 additionally adjusted for BMI. We performed multiple sensitivity analyses to exclude participants with diabetes or cardiovascular disease and to additionally adjust for VAT in Model 2. Interactions with sex and obesity (BMI ≥ 30 kg/m2) were also assessed.
We assessed the relationship of the combination of hepatic steatosis and elevated ALT with systemic inflammatory markers. An ANCOVA was used to compare differences in the mean natural log-transformed inflammatory markers between groups after adjusting for the same covariates as Model 1 described above.
An “inflammatory index” was defined as the number of inflammatory markers above the sample median value for each of hs-CRP, ICAM1, IL6, P-selectin, and urinary isoprostanes. These Inflammatory markers were chosen based on the results of the multivariable models.
Statistical tests were conducted at an α =0.05 level of significance. We performed the Benjamini-Hochberg procedure44 with false discovery rate set at 0.05 or 0.01 to account for multiple testing. All analyses were performed using SAS 9.3.
Results
Summary statistics for demographic characteristics and covariates are presented in Table 2. A total of 2,482 participants with a mean age of 51±11 years were included. Approximately 50% were women. Overall, 17% of participants had hepatic steatosis (as defined by LPR ≤ 0.33). Unadjusted inflammatory marker values are presented in Supplement Table 1. We observed statistically significant positive correlations between LPR and multiple inflammatory markers after adjusting for age and sex (Supplement Table 2). We observed a modest to strong correlation between the inflammatory markers with correlation coefficients ranging from −0.09 to +0.48 (data not shown).
Table 2:
Study sample characteristics by hepatic steatosis status
| Hepatic steatosis (n = 425) | No hepatic steatosis (n = 2057) | Overall (n = 2482) | |
|---|---|---|---|
| Demographic characteristics and covariates | |||
| Age (years) | 52±11 | 51±10 | 51±11 |
| Sex (women) | 192 (45) | 1075 (52) | 1267 (51) |
| Body Mass Index (BMI, kg/m2) | 31±6 | 27±5 | 28±5 |
| Current smoking | 47 (11) | 233 (11) | 280 (11) |
| Former smoking | 183 (43) | 773 (38) | 956 (39) |
| Cardiovascular disease | 43 (10) | 126 (6) | 169 (7) |
| Regular aspirin use | 107 (25) | 379 (18) | 486 (20) |
| Alcoholic drinks per week | 3.1±3.9 | 3.0±3.4 | 3.0±3.5 |
| Diabetes | 61 (14) | 101 (5) | 162 (7) |
| Fasting glucose (mg/dL) | 108±33 | 97±19 | 99±22 |
| Hypertension | 192/424 (45) | 486/2055 (24) | 678/2479 (27) |
| Lipid lowering therapy | 84 (20) | 249 (12) | 333 (13) |
| Total cholesterol (mg/dl) | 197±36 | 194±35 | 195±35 |
| High-density lipoprotein (HDL) cholesterol (mg/dl) | 45±14 | 54±16 | 52±16 |
| Triglycerides (mg/dl) | 180±115 | 115±75 | 126±87 |
| Alanine aminotransferase (ALT, U/L) | 34.1±25.8 | 23.1±15.8 | 25.0±18.4 |
| Visceral adipose tissue (VAT, cm3) | 2626±1049 | 1565±912 | 1747±1019 |
Mean±standard deviation for continuous variables or n (%) for categorical variables
Hepatic steatosis defined by Liver:Phantom Ratio ≤ 0.33
See Methods for covariate definitions
In Model 1, LPR was positively associated with nearly all inflammatory markers measured (with exception of CD40 ligand, Lp-PLA2 mass, MPO, and TNF-α). (Table 3). Multivariable linear regression models with adjustment for additional covariates attenuated the associations between LPR and inflammatory markers. In Model 2, LPR was significantly associated with hs-CRP (P<0.001), isoprostanes (P<0.001), IL6 (P<0.001), ICAM1 (P<0.001), and P-selectin (P=0.01). In Model 3, (with additional adjustment for BMI) these associations were further attenuated but remained statistically significant (P for all ≤ 0.05). Among these markers, a significant interaction with sex was present for only isoprostanes. When results from men and women were analyzed separately, there was no change in the direction of association, but the magnitude was greater for women. Similarly, a significant interaction with obesity was found for only CRP and IL6. The magnitude of association was greater for CRP and IL6 among obese (BMI ≥ 30) participants. (Supplement Table 3).
