Abstract
Enhancement of vasodilation of uterine arteries during pregnancy occurs through increased connexin (Cx)43 gap junction (GJ) communication supporting more frequent and sustained Ca2+ ‘bursts’. Such adaptation is lacking in subjects with preeclampsia (PE). Here we show TNF-alpha, commonly increased in PE subjects, inhibits Cx43 function and Ca2+ bursts in pregnancy-derived ovine uterine artery endothelial cells (P-UAEC) via Src and MEK/ERK phosphorylation of Cx43, and this can be reversed by PP2 or U0126. Of relevance to humans: (1) the nutraceutical Src antagonist t10,c12 CLA also recovers Ca2+ bursting in P-UAEC. (2) TNF-alpha can reduce and PP2 rescue Ca2+ bursting and NO output in human umbilical vein endothelium (HUV Endo) preparations. (3) Treatment of HUV Endo from PE subjects with PP2 alone can rescue bursting and NO output. We conclude TNF-alpha acts via Src more than MEK/ERK to inhibit GJ Cx43 function in PE subjects, and CLA may offer a potential therapy.
Keywords: TNF-alpha, gap junction, pregnancy, Src, endothelial dysfunction, Ca2+
1. Introduction
In healthy pregnancy, the uterine vasculature must adapt considerably to decrease vascular resistance relative to the systemic circulation and redirect blood flow to the growing fetus (Magness and Ford, 2014). This pregnancy adaptation depends in part upon enhancement of sustained endothelium-mediated vasodilation (Sladek et al., 1997) mediated by vasodilators such as prostacyclin and nitric oxide (NO). Both biosynthetic pathways are in turn dependent on increased intracellular Ca2+ signaling (Bird et al., 2003; Boeldt et al., 2014; Boeldt and Bird, 2017). Pregnancy-derived ovine uterine artery endothelial cells (P-UAEC) achieve adaptation at the level of multiple cell signaling responses (Bird et al., 2003). This includes enhancement of the sustained capacitative entry phase of [Ca2+]i elevation once the ER pool of Ca2+ is empty in response to a number of PLC-Beta coupled agonists, including ATP. The sustained phase response actually takes the form of sustained Ca2+ ‘bursting’ with each ‘burst’ lasting minutes in duration and mediated by periodic TRPC3/IP3R2 interaction (more fully reviewed in Boeldt et al., 2014; Boeldt and Bird, 2017). When such pregnancy adaptation of endothelial Ca2+ signaling function fails to occur in conditions such as preeclampsia (PE), vasodilation is lost and hypertension results (Bird et al., 2003; Boeldt et al., 2014; Boeldt and Bird, 2017; Sladek et al., 1997). More recent studies have shown such Ca2+ signaling adaptations in P-UAEC are dependent on establishing a high level of cell-cell contact between endothelial cells (Boeldt et al., 2014; Yi et al., 2010) through enhanced connexin 43 (Cx43) gap junction (GJ) function, even when Cx43 expression itself is unaltered (Gifford et al., 2006; Yi et al., 2010). The importance of this is shown by the finding that inhibitors of Cx43 not only block the sustained Ca2+ bursting adaptation observed in P-UAEC, but also reduce NO production, an important player in maintaining normotensive pregnancy (Boeldt et al., 2014; Boeldt et al., 2015; Yi et al., 2010).
In many cells, Cx43 function is subject to rapid inhibition by growth factors and cytokines acting through receptor-linked signaling kinases (Baum et al., 2012; Boeldt et al., 2014; Huang et al., 2003; Nimlamool et. al., 2015.). Several endocrine factors that affect kinase signaling are also altered in PE subjects, including vascular endothelial growth factor (VEGF)165 and tumor necrosis factor-alpha (TNF-alpha) (Bird et al., 2013), and their production can be increased both locally (particularly in the uteroplacental space) and systemically. Although it is commonly reported that the bioavailability of VEGF165 in the circulation of PE subjects is reduced because of increases in circulating sFlt-1, it can still be elevated locally in the decidua and/or placenta (Fan et al., 2014; Sánchez-Aranguren et al., 2014). The picture for TNF-alpha is clearer. TNF-alpha is commonly observed to be elevated in the placenta and in the circulation of PE subjects in human pregnancies (Afshari et al., 2005; Bayram et al., 2012; Benyo et al., 2001; Cackovic, 2008; Conrad et al., 1997; 1998; Kocyigit et al., 2004; Kronborg et al., 2011; Kupferminc et al., 1994; Tosun et al., 2010; Vince et al., 1995). We have previously shown 30 min VEGF165 pretreatment inhibits the subsequent ATP-induced Ca2+ bursts response in both UAEC (Boeldt et al., 2015) and intact vessels (Yi et al., 2011). Indeed, in intact vessel segments VEGF165 itself can mount a weak and transient stimulation of Ca2+ and NO production, yet the overall effect is to close Cx43 GJs and impair the much greater subsequent ATP-induced sustained Ca2+ bursting events and correspondingly sustained NO production (Yi et al., 2011). Studies of Cx43 inhibition in models of wounding (Solan and Lampe, 2014) suggest such inhibition is mediated via activation of Src kinase and MEK/ERK pathways, causing specific phosphorylation of Cx43 at known inhibitory positions s279/282 and y265. Recent studies in P-UAEC have shown VEGF165 inhibition is achieved in the same way, and blockade of Src or MEK/ERK signaling pathways achieves both reversal of these phosphorylations and corresponding rescue of the inhibitory effects of VEGF165 on Ca2+ bursts (Boeldt et al., 2015).
In contrast to these detailed studies of the action of VEGF165 on UAEC, there is little corresponding information on the effects of TNF-alpha. While TNF-alpha is a pro-inflammatory cytokine elevated during the inflammatory phase of wound healing (Singer and Clark, 1999), it is relevant that TNF-alpha infusion into animals causes significant increases in blood pressure (Alexander et al., 2002; LaMarca et al., 2005; Sunderland et al., 2011). While others have linked TNF-alpha to GJ closure in many cell types (Baum et al., 2012; Hao et al., 2005; Huang et al., 2003; Tacheau et al., 2008), there have been few studies linking TNF-alpha to the GJ closure and endothelial dysfunction in PE (Kim, 2017). Although a possible link between TNF-alpha and PE has long been suspected, much of the focus to date has been on the ability of TNF-alpha to increase reactive oxygen species (ROS) (Matsubara et al., 2015; Sanchez-Aranguren et al., 2014). Elevated ROS levels are proposed to cause PE-associated dysfunction by reacting directly with NO, thus removing bioactive NO and suppressing NO-mediated vasodilation, as well as by promoting oxidative cell damage through enhanced nitrosylation (Matsubara, et. al., 2015; Chen, 2008; Hlaing and Clement, 2014). However, these studies are typically undertaken at doses far greater than 10 ng/mL TNF-alpha (Watson and Hughes, 2012). Such extreme levels of TNF-alpha are only possible in extreme pathophysiologic conditions (such as sepsis) and are far higher than the physiologic range observed in the circulation in normal pregnancy or even PE (Ko et al., 2000; Yang et al., 2007; Kastl et al., 2014, Siwetz et al., 2016). For that reason, we focused our attention on lower, more physiologically relevant doses of TNF-alpha and considered whether inhibition of function by TNF-alpha at 10ng/mL down through 0.1 ng/ml could instead be through Cx43 GJ phosphorylation via Src and MEK/ERK activation, as we reported for VEGF165 (Boeldt et al., 2015), rather than through the ROS-based mechanisms proposed by others. To that end, we report the outcomes of our studies using P-UAEC to evaluate: (a) whether exposure to TNF-alpha causes an equal or greater acute inhibition of subsequent ATP-stimulated Ca2+ bursts in P-UAEC compared to VEGF165 treatment; (b) the effect of TNF-alpha on activation of Src and ERK pathways to phosphorylate inhibitory residues on Cx43 compared with the actions of VEGF165; and (c) whether TNF-alpha-induced endothelial dysfunction can be recovered using Src and MEK/ERK pathway inhibitors as was shown effective for VEGF165. We conclude this study by introducing a potential therapeutic agent, t10,c12 conjugated linoleic acid (CLA), a Src inhibitor (Shahzad et al., 2018) that is generally recognized as safe (GRAS) by the US Food and Drug Administration, in hopes that it can provide a nutraceutical alternative to pharmacological solutions to relieve endothelial dysfunctions found in hypertensive disorders in pregnancy, such as PE. We also demonstrate ex vivo that even the brief (1 h) application of a Src inhibitor to segments of umbilical veins of PE subjects can promote the rescue of Ca2+ bursting and NO output otherwise lacking in such vessels from PE subjects (Krupp et al., 2013).
