Abstract
Background & Aims:
Frailty is associated with mortality in patients with cirrhosis. We measured frailty using 3 simple tests and calculated liver frailty index (LFI) scores for patients at multiple ambulatory centers. We investigated associations between LFI scores, ascites, and hepatic encephalopathy (HE) and mortality.
Methods:
Adults without hepatocellular carcinoma who were on the liver transplant waitlist at 9 centers in the United States (n=1044) were evaluated using the LFI; LFI scores of 4.5 or more indicated that patients were frail. We performed logistic regression analyses to assess associations between frailty and ascites or HE and competing risk regression analyses (with liver transplantation as the competing risk) to estimate subhazard ratios (sHR) of waitlist mortality (death or removal from the waitlist).
Results:
Of study subjects, 36% had ascites, 41% had HE, and 25% were frail. The odds of frailty were higher for patients with ascites (adjusted odd ratio, 1.56; 95% CI, 1.15–2.14) or HE (OR, 2.45; 95% CI, 1.80–3.33) than without these features. Higher proportions of frail patients with ascites (29%) or HE (30%) died while on the waitlist compared to patients who were not frail (17% of patients with ascites and 20% with HE). In univariable analysis, ascites (sHR, 1.52; 95% CI, 1.14–2.05), HE (sHR, 1.84; 95% CI, 1.38–2.45), and frailty (sHR, 2.38; 95% CI, 1.77–3.20) were associated with waitlist mortality. In adjusted models, only frailty remained significantly associated with waitlist mortality (sHR, 1.82; 95% CI, 1.31–2.52)—ascites and HE were not.
Conclusions:
Frailty is a prevalent complication of cirrhosis that is observed more frequently in patients with ascites or HE and independently associated with waitlist mortality. LFI scores can be used to objectively quantify risk of death related to frailty – in excess of liver disease severity – in patients with cirrhosis.
Keywords: malnutrition, portal hypertension, end-stage liver disease, risk factor
INTRODUCTION
Current prognostic metrics for death in patients with cirrhosis have thus far focused solely on aspects of the patient that are overtly impacted by hepatic dysfunction – whether manifest as laboratory abnormalities as seen with total bilirubin or creatinine in the Model for End-Stage Liver Disease score1,2 or ascites and hepatic encephalopathy in the Child-Pugh score.3 However, cirrhosis impacts patients through more insidious ways – namely, malnutrition, muscle wasting, and decreased functional capacity – which, while widely understood by clinicians to be associated with cirrhosis, are infrequently diagnosed formally or incorporated into decision-making systematically. Emerging data have suggested that this constellation of symptoms in patients with cirrhosis can be captured conceptually by the defined syndrome of “physical frailty” and operationalized by composite metrics that measure frailty components such as physical function, functional capacity, mobility, or weakness (among many others).4–9
Through studies to date, it has become increasingly apparent that physical frailty (and its components) are only modestly correlated with liver disease severity, as measured by the Model for End-Stage Liver Disease score.4–6,10 However, the relationship between physical frailty and ascites / hepatic encephalopathy has not yet been well-characterized. While studies have suggested – and clinicians have long intuited – that physical frailty increases as the burden of ascites or hepatic encephalopathy increases,4 precise quantification of this association has been limited by the lack of an objective tool to measure frailty that is not confounded by the portal hypertensive complications themselves. Perhaps the best example of this is with the “unexplained weight loss” component of the Fried Frailty Phenotype which is confounded by those with ascites on diuretics or receiving large volume paracenteses.11
Recently, we developed a metric specifically for patients with cirrhosis, the Liver Frailty Index, to allow for objective measurement of frailty in this population and improve prognostication of mortality above and beyond MELDNa score or subjective clinician assessments alone.10,12 With this tool, we aimed to evaluate: 1) the association between physical frailty and ascites / hepatic encephalopathy, and 2) how physical frailty alters the relationship between these two portal hypertensive complications and mortality in patients with cirrhosis. Based on our clinical experience, we hypothesized that physical frailty serves as a mediator between ascites / hepatic encephalopathy and waitlist mortality in this population.
