Abstract
Polyglutamine expansion within the N-terminal region of the huntingtin protein results in the formation of intracellular aggregates responsible for Huntington’s disease, a fatal neurodegenerative condition. The interaction between TiO2 nanoparticles and huntingtin peptides comprising the N-terminal amphiphilic domain without (httNT) or with (httNTQ10) a ten-residue C-terminal polyglutamine tract, is investigated by NMR spectroscopy. TiO2 nanoparticles decrease aggregation of httNTQ10 by catalyzing the oxidation of Met7 to a sulfoxide, resulting in an aggregation-incompetent peptide. The oxidation agent is hydrogen peroxide generated on the surface of the TiO2 nanoparticles either by UV irradiation or at low steady-state levels in the dark. The binding kinetics of nonaggregating httNT to TiO2 nanoparticles is characterized by quantitative analysis of 15N dark state exchange saturation transfer and lifetime line broadening NMR data. Binding involves a sparsely populated intermediate that experiences hindered rotational diffusion relative to the free state. Catalysis of methionine oxidation within the N-terminal domain of the huntingtin protein may potentially provide a strategy for delaying the onset of Huntington’s disease.
Polyglutamine expansion within the N-terminal region of the huntingtin protein (corresponding to exon-1) favors aggregation and is responsible for Huntington’s disease, a fatal neurodegenerative condition.1 The polyglutamine domain lies downstream of the 16-residue N-terminal amphiphilic domain (httNT). Peptides comprising httNT with as few as 10 glutamines (httNTQ10) aggregate rapidly in solution and form polymorphic fibrils.2 We recently observed that oxidation of the Met7 side-chain to a sulfoxide (Met7) prevents aggregation of httNTQ10.3 In general, adsorption of fibril forming proteins and peptides on the surface of nanoparticles enhances aggregation and fibril formation by increasing the local peptide concentration and hence the probability of forming a critical initiation nucleus.4,5 Titanium oxide nanoparticles (TiO2 NPs) are unique as they possess photocatalytic properties that generate reactive oxygen species upon UV irradiation.6,7 Here we study the interaction of httNT and httNTQ10 with TiO2 NPs by NMR, and show that TiO2 NPs specifically catalyze the oxidation of Met7, thereby preventing fibril formation by reducing the concentration of the aggregation-competent, native reduced form of httNTQ10.
Spontaneous aggregation of httNTQ10 occurs over time as monitored both by an increase in Thioflavin T (ThT) fluorescence emission (Figure 1A), attributed to the formation of β-rich amyloid-like structures, and by a decrease in the intensity of the amide proton envelope of the NMR signal (Figure 1B; see SI). Reduced and oxidized monomeric httNTQ10 are NMR visible, while aggregates of reduced httNTQ10 are broadened beyond detection, resulting in a decrease in the observable amide proton envelope intensity. Addition of photoexcited TiO2 NPs (see SI and Figure S1) dramatically reduces the extent of aggregation. At 10 °C, only 8% of a 300 μM 15N-httNTQ10 sample remains NMR visible after 100 h; in the presence of photoexcited TiO2 NPs, however, aggregation plateaus out with 33% of the sample remaining NMR visible (Figure 1B). The apparent aggregation t1/2 (∼23−25 h), however, is not affected by the TiO2 NPs (Figure 1B). The reduction in the fraction of aggregating species can be attributed to TiO2 NP catalyzed oxidation of the side chain of Met7 to a sulfoxide. Several reactive oxygen species are formed on the surface of TiO2 NPs upon UV irradiation (Figure S2),6,7 but only H2O2 is stable with significant amounts generated both upon UV irradiation and in the dark (Figures 2A and S3).
Figure 1.