2. Materials and Methods
2.1. Materials
All cell culture reagents, including minimum essential medium (MEM) and fetal bovine serum (FBS), were purchased from Life Technologies (Carlsbad, CA). For [Ca2+]i imaging studies, 35-mm dishes with glass bottom coverslips were obtained from MatTek Corp (Ashland, MA). CaCl2 was from EMD Millipore (Billerica, MA), and Fura-2 AM, Pluronic F127 and the reactive oxygen species (ROS)-sensitive dye, 2’,7’-dichlorofluorescin diacetate (H2DCFDA) were purchased from Life Technologies. DAF2-DA, ATP (disodium salt), TNF-alpha, and all other chemicals, unless noted otherwise, were from Sigma (St. Louis, MO). VEGF165 was purchased from R & D Systems (Minneapolis, MN). PP2 (a known Src inhibitor) was from Enzo (Farmingdale, NY), and U0126 (a known MEK and so ERK inhibitor) was purchased from Promega Corp. (Madison, WI). CLA isomers were obtained from Matreya, LLC (Pleasant Gap, PA).
2.2. Isolation of UAEC
Uterine arteries were procured from mixed Western breed non-pregnant (NP) sheep and pregnant ewes at 120–130 days of gestation during non-survival surgery, and UAEC were prepared and stored in liquid nitrogen (Bird et al., 2000; Sullivan et al., 2006). Procedures for animal handling and protocols for experimental procedures were approved by the University of Wisconsin-Madison Research Animal Care Committees of the School of Medicine and Public Health and the College of Agriculture and Life Sciences and followed the recommended American Veterinary Medicine Association guidelines for humane treatment and euthanasia of laboratory farm animals.
2.3. Fura-2 [Ca2+]i imaging studies
Pooled P-UAEC at passage 4 were grown on glass dishes in MEM containing 20% FBS, 1% penicillin-streptomycin, and 4 ug/mL gentamicin to near 100% confluence. Imaging of cells was recorded with a digital camera (PixelFly, Cooke Corp., Romulus, MI) connected to an inverted microscope (Nikon Diaphot 150, Melville, NY), and [Ca2+]i determined by comparison to a standard curve established for the same illumination settings using buffers of known free [Ca2+] concentration, as described in detail previously (Boeldt et al., 2015; Yi et al., 2010, 2011). Briefly, cells were loaded with 10 uM Fura-2 AM for 60 min at 37°C in Krebs buffer (25 mM HEPES, pH 7.4, 125 mM NaCl, 5 mM KCl, 1mM MgSO4, 1 mM KH2PO4, 6 mM glucose, 2 mM CaCl2) in the presence of 0.05% Pluronic F127. After a 5 min recording to ensure a stable baseline, ATP (100 uM), was added and data were recorded for 30 min. In P-UAEC, 100 uM ATP is a maximally effective dose that acts on P2Y2 receptors (Gifford et al., 2006) to promote multiple distinct bursts (Yi et al., 2010). After the initial ATP treatment, the dish was washed with Krebs buffer while maintaining the focus on individually chosen cells. When applicable, a vehicle or antagonist pretreatment was added to the experimental buffer, followed by vehicle or agonist (TNF-alpha or VEGF165) treatment. Inhibitor pretreatments before agonist treatment were U0126 (10 uM), c9,t11 CLA (50 uM), t10,c12 CLA (50 uM) for 30 min or PP2 (10 uM) for 20 min. Treatment with the VEGF165 positive control (10 ng/mL) was for 30 min and TNF-alpha was at the concentration and time specified. After the allotted treatment time and a 5 min baseline recording, cells were re-stimulated with 100 uM ATP and recording resumed for another 30 min. ATP-stimulated Ca2+ bursts (each bursts having a clear maxima and two minima and achieving a peak at least twice basal level – see Yi et al., 2010) were counted for both the initial and second ATP stimulation after agonist treatment for each selected cell, and burst numbers were compared in the final data set as a percentage of the initial ATP stimulation. Individual cells that produced 3 or more Ca2+ bursts during the initial ATP stimulation (the majority) were included in the final data set for sustained Ca2+ burst experiments, as this is representative of pregnancy-adapted cells experiencing optimal cell contact (Bird et al., 2000; Yi et al., 2010).
2.4. ROS production in P-UAEC in response to TNF-alpha
After growing P-UAEC to near confluence on 35-mm glass bottom dishes, media with serum was removed, and cells were loaded with the ROS-sensitive dye H2DCFDA (5 μM in Krebs buffer) for 30 min and then were washed three times with Krebs buffer. H2DCFDA is deesterified intracellularly into a non-fluorescent polar derivative (H2DCF), and then is oxidized to its fluorescent form 2′,7′-dichlorofluorescein (DCF) by ROS (Sauer et al., 2000). Cells were recorded for 5 min to establish baseline before addition of various doses of TNF-alpha with recording continuing for 1 h. Fluorescence of DCF was monitored at 485 nm excitation and 535 emission wavelengths. Images were recorded with a digital camera (PixelFly, Cooke Corp., Romulus, MI) connected to an inverted microscope (Nikon Diaphot 150, Melville, NY). The fluorescent intensities were quantified using InCyt Im1 software (Intracellular Imaging, Inc., Cincinnati, OH) and normalized to the starting fluorescence (F/F0).
2.5. Western blot analysis
Treatment of P-UAEC on 60-mm dishes and subsequent Western blotting of extracted proteins were performed as described previously (Boeldt et al., 2015; Grummer et al., 2009; Sullivan et al., 2006). For this study, phosphorylation of Cx43 was detected on two separate membranes using the following antibodies: s279/282 Cx43 (#sc-12900-R; Santa Cruz Biotechnology, Inc., Dallas, TX) at 1:1300, and y265 Cx43 (#sc-17220-R; Santa Cruz Biotechnology) at 1:750. Phosphorylation of ERK-1/2 was detected using t202/y204 ERK-1/2 (#4377, Cell Signaling Technology, Danvers, MA) at 1:2500, and phosphorylation of Src was measured using y416 Src (#2101, Cell Signaling Technology) at 1:1300. To ensure consistent protein loading, antibodies were normalized to levels of Hsp90 (#4874, Cell Signaling Technology) at 1:2500. For all antibodies, goat anti-rabbit HRP-conjugated secondary antibody (#7074, Cell Signaling Technology) was used at 1:3000.
2.6. Human Subjects and Imaging of Ca2+ and NO in HUV Endo Preparations.
The Institutional Review Boards of the University of Wisconsin Hospital and Clinics and Meriter Hospital (both located in Madison, WI) approved this study. All subjects signed a formal consent. This study was undertaken using the same criterion and method as done previously (Krupp et al., 2013). To avoid as many confounding variables as possible in the diagnosis of PE, inclusion criteria included maternal age 18 years or older, singleton pregnancy, and clinical diagnosis of PE as before (Krupp et al., 2013). Exclusion criteria included multiple gestation, gestational age less than 29 weeks, moderate/thick meconium, maternal comorbidities including diabetes (pregestational or gestational), chronic hypertension or pre-pregnancy antihypertensive use, and chorioamnionitis. Human umbilical vein vessel segments were isolated from either healthy (HUV Endo) or PE pregnancy (PE-HUV Endo) as previously reported (Krupp et al., 2013; Yi et al., 2011). Briefly, vessel endothelium was loaded with both DAF2-DA (10 uM) and Fura2-AM (10 uM) for 90 min. The loading dye medium was then replaced, and dye hydrolysis proceeded for 30 min. Individual endothelial cells (>30 cells per field) were visualized using dual excitation (switching at 340 and 380 nm) for Fura-2 and 485 nm excitation DAF-2. Emission was uniformly measured at 525 nm for all excitation wavelengths. Vessel segments from healthy pregnancies were observed under basal conditions or after addition of ATP (100 uM) with or without pretreatment of TNF-alpha (10 ng/mL) for 1 h. In addition, PE-HUV Endo were observed under basal conditions or after pretreatment with PP2 (10 uM, 1 h). Intracellular NO production was expressed as the net increment of DAF-2 fluorescence relative to its basal value, and [Ca2+]i was calculated ratiometrically against a prerecorded standard curve (Yi et al., 2010; 2011).
