Abstract
Background:
Corin is a serine protease known to convert B-type natriuretic peptide (BNP) prohormone into BNP and its amino-terminal fragment (NT-proBNP). In mice lacking corin, high blood pressure and proteinuria were found at late gestational stages, with associated delayed trophoblast invasion and impaired spiral artery remodeling in the uterus. We hypothesize that both NT-proBNP and soluble corin elevation predict the presence of preeclampsia in pregnant patients with hypertension.
Methods:
We prospectively enrolled 149 pregnant women with a history of chronic hypertension or gestational hypertension presenting at a tertiary-care hospital. We compared plasma NT-proBNP and soluble corin concentrations based on their preeclamptic status.
Results:
In our study cohort, 62 patients with preeclampsia had lower gestational age than 87 patients without preeclampsia (33.3±3 versus 36.6±3 weeks; P<0.001), otherwise the baseline characteristics were similar. We observed higher NT-proBNP concentrations in patients with preeclampsia compared to those without preeclampsia (304.3 [96.34, 570.4] vs. 60.8 [35.61, 136.8] ng/L, P<0.001), with no differences between chronic and gestational hypertension. However, the concentration of corin was not statistically different between the two groups (1756 [1214, 2133] vs. 1571 [1171, 1961] ng/L, P=0.1087). ROC curve analysis demonstrated stronger predictive value of NT-proBNP compared to soluble corin in predicting the presence of preeclampsia in our study population (AUC 0.7406 vs. 0.5789, P<0.0001).
Conclusion:
While corin may contribute to mechanistic underpinnings of the development of preeclampsia in animal models, soluble corin likely has no diagnostic role in human pregnancies for preeclampsia beyond natriuretic peptide levels.
Keywords: Corin, NT-proBNP, Preeclampsia
INTRODUCTION
Hypertensive disease is a common finding in nearly 12-22% of pregnant females and is also responsible for 17.6% of maternal deaths in the United States.1–3 It can be further categorized as chronic hypertension, gestational hypertension, preeclampsia, and preeclampsia superimposed on chronic hypertension. Preeclampsia is a multisystem disease unique to human pregnancy, and has been responsible for significant morbidity and mortality to both mother and neonate.2, 4–7 There are various hypotheses suggesting the pathogenesis of preeclampsia, but the exact mechanism remains unknown.5,8 Although multiple factors may contribute to preeclampsia, it is evident that the placenta plays a definite role in its causation. This is supported by the fact that as soon as the baby and placenta are delivered, there is a reversal of preeclamptic features.3,5
At present, preeclampsia is diagnosed on the basis of the clinical features, and studies have been done to find markers to help identify preeclampsia in its initial phases.9 Several studies have suggested that circulating levels of natriuretic peptides increase during preeclampsia as a result of stress on ventricles of the heart, from where they are synthesized.10,14 B-type natriuretic peptide is synthesized as a ‘pre-prohormone,’ and the removal of its amino terminal signal sequences yields the prohormone pro-BNP. This has been reported to have minimal biological activity, but it serves as an immediate substrate for the production of the active hormones NT-proBNP and BNP that is cleaved by corin.15 Corin is a type II transmembrane serine protease enzyme that is expressed mainly in the heart and in the pregnant uterus.16 Yan et al. first detected corin from rat uterine decidual cells; in rat models, corin is the enzyme responsible for converting precursor natriuretic peptides into their active forms and is therefore responsible for controlling blood pressure and volume overload during pregnancy.16 Corin-deficient mice late in gestational age developed high blood pressure and proteinuria but returned to normal following delivery.17 Herein, we hypothesize that both elevated circulating NT-proBNP and soluble corin concentrations predict the presence of preeclampsia in pregnant patients presenting with hypertension.
METHODS
Approval of this study was obtained from the Cleveland Clinic Institutional Review Board (IRB) at the Clinic-affiliated Fairview General Hospital, a level III regional Perinatal Centre. We monitored all pregnant patients who presented to the hospital between 8/21/2007 to 1/15/2013. From those, we selected patients who were ≥18 years of age, provided informed consent for the study, and either had diagnosis of chronic hypertension or later developed gestational hypertension or preeclampsia. Chronic Hypertension was defined as a rise in BP (systolic ≥ 140 or diastolic ≥ 90mmHg) on or before 20 weeks of gestation, while Gestational Hypertension was defined as systolic BP ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg developing after the 20th week of pregnancy.