Table 3:
Multivariable linear regression models of the relationship between liver fat (by Liver:Phantom Ratio) and markers of inflammation and oxidative stress
| Markers of inflammation or oxidative stress | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Cluster of differentiation 40 (CD40) ligand | 0.23 (−0.52, 0.99) | 0.55 | 0.38 (−0.41, 1.17) | 0.35 | 0.47 (−0.34, 1.27) | 0.26 |
| Fibrinogen | 0.18 (0.08, 0.29) | <0.001 | 0.10 (−0.01, 0.21) | 0.07 | 0.02 (−0.08, 0.13) | 0.67 |
| High-sensitivity C-reactive protein (hs-CRP) | 3.05 (2.61, 3.48) | <0.001 | 2.20 (1.75, 2.64) | <0.001 | 1.23 (0.81, 1.65) | <0.001 |
| Intercellular adhesion molecule 1 (ICAM1) | 0.44 (0.34, 0.53) | <0.001 | 0.34 (0.24, 0.43) | <0.001 | 0.30 (0.20, 0.40) | <0.001 |
| Interleukin 6 (IL6) | 1.14 (0.88, 1.40) | <0.001 | 0.84 (0.57, 1.11) | <0.001 | 0.50 (0.23, 0.77) | <0.001 |
| Lipoprotein-phospholipase A2 (Lp-PLA2) activity | 0.09 (0.01, 0.17) | 0.03 | −0.02 (−0.09, 0.05) | 0.62 | 0.02 (−0.05, 0.09) | 0.62 |
| Lipoprotein-phospholipase A2 (Lp-PLA2) mass | −0.06 (−0.16, 0.04) | 0.27 | −0.08 (−0.19, 0.02) | 0.11 | −0.09 (−0.19, 0.02) | 0.11 |
| Monocyte chemoattractant protein 1 (MCP1) | 0.16 (0.03, 0.30) | 0.02 | 0.10 (−0.04, 0.24) | 0.16 | 0.07 (−0.08, 0.22) | 0.34 |
| Myeloperoxidase (MPO) | −0.03 (−0.38, 0.32) | 0.86 | 0.00 (−0.37, 0.37) | 0.99 | −0.13 (−0.51, 0.25) | 0.50 |
| Osteoprotegerin | 0.14 (0.02, 0.27) | 0.03 | 0.11 (−0.02, 0.24) | 0.10 | 0.13 (−0.01, 0.26) | 0.07 |
| P-selectin | 0.48 (0.32, 0.63) | <0.001 | 0.25 (0.10, 0.41) | 0.002 | 0.20 (0.04, 0.36) | 0.01 |
| TNF receptor-2 (TNFR2) | 0.21 (0.10, 0.32) | <0.001 | 0.11 (−0.01, 0.22) | 0.08 | 0.02 (−0.10, 0.14) | 0.76 |
| TNF-alpha (TNF-α) | 0.09 (−0.25, 0.43) | 0.59 | −0.09 (−0.45, 0.26) | 0.6 | −0.21 (−0.57, 0.15) | 0.25 |
| Urinary isoprostanes | 1.40 (0.99, 1.81) | <0.001 | 1.15 (0.72, 1.58) | <0.001 | 0.87 (0.43, 1.31) | <0.001 |
Estimates in table give change in loge biomarker for a 1 standard deviation increase in liver fat (measured via the liver phantom ratio) with (95% CI) and p-value
Model 1 adjusts for age, sex, smoking status, alcohol consumption, and regular aspirin use.