2.7. Statistical Analysis
Data are presented as means ± S.E.M. and analyzed by Student’s t-test or analysis of variance, as appropriate. For Ca2+ burst number experiments, paired t-tests were used to compare data for each cell with its untreated self within a single treatment group, and a Mann-Whitney rank-sum test was used when comparing against vehicle control. A value of P<0.05 was considered statistically significant.
3. Results
3.1. TNF-alpha inhibits pregnancy-adapted Ca2+ responses in P-UAEC to a level virtually identical to NP-UAEC
We have previously shown periodic Ca2+ bursting in response to ATP in P-UAEC can be impaired to the level of dysfunction of NP-UAEC by pretreatment with VEGF165 (Boeldt et al., 2015). In Fig. 1 we show the percentage of cells giving each Ca2+ bursts (1 to 5+) in response to an initial ATP stimulation in NP- and P-UAEC, and how a subsequent 10 ng/mL TNF-alpha pretreatment for 60 min changes the response to a final second ATP stimulation. As previously, to allow burst function data to be combined across cells, we expressed ATP-stimulated Ca2+ bursts after TNF-alpha treatment as a burst percentage of the same cell bursts at the initial ATP stimulation (i.e. initial ATP stimulation is 100%) (Boeldt et al., 2015). While pretreating NPUAEC with TNF-alpha for 1 h had no further inhibitory effect on the subsequent sustained Ca2+ bursting compared to the initial ATP response (Fig. 1A), pretreating the pregnancy-adapted PUAEC with TNF-alpha caused a significant reduction in the number of cells responding with Ca2+ bursts relative to the initial ATP response (Fig. 1B). In Fig 1C we show an example of such a change in burst response for a single P-UAEC challenged with ATP before (left) or after (right) treatment with 10 ng/mL TNF-alpha. Overall, Ca2+ burst numbers in TNF-alpha-treated PUAEC were reduced by TNF-alpha to a level below that of untreated NP-UAEC for more sustained bursts (bursts 4 and 5, P<0.05, comparison not shown). Thus, elevated TNF-alpha acts specifically at the level of pregnancy-adapted function that underlies pregnancy enhancement of Ca2+ signaling in P-UAEC and inhibits burst function to levels down to or below non-adapted bursting function of NP-UAEC, as first described by Yi et al. (2010).
Figure 1: Percent of UAEC showing successive bursts in response to ATP before or after TNF-alpha treatment.
Initial ATP-stimulated Ca2+ bursts were recorded for individual cells in A: NP-UAEC or B: P-UAEC treated with 100 uM ATP for 30 min (white circles). After pretreatment with 10 ng/mL TNF-alpha for 1 h, cells were again stimulated with ATP and number of bursts were again recorded on the same cells (black circles). Results are shown as mean ± S.E.M. (n=7 dishes where each dish had up to 99 individual cells recorded). *P<0.05 and **P<0.01 compared with initial ATP control. C: Graphs are an example of imaging data from a single cell in Ca2+ burst experiments. On the left is the initial ATP stimulation and on the right is the second ATP stimulation after 1 h of 10 ng/mL TNF-alpha pretreatment.
3.2. Exposure to TNF-alpha decreases sustained Ca2+ bursting at doses below that required to produce ROS in P-UAEC
To determine if the inhibitory action of TNF-alpha may relate to an excess of ROS production, we first examined the TNF-alpha dose dependency of ROS production in P-UAEC (Fig. 2A). Excess ROS production was not significantly increased below 50 ng/mL TNF-alpha, an amount substantially greater than any physiological level of TNF-alpha (0.0001–1.0 ng/mL) found in pregnant control or PE women (Afshari et al., 2005; Bayram et al., 2012; Benyo et al., 2001; Cackovic, 2008; Conrad et al., 1997; 1998; Kocyigit et al., 2004; Kronborg et al., 2011; Kupferminc et al., 1994; Tosun et al., 2010; Vince et al., 1995;;), let alone in the more extreme case of sepsis. In contrast, a detailed dose response of the effects of TNF-alpha at 1 h and 3 h pretreatment on subsequent ATP-stimulated Ca2+ bursting (Fig. 2B) revealed TNF-alpha-induced inhibition of burst function was observed at much lower doses than ROS formation (Fig. 2A). Our findings revealed the inhibitory effect of TNF-alpha was not only dose-dependent but also time-dependent. At 1 h, only 10 ng/mL TNF-alpha drastically inhibited sustained Ca2+ bursts (63.5% of initial ATP response). However, at 3 h, the low doses of TNF-alpha significantly reduced sustained Ca2+ bursting (80.1, 78.5 and 76.7% for 0.1, 1 and 10 ng/mL, respectively) compared to control cells on repeat stimulation (90% of initial ATP response).
Figure 2: Exposure of P-UAEC to high doses of TNF-alpha releases ROS but low doses of TNF-alpha also inhibit sustained Ca2+ bursts at 1 and 3 h.
A: P-UAEC were loaded with profluorescent H2DCFDA and then exposed to various doses of TNF-alpha for 1 h. Oxidation to fluorescent DCF by intracellular ROS is expressed as final vs initial fluorescence (n=4 dishes per dose). B: P-UAEC were stimulated with 100 uM ATP for 30 min while Ca2+ burst numbers were recorded, then cells were washed, incubated with various TNF-alpha doses (0.1, 1, or 10 ng/mL) for 1 or 3 h, and then the same cells were re-stimulated with ATP and bursts were counted. Data is presented as a percentage of the bursts in response to ATP after TNF-alpha treatment relative to the ATP stimulation before TNF-alpha treatment. Data are shown as mean burst % ± S.E.M. and only include cells that give 3 or more bursts upon initial ATP stimulation. Statistics were performed on raw data (n=4–9 dishes for each treatment where each dish had up to 99 individual cells). *P<0.01 and **P<0.001 compared with unstimulated control.
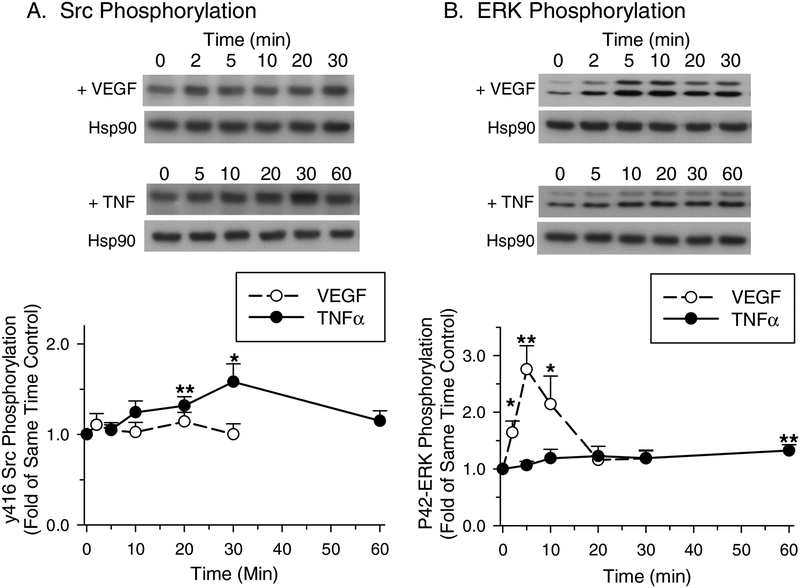
3.3. Time Course of Src and ERK phosphorylation after treatment with VEGF165 or TNF-alpha
The time courses of P-ERK and P-Src in P-UAEC in response to VEGF165 or TNF-alpha stimulation are shown in Fig. 3. The time-dependent phosphorylation of Src y416 in TNF-alpha-treated cells increased progressively to a maximum at 30 min and achieved significance at 20 and 30 min (Fig. 3A). The time-dependent phosphorylation of P-ERK was minimal in response to 10 ng/mL TNF-alpha, lacking any substantial early rise and only showing a small, but significant increase by 60 min (Fig. 3B). In contrast, VEGF165 showed no detectable increase in Src y416 phosphorylation through 30 min (Fig. 3A) while ERK phosphorylation peaked at 5 min, remained elevated through 10 min, and returned to near control levels by 20 min (Fig. 3B). These findings suggest that TNF-alpha preferentially signals through Src and less through ERK in P-UAEC, and that Src activation is sustained This is in contrast to VEGF165, which acts strongly, but transiently through ERK, and less so through Src.
