Abstract
Successive nucleophilic and electrophilic allylation mediated by the bis-Boc-carbonate derived from 2-methylene−1,3-propane diol enables formation of enantiomerically enriched 2,4-disubstituted pyrrolidines. An initial enantioselective iridium-catalyzed transfer hydrogenative carbonyl C-allylation is followed by Tsuji-Trost N-allylation using 2-nitrobenzenesulfonamide. Subsequent Mitsunobu cyclization provides the N-protected 2,4-disubstituted pyrrolidines.
Graphical Abstract

The development of catalytic asymmetric methods for the synthesis of saturated N-heterocycles1,2 is driven by the frequency with which such structural motifs occur as substructures in FDA approved drugs,3 and the growing appreciation that stereochemical complexity improves prospects for clinical success.4,5 Our exploration of hydrogen-mediated reductive coupling6 has enabled diverse methods for catalytic enantioselective C-C bond formation, including carbonyl allylation.6d,7 In these processes, primary alcohol oxidation is balanced by C-O reductive cleavage of an allylic acetate pronucleophile resulting in the formation of a transient aldehyde-allylmetal pair, which combine to form secondary homoallylic alcohols. Based on this reactivity pattern, we envisioned an approach to N-protected 2,4-disubstituted pyrrolidines wherein the bis-Boc-carbonate derived from 2-methylene-1,3-propane diol is subjected to successive nucleophilic and electrophilic allylation (Figure 1).8,9 While numerous bifunctional allylmetal reagents based on tin, boron or silicon have been described,10 the use of such reagents for pyrrolidine synthesis is uncommon and is soley manifested by the pioneering efforts of Trost vis-à-vis trimethylenemethane (TMM) cycloadditions of imines.10a,11,12 Catalytic enantioselective cycloadditions of this type have been reported,11c-f but require tailored chiral phosphoramidite ligands and are largely restricted to aryl-substituted imines.11c,e To our knowledge, highly enantioselective syntheses of 2-alkyl-4-methylenepyrrolidines remain a largely unmet challenge. Here, we report a catalytic protocol for the synthesis of 2-alkyl-4-methylenepyrrolidines that utilizes inexpensive SEGPHOS ligands and avoids moisture sensitive imine reactants.
Figure 1.
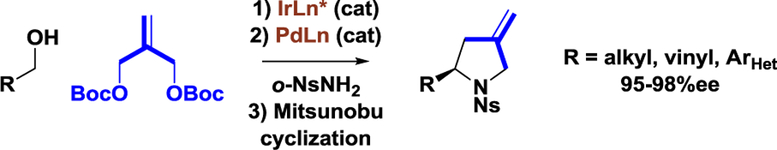
Enantioselective pyrrolidine synthesis via successive nucleophilic and electrophilic allylation.
In an initial experiment, 4-bromobenzyl alcohol 1a (100 mol %) was exposed to bis-Boc-carbonate 2a13 (200 mol %) in the presence of the π-allyliridium C,O-benzoate complex derived from 4-cyano-3-nitrobenzoic acid and (S)-DM-SEGPHOS and K3PO4 (100 mol %) in DME (0.4 M) at 80 °C. The homoallylic alcohol 3a was generated in 58% yield and 89% ee (Table 1, entry 1). Decreased loadings of K3PO4 (10 mol %) led to a higher isolated yield of 3a (Table 1, entry 3). Different chiral phosphine ligands were evaluated (Table 1, entries 6–9). Optimal enantioselectivities were obtained using (S)-DM-SEGPHOS or (S)-SEGPHOS (Table 1, entries 3 and 9). It was found that a slight decrease in reaction temperature (70 °C) improved enantioselectivity without diminishing the isolated yield of 3a (Table 1, entry 11). Similar efficiencies were observed with the catalyst incorporating the 3,4-dinitro-C,O-benzoate moiety (Table 1, entry 12).
Table 1.