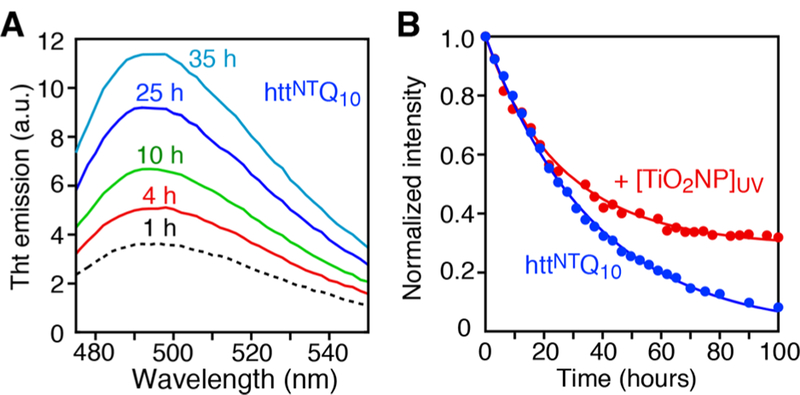
Effect of TiO2 NPs on aggregation of httNTQ10 monitored by NMR at 10 °C. Time course of (A) ThT emission of 25 μM httNTQ10 in the absence of TiO2 NPs, and (B) of the intensity of the amide proton envelope of 300 μM 15N-labeled httNTQ10 in the absence (blue circles) and presence (red circles) of 5 g·L−1 TiO2 NPs photoexcited by exposure to UV light prior to addition to the peptide solution. The solid lines represent best fits where the disappearance of reduced, NMR visible httNTQ10 (kagg ∼ 0.03 h−1) due to aggregation competes with oxidation to aggregation-incompetent Met7O-httNTQ10 (see Scheme 1). The normalized intensities are corrected for sample to sample variations in the amounts of Met7O-httNTQ10 present at t = 0 (see SI).
Figure 2.
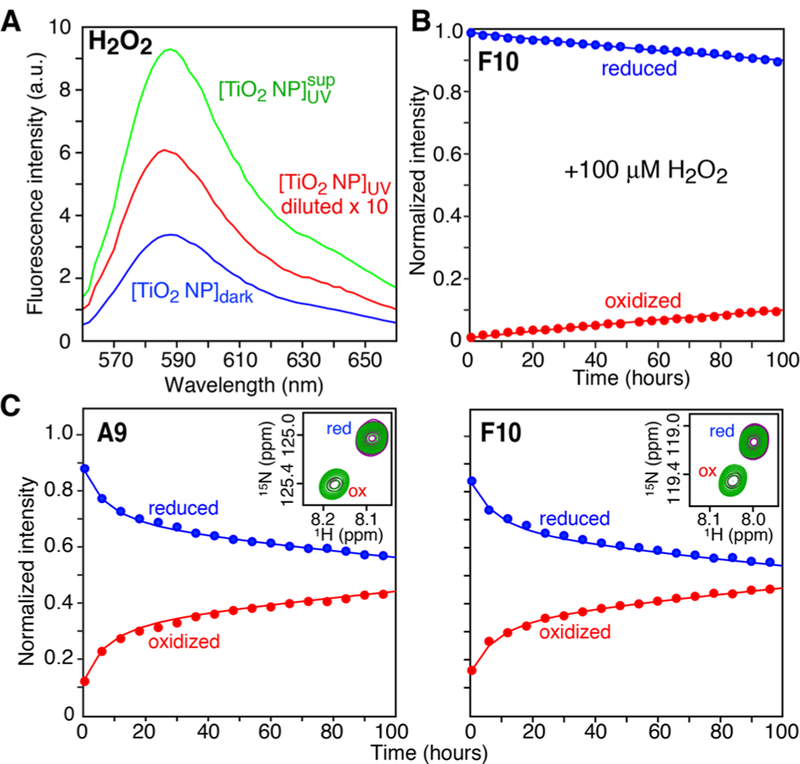
TiO2-catalyzed oxidation of httNT. (A) Amplex Red assay for H2O2 generated by 5 g·L−1 TiO2 NPs in the dark (blue, ∼4 μM H2O2) and upon UV exposure for 3 h (red, NP suspension, ∼76 μM H2O2; green, supernatant after removal of NPs, ∼11 μM H2O2). (B) Time course of Met7 oxidation of 300 μM 15N-labeled httNT following addition of (B) 100 μM H2O2 and (C) UV-irradiated TiO2 NPs (5 g·L−1, 3 h UV exposure)monitored by the reduction and corresponding increase in intensities of Ala9 and Phe10 cross-peaks arising from reduced (blue) and Met7O oxidized (red) httNT, respectively, in a series of 1H−15N HSQC spectra. The insets in (C) show the cross-peaks corresponding to the reduced (upfield) and oxidized (downfield) states at t = 0 (purple) and 80 (green) hours. The experimental data in panels B and C are shown as circles and the best-fit curves obtained by nonlinear optimization and integration of the differential equations (eq S1) describing the oxidation process in Scheme 1 are represented by solid lines. The different ratios of oxidized to reduced httNT at time zero in panels (B) and (C) reflect sample to sample variations. Data were collected at 10 °C and a spectrometer frequency of 600 MHz.