Figure 3: Src and ERK phosphorylation after treatment with VEGF165 or TNF-alpha.
PUAEC were treated for up to 30 min with 10 ng/mL VEGF165 or up to 60 min with 10 ng/mL TNF-alpha. Results were normalized to Hsp90 protein levels and expressed as fold change over unstimulated control. A: Time course of y416 Src phosphorylation by VEGF165 or TNF-alpha. B: Time course of P42-ERK phosphorylation by VEGF165 or TNF-alpha. Values shown are mean ± S.E.M. of 6–7 independent experiments. *P<0.05 and **P<0.001 compared with control.
3.4. Src- and ERK-mediated phosphorylation of Cx43 at Ser-279/282
Having established Src and ERK activation time courses, we next examined the ability of TNF-alpha to promote phosphorylation of an MEK/ERK-specific target site on Cx43 (s279/282) at 30 min, the time of maximal Src activity (above) and the same time used previously for VEGF165 (Boeldt et al., 2015). In Fig. 4 we show that VEGF165 and TNF-alpha both significantly increased phosphorylation at s279/282. The Src inhibitor PP2 was able to prevent the significant increase in s279/282 phosphorylation by VEGF165 and TNF-alpha (Fig. 4A) but failed to fully return phosphorylation levels to basal. While the MEK/ERK inhibitor U0126 fully reversed the significant increase in s279/282 phosphorylation by VEGF165 to a level indistinguishable from basal, U0126 did not reduce TNF-alpha-stimulated s279/282 phosphorylation to basal levels.
Figure 4: Effect of VEGF165 or TNF-alpha and the inhibition by Src and ERK inhibitors PP2 or U0126 on s279/282 Cx43 phosphorylation.
P-UAEC were pretreated for 20 min with (A) 10 uM PP2 or (B) 10 uM U0126, followed by a 30 min treatment with 10 ng/mL VEGF165 or 10 ng/mL TNF-alpha. Results were normalized to Hsp90 protein levels and expressed as fold change over unstimulated control. Values shown are mean ± S.E.M. of 7 independent experiments. *P<0.05 and **P<0.01 compared with unstimulated control; +P<0.05 compared with same agonist control.
3.5. Src- and ERK-mediated phosphorylation of Cx43 at Tyr-265
In the parallel analysis of the Src-sensitive site y265 on Cx43, VEGF165 and TNF-alpha treatments each induced a significant phosphorylation at y265 at 30 min (Fig. 5). On pretreatment with PP2, P-UAEC treated with vehicle (control), VEGF165, or TNF-alpha all showed a significant reversal in y265 phosphorylation to a level below basal (Fig. 5A), suggesting a strong dependence of y265 phosphorylation on basal, as well as agonist-stimulated, Src activity. In contrast, U0126 was unable to reduce basal, or reverse the VEGF165-or TNF-alpha-induced y265 phosphorylation (Fig. 5B).
Figure 5: Effect of VEGF165 or TNF-alpha and the inhibition by Src and ERK inhibitors PP2 or U0126 on y265 Cx43 phosphorylation.
P-UAEC were pretreated for 20 min with (A) 10 uM PP2 or (B) 10 uM U0126, followed by a 30 min treatment with 10 ng/mL VEGF165 or 10 ng/mL TNF-alpha. Results were normalized to Hsp90 protein levels and expressed as fold change over unstimulated control. Values shown are mean ± S.E.M. of 7 independent experiments; *P<0.05 and **P<0.001 compared with unstimulated control; ++P<0.01 compared with same agonist control.
3.6. Src-mediated phosphorylation of ERK
Because of the ability of PP2 to block the observed VEGF165-stimulated phosphorylation of the ERK-sensitive s279/282 site (Fig. 4), we also looked for possible crosstalk between Src and MEK/ERK in P-UAEC, as is known to occur in other cells (reviewed in Thévenin et al., 2013), by evaluating ERK phosphorylation in response to VEGF165 or TNF-alpha together with the Src family kinase inhibitor PP2 or MEK/ERK inhibitor U0126. Treatment with PP2 was able to partly but significantly reduce basal ERK phosphorylation lower, and PP2 also prevented significant ERK phosphorylation above control levels with VEGF165 or TNF-alpha (Fig. 6A). As expected, because of the known strong ability of U0126 to block even basal MEK/ERK activation, ERK phosphorylation was significantly reduced by U0126 to far below basal control levels regardless of treatment (Fig. 6B).
Fig. 6: Effect of VEGF165 or TNF-alpha and the inhibition by Src and ERK inhibitors PP2 or U0126 on ERK phosphorylation.
P-UAEC were pretreated for 20 min with (A) 10 uM PP2 or (B) 10 uM U0126, followed by a 30 min treatment with 10ng/mL VEGF165 or 10ng/mL TNF-alpha. Results were normalized to Hsp90 protein levels and expressed as fold change over unstimulated control. Graph illustrates p42-ERK fold values shown as mean ± S.E.M. of 7 independent experiments; *P<0.05 and **P<0.001 compared with unstimulated control; +P<0.05 and ++P<0.001 compared with same agonist control.
3.7. Effect of blockade of MEK/ERK and Src signaling pathways on TNF-alpha inhibition of ATP-stimulated Ca2+ bursts
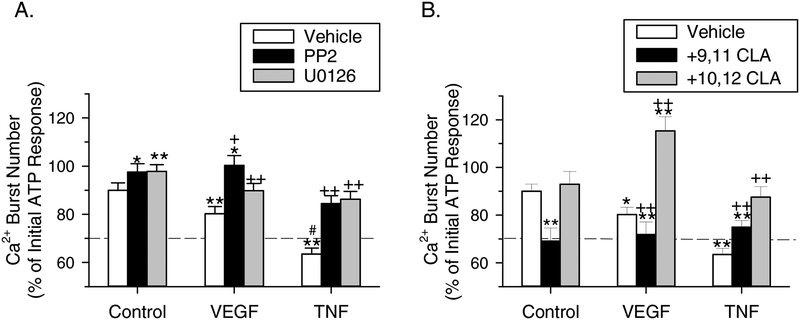
Having shown via Western analyses that TNF-alpha-stimulated phosphorylation of inhibitory sites on Cx43 involves both Src- and ERK-mediated pathways, we wanted to demonstrate these events mediate the functional inhibition of sustained Ca2+ bursting. Fig. 7A shows the effects of PP2 or U0126 pretreatment on rescuing P-UAEC from acute inhibition of ATP-stimulated Ca2+ bursts when added before VEGF165 or TNF-alpha. Repeat ATP stimulation gave a small decrease in sustained Ca2+ bursting (90% of initial ATP response), consistent with our prior studies that show ATP has a weak stimulatory effect on the phosphorylation of inhibitory sites on Cx43 (Fig. 2B) as reported previously (Boeldt et al., 2015). Conversely, when used alone between ATP treatments, PP2 or U0126 prevented this reduction in burst levels (97.6% and 97.7% of initial ATP response, respectively). Consistent with results published by Boeldt et al. (2015), VEGF165 inhibited sustained Ca2+ bursting significantly (80% of initial ATP response), but this inhibition could be recovered to the repeat ATP stimulation level when P-UAEC were pretreated with U0126 (89.8%) or even improved to the initial ATP stimulation level with PP2 (100.3%). Treatment with TNF-alpha was more effective at inhibiting Ca2+ bursts than VEGF165 (P<0.001), and after pretreating cells with the rescue agents PP2 or U0126, the sustained Ca2+ bursts were each recovered back to levels similar to repeat ATP stimulation levels (84.4% and 86.3%, respectively).
Figure 7: Effect of VEGF165 or TNF-alpha and the Src and ERK inhibitors PP2 or U0126 on acute inhibition of ATP-stimulated Ca2+ bursts.