Model 2 additionally adjusts Model 1 for hypertension, lipid treatment, total/high density lipoprotein cholesterol ratio, triglycerides, diabetes, and prevalent cardiovascular disease.
Model 3 additionally adjusts Model 2 for body mass index
See Methods for covariate definitions
Using the Benjamini-Hochberg procedure, we evaluated for multiple testing at a false discovery rate threshold of 0.05 and 0.01. Using either threshold, all of the unadjusted p-values that were statistically significant in Models 1–3 remained statistically significant after adjustment for multiple testing.
We conducted a sensitivity analysis to adjust for radiographic VAT in place of BMI in Model 3. Results were consistent with the primary analysis (Supplement Table 4). We also repeated Model 3 after excluding participants with diabetes or cardiovascular disease (287 excluded). Results were consistent in strength and direction of association (Supplement Table 5).
We performed an analysis to compare participants with hepatic steatosis with elevated ALT (n=245) to participants with hepatic steatosis without elevated ALT (n=180). After adjustment for the variables included in Model 1, we found statistically significantly greater ICAM1 (P<0.0001), LP-PLA2 activity (P=0.0002), P-selectin (P=0.0001), and TNFR2 (P=0.0001) among those with elevated ALT (Supplement Table 6).
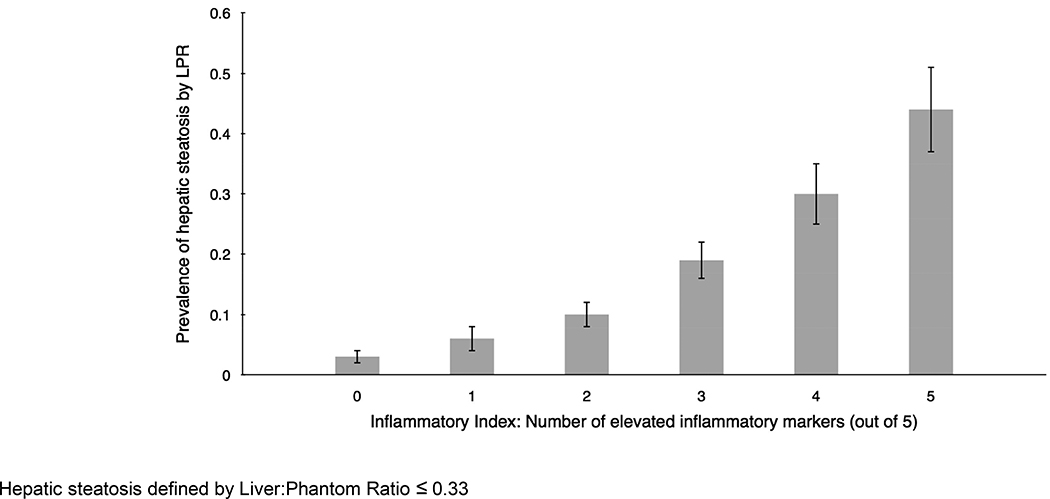
An inflammatory index was calculated for participants with measurements for hs-CRP, ICAM1, IL6, P-selectin, and urinary isoprostanes (n=2,281,). The prevalence of hepatic steatosis was greater among participants with a higher inflammatory index (Figure 2). For participants with an inflammatory index of 0, the prevalence of hepatic steatosis was 3%, whereas for participants with an inflammatory index of 5 the prevalence of hepatic steatosis was 44%.