Patients were identified and blood was drawn at the time of enrollment for the study. Participants were provided with a questionnaire by the research staff, and 24 cc of blood was drawn for analysis of corin and NT-proBNP analysis. A urine sample was obtained and tested for microalbuminuria/proteinuria. Patients were divided into two groups based on the presence or absence of preeclampsia. Preeclampsia was defined as systolic BP ≥140mm Hg and/or diastolic BP ≥ 90 mm Hg developing after the 20th week of pregnancy with proteinuria ≥ 300 grams/24 hours. NT-proBNP and corin concentrations were compared between the two groups.
Amino-terminal pro-B-type natriuretic peptide (NT-proBNP) was determined by the Roche Elecsys proBNP assay (Roche Diagnostics, Indianapolis, Indiana). The NT-proBNP assay has a reportable range of 5-35,000 pg/mL, with an analytical sensitivity of ≤5 pg/mL and a robust analytical specificity (<1% interference), according to CLSI EP7-A standards. Interassay coefficients of variation (CVs) were 8%-15%; intra-assay CVs were 6%- 8%. The corin assay was done with the Quantikine Human Corin Immunoassay from R&D Systems (Minneapolis, MN). The assay is a solid phase ELISA designed to measure human corin in cell culture supernatants, tissue homogenates, serum, and plasma. It contains NS0-expressed recombinant human corin and has been shown to accurately quantitate the recombinant factor. Results obtained using natural human corin showed linear curves that were parallel to the standard curves obtained using the Quantikine kit standards. These results indicate that this kit can be used to determine relative mass values for naturally occurring corin.
NT-proBNP and corin concentrations are reported as median [IQR], with normality assessed by the Sharpiro-Wilk test. Comparison of NT-proBNP and corin values among groups was performed using the Mann-Whitney U test. Subject characteristics were compared across diagnostic groups using Independent T-test and Chi-square tests, as appropriate. Statistical analyses among subject characteristics were performed using SPSS v20.0 (IBM, Armonk, NY). Furthermore, the Pearson’s correlation coefficient (ρ) was used as a nonparametric measure of association between log-transformed NT-proBNP and corin concentrations. The distribution of NT-proBNP concentrations was right-skewed, but logarithmic transformation resulted in an approximately normal distribution. Statistical analyses, including receiver operating characteristic (ROC) analysis and area under curve (AUC) calculations, were performed using JMP Pro v9.0 (SAS Institute, Cary, NC). Graphs were generated with GraphPad Prism v7.04 (La Jolla, CA). All the authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A two-sided P-value of <0.05 was considered to be statistically significant.
RESULTS
A total of 149 patients were prospectively enrolled for the study. In our study cohort, 62 (42%) patients were diagnosed with preeclampsia. In the preeclampsia group, 15 (24%) patients had chronic hypertension while 47 (76%) patients had gestational hypertension. In the non-preeclamptic group, 72 (83%) patients had chronic hypertension while 15 (17%) patients had gestational hypertension.
Baseline characteristics were analyzed between the two groups. Patients with preeclampsia had lower gestational age than patients without preeclampsia (33.3±3 versus 36.6±3 weeks, P<0.001), otherwise they had similar baseline characteristics. No statistical differences were found between the two groups with respect to blood pressure, age, parity, hemoglobin concentration, height and weight. Also, there was no difference in the history of preeclampsia from previous pregnancies, smoking status, parity, multiple pregnancies, and family history of hypertension between the two groups (Table 1).