Ca2+ burst experiments were performed as described in Fig. 2C, except (A) the Src inhibitor PP2 (10 uM, 20 min) or U0126 (10 uM, 30 min) or (B) 9,11 CLA or 10,12 CLA (50 uM, 30 min) were added after the initial ATP stimulation and remained in the dish during the specified treatment (VEGF165: 10 ng/mL, 30 min; TNF: 10 ng/mL, 60 min). For reference, the dashed line indicates the equivalent level of Ca2+ bursts in NP-UAEC upon ATP stimulation. Values shown are mean ± S.E.M. (n=4–9 dishes). *P<0.01 and **P<0.001 compared with ATP alone control; +P<0.01 and ++P<0.001 compared with same agonist control; #P<0.001 compared with VEGF165.
We then tested t10,c12 CLA, a nutraceutical compound capable of inhibiting Src because this is a possible GRAS therapeutic option for rescuing sustained Ca2+ signaling in endothelial cells in humans. In order to determine if PP2-like recovery from TNF-alpha inhibition of Ca2+ bursts could be achieved as it was for VEGF165-mediated inhibition (Boeldt et al., 2015), P-UAEC were pretreated with t10,c12 CLA before adding TNF-alpha (Fig. 7B). Pretreatment with t10,c12 CLA rescued ATP-stimulated Ca2+ burst responses in the presence of TNF-alpha back to repeat stimulation control levels (87.5%). Although VEGF165-induced inhibition of Ca2+ burst response could be rescued to an even higher level by t10,c12 CLA (115.3%) (Boeldt et al., 2015), it should be noted TNF-alpha inhibition is more dramatic than for VEGF165, and we obtained a similar rescue of Ca2+ bursts with PP2, suggesting CLA action mimics that of PP2. In contrast, the c9,t11 isomer of CLA, which is not a Src inhibitor (Shahzad et al., 2018) and thus used as a negative control, only slightly reversed TNF-alpha-induced inhibition and could not substantially recover Ca2+ bursting to control levels in the presence of VEGF165 (71.9%) or TNF-alpha (75%). When used alone, t10,c12 CLA gave a similar response to ATP alone (92.4%), whereas c9,t11 CLA significantly decreased sustained bursting compared to control (69.1%).
3.8. Changes in Ca2+ response and NO production in HUV Endo after TNF-alpha treatment
Because there is little research on the effects of TNF-alpha on [Ca2+]i and NO responses in human vessels and particularly during pregnancy, ex vivo studies were undertaken using HUV Endo preparations from healthy and PE pregnancies (referred to as PE-HUV Endo) as described by Krupp et al. (2013). In Fig. 8A we show the real-time effect of TNF-alpha on HUV Endo in control patients. As reported in Krupp et al. (2013), there is immediate periodic [Ca2+]i bursting in the endothelium of vessels from control patients, and a substantial increase in NO production throughout 30 min of ATP treatment. With TNF-alpha pre-treatment (Fig. 8B) however, NO output was severely decreased and the sustained [Ca2+]i bursting beyond the initial peak was clearly diminished, representing a tracing similar to UA-Endo vessels from non-pregnant sheep (Yi et al., 2005; 2011) and HUV vessels from PE patients (Krupp et al., 2013) (Fig. 8C). While PE-HUV show a diminished response (as first reported by Krupp et al., 2013), PP2 pretreatment for just 1 h promotes partial recovery of bursting and NO output (Fig. 8D). While panels A through D show images on individual cells in each case, TNF-alpha treatment also causes the number of cells responding to drop. The quantification of the percentage of cells showing each burst is given in Fig. 8E, 8F. TNF-alpha treatment causes otherwise normal control subjects cells in the HUV Endo monolayer to burst at a significantly lower rate compared to cells from normal cords treated with vehicle alone (Fig. 8E). When we observed cells in the HUV Endo monolayer of PE-derived cord segments, the burst percentage of cells responding was very low, as expected. On pretreatment with 10 ng/mL PP2 for just 1 h we observed a clear improvement in Ca2+ bursting and NO output (Fig. 8F), and the magnitude of this recovery was similar to the inhibitory effect of TNF-alpha in Fig 8E.
Figure 8: Real-time changes in [Ca2+]i and NO in HUV Endo.
Intact vessels dissected from cords from control subjects were (A) treated with vehicle and then ATP (100 uM), or (B) pretreated with TNF-alpha (10 ng/mL, 1 h) and then ATP. Intact vessels dissected from cords from PE subjects were (C) treated with vehicle and then ATP (100 uM), or (D) pretreated with PP2 (10 uM, 1 h) and then ATP. Graphs are representative tracings of individual cells from HUV Endo. The percentage of total imaged cells that show each successive Ca2+ burst was quantified in (E) for control cords treated with vehicle control or TNF-alpha (as represented in A and B) or in (F) PE-derived cords treated with vehicle or PP2 (as represented in C and D). Values are for n=5 or more subjects in each group expressed as mean ± S.E.M with *P<0.05 relative to the paired no TNF-alpha (E) or no PP2 (F) control.
So far we have analyzed the changes in burst number for individual cells and or average bursts per cell, but not averaged the response of the vessel (all cells) as a whole. Fig. 9A–D is the combined average data from the studies shown in Fig. 8A–D. Panels represent HUV Endo from control cords pre-treated with vehicle (Fig. 9A) or TNF-alpha (Fig. 9B) or PE cords treated with vehicle (Fig. 9C) or PP2 (Fig. 9D). Note the individual bursts are no longer apparent in averaged data, but the difference in response is still clear. In HUV Endo from control cords, the initial [Ca2+]i peak is broad and declines slowly through the sustained phase, only reaching baseline at 30 min (Fig. 9A). Providing [Ca2+]i remains elevated, NO production continues as indicated by a progressive rise in F/F0 for DAF signal. Pretreatment with TNF-alpha (Fig. 9B) speeds the decline in sustained phase [Ca2+]i response to ATP, and there is a corresponding reduction in NO output. In contrast, PE-HUV (Fig. 9C) shows a poor initial ability to maintain Ca2+ above baseline even beyond 10 min and NO output is correspondingly very low (as reported by Krupp et al 2013). Nonetheless, pretreatment of PE-HUV Endo segments with PP2 for 1 h (Fig. 9D) both improves the ability to maintain elevated [Ca2+]i during the sustained phase and there is a corresponding improvement in overall NO output.
Figure 9: Averaged Change in [Ca2+]i and NO in HUV Endo.
The individual cell data shown in Fig. 8 were averaged together to determine the overall changes in levels of [Ca2+]i and NO. Intact vessels dissected from cords from control subjects were (A) treated with vehicle and then ATP (100 uM), or (B) pretreated with TNF-alpha (10 ng/mL, 1 h) and then ATP. Intact vessels dissected from cords from PE subjects were (C) treated with vehicle and then ATP (100 uM), or (D) pretreated with PP2 (10 uM, 1 h) and then ATP. The changes above baseline of [Ca2+]i at the initial peak of the Ca2+ response, at 900 s in the sustained phase, and in the NO output at 1500 s were quantified in (E) control cords treated with vehicle or TNF-alpha (as demonstrated in A and B) or in (F) PE-derived cords treated with vehicle control or PP2 (as demonstrated in C and D). Values are for n=5 or more subjects in each group expressed as mean ± S.E.M with *P<0.05 relative to the paired no TNF-alpha (E) or no PP2 (F) control.
In Fig. 9E–F, we again quantify the change above basal of the initial peak of [Ca2+]i, the sustained phase [Ca2+]i response at 900s, and the overall NO output at 1500s, as in Krupp et al. (2013). Activation of eNOS is known to be most dependent on sustained Ca2+ responses, so it was no surprise when there was no change at the initial [Ca2+]i peak in each case, but the reduction in sustained phase [Ca2+]i at 900s in control HUV treated with TNF-alpha (left) and the increase in sustained phase [Ca2+]i at 900s on treatment of PE-HUV with PP2 (right) each corresponded closely to the observed change in NO in each case.