METHODS
Patients
This study was conducted as part of the Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study which includes 9 liver transplant centers in the United States: University of California, San Francisco (n=770), Baylor University Medical Center (n=51), Columbia University Medical Center (n=50), Duke University (n=40), University of Pittsburgh (n=37), Johns Hopkins Medical Institute (n=38), Loma Linda University (n=32), University of Arkansas for Medical Sciences (n=20), Northwestern (n=6). The complete FrAILT Study protocol has been published in full.10 The FrAILT Study enrolled patients with cirrhosis who were listed or eligible for listing for liver transplantation at the Multi-Center FrAILT centers and seen as outpatients. Excluded were patients who were listed with MELDNa exception points, as the time that these patients spend on the waitlist are not dependent on their native liver disease function, and those who did not speak English or Spanish, as the consent forms are not currently available in non-English or non-Spanish languages. Once enrolled, patients outcomes were ascertained prospectively.
Study procedures
At enrollment, all patients underwent a single, baseline objective measurement of frailty using:
Grip strength11: the average of three trials, measured in the subject’s dominant hand using a hand dynamometer;
Timed chair stands13: measured as the number of seconds it takes to do five chair stands with the subject’s arms folded across the chest;
Balance testing13: measured as the number of seconds that the subject can balance in three positions (feet placed side-to-side, semi-tandem, and tandem) for a maximum of 10 seconds each.
These three tests were administered by trained study personnel. With these three individual tests of frailty, the Liver Frailty Index was calculated using the following equation3 (calculator available at: http://liverfrailtyindex.ucsf.edu):
The classifications of frailty were determined by using previously established cut-offs of the Liver Frailty Index with “frail” defined as Liver Frailty Index ≥4.5.3
Ascites was ascertained at the baseline study visit from the hepatologists’ recorded physical exam or the management plan associated with the clinic visit that occurred on the same day at the assessment of frailty. Ascites was categorized as “absent” if ascites was not present on physical exam or “present” if ascites was present on exam and/or the patient was noted to be undergoing large volume paracenteses. Hepatic encephalopathy was determined at the baseline study visit from the time to complete the Numbers Connection Test A14 performed at the time of the frailty measurement. Hepatic encephalopathy was categorized as “present” if the patient took ≥45 seconds to complete the task. This cut-off of 45 seconds was selected based on normative data determined from healthy controls and compared with individuals with and without hepatic encephalopathy.14 Separately, data regarding ascites and hepatic encephalopathy were collected from the medical record based on history (as mentioned in the progress note) or by medication use. In sensitivity analyses using different definitions to define ascites or hepatic encephalopathy, the primary association of interest (between frailty and waitlist mortality) was not substantively changed.
Data regarding demographics were extracted from the clinic visit note from the same day as the objective frailty measurement. Patients were considered to have a diagnosis of hypertension, diabetes, or coronary artery disease if was reported in their electronic health record.
Statistical analysis
Baseline demographics were presented as medians [interquartile ranges (IQR)] for continuous variables or percentages for categorical variables and compared by frailty status using Wilcoxon rank sum or chi-square tests. Logistic regression was used to assess associations between frailty and ascites or hepatic encephalopathy. The primary outcome was waitlist mortality, defined as the combined outcome of death or delisting for being too sick for liver transplantation. Follow up time for those who did not achieve a terminal waitlist event was censored on April 13, 2018. Life tables were used to estimate the cumulative incidence of waitlist mortality at 12-months from study entry; logrank p-values were used to test for statistical significance between groups (e.g., frail versus non-frail, ascites versus no ascites). We modeled the cumulative incidence function with Fine and Gray competing risk regressions to estimate the risk of waitlist mortality associated with each variable.15 Risk estimates were described as subhazard ratios (SHR) with 95% confidence intervals (CI). In these competing risk models, deceased donor liver transplantation was treated as the competing risk; patients who underwent living donor liver transplantation were censored on the day of their liver transplantation. Patients who were removed for reasons other than being too sick (i.e., for social reasons) were also censored on the day of their removal from the waitlist. For the regression models, all variables associated with waitlist mortality with a p-value of 0.2 in univariable analysis were evaluated for inclusion in the final multivariable model. Backwards stepwise regression was then performed to derive the final multivariable model which included only variables associated with a p-value <0.05. We assessed interactions between ascites or hepatic encephalopathy and frailty.