Figure 2:
Prevalence of hepatic steatosis is greater among those with higher inflammatory index
Discussion
In a cohort of community-dwelling participants without pre-selection for the presence of liver disease, we observed a statistically significant association between liver fat and markers of systemic inflammation and oxidative stress. After accounting for multiple covariates, including BMI, liver fat was positively associated with hs-CRP, isoprostanes, IL6, ICAM1, and P-selectin. Of these, ICAM1 and P-selectin were significantly greater in participants with hepatic steatosis and elevated ALT compared to those with hepatic steatosis without elevated ALT. Among those with greater inflammation, we observed a stepwise greater prevalence of hepatic steatosis.
The mechanisms linking hepatic steatosis to systemic inflammation independent of other cardiometabolic risk factors remain uncertain. In a prior FHS analysis, VAT, not subcutaneous fat, was associated with hs-CRP, IL6, and urinary isoprostanes, after accounting for other measures of obesity.42 Our findings expand on this prior study to suggest that hepatic fat remains associated with multiple inflammatory markers after accounting for general adiposity (BMI) or central adiposity (VAT). Prior studies have shown that exposure of hepatocytes to fatty-acids induces expression of TNF-α,45,46 IL6,21,46 ICAM1,47 and isoprostanes,48 possibly mediated via nuclear factor-κB.49 Chronic hepatic activation of the nuclear factor-κB pathway promotes IL6-mediated insulin resistance50 and inhibition of TNF-α attenuates hepatic fatty acid oxidation and insulin resistance driven by Kupffer cell activation.51
The associations we observed reflect relationships between certain inflammatory biomarkers and atherosclerotic risk, which is more common among individuals with NAFLD.52 P-selectin is associated with increased risk of coronary heart disease53 and metabolic syndrome.54 IL655 and ICAM156 also appear to have significant roles in atherogenesis. Elevated isoprostanes are associated with increased carotid artery stiffness,57 and hs-CRP is associated with incident cardiovascular events.8
Prior studies have shown a similar association between hepatic steatosis and systemic inflammation.10,14,23–31,33,15,35,58,16–22 In smaller studies of specific populations, radiographic hepatic steatosis is positively associated with hs-CRP, IL6, and ICAM1, consistent with our results.30,58 Small studies also show greater hs-CRP among those with hepatic steatosis and nonalcoholic steatohepatitis (NASH).17–19 IL6 is associated with the degree of hepatic inflammation and fibrosis among smaller cohorts of subjects with NAFLD.18,21 Unfortunately, the use of a subjects selected for NAFLD or NASH may generate referral bias which limits the generalizability of most prior reports. Our study confirms these results, and because we studied a cohort without pre-selection for liver disease, our findings show that even asymptomatic hepatic steatosis could have consequences for the overall health of all patients. We hypothesize that greater levels of systemic inflammation may be associated with more rapid liver disease progression and a higher incidence of liver-related clinical events; longitudinal studies are warranted to test these hypotheses. Similarly, interventional studies should be pursued to determine if efforts to ameliorate NAFLD (e.g. weight loss or exercise) reduce systemic inflammatory burden. These markers may be useful measures of the effectiveness of interventions to reduce hepatic steatosis and as has been shown in rodent models, may serve as potential targets for treatments to reduce the systemic impact of metabolic syndrome, though future study is needed.50,51
The major strength of our study was the enrollment of a moderately large number of participants from within a well-characterized cohort without selection based on a known diagnosis of liver disease. Nonetheless, limitations should be considered. Our sample was largely middle-aged and older adults of European descent, which may limit applicability to other populations. While computed tomography assessment of hepatic steatosis is an accurate modality in common clinical use, results may differ from histological findings. Importantly, computed tomography does not permit differentiation of simple steatosis from NASH. Hence, in our study, we were unable to examine the systemic inflammatory burden among individuals with simple steatosis compared to NASH or NASH with fibrosis. Prior research estimates the prevalence of advanced fibrosis among those with NAFLD to be near 10%59, thus we expect the overall prevalence of advanced fibrosis to be low in our sample. Additional studies in participants with more advanced disease are needed to determine the systemic inflammatory burden across the spectrum of NAFLD. The biomarkers studied have a role in other non-cardiac disease states, but it is possible that others not included here may have important associations with NAFLD. The sample size for each biomarker was slightly different and CD40, fibrinogen, MPO, and TNF-α had less available data and had less statistical power for identifying associations. Our study was cross-sectional and observational, and we cannot exclude residual confounding nor establish a causal relation between liver fat and systemic inflammation.