4. Discussion
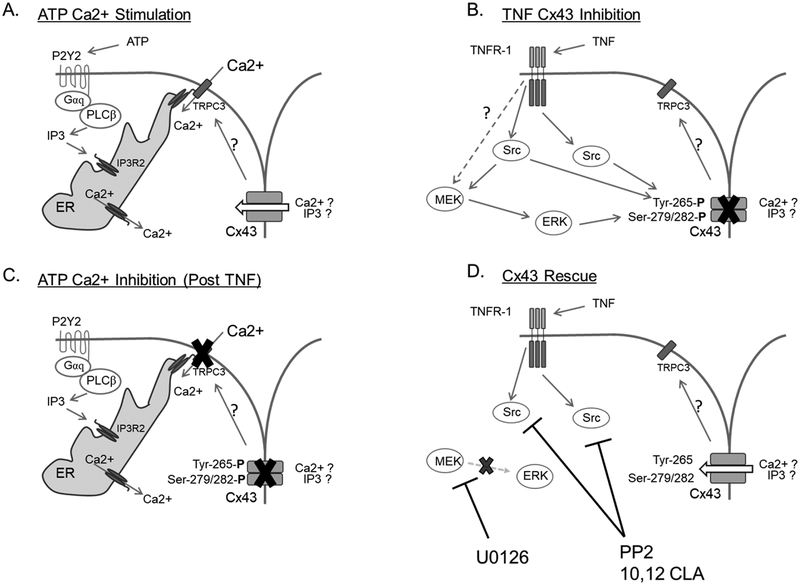
Our findings support the proposal that TNF-alpha plays a significant role in causing GJ dysfunction via phosphorylation of inhibitory residues on Cx43. While others have proposed that TNF-alpha inhibition of GJ function occurs through ROS production and resulting oxidative stress (reviewed in Chen et al., 2008), we were unable to measure a significant amount of ROS generation by P-UAEC until 50 ng/mL TNF-alpha was used, a dose far beyond a physiologic range except perhaps in sepsis. This finding fully agrees with the observation that clinical antioxidant therapy is largely ineffective in PE subjects (Gülmezoğlu et al., 1997; Spinnato 2nd et al., 2007). The results of this study are also consistent with reported physiologic levels of TNF-alpha in the blood of control and PE subjects in pregnancy (Afshari et al., 2005; Bayram et al., 2012; Benyo et al., 2001; Cackovic M, 2008; Conrad et al., 1997; 1998; Kocyigit et al., 2004; Kronborg et al., 2011; Kupferminc et al., 1994; Tosun et al., 2010; Vince et al., 1995;). Thus, it seems highly likely that rapid and direct inhibition of GJ function is the true action of local or systemic TNF-alpha in PE subjects, and this action is primarily mediated via Src and secondary to ERK kinase activation (Fig. 10), rather than by ROS accumulation.
Figure 10. Proposed mechanism for ATP stimulation, TNF-alpha inhibition, and subsequent rescue of sustained Ca2+ responses in P-UAEC.
Panel A depicts a typical ATP-stimulated Ca2+ response. Most notably, the initial Ca2+ response consists of IP3-regulated endoplasmic reticulum Ca2+ release into the cytosol. When endoplasmic reticulum Ca2+ levels are sufficiently low, IP3R2 interacts with TRPC3 at the cell surface, opening the channel and allowing extracellular Ca2+ influx. This process is permitted/potentiated by Cx43 function through an as yet unknown mechanism. Panel B shows the TNF-stimulated signaling pathways responsible for acute inhibitory phosphorylation of Cx43 at Tyr-265 and Ser-279/282. Src family kinase signaling originates from activation of TNFR, likely the R1 isoform. Our data suggests MEK/ERK signaling also occurs possibly from TNFR1 but certainly secondary to Src kinase activation. Panel C shows the consequence of TNF-stimulated Cx43 phosphorylation on the subsequent ATP-stimulated Ca2+ responses. The phosphorylation of Cx43 at Tyr-265 and Ser-279/282 render Cx43 non-functional and thus the transmission of signals necessary for sustained phase Ca2+ burst responses via TRPC3 is lost. The result is a normal initial Ca2+ response to ATP stimulation but a significant reduction in sustained-phase Ca2+ bursts. Panel D shows the sites of action for pharmacological inhibitors of Src family kinases (PP2 and 10:12 CLA) and MEK/ERK (U0126). Blockage of Src and MEK/ERK kinases prevents inhibitory phosphorylation of Cx43 at Tyr-265 andSer-279/282 and therefore rescues cells from the inhibitory activity of TNF-alpha pretreatment on ATP-stimulated sustained-phase Ca2+ bursts.
TNF-alpha is well-known as a sustained activator of Src (Bode JG, 2003; Page, 2009), and our proposal that the actions of TNF-alpha are mediated through Src and MEK/ERK kinases is consistent with observations in other cell types (Bird, et al. 2013). Our findings herein show TNF-alpha can blunt specifically the pregnancy-enhanced Ca2+ bursting response in P-UAEC to a level found in non-adapted NP-UAEC in a manner directly related to Src and MEK/ERK mediated Cx43 phosphorylation. We have also shown TNF-alpha signals far more prominently through Src and can promote a far greater decrease in ATP-stimulated bursts numbers in P-UAEC beyond the action of VEGF165. Clearly, TNF-alpha is not simply mimicking VEGF165 actions, and its greater inhibitory action is consistent with its more Src focused cell signaling abilities (Fig. 10).
The results of this study have increased our understanding of basic cellular and molecular signaling that can lead us directly to potential and much needed therapeutics for PE subjects. Given the current evidence in P-UAEC that TNF-alpha activates Src- and (through cross talk) ERK-targeted inhibitory sites on Cx43, and our demonstrated ability to rescue the pregnancy-adapted Ca2+ burst responses with Src- or MEK/ERK-selective inhibitors, we suggest that in vivo targeting of these kinases to prevent over-activity of Cx43 and so retain GJ function and Ca2+ burst signaling could be beneficial in human PE subjects. However, we must first ask what Cx43 phosphorylation target is best to protect against TNF-alpha action in P-UAEC? While VEGF165 and TNF-alpha both phosphorylate the ERK- and Src-sensitive sites on Cx43, the MEK/ERK inhibitor U0126 fully reversed VEGF165-induced phosphorylation at s279/282, but was ineffective for TNF-alpha-induced phosphorylation. This is consistent with the high selectivity of U0126 for the MEK/ERK pathway and the relatively weaker action TNF-alpha has on ERK phosphorylation, and suggests that TNF-alpha, although capable of acting weakly through ERK, does not rely as heavily on this single pathway as does VEGF165. With regard to the Cx43 residue y265, both VEGF165 and TNF-alpha increase phosphorylation in a manner consistent with inhibition of Ca2+ bursting. Our findings show PP2 alone blocks basal phosphorylation at y265 to below that of control cells without such a parallel effect on s279/282 phosphorylation. So, in this respect, PP2 is relatively selective, and its action to inhibit y265 phosphorylation to below basal levels is consistent with the unique ability of PP2 to raise P-UAEC bursting function above control when used alone and suggests even basal Src activity is enough to influence basal Ca2+ bursting. Thus, it may be expected that by inhibiting Src, we could also reverse VEGF165- and TNF-alpha-induced phosphorylation and potentially restore GJ function in the form of Ca2+ bursting back to the initial ATP response level (100%).
While this over-recovery was observed with PP2 treatment with VEGF165 in the Ca2+ burst studies in P-UAEC, it was not the case for TNF-alpha. Both PP2 and U0126 can recover sustained bursting in TNF-alpha-treated cells back to control levels equivalent to repeat ATP stimulation, but PP2 does not restore bursting further to the level of the initial ATP response as it does with VEGF165. Precisely why this does not occur with TNF-alpha may relate to that fact VEGF165 does not inhibit Ca2+ bursting to the degree that TNF-alpha inhibits, and this added potency of TNF-alpha may simply be more difficult to overcome. Further supporting evidence that ERK is not solely responsible for TNF-alpha-mediated inhibition of bursting is given by the simple fact that we see a far greater inhibition in Ca2+ signaling by TNF-alpha (which clearly activates Src) than by VEGF165 (which acts predominantly via ERK), and we see similar reversals of TNF-alpha inhibition of sustained bursting when using either Src or MEK/ERK inhibitors. It remains to be determined if the incomplete rescue of the inhibitory effects of TNF-alpha compared to VEGF165 are due more to sustained Src signaling by TNF-alpha alone, which is cross-talking to ERK and thus more difficult to block, or if other, unidentified signaling pathways that are neither Src- nor ERK-mediated are responsible. This especially becomes more relevant as we move from 1- to 3-h treatment since at this time acute phosphorylation of Cx43 may no longer be the only mechanism by which TNF-alpha promotes endothelial dysfunction. Extended exposure to TNF-alpha can also result in changes in cell-cell junctional breakdown (Shivanna et al., 2010) and this is perhaps why the action of TNF-alpha on Ca2+ bursting at 3 h is observed at lower doses than at 1 h, and with altered dose dependency. This long-term response to TNF-alpha is currently under investigation in this laboratory.