Statistical analyses were performed using Stata (v14, College Station, TX). The Institutional Review Boards at each of the participating sites approved this study.
RESULTS
Characteristics of the entire patient population (Table 1)
Table 1.
Characteristics of the 1,044 patients with cirrhosis included in this study.
| Characteristics | All n=1,044 | By Frailty Status | p-value | ||
|---|---|---|---|---|---|
| Not Frail n=779 (75%) | Frail n=265 (25%) | ||||
| Age, years | 57 (49–63) | 57 (49–63) | 58 (51–63) | 0.07 | |
| Female | 43% | 41% | 46% | 0.16 | |
| Race/Ethnicity | Non-Hispanic White | 61% | 60% | 63% | 0.72 |
| Black | 4% | 4% | 5% | ||
| Hispanic | 23% | 24% | 21% | ||
| Asian/Pacific Islander | 4% | 5% | 3% | ||
| Other | 8% | 7% | 8% | ||
| Body mass index, kg/m2 | 28 (25–33) | 28 (25–33) | 28 (25–33) | 0.95 | |
| Etiology of liver disease | Chronic hepatitis C | 29% | 31% | 21% | <0.001 |
| Alcohol | 25% | 24% | 29% | ||
| Non-alcoholic steatohepatitis | 17% | 15% | 23% | ||
| Autoimmune/cholestatic | 16% | 18% | 11% | ||
| Other | 13% | 11% | 16% | ||
| Hypertension | 38% | 37% | 38% | 0.71 | |
| Diabetes | 30% | 26% | 42% | <0.001 | |
| Coronary artery disease | 5% | 6% | 3% | 0.11 | |
| MELDNa | 18 (15–22) | 18 (14–22) | 21 (16–25) | <0.001 | |
| Total bilirubin, mg/dL | 2.5 (1.6–4.2) | 2.5 (1.6–4.0) | 2.6 (1.5–4.8) | 0.68 | |
| Creatinine, mg/dL† | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 1.0 (0.8–1.4) | <0.001 | |
| INR | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.4 (1.3–1.7) | 0.15 | |
| Sodium, mEq/L | 137 (134–139) | 137 (134–139) | 136 (133–139) | 0.003 | |
| Albumin, g/dL | 3.1 (2.6–3.5) | 3.1 (2.6–3.5) | 3.0 (2.6–3.4) | 0.07 | |
| Dialysis | 5% | 3% | 11% | <0.001 | |
| Ascites | 36% | 32% | 48% | <0.001 | |
| Hepatic encephalopathy | 41% | 36% | 57% | <0.001 | |
| Outcome | Waiting | 44% | 47% | 35% | <0.001 |
| Death/delisted for being too sick for transplant | 18% | 15% | 26% | ||
| Deceased donor liver transplant | 27% | 26% | 27% | ||
| Other | 11% | 12% | 12% | ||
Median (interquartile range) or %
Among those who were not on dialysis
A total of 1,044 patients with cirrhosis were included in this study. Baseline characteristics of the cohort are shown in Table 1. To briefly summarize, median age was 57 years, 43% were female, 61% were non-Hispanic White, and median body mass index was 28 kg/m2. Twenty-nine percent had chronic hepatitis C as their primary etiology of liver disease, 25% alcoholic liver disease, and 17% non-alcoholic steatohepatitis. Rates of hypertension, diabetes, and coronary artery disease were 38%, 30%, and 5%, respectively. In this outpatient cohort, median MELDNa was 18 and albumin was 3.1 g/dL. By the end of follow up, 182 (18%) experienced the primary outcome of death/delisting for being too sick for liver transplantation, 277 (27%) underwent deceased donor liver transplantation, and 91 (9%) underwent living donor liver transplantation (and were censored at the time of their transplant for the analyses). Cumulative incidences of mortality at 3-, 6-, and 12-months were 6%, 11%, and 19%. Median (IQR) follow up time was 381 days (148–944).