In conclusion, we found that liver fat is positively associated with markers of systemic inflammation among participants without pre-selection for clinical liver disease. This suggests that even without a known diagnosis of NAFLD or NASH, hepatic steatosis may have important systemic consequences. Further studies to characterize these relationships and the underlying causal mechanisms are warranted.
Supplementary Material
What you need to know:
Background:
Patients with clinically apparent non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) have higher levels of systemic inflammation. It is unknown whether this is also true in the community setting among patients without pre-selection for liver disease.
Findings:
Among 2,482 participants without pre-selection for liver disease, we observed a positive association between more liver fat (measures by computed tomography) and multiple markers of systemic inflammation, even after accounting for covariates including body mass index.
Implications for patient care:
Individuals with NAFLD, even without clinically apparent liver disease, may have increased systemic inflammation, not explained by common comorbidities. This may increase risk of cardiovascular or other diseases.
Acknowledgments
Grant Support
Dr. Fricker is supported in part by the Boston University Clinical and Translational Science Institute (grant UL1-TR000157). Dr. Vasan is supported by the Evans Medical Foundation and the Jay and Louis Coffman Endowment. Dr. Benjamin is supported in part by NIH 1R01HL128914, 2R01 HL092577, 1RO1 HL64753; R01 HL076784; 1 R01 AG028321. Dr. Long is supported in part by NIH grant K23 DK113252, the Boston University School of Medicine Department of Medicine Career Investment Award and the Boston University Clinical Translational Science Institute UL1 TR001430. This work was supported by the Boston University School of Medicine and the National Heart, Lung, and Blood Institute’s (NHLBI) contracts N01-HC-25195 and HHSN268201500001I (RSV), R01 HL 71039, and K24 HL 4334 (RSV), and the Division of Intramural Research of the National Heart, Lung, and Blood Institute.
Abbreviations:
- ALT
Alanine aminotransferase
- BMI
Body mass index
- CD40
Cluster of differentiation 40
- FHS
Framingham Heart Study
- hs-CRP
High-sensitivity C-reactive protein
- ICAM1
Intercellular adhesion molecule 1
- IL6
Interleukin 6
- LP-PLA2
Lipoprotein-phospholipase A2
- LPR
Liver:Phantom ratio
- MCP-1
Monocyte chemoattractant protein 1
- MPO
Myeloperoxidase
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- TNF
Tumor necrosis factor
- TNFR2
Tumor necrosis factor receptor 2
- VAT
Visceral adipose tissue
Footnotes
Disclosures:
Alison Pedley is an employee at Merck. To the best of our knowledge, there exist no other conflicts of interest (personal, professional, or financial) among the remaining authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017;67:328–357. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017;66:323–329. [DOI] [PubMed] [Google Scholar]
- 6.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The coronary artery risk development in young adults study. Atherosclerosis 2014;235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long MT, Wang N, Larson MG, et al. Nonalcoholic fatty liver disease and vascular function: Cross-sectional analysis in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2015;35:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012;367:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Fuster JJ, Walsh K. Adipokines: A link between obesity and cardiovascular disease. J Cardiol 2014;63:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suomela E, Oikonen M, Pitkänen N, et al. Childhood predictors of adult fatty liver. The Cardiovascular Risk in Young Finns Study. J Hepatol 2016;65:784–790. [DOI] [PubMed] [Google Scholar]
- 11.Monzo-Beltran L, Vazquez-Tarragón A, Cerdà C, et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol 2017;12:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. Diabetes Mellitus and Inflammation. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Day CP, Bonora E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. N Engl J Med 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 14.Niu Y, Zhang W, Yang Z, et al. Plasma osteoprotegerin levels are inversely associated with nonalcoholic fatty liver disease in patients with type 2 diabetes: A case-control study in China. Metabolism 2016;65:475–481. [DOI] [PubMed] [Google Scholar]