The oxidation kinetics of httNTQn huntingtin peptides (where n = number of glutamines in the polyglutamine tract) in the presence of TiO2 NPs can be described by three parallel reactions (Scheme 1): two second-order processes involving oxidation of httNTQn (Pred) to Met7O-httNTQn (Poxi) by H2O2, generated upon UV irradiation, either dissolved in solution (k1) or on the surface of the TiO2 NPs (k2), and a pseudo-first-order process occurring in the dark that involves a low steady-state (i.e., continuously generated) level of H2O2 on the surface of the TiO2 NPs.8 These reactions occur concomitantly with aggregation of Pred which, for simplicity, is described as a unimolecular process with a rate constant kagg. Our treatment of the kinetics of all oxidative processes assumes that oxidation proceeds on a time-scale much slower than that of binding to TiO2 NPs, as is amply confirmed experimentally (see below).
Scheme 1.
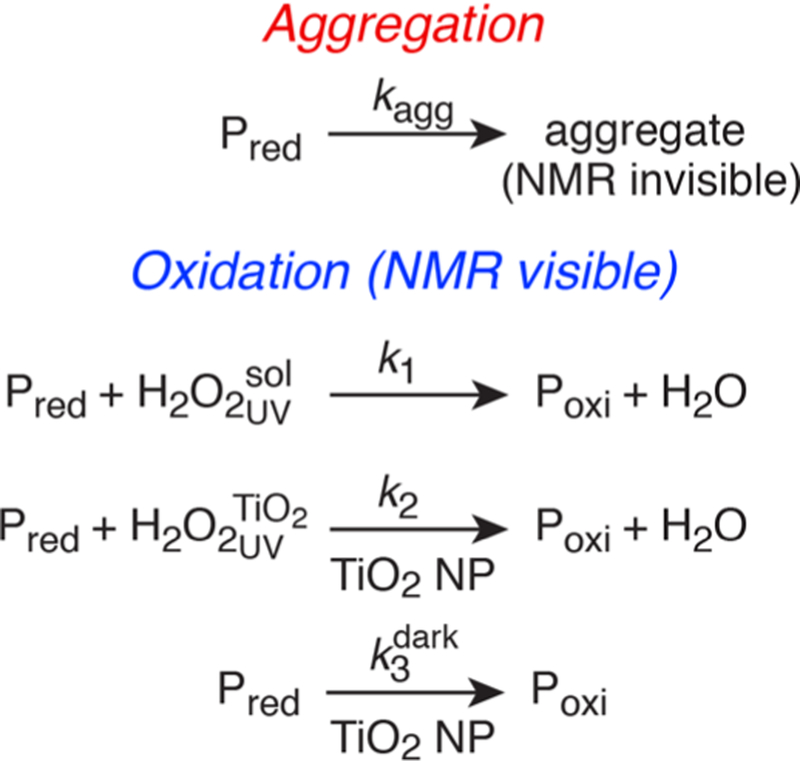
Parallel Reactions Describing Aggregation and TiO2 NP-Catalyzed Oxidation of httNTQn Peptides
To study the kinetics of TiO2 catalyzed oxidation of huntingtin peptides in the absence of aggregation (kagg = 0 in Scheme 1), we made use of a peptide comprising only the N-terminal amphiphilic domain, httNT, which remains stable in the presence of TiO2 NPs for many days (Figure S4). Oxidation of httNT by H2O2 free in solution makes an insignificant contribution to the kinetics of httNT oxidation in the presence of 5 g·L−1 TiO2 NPs as the concentration of dissolved H2O2 generated upon UV irradiation (∼11 μM, Figure 2A) is 10-fold lower than that used in the experiment with added H2O2 shown in Figure 2B. The time course of oxidation in the presence of UV irradiated TiO2 is biphasic (Figure 2C). The first phase arises from second-order (k2 ∼ 500 M−1 h−1) oxidation of Met7 by the substantial amount of H2O2 located on the surface of the TiO2 NPs generated by UV irradiation. Oxidation on the TiO NP surface is accelerated ∼45-fold relative to that free in solution. Once H2O2 generated by UV irradiation is consumed, a slower apparent first order oxidation process occurs owing to the steady-state level of H2O2 present on the TiO2 NP surface in the dark. With these values of k2 and , Scheme 1 quantitatively accounts for the disappearance of NMR visible httNTQ10 in the presence of photoactivated TiO2 NPs with kagg ∼ 0.03 h−1 which remains unchanged in the absence of TiO2 NPs (Figure 1B).