Comparison of baseline characteristics by frailty status (Table 1)
Of the 1,044 patients included in this study, 265 (25%) were classified as frail. Compared to non-frail patients, frail patients tended to be older (58 vs. 57 years). They were less likely to have chronic hepatitis C (21 vs. 31%) or autoimmune diseases (11 vs. 18%) as their etiologies of cirrhosis but more likely to have non-alcoholic steatohepatitis (23 vs. 15%) or diabetes (42 vs. 26%). However, frail and non-frail patients were similar by gender, race/ethnicity, BMI, hypertension, and coronary artery disease. Median MELDNa was 21 in frail and 18 in non-frail patients. More frail than non-frail patients were on dialysis (11 vs. 3%).
Overall rates of mortality for the cohort were 26% (69 / 265) among patients who were frail and 15% (114 / 779) who were non-frail [p<0.001]. Cumulative incidences of waitlist mortality at 3-, 6-, and 12-months were 13%, 22%, and 35% among those who were frail versus 2%, 6%, and 11% among those who were not frail [logrank p<0.001].
Associations between ascites, frailty, and mortality
Ascites was present in 36% of patients. Rates of ascites were higher in frail compared to non-frail patients (48 vs. 32%). In univariable logistic regression, the odds of being frail were 2-fold higher among those with ascites compared to those without (OR 1.97, 95% 1.48–2.62; p<0.001). Other variables that were also associated with frailty with a p-value <0.2 were age (OR 1.01 per year; 95% CI 1.00–1.03; p=0.07), female sex (OR 1.22; 95% CI 0.92–1.62; p=0.16), chronic hepatitis C (OR 0.57; 95% CI 0.41–0.80; p=0.001), diabetes (OR 2.06; 95% CI 1.54–2.77; p<0.001), MELDNa (OR 1.09 per point; 95% CI 1.06–1.12; p<0.001), and albumin (OR 0.76 per g/dL; 95% CI 0.61–0.95; p=0.02). In final multivariable analysis, after adjustment for HCV, diabetes, and MELDNa, ascites remained significantly associated with frailty (OR 1.56; 95% CI 1.15–2.14; p=0.002).
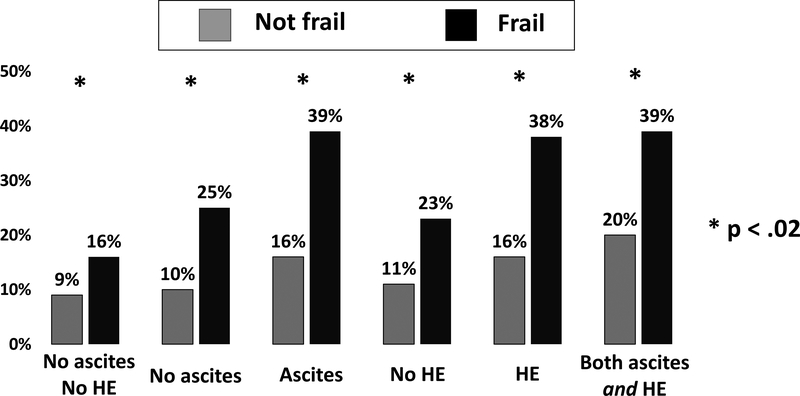
Waitlist mortality occurred within 12 months in 23% of those with ascites and 13% of those without (p<0.001). When stratified by frailty status, rates of waitlist mortality were significantly higher among those who were frail regardless of ascites status (Figure). In univariable competing risks analysis, ascites was strongly associated with waitlist mortality (HR 1.52; p=0.004). After adjustment for frailty, this association weakened (HR 1.33; p=0.06); there was no significant interaction between ascites and frailty (interaction p=0.80). After adjustment for additional potential confounders, including MELDNa, age, albumin, and Hispanic race, ascites was no longer associated with waitlist mortality (Table 2).
Figure.
Rates of waitlist mortality at 12 months, defined as death or delisting for being too sick for liver transplantation, by ascites or hepatic encephalopathy and frailty.
Table 2.