Table 1:
Baseline Characteristics
| No-Preeclampsia (n=87) | Preeclampsia (n=62) | P value | |
|---|---|---|---|
| Maternal Age ( years) | 32.35 ± 5.67 | 31.09 ± 6.03 | 0.094 |
| Gestational Age (weeks) | 36.59 ± 2.64 | 33.25 ± 3.40 | <0.001 |
| Maternal Height (m) | 1.64 ± .07 | 1.65 ± 0.08 | 0.307 |
| Maternal Weight (kg) | 81.66 ± 23.69 | 87.43 ± 27.55 | 0.167 |
| Hemoglobin Concentration (g/dL) | 11.74 ±1.21 | 11.79 ±1.16 | 0.724 |
| Systolic BP (mm Hg) | 139.37± 16.81 | 144.11 ± 25.82 | 0.363 |
| Diastolic BP (mm Hg) | 80.78 ± 13.22 | 79.58 ± 17.24 | 0.637 |
| Gravidity>1 | 51(58.6%) | 30(48.4%) | 0.493 |
| Multiple Pregnancy | 5(5.7%) | 7(11.5%) | 0.234 |
| Patients on Antihypertensive | 36(41.4%) | 26(41.9%) | 1.000 |
| History of Preeclampsia | 14(16.1%) | 9(14.5%) | 0.823 |
| Family History of Hypertension | 70(80.5%) | 51(82.3%) | 0.834 |
| Current Smoker | 4(4.6%) | 3(4.8%) | 1.000 |
| History of Smoking | 44(50.6%) | 29(46.8%) | 0.740 |
Figure 1 presents the distribution of NT-proBNP and soluble corin among our study population. We observed higher NT-proBNP concentrations in patients with preeclampsia compared to those without preeclampsia (304.3 [96.34, 570.4] vs. 60.8 [35.61, 136.8] ng/L, P<0.0001, Figure 1A). Subgroup analysis in patients without preeclampsia showed no significant difference in the NT-proBNP concentration between patients with chronic hypertension and gestational hypertension (59.21 [50.01, 90.97] vs. 68.01 [35.33, 155.8] ng/L, P=0.5904). Similarly, there was no significant difference in NT-proBNP concentration between patients with chronic hypertension and gestational hypertension in the preeclamptic cohort (308.6 [61.3, 598] vs. 295.6 [99.02, 561.8] ng/L, P=0.8619). Interestingly, there were no significant differences in blood pressure between those with and without preeclampsia (systolic BP: 139±17 vs. 144 ± 26 mmHg, P=0.36; diastolic BP: 81±13 vs. 80±17 mmHg, P=0.64), despite differences in NT-proBNP concentration. Furthermore, analysis between chronic hypertension patients versus gestational hypertension patients revealed no differences in blood pressure (systolic BP: 146±22 vs. 140±21 mmHg, P=0.29; diastolic BP: 76±15 vs. 81±15 mmHg, P=0.89). Finally, gestational age was found to negatively correlate to NT-proBNP concentration among the entire study cohort (ρ=−0.5487, P=0.0082).
Figure 1:
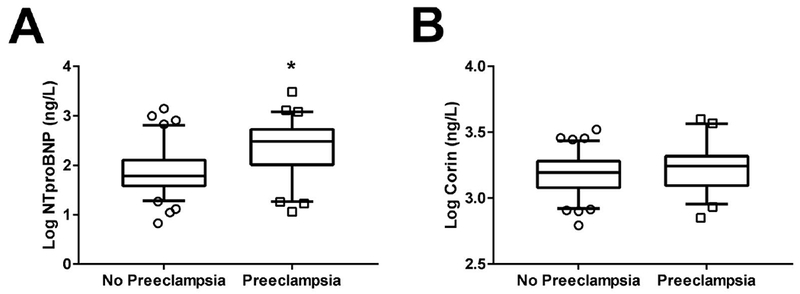
Comparison of NT-proBNP and Corin Levels between Patients with versus without Preeclampsia. (A) Logarithmic-adjusted values of NT-proBNP concentrations are larger in preeclampsia vs. non-preeclampsia subjects. (B) Logarithmic-adjusted values of corin concentrations do not differ between preeclampsia and non-preeclampsia subjects. *P<0.0001 vs. No Preeclampsia.
