Introduction:
Recurrent hepatic encephalopathy (HE) leads to significant disability and mortality despite standard of care (SOC) i.e. lactulose and add-on rifaximin1. It is associated with impairment of the gut-liver-brain axis and fecal microbiota transplant (FMT) may be useful. In recurrent C. difficile, a single FMT has sustained cure rates of >80% with a tolerable long-term safety profile2. In a published safety trial, 10 recurrent HE participants each were randomized to pre-treatment antibiotics and FMT, or standard of care (SOC), which was safe. As a secondary outcome there was an improvement in short-term cognitive function and hospitalizations3. However, the long-term impact of FMT is unclear. Our aim was to determine the long-term impact of FMT on cognition, hospitalizations and HE by extending results of this trial3.
Methods:
For rational donor selection, the microbiome profile of HE patients and healthy controls were assessed. HE patients demonstrated a relative reduction of Lachnospiraceae and Ruminococcaceae compared to controls. Leveraging the cross-sectional microbiome data, a donor was selected from the donor database using Random Forrest analysis, to complement the relative deficiencies of the patient microbiota3. The same donor was used for all FMT-assigned participants. These participants underwent five days of pre-FMT antibiotics after which 90ml (27 grams of stool) of the donor material containing approximately 2.7E12 CFU was administered via enema. SOC participants did not undergo any interventions or antibiotic therapy. All participants were followed in keeping with the visits reported in Figure 1A. There were no FMT-related serious adverse events in the five months post-study as judged by the data safety monitoring board. For the long-term study, all participants were followed prospectively at least every 2 months through chart review, phone calls or in-person with specific focus on hospitalizations and HE episodes. Eligibility, cognitive testing, further donor selection and methods are in the supplement. At the long-term visit, we re-administered cognitive tests (Psychometric hepatic encephalopathy score, PHES and EncephalApp Stroop) and obtained stool (Figure 1A)3. Data were compared to pre-FMT, post-antibiotics and early post-FMT values. Microbial composition was studied using 16S rRNA sequencing. Principal component (PCO) and Linear discriminant analysis effect size (LEFSe) analysis were performed.
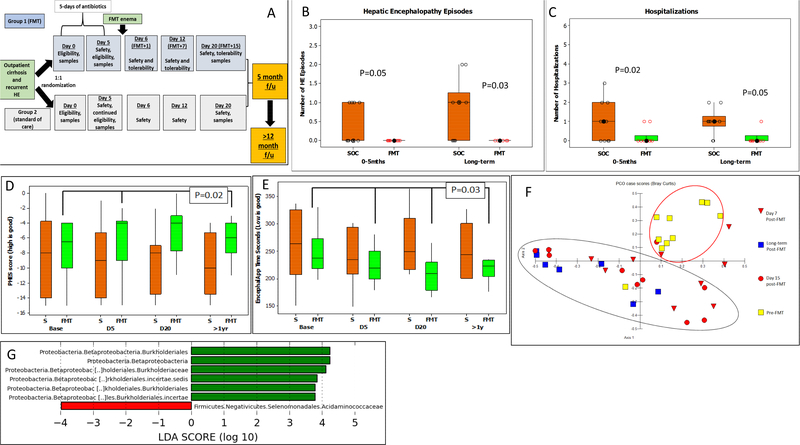
Figure 1.
1A: Schematic of study follow-up, 1B/C: Hepatic encephalopathy episodes and total hospitalizations in SOC compared to FMT in the original study (0–5 months) compared to long-term (>12 months). Comparison is made using Kruskal-Wallis tests between groups. 1D: Median and 95% CI of total Psychometric hepatic encephalopathy score (PHES) at baseline, Day 5 (D5, pre-FMT but post-antibiotics), Day 20 (15 days post-FMT) and >1 yr in the FMT group (green bars) compared to SOC (S, orange bars) showed a better (higher) PHES score post-FMT in the FMT groups compared to SOC at D20 and long-term. 1E: Median and 95% CI of EncephalApp OffTime+OnTime at baseline, day 5 (D5, pre-FMT but post-antibiotics), Day 20 (15 days post-FMT) and >1 yr in the FMT group (green bars) compared to SOC (S, orange bars) showed a better (lower) time required for EncephalApp post-FMT in the FMT groups compared to SOC at D20 and long-term. 1F: Principal component analysis of microbiota in the FMT group pre-FMT, Day 7, Day 15 and long-term follow up showed relative clustering between the post-FMT group microbiota (black oval) compared to pre-FMT (red oval). 1G: On LEFSe, there was a significant increase in relative abundance of Burkholderiaceae and decrease of Acidaminococcaceae in FMT compared to SOC group at study end. SOC group is in red and FMT group is in green
Results:
All participants were on lactulose, rifaximin and proton pump inhibitors (PPI) at trial initiation and continued these throughout. All participants were followed for >12 months and up to 15 months (12.9±2.9 months FMT and 12.8±3.7 months SOC) but three were excluded. Two from SOC (1 died, 1 liver transplant) and one from FMT who died (p=1.0 between groups) with these events occurring 11.3±2.9 months post-randomization. Overall, in those assigned to FMT, the intervention was well-tolerated and had a non-concerning long-term safety profile, without infections or need for new antibiotic initiation. There was a significantly higher number of hospitalizations and HE episodes in the SOC arm compared to the FMT arm during the long-term