Univariable and step-wise additive multivariable models to assess associations between co-variables with waitlist mortality using competing risks models ((with deceased donor liver transplant as the competing risk) for the primary predictor of ascites.
| Subhazard Ratios (95% CI) p-value | ||||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable models | |||||
| Ascites | 1.52 (1.14–2.05) p=0.004 |
1.33 (0.99–1.80) p=0.06 |
1.22 (0.90–1.65) p=0.21 |
1.23 (0.90–1.67) p=0.19 |
1.17 (0.86–1.60) p=0.31 |
1.22 (0.89–1.66) p=0.22 |
| Frail | 2.38 (1.77–3.20) p<0.001 |
2.18 (1.61–2.96) p<0.001 |
1.94 (1.42–2.66) p<0.001 |
1.83 (1.33–2.52) p<0.001 |
1.86 (1.35–2.55) p<0.001 |
1.89 (1.38–2.59) p<0.001 |
| MELDNa, per point | 1.06 (1.04–1.09) p<0.001 |
1.05 (1.02–1.07) p=0.001 |
1.05 (1.02–2.52) p<0.001 |
1.04 (1.01–1.08) p=0.006 |
1.04 (1.01–1.07) p=0.02 |
|
| Age | 1.03 (1.01–1.05) p=0.003 |
1.03 (1.01–1.05) p=0.002 |
1.03 (1.01–1.05) p=0.001 |
1.03 (1.01–1.05) p=0.001 |
||
| Albumin, per g/dL | 0.64 (0.50–0.81) p<0.001 |
0.71 (0.54–0.93) p=0.01 |
0.72 (0.55–0.96) p=0.02 |
|||
| Hispanic race | 1.41 (1.04–1.92) p=0.03 |
1.46 (1.06–2.01) p=0.02 |
||||
Variables evaluated for inclusion in the multivariable models were (because of p-value<0.2 in univariable analysis): age, female sex, Hispanic race, height, weight, dialysis, albumin. Only variables associated with a p-value <0.05 were retained in the final models
Associations between hepatic encephalopathy, frailty, and mortality
Hepatic encephalopathy was present in 41% of patients. Rates of hepatic encephalopathy at 12 months were higher in frail compared to non-frail patients (57 vs. 36%). In univariable logistic regression, the odds of being frail were over 2-fold higher among those with hepatic encephalopathy than those without (OR 2.38; 95% CI, 1.78–3.17; p<0.001). In final multivariable analysis, after adjustment for Hispanic race, HCV, diabetes, and MELDNa, hepatic encephalopathy remained significantly associated with frailty (OR 2.45; 95% CI 1.80–3.33; p<0.001).
Waitlist mortality occurred within 12 months in 23% of those with hepatic encephalopathy and 13% of those without (p<0.001). When stratified by frailty status, rates of waitlist mortality were significantly higher among those who were frail regardless of hepatic encephalopathy status (Figure). In univariable competing risks analysis, hepatic encephalopathy was strongly associated with waitlist mortality (HR 1.84; p<0.001). After adjustment for frailty, this association remained significant (HR 1.62; p<0.001), but there was no significant interaction between hepatic encephalopathy and frailty (interaction p=0.80). Even after adjustment for additional potential confounders, including MELDNa, age, albumin, and Hispanic race, both hepatic encephalopathy and frailty remained significantly associated with waitlist mortality (Table 3).
Table 3.
Univariable and step-wise additive multivariable models to assess associations between co-variables with mortality using competing risks models ((with deceased donor liver transplant as the competing risk) for the prims predictor of hepatic encephalopathy.