Selected optimization experiments in the enantioselective coupling of alcohol 1a and bis-Boc-carbonate 2a via alcohol-mediated hydrogen transfer.a
 |
Yields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
As 3,4-dinitrobenzoic acid is commercially available (and 4-cyano-3-nitrobenzoic acid is not), the optimized conditions employing (S)-Ir-III (Table 1, entry 12) were applied to the coupling of alcohols 1a–1j with bis-Boc-carbonate 2a (Table 2). Benzylic alcohols 1a–1e, the allylic alcohol geraniol 1f, and aliphatic alcohols 1g–1j delivered the respective adducts 3a–3j in good yield with excellent levels of enantioselectivity. The absolute stereochemistry of adducts 3a–3j was assigned in analogy to adduct 3i, which was determined by single crystal X-ray diffraction analysis. The conversion of alcohols 1a–1j to adducts 3a–3j represent redox-neutral processes. As illustrated by the conversion of aldehydes dehydro-1e, dehydro-1f and dehydro-1h to adducts 3e, 3f and 3h, 2-propanol-mediated reductive couplings of aldehyde reactants also proceed efficiently with high levels of enantioselectivity under identical conditions (Scheme 1).
Table 2.
Redox-neutral coupling of alcohols 1a–1j with bis-Boc-carbonate 2a to form adducts 3a–3j.a
 |
Yields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
(S)-Ir-II (5 mol %).
DME (0.2 M), 60 °C.
Scheme 1.
Reductive coupling of aldehydes dehydro-1e, dehydro-1f and dehydro-1h with bis-Boc-carbonate 2a to form adducts 3e, 3f and 3h.a

aYields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
The conversion of adducts 3a–3j to the 2-substituted 4-methylenepyrrolidines 5a–5j was achieved via Tsuji-Trost allylation followed by Mitsunobu cyclization (Table 3). Whereas Tsuji-Trost allylation of para-nitrobenzenesulfonamide resulted in significant quantities of over-alkylation, corresponding reactions of ortho-nitrobenzenesulfonamide were more selective, providing the highly tractable ortho-nosyl containing adducts 4a–4j in good yield.14 Cyclization of adducts 4a–4j under Mitsunobu conditions proceeded smoothly to deliver the 2-substituted 4-methylenepyrrolidines 5a–5j.15 The enantiomeric purity of 2 pyrrolidines 5d and 5j was evaluated, which revealed no erosion in enantiomeric purity occurred upon Mitsunobu cyclization
Table 3.
Conversion of adducts 3a–3j to 4-methylenepyrrolidines 5a–5j via Tsuji-Trost allylation-Mitsunobu cyclization.a
 |
Yields are of material isolated by silica gel chromatography. See Supporting Information for further experimental details.
After the first reaction was done, PPh3 (500 mol%), DIAD (500 mol%) and THF (0.05 M) were added at 25 °C, 12 h.
To illustrate the utility of 4-methylenepyrrolidines 5a–5j, compounds 5d and 5h were subjected to a series of functional group manipulations (Scheme 2). The synthesis of carboxamide 7d from compound 5d demonstrates facile removal of the ortho-nosyl protecting group and corroborates the anticipated inversion of stereochemistry in the Mitsunobu cyclization.15 4-Methylenepyrrolidine 5h is readily converted to the spirocyclopropane 6h,16 which embodies a structural motif evident in the FDA approved drug for the treatment of hepatitis C.17 Finally, oxidative cleavage18 of 4-methylenepyrrolidine 5h followed by exposure of the resulting ketone 7h to Deoxy-Fluor®19 delivers the gem-difluoride 8h.
Scheme 2.
Derivatization of 4-methylenepyrrolidines 5d and 5h.a

aYields are of material isolated by silica gel chromatography. See Supporting Information for further experimental details.
In conclusion, we report the enantioselective synthesis of 2-substituted-4-methylenepyrrolidines through successive nucleophilic and electrophilic allylation of bis-Boc-carbonate 2a. Whereas prior methods for the enantioselective synthesis of 2-substituted-4-methylenepyrrolidines involve TMM cycloadditions of moisture sensitive imine reactants and are restricted to 2-aryl-substituted adducts, the present protocol enables facile access to 2-alkyl-4-methylenepyrrolidines from highly tractable alcohol reactants.
Supplementary Material
Acknowledgments.
Acknowledgment is made to the Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445). The China Scholarship Council is acknowledged for predoctoral scholarship support (G.L. CSC.201707060020).
Footnotes
Notes.
The authors declare no competing financial interest.