The efficiency of heterogeneous catalysis is dependent upon the strength of interaction between the adsorbate (httNT) and the surface of the catalyst.9 We therefore characterized the kinetic and mechanistic details of 15N-labeled httNT adsorption on the surface of TiO2 NPs using 15N dark state exchange saturation transfer (DEST) and lifetime line broadening (ΔR2) which enable one to quantitatively analyze exchange processes between an NMR visible species and very high molecular weight, NMR invisible “dark” states.10–13 Although binding of httNTQ10 to TiO2 NPs cannot be studied quantitatively owing to httNTQ10 aggregation during the time course of the DEST experiment (several days), ΔR2 measurements are sufficiently fast (a few hours) to demonstrate significant 15N lifetime line broadening (∼10 s−1), and hence binding, of httNTQ10 in the presence of TiO NPs (Figure S5).
Examples of 15N-DEST profiles and a plot of 15N-ΔR2 as a function of residue for reduced httNT in the presence of TiO2 NPs in the dark are shown in Figure 3A,B, respectively. These conditions correspond to a quasi-equilibrium state where Met7 oxidation by the small amount of steady-state H2O2 generated on the TiO2 NP surface in the dark proceeds very slowly (t1/2 ∼ 350 h). The shapes of the 15N-DEST profiles are unusual and characterized by a narrow component superimposed on a broad one (Figure 3A) indicative of a three-state exchanging system in which the slow exchange process due to httNT binding to the TiO2 NP surface is accompanied by a second exchange process occurring on a much faster time scale. The 15N-DEST and R2 data were fit simultaneously to the three-state exchange model shown in red in Figure 3B via propagation of a set of Bloch-McConnell14 equations.
Figure 3.
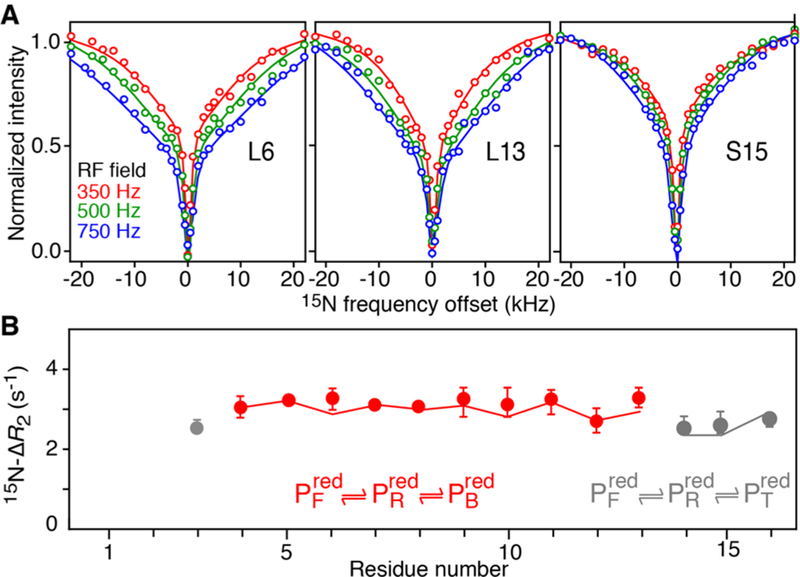
Kinetics of httNT binding to TiO2 NPs characterized by relaxation-based NMR. (A) Examples of 15N-DEST profiles and (B) 15N-ΔR2 as a function of residue observed for 300 μM 15N-labeled httNT in the presence of 5 g·L−1 TiO2 NPs in the dark. Circles represent experimental data, and solid lines are best fits to a three-state exchange model. Residues requiring a separate treatment are shown in gray. Data were recorded at 600 MHz and 10 °C.