| Subhazard Ratios (95% CI) p-value | |||||
| Univariable | Multivariable models | ||||
| Hepatic encephalopathy | 1.84 (1.38–2.45) p<0.001 |
1.62 (1.21–2.19) p=0.001 |
1.60 (1.18–2.16) p=0.002 |
1.40 (1.03–1.91) p=0.03 |
1.31 (0.96–1.79) p=0.08 |
| Frail | 2.38 (1.77–3.20) p<0.001 |
2.11 (1.55–2.85) p<0.001 |
1.85 (1.35–2.55) p<0.001 |
1.82 (1.32–2.51) p<0.001 |
1.83 (1.33–2.52) p<0.001 |
| MELDNa, per point | 1.06 (1.04–1.09) p<0.001 |
1.04 (1.01–1.07) p=0.003 |
1.05 (1.02–1.08) p=0.001 |
1.04 (1.01–1.07) p=0.01 |
|
| Age | 1.03 (1.01–1.05) p=0.003 |
1.03 (1.01–1.05) p=0.01 |
1.03 (1.01–1.05) p=0.005 |
||
| Albumin, per g/dL | 0.64 (0.50–0.81) p<0.001 |
0.72 (0.54–0.94) p=0.02 |
|||
Variables evaluated for inclusion in the multivariable models were (because of p-value<0.2 in univariable analysis): age, female sex, Hispanic race, height, weight, dialysis, albumin. Only variables associated with a p-value <0.05 were retained in the fit models
Associations between ascites, hepatic encephalopathy, frailty, and mortality
A total of 396 (38%) had neither ascites nor hepatic encephalopathy at the time of frailty assessment; 163 (16%) had both ascites and hepatic encephalopathy. When stratified by frailty status, rates of waitlist mortality a 12 months were significantly higher among those who were frail, regardless of whether they had neither ascites nor hepatic encephalopathy (p=0.01) or both ascites and hepatic encephalopathy (p=0.02) [Figure].
Table 4 displays the associations with mortality when ascites, hepatic encephalopathy, and frailty were considered together in a single model. While ascites (HR 1.48; p=0.009) and hepatic encephalopathy (HR 1.76; p<0.001) were both significantly associated with waitlist mortality in bivariate analysis, ascites lost significance (but there remained a trend, HR 1.32; p=0.08) when adjusted for frailty. The association between ascites and waitlist mortality continued to weaken with increasing adjustment for confounders, including MELDNa, age, albumin, and Hispanic race (Table 4). Hepatic encephalopathy, on the other hand, remained significantly associated with waitlist mortality after adjustment for frailty and MELDNa score, but was no longer significant in the full adjusted multivariable model. Frailty remained significantly associated with waitlist mortality independent of all other co-variables. A sensitivity analysis evaluating this effect among the centers demonstrated a similar qualitative association between frailty, ascites, and hepatic encephalopathy in the dominant center versus all other centers (data not shown).
Table 4.
Univariable and step-wise additive multivariable models to assess associations between co-variables with waitlist mortality using competing risks models ((with deceased donor liver transplant as the competing risk) for the primary predictors of ascites and hepatic encephalopathy together.
| Subhazard Ratios (95% CI) p-value | ||||||
| Multivariable models | ||||||
| Ascites | 1.48 (1.10–1.99) p=0.009 |
1.32 (0.97–1.78) p=0.08 |
1.21 (0.89–1.65) p=0.23 |
1.23 (0.90–1.67) p=0.20 |
1.18 (0.87–1.62) p=0.29 |
1.21 (0.88–1.65) p=0.24 |
| Hepatic encephalopathy | 1.76 (1.31–2.35) p<0.001 |
1.58 (1.17–2.13) p=0.003 |
1.56 (1.15–2.11) p=0.004 |
1.37 (1.00–1.87) p=0.05 |
1.30 (0.95–1.77) p=0.10 |
1.22 (0.89–1.67) p=0.21 |
| Frail | 1.94 (1.42–2.66) p<0.001 |
1.76 (1.27–2.44) p=0.001 |
1.73 (1.24–2.40) p=0.001 |
1.75 (1.27–2.43) p=0.001 |
1.82 (1.31–2.52) p<0.001 |
|
| MELDNa, per point | 1.04 (1.01–1.07) p=0.007 |
1.04 (1.01–1.07) p=0.003 |
1.04 (1.01–1.07) p=0.02 |
1.04 (1.00–1.07) p=0.03 |
||
| Age | 1.02 (1.01–1.04) p=0.01 |
1.03 (1.01–1.05) p=0.007 |
1.03 (1.01–1.05) p=0.004 |
|||
| Albumin, per g/dL | 0.73 (0.55–0.96) p=0.03 |
0.74 (0.56–0.97) p=0.03 |
||||
| Hispanic race | 1.41 (1.02–1.94) p=0.04 |
|||||
Variables evaluated for inclusion in the multivariable models were (because of p-value<0.2 in univariable analysis): age, female sex, Hispanic race, height, weight, dialysis, albumin. Only variables associated with a p-value <0.05 were retained in the final mode
DISCUSSION
Cirrhosis most commonly leads to premature death through complications of portal hypertension. However, manifestations of hepatic synthetic dysfunction and portal hypertension present in an often unpredictable pattern: while one patient may develop ascites without ever experiencing hepatic encephalopathy, another may develop ascites, hepatic encephalopathy, and bleeding esophageal varices within a period of months. Similarly, physical frailty, an overt extrahepatic manifestation of cirrhosis, presents variably in patients with cirrhosis, with some patients experiencing muscle wasting and functional impairment along with their portal hypertensive complications, and others experiencing advanced frailty out of proportion to their burden of portal hypertensive complications. The importance of physical frailty in the context of portal hypertension in patients with cirrhosis has not been previously explored.