follow-up (Figure 1B/C). In the SOC arm, there was a total of 10 hospitalization (2 participants: 2 events; 6 participants: 1 event) compared to 1 in the FMT arm (p=0.05). The majority of the hospitalizations were liver-related (SOC: HE 4, infection 2, ascites 2 vs FMT: ascites 1). The two SOC participants with infections (one C.difficile and one pneumonia) required short courses of broad-spectrum antibiotics. In the SOC arm, there was a total of 8 HE events (2 participants: 2 events, 4 participants: 1 event) compared to 0 in the FMT arm (p=0.03). In both arms, MELD score change between pre-intervention and long-term post-intervention for the surviving patients were similar (SOC: 2.78±4.7 vs FMT: 2.8±4.5, p=0.9). Cognitive function, which had improved in the FMT arm at Day 20 post-FMT, remained significantly better in the FMT arm compared to SOC on both tests on long-term testing (Figure 1D/E).
Microbiota composition:
Stool samples were obtained from 7 in the FMT arm and in the 6 SOC arm at the long-term visit the same time as cognitive testing. The remainder of patients had expired, (n=2), were transplanted (n=1) or refused to provide samples (n=4). There was an increase in relative abundance of Burkholderiaceae and decreased Acidaminoccocaceae in the FMT arm long-term (Figure 1F) on LEFSe. Lachnospiraceae and Ruminococcaceae relative abundance remained similar between groups. On PCO, there was a clustering of the long-term microbiota with microbiota obtained at Day 7 and 15 post-FMT compared to pre-FMT microbiota (Figure 1G), indicating that the microbiota were similar post-FMT regardless of short or long-term follow-up compared to pre-FMT microbial composition.
Discussion:
The current study extends the experience of the first randomized clinical trial of FMT after antibiotics in cirrhosis and recurrent HE over more than 12 months. The results suggest long-term safety as well as sustained improvement in clinical and cognitive function parameters among patients who received FMT with pre-treatment antibiotics4. The most striking improvement was in the prevention of HE recurrence and liver-related hospitalizations in this recalcitrant population, which was associated with improved cognition compared to those on SOC. This occurred despite continuation of lactulose, rifaximin and PPI across groups. Prior studies have demonstrated cognitive impairment as a predictor for future HE episodes, and the alteration of gut-liver-brain axis with this intervention could interrupt this cycle of readmissions5. The improvement in all-cause hospitalizations reflects prior data in C. difficile and alcoholic hepatitis2, 6. From a microbiome perspective there were differences in profiles between the SOC and FMT arms at long-term analysis, but not in Lachnospiraceae and Ruminococcaceae relative abundance. These results are unlikely due to mitigation of PPI microbiome effect and extend prior FMT studies where the ultimate microbial profile may not precisely reflect the donor to result in benefit 7, 8. Functional evaluation is therefore needed to understand the potential mechanisms of action of microbiome therapeutics. Our study is limited by a small sample size as well as the use of pre-treatment antibiotics without an FMT alone arm; however, data suggests antibiotics promote FMT engraftment4. Overall, this preliminary experience demonstrates that FMT after antibiotics may be safe and potentially effective in preventing long-term recurrence of HE. However, larger controlled trials with HE as the primary outcome are needed.
Supplementary Material
Acknowledgments
Funding: Partly supported by VA Merit Review grant I0CX001076, NCATS R21TR02024 and McGuire Research Institute to JSB.
Footnotes
Conflict of Interest: ZK is an employee and shareholder of Finch Therapeutics Group, as well as an advisor for OpenBiome; no other relevant conflicts exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Vilstrup H et al. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CR et al. Gastroenterology 2015;149:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj JS et al. Hepatology 2017. [Google Scholar]
- 4.Ji SK et al. Front Microbiol 2017;8:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patidar KR et al. Am J Gastroenterol 2014;109:1757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philips CA et al. Clin Gastroenterol Hepatol 2017;15:600–602. [DOI] [PubMed] [Google Scholar]
- 7.Khanna S et al. Microbiome 2017;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao D et al. Hepatology 2016;63:339–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.