Exchange between free and TiO2 NP-bound httNT monomer proceeds via an intermediate whose rotational diffusion is only partially restricted, presumably due to initial binding to the hydration layer surrounding the TiO2 NP; the final bound state experiences the same rotational diffusion as the TiO2 NP itself (Figure 4). This binding mechanism is similar to that observed for the adsorption of cholic acid to ceria NPs.15
Figure 4.
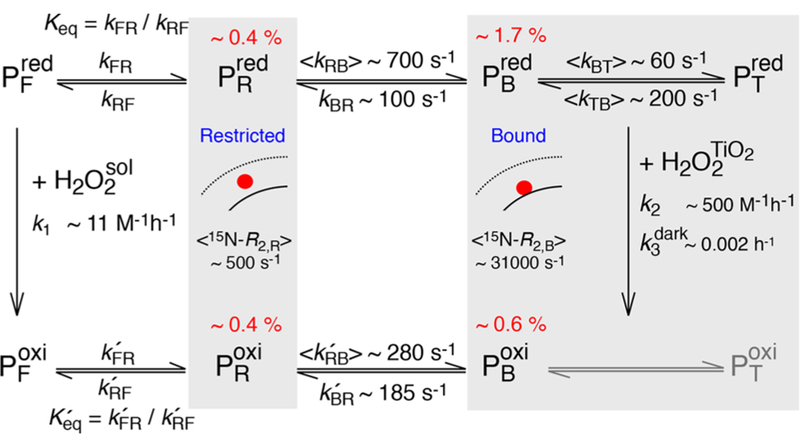
Overall kinetic scheme for the binding httNT to TiO2 NPs coupled with oxidation of httNT to the Met7 sulfoxide form either in solution or on the NP surface. States in contact with TiO2 NPs are shaded.
The population of TiO2 NP-bound reduced httNT, the rate constant kBR (97 ± 5 s−1) for the transition from fully bound to partially restricted states, and the transverse relaxation rates in the bound state are well-defined from the fits. The population of the intermediate state , however, cannot be determined with certainty without assumptions regarding the magnitude of its transverse spin relaxation rate, . This is due to fast interconversion between and on the relaxation time-scale , so that extraction of kFR, kRF and is not possible without assumptions regarding these parameters. Thus, only ranges of and could be established (see SI): for , varies from 0.5 to 0.2%. Although populated at less than 1%, the inclusion of into the analysis is essential to reproduce the experimental 15N-DEST profiles.
The 15N-DEST/ΔR2 data for Leu3 and the C-terminal three residues of httNT required a separate treatment and were fitted to the three-state model shown in gray in Figure 3B. Although formally the same overall three-state exchange model is used for these residues , the exchange process subsumes initial binding to the NP surface followed by reversible detachment from the surface (state , where “T” denotes “tethered”).13 The rate constants kBT and kTB were calculated a-posteriori (see SI and Figure 4) and fall in the ranges 40 to 75 s−1 and 160 to 240 s−1, respectively, depending on the assumed population of state . It follows that reversible detachment of these 4 residues from the NP surface occurs ∼3-fold slower than the binding event proper . While the central residues of httNT are likely to form an ordered helical structure when bound to TiO2 NPs, as observed for httNTQn peptides bound to lipid micelles,3 the parts of the peptide that remain unstructured in the bound state retain the flexibility of the free peptide and can transiently detach from the NP surface (i.e., they are “tethered”). The same phenomenon was also observed in the interaction of httNTQn with small unilamellar lipid vesicles.13
The binding of Met7O-httNT to TiO2 NPs was also characterized by analysis of 15N-DEST and ΔR2 data (Figure S6). The population of the bound state is ∼0.6%, ∼3-fold lower than that of the reduced form, indicating that oxidation of Met7 reduces the binding affinity to TiO2 NPs, in agreement with previous studies on the interaction of httNTQ7 with lipid micelles.3
In conclusion, the current work provides a mechanistic basis for understanding the interaction of huntingtin peptides with TiO2 NPs coupled with surface-catalyzed oxidation, and suggests that targeted catalysis of Met7 oxidation within the httNT domain of the huntingtin protein may provide a strategy for delaying the onset of Huntington’s disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank James Baber, Dan Garrett and Jinfa Ying for NMR and computational support. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (to G.M.C.).