Supporting Information Available. Spectral data for all new compounds (1H NMR, 13C NMR, IR, HRMS). Single crystal X-ray diffraction data for compounds 3i and 7d. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- (1).For selected reviews on metal catalysis for the synthesis of saturated N-heterocycles, including pyrrolidines, see: (a) Mitchinson A; Nadin A J. Chem. Soc., Perkin Trans 1, 2000, 2862–2892. [Google Scholar]; (b) Wolfe JP Stereoselective Synthesis of Saturated Heterocycles via Palladium-Catalyzed Alkene Carboetherification and Carboamination Reactions Synlett 2008, 2913–2937. [DOI] [PMC free article] [PubMed]; (c) Schultz DM; Wolfe JP Recent Developments in Palladium- Catalyzed Alkene Aminoarylation Reactions for The Synthesis of Nitrogen Heterocycles. Synthesis 2012, 44, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vo C-VT; Bode JW Synthesis of Saturated N-Heterocycles. J. Org. Chem 2014, 79, 2809–2815. [DOI] [PubMed] [Google Scholar]; (e) Race NJ; Hazelden IR; Faulkner A; Bower JF Recent Developments in the Use of aza-Heck Cyclizations for The Synthesis of Chiral N-Heterocycles. Chem. Sci 2017, 8, 5248–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).For selected reviews covering alternate approaches to the catalytic enantioselective synthesis of substituted pyrrolidines, see: (a) Han M-Y; Jia J-Y; Wang W. Recent Advances in Organocatalytic Asymmetric Synthesis of Polysubstituted Pyrrolidines. Tetrahedron Lett 2014, 55, 784–794. [Google Scholar]; (b) Adrio J; Carretero JC Recent Advances in The Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides. Chem. Commun 2014, 50, 12434–12446. [DOI] [PubMed] [Google Scholar]
- (3).For selected reviews highlighting the recurrence of N-heterocycles among FDA approved drugs, see: (a) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]; (b) Aldeghi M; Malhotra S; Selwood DL; Chan AWEC Two- and Three-dimensional Rings in Drugs. Chem. Biol. Drug Des 2014, 83, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- (4).(a) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (b) Lovering F Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Comm 2013, 4, 515–519. [Google Scholar]
- (5).The importance of stereochemical complexity is evident in a survey of recently approved drugs. Notably, pyrrolidine substructures appeared in 7 of the 19 drugs approved in 2016: Flick AC; Ding HX; Leverett CA; Fink SJ; O’Donnell CJ Synthetic Approaches to New Drugs Approved During 2016. J. Med. Chem 2018, 61, 7004–7031. [DOI] [PubMed] [Google Scholar]
- (6).For selected reviews on catalytic reductive C-C coupling via hydrogenation, transfer hydrogenation and hydrogen auto-transfer, see: (a) Hassan A; Krische MJ Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods. Org. Proc. Res. Devel 2011, 15, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem. Int. Ed 2014, 53, 9142–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim SW; Zhang W; Krische MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res 2017, 50, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).For selected reviews on enantioselective carbonyl allylation, see: (a) Ramachandran PV Pinane-based versatile “allyl” boranes. Aldrichim. Acta 2002, 35, 23–35. [Google Scholar]; (b) Denmark SE; Fu J Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem. Rev 2003, 103, 2763–2794. [DOI] [PubMed] [Google Scholar]; (c) Yu C-M; Youn J; Jung H-K Regulation of Stereoselectivity and Reactivity in the Inter- and Intramolecular Allylic Transfer Reactions. Bull. Korean Chem. Soc 2006, 27, 463–472. [Google Scholar]; (d) Marek I; Sklute