In this prospective study including over 1,000 patients with cirrhosis awaiting liver transplantation from 9 liver transplant centers in the U.S., we investigated the relationships between ascites, hepatic encephalopathy, and physical frailty – and how the presence of each impacts waitlist mortality. Of note, we specifically evaluated two portal hypertensive complications, and not gastroesophageal varices, given the scientifically plausible mechanisms by which ascites and hepatic encephalopathy might lead to physical frailty (e.g., through early satiety, protein wasting, anorexia, decreased physical activity). As expected, we observed strong associations between ascites / hepatic encephalopathy and physical frailty. In fact, patients with ascites or hepatic encephalopathy were at least 2-fold more likely to be frail than those without these manifestations. Furthermore, patients who met criteria for frail had a significantly higher median MELDNa – largely driven by a greater degree of renal dysfunction – which is likely to be related to their higher degree of ascites. However, while both ascites and hepatic encephalopathy were associated with waitlist mortality in univariable analysis, adjustment for physical frailty attenuated these associations and, in patients with ascites, adjustment for physical frailty entirely accounted for the mortality risk. Lastly, when considering all three factors – ascites, hepatic encephalopathy, and physical frailty – together in adjusted models, only physical frailty remained significantly associated with waitlist mortality.
Although patients with ascites or HE who were frail had significantly higher rates of waitlist mortality than those who were not frail – we did not observe a statistically significant interaction between ascites and frailty or HE and frailty. While this might suggest that portal hypertensive complications lead to mortality completely independently of frailty, we offer an alternate explanation. In the field of geriatrics, physical frailty has been described as “an aggregate expression of risk resulting from age- or disease-associated physiologic accumulation of subthreshold decrements affecting multiple physiologic systems”.16 Refining this statement for the patient whose “disease-associated physiologic decrement” is dominated by cirrhosis, frailty likely represents the end manifestation of chronic hepatic synthetic dysfunction with muscle wasting and under-nutrition, and may be worsened by the presence of overt portal hypertensive complications. It is therefore possible that the impact of ascites/HE on waitlist mortality is captured, at least in part, by the impact that frailty has on mortality in this population.
We acknowledge the following limitations to our study. First, we categorized patients as having ascites or hepatic encephalopathy if either was present on the day of frailty assessment, not based on past medical history or use of medications. Nor did we account for subsequent development of ascites or hepatic encephalopathy in our models. This decision was based on our hypothesis that ascites / hepatic encephalopathy lead to frailty largely through under-nutrition, decreased activity, and muscle wasting that may not be plausible if the ascites and hepatic encephalopathy were controlled. Furthermore, sensitivity analyses using various definitions of ascites and hepatic encephalopathy (e.g., documented history, medication use) did not change the significant association between frailty and waitlist mortality. Second, ascites and hepatic encephalopathy were treated as dichotomous variables in our analyses rather than as categorical variables. We did this to improve the accuracy of ascertainment of these primary predictors; identifying various degrees of ascites and hepatic encephalopathy may be limited by relative subjectivity.17 A sensitivity analysis evaluating ascites and hepatic encephalopathy as categorical variables (none, mild/moderate, severe/refractory) did not change the associations between frailty and waitlist mortality (data not shown). Third, enrollment of patients into the FrAILT study was not consecutive at all the sites; however, recruitment at the non-consecutive sites was based on study coordinator availability, rather than any specific patient characteristic, minimizing potential selection bias. Lastly, the multi-center nature of this study exposes our analyses to heterogeneity related to center-specific effects that might not have been accounted for in our analyses. However, the multi-center nature of our study enhances the generalizability of our results, as the centers in this study represent multiple different regions with a wide range of transplant MELDNa scores and populations of transplant patients.