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Andresen JM; Gayan J; Djousse L; Roberts S; Brocklebank D; Cherny SS; Cardon LR; Gusella JF; MacDonald ME; Myers RH; Housman DE; Wexler NS The Relationship between Cag Repeat Length and Age of Onset Differs for Huntington’s Disease Patients with Juvenile Onset or Adult Onset. Ann. Hum. Genet 2007, 71, 295–301. [DOI] [PubMed] [Google Scholar]
- (2).Jayaraman M; Kodali R; Sahoo B; Thakur AK; Mayasundari A; Mishra R; Peterson CB; Wetzel R Slow Amyloid Nucleation Via α Helix-Rich Oligomeric Intermediates in Short Polyglutamine-Containing Huntingtin Fragments. J. Mol. Biol 2012, 415, 881–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ceccon A; Schmidt T; Tugarinov V; Kotler SA; Schwieters CD; Clore GM Interaction of Huntingtin Exon-1 Peptides with Lipid-Based Micellar Nanoparticles Probed by Solution NMR and Q-Band Pulsed EPR. J. Am. Chem. Soc 2018, 140, 6199–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Linse S; Cabaleiro-Lago C; Xue WF; Lynch I; Lindman S; Thulin E; Radford SE; Dawson KA Nucleation of Protein Fibrillation by Nanoparticles. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 8691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wu WH; Sun X; Yu YP; Hu J; Zhao L; Liu Q; Zhao YF; Li YM TiO2 Nanoparticles Promote β-Amyloid Fibrillation in Vitro. Biochem. Biophys. Res. Commun 2008, 373, 315–8. [DOI] [PubMed] [Google Scholar]
- (6).Schneider J; Matsuoka M; Takeuchi M; Zhang J; Horiuchi Y; Anpo M; Bahnemann DW Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev 2014, 114, 9919–86. [DOI] [PubMed] [Google Scholar]
- (7).Nosaka Y; Nosaka AY Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev 2017, 117, 11302–11336. [DOI] [PubMed] [Google Scholar]
- (8).Jayaram DT; Runa S; Kemp ML; Payne CK Nanoparticle-Induced Oxidation of Corona Proteins Initiates an Oxidative Stress Response in Cells. Nanoscale 2017, 9, 7595–7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Schauermann S; Nilius N; Shaikhutdinov S; Freund HJ Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res 2013, 46, 1673–1681. [DOI] [PubMed] [Google Scholar]
- (10).Fawzi NL; Ying J; Torchia DA; Clore GM Kinetics of Amyloid Beta Monomer-to-Oligomer Exchange by NMR Relaxation. J. Am. Chem. Soc 2010, 132, 9948–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Fawzi NL; Ying J; Ghirlando R; Torchia DA; Clore GM Atomic-Resolution Dynamics on the Surface of Amyloid β Protofibrils Probed by Solution NMR. Nature 2011, 480, 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ceccon A; Tugarinov V; Bax A; Clore GM Global Dynamics and Exchange Kinetics of a Protein on the Surface of Nanoparticles Revealed by Relaxation-Based Solution NMR Spectroscopy. J. Am. Chem. Soc 2016, 138, 5789–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ceccon A; Clore GM; Tugarinov V Decorrelating Kinetic and Relaxation Parameters in Exchange Saturation Transfer NMR: A Case Study of N-Terminal Huntingtin Peptides Binding to Unilamellar Lipid Vesicles. J. Phys. Chem. B 2018, 10.1021/acs.jpcb.8b07112. [DOI] [PMC free article] [PubMed]
- (14).McConnell HM Reaction Rates by Nuclear Magnetic Resonance. J. Chem. Phys 1958, 28, 430–431. [Google Scholar]
- (15).Egner TK; Naik P; Nelson NC; Slowing II; Venditti V Mechanistic Insight into Nanoparticle Surface Adsorption by Solution NMR Spectroscopy in an Aqueous Gel. Angew. Chem., Int. Ed 2017, 56, 9802–9806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