G Creation of quaternary stereocenters in carbonylallylation reactions. Chem. Commun 2007, 1683–1691. [DOI] [PubMed]; (e) Hall DG Lewis and Brønsted Acid Catalyzed Allylboration of Carbonyl Compounds: From Discovery to Mechanism and Applications. Synlett 2007, 11, 1644–1655. [Google Scholar]; (f) Hargaden GC; Guiry PJ The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction. Adv. Synth. Catal 2007, 349, 2407–2424. [Google Scholar]; (g) Lachance H; Hall DG Allylboration of Carbonyl Compounds. Org. React 2008, 73, 1–596. [Google Scholar]; (h) Han SB; Kim IS; Krische MJ Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency. Chem. Commun 2009, 7278–7287. [DOI] [PMC free article] [PubMed]; (i) Yus M; González-Gómez JC; Foubelo F Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev 2011, 111, 7774–7854. [DOI] [PubMed] [Google Scholar]; (j) Moran J; Krische MJ Enantioselective Carbonyl Allylation and Crotylation from the Alcohol Oxidation Level via C-C Bond Forming Transfer Hydrogenation. Asymmetric Synthesis Ii: More Methods and Applications 2012, 187–196.; (k) Yus M; Gonzalez-Gomez JC; Foubelo F Diastereoselective Allylation of Carbonyl Compounds and Imines: Application to the Synthesis of Natural Products. Chem. Rev 2013, 113, 5595–5698. [DOI] [PubMed] [Google Scholar]; (l) Huo H-X; Duvall JR; Huang M-Y; Hong R Catalytic asymmetric allylation of carbonyl compounds and imines with allylic boronates. Org. Chem. Front 2014, 1, 303–320. [Google Scholar]; (m) Kumar P; Tripathi D; Sharma BM; Dwivedi N Transition metal catalysis—a unique road map in the stereoselective synthesis of 1,3-polyols. Org. Biomol. Chem 2017, 15, 733–761. [DOI] [PubMed] [Google Scholar]; (n) Spielmann K; Niel G; de Figueiredoa RM; Campagne JM Catalytic nucleophilic ‘umpoled’ π-allyl reagents. Chem. Soc. Rev 2018, 47, 1159–1173. [DOI] [PubMed] [Google Scholar]
- (8).For a related approach to the asymmetric synthesis of substituted pyrans, see: Shin I; Wang G; Krische MJ Catalyst-Directed Diastereo- and Site-Selectivity in Successive Nucleophilic and Electrophilic Allylations of Chiral 1,3-Diols: Protecting Group-Free Synthesis of 4-Hydroxy-2,6-cis- or trans-Pyrans. Chem. Eur. J 2014, 20, 13382–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).During the preparation of our manuscript, there appeared a report on the use of our π-allyliridium-C,O-benzoate catalysts in bidirectional carbonyl allylations using 3-chloro-2-chloromethyl-1-propene: Quintard A; Rodriguez J Enantioselective Ir-Catalyzed Bidirectional Reductive Coupling. Org. Lett 2019, 21, 453–456. [DOI] [PubMed] [Google Scholar]
- (10).For selected examples of related bifunctional reagents incorporating allylstannane, allylborane or allylsilane moieties: (a) Trost BM; King SA A Two Catalyst System for Cycloadditlon of a Trimethylenemethane Fragment to Aldehydes. Tetrahedron Lett 1986, 27, 5971–5974. [Google Scholar]; (b) Degl’Innocenti A; Dembech P; Mordini A; Ricci A; Seconi G [3 + n] Annulation Reactions by Means of 3-Trimethylstannyl-2-[(trimethylstannyl)methyl]propene, an Isobutene Dianion Synthetic Equivalent. Synthesis 1991, 267–269.; (c) D’Aniello F; Mattii D; Taddei M 2-(Chloromethyl)-2-(trimethylsilyl)-1-propene: A Convenient Reagent for the Synthesis of Methylenetetrahydrofurans and Methylenebutyrolactones. Synlett 1993, 2, 119–121. [Google Scholar]; (d) D’Aniello F; Mann A; Mattii D; Taddei M Stereoselective “Ene” Reaction of Allylsilanes with Amino Aldehydes. An Application to the Synthesis of Potential HIV-1 Protease Inhibitors. J. Org. Chem 1994, 59, 3762–3768. [Google Scholar]; (e) Ryter