In contrast, plasma corin concentrations were not significantly different between the non-preeclampsia and preeclampsia groups (1756 [1214, 2133] vs. 1571 [1171, 1961] ng/L, P=0.1087, Figure 1B). Contrary to NT-proBNP, gestational age did not correlate to soluble corin concentrations (P=0.3165). We did not observe a correlation between corin and NT-proBNP log- transformed concentrations (ρ=−0.0548, P=0.5142, Figure 2). Also, receiver operating characteristic (ROC) curve analysis was performed for NT-proBNP and corin against preelamptic status (Figure 3). NT-proBNP demonstrated a significant association in predicting the presence of preeclampsia (AUC 0.7406, 95% CI 0.6543-0.827, P<0.0001, Figure 3A). However, corin did not displayed any predictive value for the presence of preeclampsia (AUC 0.5789, 95% CI 0.4817-0.676, P=0.1081, Figure 3B).
Figure 2:
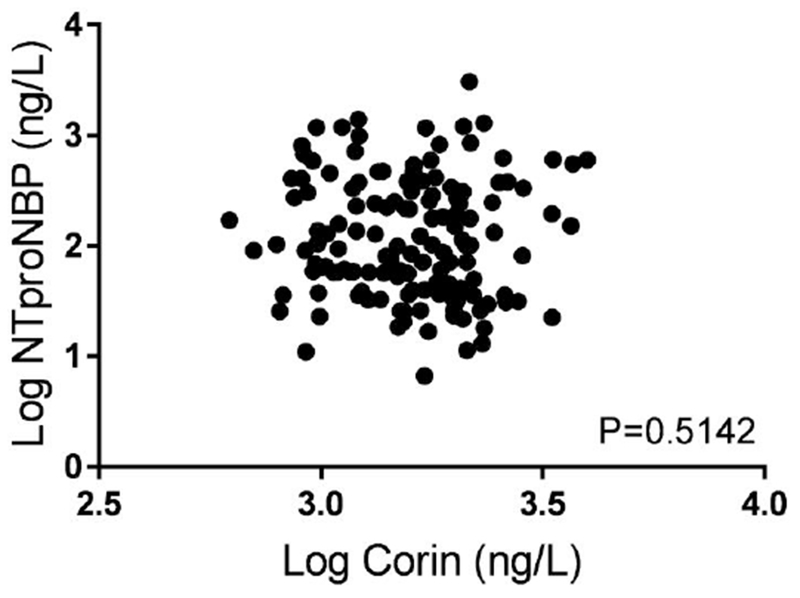
Scatter Plot between NT-proBNP and Corin Concentrations
Figure 3:

Predictive Value of Plasma NT-proBNP and Plasma Corin for Preeclampsia Status. (A) Receiver Operating Characteristic (ROC) analysis of NT-proBNP reveals a significant relationship to preeclampsia status. (B) ROC analysis of corin shows no significant sensitivity nor sensitivity to preeclampsia status. *P=0.0001.
DISCUSSION
We observed that in a large series of pregnant patients presenting with hypertension, systemic levels of NT-proBNP were significantly elevated in the patients with preeclampsia when compared to those patients without preeclampsia. However, soluble corin concentrations did not differ between those with and without preeclampsia.
The preeclamptic condition is considered to be associated with insufficient trophoblastic invasion and impaired spiral artery remodeling.18–20 This in turn causes placental insufficiency and the subsequent stress leads to the clinical features of preeclampsia. Experimental studies were done to find out the association between preeclamptic condition and corin in murine models. In those experiments, using knockout mice models, it was found that corin deficiency is associated with the rise of blood pressure and proteinuria in the later part of gestation - features that mimic preeclampsia in humans.22, 25 However, this finding in murine models needs to be considered carefully in humans, as the utero-placental structure of mouse and human are quite different with respect to the basic developmental process. For example, trophoblastic invasion and remodeling of uterine spiral arteries are found to be less extensive in rat models than in humans.21, 22
It is now known that corin is secreted from various tissues, mainly cardiac tissues and the pregnant uterus.16, 23, 24 According to a study by Zhou et al., it could be hypothesized that the preeclamptic condition can be attributed to corin deficiency. Previous studies have demonstrated that higher circulating levels of soluble corin are observed in patients with pregnancy-induced hypertension as compared to non-pregnant patients.25–27 This paradoxical increase can be explained by the fact that corin is secreted not from the placenta, but from the heart in response to the high blood pressure in pregnancy induced hypertension - causing extensive enzyme shedding from the cell surface (it is a serine type II transmembrane protease).27–30 Furthermore, this can also explain the presence of the similar blood concentrations of soluble corin in our study groups (1756 [1214, 2133] vs. 1571 [1171, 1961] ng/L, P=0.1087), irrespective of preeclamptic status. This observation may point towards the possibility that corin is secreted from the heart in response to high blood pressure, consistent with findings from Zaki et al. also showing that increased circulating corin is associated with higher blood pressure in pregnancy-induced hypertensive patients.31 However, Gu et al. have demonstrated higher plasma concentrations of corin in both severe and mild preeclampsia patients, as compared to both pregnant normotensive controls and a chronic hypertensive group.32 While the scope of our study serves specifically to compare NT-proBNP to corin as a preeclamptic marker, the elevation of corin among other studies highlights the complex multitude of mechanisms involved in preeclampsia.