Despite these limitations, this work has important implications for the evaluation and management of patients with cirrhosis, particularly those awaiting liver transplantation. Physical frailty is prevalent in patients with cirrhosis, is observed more frequently in patients with ascites or hepatic encephalopathy, and is independently associated with waitlist mortality. Through this study, we offer the hepatology community the Liver Frailty Index, which has strong advantages over other metrics in that it was developed specifically for patients with cirrhosis and is purely performance-based (making it more objective than metrics using survey-based components). Future studies will focus on longitudinal changes in physical frailty in this population and the relationship between the progression of portal hypertensive complications and the trajectory of frailty. If we incorporate frailty measurement into routine transplant practice, we can use it to identify those who are most vulnerable to death and therefore in need of an accelerated path to transplant to reduce waitlist mortality – whether that be via living donor liver transplantation, accepting increased-risk donor livers, or seeking transplantation at centers with lower transplant MELDNa scores. Such options are frequently difficult to pursue for patients and their caregivers, but information about their added risk of death due to frailty might provide motivation. Furthermore, our work lays the foundation for the development of activity- and nutrition-based interventions targeting specific components of physical frailty in attempt to mitigate risk of adverse outcomes. Our multi-center study, demonstrating the importance of physical frailty above and beyond traditional portal hypertensive complications, provides strong justification for widespread implementation of physical frailty measurement in clinical practice.
Need to Know.
Background:
We evaluated associations between frailty, ascites, or hepatic encephalopathy (HE) and mortality in patients with cirrhosis using the liver frailty index (LFI) scores, measured using 3 simple tests performed at 9 liver transplant centers.
Findings:
In patients with cirrhosis, frailty is prevalent and observed more frequently among patients with ascites or HE but is independently associated with waitlist mortality.
Implications for Patient Care:
LFI scores can be used to calculate risk of death related to frailty – in excess of liver disease severity – in patients with cirrhosis.
Acknowledgements:
Financial support: This study was funded by NIH K23AG048337 (Lai), NIH P30AG044281 (Lai), NIH F32AG053025 (Haugen), NIH P30DK026743 (Lai, Dodge), NIH K24DK101828 (Segev). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
List of abbreviations:
- BMI
body mass index
- HCV
hepatitis C virus
- HR
hazard ratio
- IQR
interquartile range
- MELDNa
Model for End-Stage Liver Disease
- OR
odds ratio
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by Gastroenterology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kamath P A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N Engl J Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Child CG, Turcotte JG. Surgery and portal hypertension In: Child CG, ed. The Liver and Portal Hypertension. Saunders; 1964:50–64. [Google Scholar]
- 4.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty Predicts Waitlist Mortality in Liver Transplant Candidates. American journal of transplantation. 2014;14(8):1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplantation. 2010;16(12):1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 6.Tandon P, Reddy KR, O’Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65(1):217–224. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 7.Tandon P, Tangri N, Thomas L, et al. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients With Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am J Gastroenterol. 2016;111(12):1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 8.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584–590. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol. 2016;111(12):1768–1775. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 10.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–574. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. [DOI] [PubMed] [Google Scholar]
- 12.Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Am J Gastroenterol. 2017;10:1–8. doi: 10.1038/ajg.2017.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):85–94. [DOI] [PubMed] [Google Scholar]
- 14.Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol. 1998;28(4):646–653. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 16.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 17.Reuter B, Walter K, Bissonnette J, et al. Assessment of the spectrum of hepatic encephalopathy: A multicenter study. Liver Transplantation. 2018;24(5):587–594. doi: 10.1002/lt.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]