K; Livinghouse T [2-((Trimethylsilyl)methyl)prop-2-enyl]- lithium. A Versatile Reagent for the Synthesis of 2-Substituted Propenylsilanes. J. Org. Chem 1997, 62, 4842–4844. [Google Scholar]; (f) Kang K-T; Sung TM; Kim JK; Kwon YM Synthesis of Functionalized Allylsilanes Using 3-(Stannyl)-2-(silylmethyl)propene. Synth. Commun 1997, 27, 1173–1181. [Google Scholar]; (g) Barrett AGM; Braddock DC; de Koning PD Bidirectional Asymmetric Allylboration and The Synthesis of C2 Symmetric 3-Methylenepentane-1,5-Diols. Chem. Commun 1999, 459–460. [DOI] [PubMed]; (h) Barrett AGM; Braddock DC; de Koning PD; White AJP; Williams DJ Bidirectional Asymmetric Allylboration. A Convenient Asymmetric Synthesis of C2-Symmetric 3-Methylenepentane-1,5-diols and Rapid Access to C2-Symmetric Spiroketals. J. Org. Chem 2000, 65, 375–380. [DOI] [PubMed] [Google Scholar]; (i) Yu C-M; Lee J-Y; So B; Hong J Sequential Catalytic Asymmetric Allylic Transfer Reaction: Enantioselective and Diastereoselective Construction of Tetrahydropyran Units. Angew. Chem. Int. Ed 2002, 41, 161–163. [DOI] [PubMed] [Google Scholar]; (j) Keck GE; Yu T; McLaws MD Enantio- and Diastereoselective Additions to Aldehydes Using the Bifunctional Reagent 2-(Chloromethyl)-3- (tributylstannyl)propene: Application to a Synthesis of the C16- C27 Segment of Bryostatin 1. J. Org. Chem 2005, 70, 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Heumann LV; Keck GEA A New Construction of 2-Alkoxypyrans by an Acylation-Reductive Cyclization Sequence. Org. Lett 2007, 9, 1951–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Trost BM; Bringley DA; Silverman SM Asymmetric Synthesis of Methylenetetrahydrofurans by Palladium-Catalyzed [3 + 2] Cycloaddition of Trimethylenemethane with Aldehydes – A Novel Ligand Design. J. Am. Chem. Soc 2011, 133, 7664–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Williams DR; Claeboe CD; Liang B; Zorn N; Chow NSC A Bidirectional SE′ Strategy for 1,5-syn and 1,5-anti Stereocontrol toward the Synthesis of Complex Polyols. Org. Lett 2012, 14, 3866–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Ahlers A; de Haro T; Gabor B; Fürstner, A. Concise Total Synthesis of Enigmazole A. Angew. Chem. Int. Ed 2016, 55, 1406–1411. [DOI] [PubMed] [Google Scholar]; (o) Tekle-Smith MA; Williamson KS; Hughes IF; Leighton JL Direct, Mild, and General n-Bu4NBr-Catalyzed Aldehyde Allylsilylation with Allyl Chlorides. Org. Lett 2017, 19, 6024–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For the synthesis of 4-methylenepyrrolidines via TMM-imine cycloaddition, see: (a) Jones MD; Kemmitt RDWJ Reactions between Trimethylenemethane Metal Complexes and The Carbon–Nitrogen Double Bond: Nickel and Palladium Catalysed Synthesis of Pyrrolidines. J. Chem. Soc. Chem. Commun 1986, 1201–1203.; (b) Trost BM; Marrs CM A [3+2] Cycloaddition and [4+3] Cycloaddition Approach to N-Heterocycles via Palladium-Catalyzed TMM Reactions with Imines. J. Am. Chem. Soc 1993, 115, 6636–6645. [Google Scholar]; (c) Trost BM; Silverman SM; Stambuli JP Palladium-Catalyzed Asymmetric [3+2] Cycloaddition of Trimethylenemethane with Imines. J. Am. Chem. Soc 2007, 129, 12398–12399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Trost BM; Silverman SM Enantioselective Construction of Highly Substituted Pyrrolidines by Palladium-Catalyzed Asymmetric [3+2] Cycloaddition of Trimethylenemethane with Ketimines. J. Am. Chem. Soc 2010, 132, 8238–8240. [DOI] [PubMed] [Google Scholar]; (e) Trost BM; Silverman SM Enantioselective Construction of Pyrrolidines by Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition of Trimethylenemethane with Imines. J. Am. Chem. Soc 2012, 134, 4941–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Trost BM; Lam TM; Herbage MA Regio- and Enantioselective Synthesis of Pyrrolidines Bearing a Quaternary Center by Palladium-Catalyzed Asymmetric [3 + 2] Cycloaddition of Trimethylenemethanes. J. Am. Chem. Soc 2013, 135, 2459–2461. [DOI] [PubMed] [Google Scholar]