- 15.Monseu M, Dubois S, Boursier J, et al. Osteoprotegerin levels are associated with liver fat and liver markers in dysmetabolic adults. Diabetes Metab 2016;42:364–367. [DOI] [PubMed] [Google Scholar]
- 16.Musso G, Gambino R, Michieli F De, et al. Nitrosative stress predicts the presence and severity of nonalcoholic fatty liver at different stages of the development of insulin resistance and metabolic syndrome: possible role of vitamin A intake. Am J Clin Nutr 2007;86:661–671. [DOI] [PubMed] [Google Scholar]
- 17.Targher G Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol 2006;45:879–882. [DOI] [PubMed] [Google Scholar]
- 18.Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167–1174. [DOI] [PubMed] [Google Scholar]
- 19.Colak Y, Senates E, Ozturk O, et al. Association of serum lipoprotein-associated phospholipase A2 level with nonalcoholic fatty liver disease. Metab Syndr Relat Disord 2012;10:103–109. [DOI] [PubMed] [Google Scholar]
- 20.du Plessis J, van Pelt J, Korf H, et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 2015;149:635–648. [DOI] [PubMed] [Google Scholar]
- 21.Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103:1372–1379. [DOI] [PubMed] [Google Scholar]
- 22.Mueller JL, Feeney ER, Zheng H, et al. Circulating soluble CD163 is associated with steatohepatitis and advanced fibrosis in nonalcoholic fatty liver disease. Clin Transl Gastroenterol 2015;6:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajmera V, Perito ER, Bass NM, et al. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology 2017;65:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology 2004;40:46–54. [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Durazzo M, et al. Adipokines in NASH: Postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 2005;42:1175–1183. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda M, Mawatari H, Fujita K, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 2007;42:573–582. [DOI] [PubMed] [Google Scholar]
- 27.Jarrar MH, Baranova A, Collantes R, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2008;27:412–421. [DOI] [PubMed] [Google Scholar]
- 28.Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obes (Silver Spring) 2008;16:1394–1399. [DOI] [PubMed] [Google Scholar]
- 29.Kugelmas M, Hill DB, Vivian B, et al. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003;38:413–419. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Bertolini L, Scala L, et al. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med 2005;22:1354–1358. [DOI] [PubMed] [Google Scholar]
- 31.Ercin CN, Dogru T, Tapan S, et al. Levels of soluble CD40 ligand and p-selectin in nonalcoholic fatty liver disease. Dig Dis Sci 2010;55:1128–1134. [DOI] [PubMed] [Google Scholar]
- 32.Price JC, Wang R, Seaberg EC, et al. The association of inflammatory markers with nonalcoholic fatty liver disease differs by HIV serostatus. Open Forum Infect Dis 2017;4:ofx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi M, Iwasa M, Araki J, et al. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J Gastroenterol Hepatol 2006;21:1821–1825. [DOI] [PubMed] [Google Scholar]
- 34.Hamirani YS, Katz R, Nasir K, et al. Association between inflammatory markers and liver fat: The Multi-Ethnic Study of Atherosclerosis. J Clin Exp Cardiolog 2014;5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sookoian S, Castã No GO, Burgu No AL, et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 2010;209:585–591. [DOI] [PubMed] [Google Scholar]
- 36.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DAWBER TR, KANNEL WB, LYELL LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci 1963;107:539–556. [DOI] [PubMed] [Google Scholar]
- 38.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 2010;51:307–328. [DOI] [PubMed] [Google Scholar]
- 39.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham heart study. Hepatology 2010;51:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keaney JF, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol 2003;23:434–439. [DOI] [PubMed] [Google Scholar]