Our results are consistent with other studies, which indicate that NT-proBNP concentration is higher in patients with preeclampsia, but those studies compared preeclamptic patients with normal pregnant population and not with non-preeclamptic hypertensive patients.10–12, 14 Tihtonen et al. showed increased NT-proBNP in preeclamptic patients as compared to pregnant patients with chronic hypertension and normal pregnancy, albeit with a smaller sample size compared to our study cohort (19 vs. 62 preeclamptic patients).13 It is important to highlight the fact that during preeclampsia, there is a marked increase in peripheral resistance, which results in an increase in afterload. This increase in afterload results in increased ventricular stress, which in turn is responsible for increased secretion of NT-proBNP as a compensatory mechanism. Natriuretic peptides, like NT-proBNP, which are secreted in response to high blood pressure, work to bring blood pressure back to normal through natriuresis, diuresis, and vasodilation. This seems to indicate that blood pressure drives the production of NT-proBNP, with similar conclusions being drawn from other studies.10, 13, 33In contrast, our study reveals the etiological significance of preeclampsia rather than hypertensive status as the cause of increased NT-proBNP. Moreover, it is conceivable that plasma volume expansion and venous congestion can be key drivers of NT-proBNP increase.34, 35 However, hemodynamic stress in preeclamptic human patients is multifactorial. While examining preelampsia through the window of NT-proBNP provides useful insight, it is only one prism with which to understanding this phenomenon. The entirety of the disease spectrum consists of multiple dynamic equilibria, along with many compensatory mechanisms with which the body battles physiological changes.
CONCLUSIONS
In patients with pregnancy-associated hypertension, increased circulating NT-proBNP is associated with underlying preeclampsia. Despite the potential mechanistic contributions of corin in the development of preeclampsia in animal models, systemic levels of corin do not reflect preeclamptic status.
Acknowledgments
Support/Disclosure.
This research was supported by the Community West Foundation and by the National Institutes of Health grant R01HL103931. There are no relationships to disclose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acog practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, january 2002. Obstet Gynecol. 2002;99:159–167 [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799 [DOI] [PubMed] [Google Scholar]
- 3.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Current opinion in obstetrics & gynecology. 2013;25:124–132 [DOI] [PubMed] [Google Scholar]
- 4.Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull. 2003;67:161–176 [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56 [DOI] [PubMed] [Google Scholar]
- 6.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192 [DOI] [PubMed] [Google Scholar]
- 7.Bakacak M, Serin S, Ercan O, Kostu B, Bakacak Z, Kiran H. Association of serum n- terminal pro-brain natriuretic peptide levels with the severity of preeclampsia. J Matern Fetal Neonatal Med. 2016;29:2802–2806 [DOI] [PubMed] [Google Scholar]
- 8.Walker JJ. Pre-eclampsia. Lancet. 2000;356:1260–1265 [DOI] [PubMed] [Google Scholar]
- 9.Polsani S, Phipps E, Jim B. Emerging new biomarkers of preeclampsia. Adv Chronic Kidney Dis. 2013;20:271–279 [DOI] [PubMed] [Google Scholar]
- 10.Kale A, Kale E, Yalinkaya A, Akdeniz N, Canoruc N. The comparison of amino-terminal probrain natriuretic peptide levels in preeclampsia and normotensive pregnancy. J PerinatMed. 2005;33:121–124 [DOI] [PubMed] [Google Scholar]
- 11.Pouta AM, Vuolteenaho OJ, Laatikainen TJ. An increase of the plasma n-terminal peptide of proatrial natriuretic peptide in preeclampsia. Obstet Gynecol. 1997;89:747–753 [DOI] [PubMed] [Google Scholar]