- (12).For approaches to racemic 4-methylenepyrrolidines beyond TMM-imine cycloaddition, see: (a) Scarborough CC; Stahl SS Synthesis of Pyrrolidines via Palladium(II)-Catalyzed Aerobic Oxidative Carboamination of Butyl Vinyl Ether and Styrenes with Allyl Tosylamides. Org. Lett 2006, 8, 3251–3254. [DOI] [PubMed] [Google Scholar]; (b) Martínez C; Muniz K An Iodine-Catalyzed Hofmann–Löffler Reaction. Angew. Chem. Int. Ed 2015, 54, 8287–8291. [DOI] [PubMed] [Google Scholar]
- (13).For use of bis-Boc-carbonate 2a in double Tsuji-Trost reactions of stabilized C-nucleophiles, see: Grenning AJ; Boyce JH; Porco JA Jr. Rapid Synthesis of Polyprenylated Acylphloroglucinol Analogs via Dearomative Conjunctive Allylic Annulation. J. Am. Chem. Soc 2014, 136, 11799–11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).For selected examples of Tsuji-Trost allylic amination employing sulphonamide nucleophiles, see: (a) Byström SE; Aslanian R; Bäckvall J-E Synthesis of Protected Allylamines via Palladium-Catalyzed Amide Addition to Allylic Substrates. Tetrahedron Lett 1985, 26, 1749–1752. [Google Scholar]; (b) Bäckvall J-E; Renko ZD; Byström SE Tetrahedron Lett 1987, 28, 4199–4202. [Google Scholar]; (c) Bäckvall J-E; Schink HE; Renko ZD A Stereocontrolled Organopalladium Route to 2,5-Disubstituted Pyrrolidine Derivatives. Application to the Synthesis of a Venom Alkaloid of the Ant Species Monomorium latinode. J. Org. Chem 1990, 55, 826–831. [Google Scholar]; (d) Jumnah R; Williams JMJ; Williams AC Synthesis of N-Protected Amino Esters via Palladium Catalysed Allylic Substitution. Tetrahedron Lett 1993, 34, 6619–6622. [Google Scholar]; (e) Lotz M; Kramer G; Knochel P Facile Axial Chirality Control by Using a Precursor with Central Chirality. Application to the Preparation of New Axially Chiral Diphosphine Complexes for Asymmetric Catalysis. Chem. Commun 2002, 2546–2547.; (f) Feng B; Cheng H-G; Chen J-R; Deng Q-H; Lu L-Q; Xiao W-J Palladium/Sulfoxide–Phosphine-Catalyzed Highly Enantioselective Allylic Etherification and Amination. Chem. Commun 2014, 9550–9553. [DOI] [PubMed]
- (15).For a review encompassing the Mitsunobu reaction of nitrobenzenesulfonamides, see: Kan T; Fukuyama T Ns Strategies: A Highly Versatile Synthetic Method for Amines. Chem. Commun 2004, 353–359. [DOI] [PubMed]
- (16).Tymtsunik AV; Bilenko VA; Ivon YM; Grygorenko OO; Komarov IV Tetrahedron Lett 2012, 53, 3847–3849. [Google Scholar]
- (17).Gentile I; Buonomo AR; Borgia F; Castaldo G; Borgia G Ledipasvir: A Novel Synthetic Antiviral for The Treatment of HCV Infection. Expert Opin. Investig. Drugs 2014, 23, 561–571. [DOI] [PubMed] [Google Scholar]
- (18).Watson DW; Gill M; Kemmitt P; Lamont SG; Popescu MV; Simpson I An Investigation into The Role of 2,6-Lutidine as an Additive for The RuCl3-NaIO4 Mediated Oxidative Cleavage of Olefins to Ketones. Tetrahedron Lett 2018, 59, 4479–4482. [Google Scholar]
- (19).Reddy KM; Bhimireddy E; Thirupathi B; Breitler S; Yu S; Corey EJ Cationic Chiral Fluorinated Oxazaborolidines. More Potent, Second-Generation Catalysts for Highly Enantioselective Cycloaddition Reactions. J. Am. Chem. Soc 2016, 138, 2443–2453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