- 41.Maurovich-Horvat P, Massaro J, Fox CS, et al. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes 2007;31:500–506. [DOI] [PubMed] [Google Scholar]
- 42.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume. Neurology 2007;68:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- 45.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-α expression via a lysosomal pathway. Hepatology 2004;40:185–194. [DOI] [PubMed] [Google Scholar]
- 46.Chávez-Tapia NC, Rosso N, Uribe M, et al. Kinetics of the inflammatory response induced by free fatty acid accumulation in hepatocytes. Ann Hepatol 2014;13:113–120. [PubMed] [Google Scholar]
- 47.Chong L-W, Hsu Y-C, Lee T-F, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol 2015;15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller C, Gardemann A, Keilhoff G, et al. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine 2012;19:395–401. [DOI] [PubMed] [Google Scholar]
- 49.Baker RG, Hayden MS, Ghosh S. NF-kB, Inflammation, and Metabolic Disease. Cell Metab 2011;13:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 2005;11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang W, Metlakunta A, Dedousis N, et al. Depletion of Liver Kupffer Cells Prevents the Development of Diet-Induced Hepatic Steatosis and Insulin Resistance. Diabetes 2010;59:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Airaghi L, Rango M, Maira D, et al. Subclinical cerebrovascular disease in NAFLD without overt risk factors for atherosclerosis. Atherosclerosis 2018;268:27–31. [DOI] [PubMed] [Google Scholar]
- 53.Bielinski SJ, Berardi C, Decker PA, et al. P-selectin and subclinical and clinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015;240:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dallmeier D, Larson MG, Vasan RS, et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol Metab Syndr 2012;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma AK, Singh JP, Heist EK. Stress cardiomyopathy: diagnosis, pathophysiology, management, and prognosis. Crit Pathw Cardiol 2011;10:142–147. [DOI] [PubMed] [Google Scholar]
- 56.Kitagawa K, Matsumoto M, Sasaki T, et al. Involvement of ICAM-1 in the progression of atherosclerosis in APOE-knockout mice. Atherosclerosis 2002;160:305–310. [DOI] [PubMed] [Google Scholar]
- 57.Ren H, Zhou X, Luan Z, et al. The relationship between carotid atherosclerosis, inflammatory cytokines, and oxidative stress in middle-aged and elderly hemodialysis patients. Int J Nephrol 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price JC, Seaberg EC, Latanich R, et al. Risk Factors for Fatty Liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol 2014;109:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le MH, Devaki P, Ha NB, et al. Prevalence of non-Alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mach F, Schönbeck U, Sukhova GK, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A 1997;94:1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoamanesh A, Preis SR, Beiser AS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham heart study. Neurology 2015;84:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheller J, Garbers C, Rose-John S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 2014;26:2–12. [DOI] [PubMed] [Google Scholar]
- 63.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in communities (ARIC) study. Cir 2004;109:837–842. [DOI] [PubMed] [Google Scholar]
- 64.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl 2005;6:21–29. [DOI] [PubMed] [Google Scholar]
- 65.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005;25:1102–1111. [DOI] [PubMed] [Google Scholar]
- 66.Stepień E, Fedak D, Klimeczek P, et al. Osteoprotegerin, but not osteopontin, as a potential predictor of vascular calcification in normotensive subjects. Hypertens Res 2012;35:531–538. [DOI] [PubMed] [Google Scholar]
- 67.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HL, Lee JP, An JN, et al. Soluble tumor necrosis factor receptors and arterial stiffness in patients with coronary atherosclerosis. Am J Hypertens 2017;30:313–318. [DOI] [PubMed] [Google Scholar]
- 69.Roberts LJ, Morrow JD. Measurement of F2-Isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 2000;28:505–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.