As our data stands, were we to choose a kinase to target as a basis for future therapy to keep Cx43 channels open by preventing Cx43 inhibitory phosphorylation, that kinase would be Src. To bring our work full circle to human disease, we also studied the ability of TNF-alpha to inhibit Ca2+ bursting and NO output in isolated HUV Endo preparations, exactly as undertaken by Krupp et al. (2013). Our findings established there is clear inhibition of both [Ca2+]i bursting and NO output in healthy umbilical vein vessel segments on exposure to TNF-alpha for 1 h. Perhaps more importantly as a proof of principle that targeting Src over-activity may be therapeutic, we have also shown that vessels from PE subjects that lack sustained Ca2+ bursting and NO output can show improved burst function in response to ATP when pretreated with PP2 for as little as 1 h. The fact we see any recovery at all in that short time with PP2 alone is very encouraging, given the development of cord dysfunction in PE is a chronic state. That said, it is clear the pharmacologic inhibitor PP2, which targets a wide range of Src family kinase members (McLauchlan et al., 2003; Bain et al., 2007), would most likely be entirely unsuitable for pregnant women. What is needed is a far more tolerable and safe Src inhibitor and most likely one that functions at submaximal doses. Our studies in isolated P-UAEC have identified that this compound could indeed be t10,c12 CLA, because it successfully rescued VEGF165 inhibition of Ca2+ bursting in P-UAEC at doses of 5 uM and above (Boeldt et al., 2015). Now we have also shown here that t10,c12 CLA can rescue P-UAEC from the inhibitory actions of TNF-alpha, and this action is stereo-isoform-specific, it may be a candidate for use in PE therapy. Of note, Clarinol, a 1:1 formulation of predominantly t10,c12 and c9,t11 CLA is currently approved for use at doses up to 3.4 g/day in pregnancy (GRAS by the FDA).
Conclusion
There are many questions still to answer, but the major findings of this study are the clear demonstration that TNF-alpha can indeed mediate endothelial damage acutely through Cx43 inhibitory phosphorylation (rather than through excess ROS production), and at physiologically relevant doses. The results suggest that longer treatment at physiologic levels of TNF-alpha seen in PE subjects may explain the observed loss of vasodilatory function in PE subjects. The fact that TNF-alpha can impair P-UAEC function through multiple kinase-based mechanisms and to a far greater extent than VEGF165, supports our proposal that TNF-alpha may play a key role in causing endothelial damage in PE subjects. This initial study on TNF-alpha, as well as on therapeutic agents that may rescue P-UAEC treated with TNF-alpha, and indeed vessels from PE subjects from such damage, is an important first step towards a new mechanistic understanding of how endothelial cell function is impaired in PE pregnancy and ultimately finding a therapy to treat it. Pharmacologic inhibitors of Src clearly work but may not be suitable to use in human pregnancy. Nutraceutical Src inhibitors such as t10,c12 CLA show considerable promise, but while clearly safe, we need further study to determine if they are effective and at what optimal dose.
Highlights:
We have previously shown pregnancy is associated with enhanced Ca2+ signaling in uterine artery endothelial cells mediated by enhanced Cx43 connectivity.
Here we show TNF‐alpha inhibits Cx43 function and so inhibits periodic Ca2+ bursts in pregnancy‐ derived ovine uterine artery endothelial cells (P‐UAEC).
TNF acts primarily via Src to inhibit Cx43 by causing phosphorylation directly at positions 265 and indirectly at position 279/282.
TNF action can be reversed by the Src inhibitor PP2 or MEK/ERK inhibitor U0126. The nutraceutical Src antagonist t10,c12 CLA also recovers Ca2+ bursting in P‐UAEC.
Imaging studies in intact endothelium of normal umbilical vein suggests TNF‐alpha exposure inhibits GJ Cx43 function and this is paralleled by a reduction in NO production down to a level similar to that of preeclampsia. In vessels already impaired by preeclampsia, Src inhibitors offer a potential therapy.
Acknowledgements
The authors thank Jason Austin for assistance in cell preparation and lab support.
Funding
This work has been supported by NIH awards HD038843 (IMB, RRM) and HL117341 (RRM) including the use of CLA isoforms as shown here. This paper reports partial fulfillment of the requirements for ACA towards a PhD in the University of Wisconsin Endocrinology and Reproductive Physiology Training Program. ACA was also supported by T32 predoctoral training award (T32HD41921).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
IMB and DSB are patent applicants on the use of t10,c12 CLA as a therapeutic agent during pregnancy based on this and prior data cited in this paper. All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Afshari JT, Ghomian N, Shameli A, Shakeri MT, Fahmidehkar MA, Mahajer E, Khoshnavaz R, Emadzadeh M, 2005. Determination of Interleukin-6 and Tumor Necrosis Factor-alpha concentrations in Iranian-Khorasanian patients with preeclampsia. BMC Pregnancy Childbirth. 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP, 2002. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 15, 170–175. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P, 2007. The selectivity of protein kinase inhibitors: a further update. Biochem J. 408(3), 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum JR, Dolmatova E, Tan A, and Duffy HS, 2012. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol 3, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram M, Bostanci MS, Celtemen MB, Bagrlaclk EU, Yaman M, Civil F, 2012. Maternal Inflammatory Response in Severe Preeclamptic and Preeclamptic Pregnancies. J Clin Gynecology and Obstetrics. 1:2–3, 40–45. [Google Scholar]
- Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP, 2001. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 86, 2505–2512. [DOI] [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR 2000. Pregnancy-Dependent Changes in Cell Signaling Underlie Changes in Differential Control of Vasodilator Production in Uterine Artery Endothelial Cells. Endocrinology. 141(3), 1107–1117. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zhang L, Magness RR, 2003. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Phyiol 284, R245. [DOI] [PubMed] [Google Scholar]
- Bird IM, Boeldt DS, Krupp J, Grummer MA, Yi FX, and Magness RR, 2013. Pregnancy, Programming and Preeclampsia: Gap Junctions at the Nexus of Pregnancy-induced Adaptation of Endothelial Function and Endothelial Adaptive Failure in PE. Current Vascular Pharmacology. 11, 712–729. [DOI] [PubMed] [Google Scholar]
- Bode JG, Schweigart J, Kehrmann J, et al. 2003. TNF-alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol. 171:257–266. [DOI] [PubMed] [Google Scholar]
- Boeldt DS, Hankes AC, Alvarez RE, Khurshid N, Balistreri M, Grummer MA, Yi FX, Bird IM, 2014. Pregnancy programming and preeclampsia: identifying a human endothelial model to study pregnancy-adapted endothelial function and endothelial adaptive failure in preeclamptic subects. Adv. Exp. Med. Biol 814, 27–47. [DOI] [PubMed] [Google Scholar]
- Boeldt DS, Grummer MA, Yi FX, Magness RR, and Bird IM, 2015. Phosphorylation of Ser-279/282 and Tyr-265 positions on Cx43 as possible mediators of VEGF-165 inhibition of pregnancy-adapted Ca2+ burst function in ovine uterine artery endothelial cells. Mol. Cell Endo. 412 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeldt DS and Bird IM, 2017. Vascular adaptation in pregnancy and endothelial dysfunction in PE. J. Endo 232, R27–R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, Lockwood CJ, Buhimschi IA, 2008. Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet. Gynecol 112(1), 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Andersen BT, Hill M, Zhang J, Booth F, Zhang C, 2008. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Curr Hypertens Rev. 4:4:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP and Benyo DF, 1997. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 37, 240–9. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Miles TM, Benyo DF, 1998. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 40, 102–11. [DOI] [PubMed] [Google Scholar]
- Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. 2014. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 124, 4941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford SM, Yi FX, and Bird IM, 2006. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling.” Journal of Endocrinology. 190, 373–384. [DOI] [PubMed] [Google Scholar]
- Grummer MA, Sullivan JA, Magness RR, and Bird IM, 2009. Vascular endothelial growth factor acts through novel, pregnancy-enhanced receptor signalling pathways to stimulate endothelial nitric oxide synthase activity in uterine artery endothelial cells. Biochem J. 417, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülmezoğlu AM, Hofmeyr GJ, and Oosthuisen MM, 1997. Antioxidants in the treatment of severe pre-eclampsia: an explanatory randomized controlled trial. Br J Obstet Gynaecol. 104(6), 689–96. [DOI] [PubMed] [Google Scholar]
- Hao JL, Suzuki K, Lu Y, Hirano S, Fukuda K, Kumagai N, Kimura K, Nishida T, 2005. Inhibition of gap junction-mediated intercellular communication by TNF-alpha in cultured human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci 46, 1195–1200. [DOI] [PubMed] [Google Scholar]
- Hlaing KH and Clement MV, 2014. Formation of protein S-nitrosylation by reactive oxygen species. Free Radic Res. 48(9), 996–1010. [DOI] [PubMed] [Google Scholar]
- Huang S, Dudez T, Scerri I, Thomas MA, Giepmans BN, Suter S, Chanson M, 2003. Defective activation of c-Src in cyctic fibrosis airway epithelial cells results in loss of tumor necrosis factor alpha-induced-gap junction regulation. J Biol Chem. 278(10), 8326–32. [DOI] [PubMed] [Google Scholar]
- Kastl L, Sauer SW, Ruppert T, Beissbarth T, Becker MS, Suss D, Krammer PH, Gulow K 2014. TNF-a mediates mitochondrial uncoupling and enhances ROS-dependent cell migration via NF-kB activation in liver cells. FEBS letters. 588:1, 175–183. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee KS, Kim JH, Lee DK, Park M, Choi S, Park W, Kim S, Choi YK, Hwang JY, Choe J, Won MH, Jeoung D, Lee H, Ryoo S, Ha KS, Kwon YG, Kim YM, 2017. Aspirin prevents TNFa induced endothelial cell dysfunction by regulating the NF-kB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol Med. 104, 185–198. [DOI] [PubMed] [Google Scholar]
- Ko S, Kwok TT, Fung KP, Choy YM, Lee CY, Kong SK, 2000. Slow rise of Ca2+ and slow release of ROS are two cross-talked events important in tumor necrosis factor-alpha-mediated apoptosis. Free Radic Res. 33(3), 295–304. [DOI] [PubMed] [Google Scholar]
- Kocyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z, 2004. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 19, 267–273. [DOI] [PubMed] [Google Scholar]
- Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK,Allen J, Knudsen UB, 2011. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 90, 791–796. [DOI] [PubMed] [Google Scholar]
- Krupp J, Boeldt DS, Yi FX, Grummer MA, Bankowski Anaya HA, Shah DM, Bird IM, 2013. The loss of sustained Ca2+ signaling underlies suppressed endothelial nitric oxide production in preeclamptic pregnancies: implications for new therapy. Am J Physiol Heart Circ. Physiology 305(7), H969–H989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML, 1994. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 170, 1752–1757. [PubMed] [Google Scholar]
- LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP, 2005. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of tumor necrosis factoralpha. Hypertension. 46, 1022–1025. [DOI] [PubMed] [Google Scholar]
- Magness RR and Ford SP, 2014. Maternal Cardiovascular Adaptation and Uterine Circulation-Physiology and Pathophysiology In: Stress and Developmental Programming of Health and Disease: Beyond Phenomenology. Editors Zhang LL and Longo LD Nova Science Publishers, Inc. Hauppauge, NY: Chapter 7, pp.341–374 [Google Scholar]
- Matsubara K, Higaki T, Matsubara Y, Nawa A. 2015. Nitric Oxide and Reactive Oxygen Species in the Pathogenesis of Preeclampsia. Int J Mol Sci.16(3), 4600–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan H, Elliott M, Cohen P, 2003. The specificities of protein kinase inhibitors: an update. Biochemical Journal. 371(1), 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimlamool W, Andrews RM, Falk MM, 2015. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol. Biol. Cell 26(15), 2755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. 2009. Tyrosine kinases and inflammatory signaling. Curr Mol Med. 9:69–85. [DOI] [PubMed] [Google Scholar]
- Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M, 2014. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 5, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Gunther J, Hescheler J, Wartenberg M, 2000. Thalidomide inhibits angiogenesis in embryoid bodies by the generation of hydroxyl radicals. Am J Pathol. 156, 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad MMK, Felder M, Ludwig K, Van Galder HR, Anderson ML, Kim J, Cook ME, Kapur AK, Patankar MS, 2018. Trans10,cis12 conjugated linoleic acid inhibits proliferation and migration of ovarian cancer cells by inducing ER stress, autophagy, and modulation of Src. PLoS ONE. 13(1), e0189524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna M, Rajashekhar G, and Srinivas SP. 2010. Barrier Dysfunction of the Corneal Endothelium in Response to TNF-α: Role of p38 MAP Kinase. Invest Ophthalmol Vis Sci. 51: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA, 1999. Cutaneous wound healing. N Engl J Med. 341, 738–46. [DOI] [PubMed] [Google Scholar]
- Siwetz M, Blaschitz A, El-Heliebi A, Hiden U, Desoye G, Huppertz B, Gauster M, 2016. TNF-α alters the inflammatory secretion profile of human first trimester placenta. Lab Invest. 96, 428–438. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP, 1997. Nitric oxide and pregnancy. Am J Physiology. 272, R441–R463. [DOI] [PubMed] [Google Scholar]
- Solan JL and Lampe PD, 2014. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 588(8), 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinnato JA 2nd, Freire S, Pinto E, Silva JL, et al. 2007. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 110(6), 1311–8. [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Grummer MA, Yi FX, Bird IM, 2006. Pregnancy-Enhanced Endothelial Nitric Oxide Synthase (eNOS) Activation in Uterine Artery Endothelial Cells Shows Altered Sensitivity to Ca2, U0126, and Wortmannin But Not LY294002—Evidence that Pregnancy Adaptation of eNOS Activation Occurs at Multiple Levels of Cell Signaling. Endocrinology. 147(5), 2442–2457. [DOI] [PubMed] [Google Scholar]
- Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A, 2011. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine. 56, 192–199. [DOI] [PubMed] [Google Scholar]
- Tacheau C, Laboureau J, Mauviel A, Verrecchia F, TNF-alpha Represses connexin43 expression in HaCat keratinovytes via activation of JNK signaling. 2008. J Cell. Phys 216(2), 438–44. [DOI] [PubMed] [Google Scholar]
- Thévenin AF, Kowal TJ, Fong JT, Kells RM, Fisher CG, Falk MM 2013. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology (Bethesda). 28(2), 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioğlu E, 2010. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med.23, 880–6. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW, 1995. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with preeclampsia. Br J Obstet Gynaecol. 102, 20–25. [DOI] [PubMed] [Google Scholar]
- Watson AJM and Hughes KR, 2012. TNF-a-induced intestinal epithelial cell shedding: implications for intestinal Barrier function. Annals of the New York Academy of Sciences. 1258, 1–8. [DOI] [PubMed] [Google Scholar]
- Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM, 2007. Pro-inflammatory Cytokines increase reactive oxygen species through mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp Eye Res. 85(4), 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi FX, Magness RR, Bird IM, 2005. Simultaneous imaging of [Ca2+]I and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol. 288, 140–148. [DOI] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, and Bird IM, 2010. Pregnancy Enhances Sustained Ca2+ Bursts and Endothelial Nitric Oxide Synthase Activation in Ovine Uterine Artery Endothelial Cells Through Increased Connexin 43 Function. Biol. Reprod 82, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Magness RR, Bird IM, 2011. [Ca2+]i signaling vs. eNOS expression as determinants of NO output in uterine artery endothelium: relative roles in pregnancy adaptation and reversal by VEGF165. Am J Physiol Heart Circ Physiol. 300, H1182–H1193. [DOI] [PMC free article] [PubMed] [Google Scholar]