- 12.Seong WJ, Kim SC, Hong DG, Koo TB, Park IS. Amino-terminal pro-brain natriuretic peptide levels in hypertensive disorders complicating pregnancy. Hypertens Pregnancy .30:287–294 [DOI] [PubMed] [Google Scholar]
- 13.Tihtonen KM, Koobi T, Vuolteenaho O, Huhtala HS, Uotila JT. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol. 2007;196:328 e321-327 [DOI] [PubMed] [Google Scholar]
- 14.Itoh H, Sagawa N, Mori T, Mukoyama M, Nakao K, Imura H. Plasma brain natriuretic peptide level in pregnant women with pregnancy-induced hypertension. Obstet Gynecol. 1993;82:71–77 [PubMed] [Google Scholar]
- 15.Xu-Cai YO, Wu Q. Molecular forms of natriuretic peptides in heart failure and their implications. Heart.96:419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cdna from human heart. J Biol Chem. 1999;274:14926–14935 [DOI] [PubMed] [Google Scholar]
- 17.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Wu Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta. 2013;34:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7 [DOI] [PubMed] [Google Scholar]
- 20.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta. 2006;27:939–958 [DOI] [PubMed] [Google Scholar]
- 21.Carter AM, Enders AC, Jones CJ, Mess A, Pfarrer C, Pijnenborg R, Soma H. Comparative placentation and animal models: Patterns of trophoblast invasion -- a workshop report. Placenta. 2006;27 Suppl A:S30–33 [DOI] [PubMed] [Google Scholar]
- 22.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19 [DOI] [PubMed] [Google Scholar]
- 23.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC, Jr. Corin is present in the normal human heart, kidney, and blood, with pro-b-type natriuretic peptide processing in the circulation. Clin Chem.57:40–47 [DOI] [PubMed] [Google Scholar]
- 24.Polzin D, Kaminski HJ, Kastner C, Wang W, Kramer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int.78:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Current hypertension reports. 2014;16:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armaly Z, Assady S, Abassi Z. Corin: A new player in the regulation of salt-water balance and blood pressure. Current opinion in nephrology and hypertension. 2013;22:713–722 [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190 [DOI] [PubMed] [Google Scholar]
- 29.Shrestha K, Troughton RW, Borowski AG, Yandle TG, Richards AM, Klein AL, Tang WH. Plasma corin levels provide minimal prognostic utility incremental to natriuretic peptides in chronic systolic heart failure. J Card Fail .16:621–627 [DOI] [PubMed] [Google Scholar]
- 30.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748 [DOI] [PubMed] [Google Scholar]
- 31.Zaki MA, El-Banawy Sel D, El-Gammal HH. Plasma soluble corin and n-terminal pro-atrial natriuretic peptide levels in pregnancy induced hypertension. Pregnancy Hypertens. 2012;2:48–52 [DOI] [PubMed] [Google Scholar]
- 32.Gu Y TD, Xu J, Lewis DF, Morgan JA, Cooper DB, McCathran CE, Wang Y. Aberrant pro-atrial natriuretic peptide/corin/natriuretic peptide receptor signaling is present in maternal vascular endothelium in preeclampsia. Pregnancy Hypertens. 2018;11:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speksnijder L, Rutten JH, van den Meiracker AH, de Bruin RJ, Lindemans J, Hop WC, Visser W. Amino-terminal pro-brain natriuretic peptide (nt-probnp) is a biomarker of cardiac filling pressures in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol.153:12–15 [DOI] [PubMed] [Google Scholar]
- 34.Gyselaers W, Mullens W, Tomsin K, Mesens T, Peeters L. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: A review. Ultrasound Obstet Gynecol. 2011;38:123–129 [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga N, Kanayama N, Sugimura M, Kobayashi T, Terao T. Dilatation of the left renal vein in preeclampsia. J Matern Fetal Med. 2000;9:356–359 [DOI] [PubMed] [Google Scholar]


