Abstract
Objectives:
To determine the distribution of semen parameters among adolescent and adult males presenting for fertility preservation.
Methods:
A retrospective, cross-sectional cohort study of adolescent males age 11–19 who underwent semen analysis (SA) for fertility preservation at three centers in two countries with a comparison cohort of adult men presenting for fertility preservation. Prevalence of azoospermia and distribution of semen parameters was compared across groups.
Results:
A total of 197 adolescents and 95 adults underwent SA for fertility preservation. Azoospermia was present in 17 (8.6%) adolescents and 3 (3.2%) adults. There was decline in the prevalence of azoospermia with increasing age. After exclusion of patients with azoospermia, the adolescent and adult cohorts were comprised of 180 and 92 patients, respectively. Median age at presentation among adolescents versus adults was 16.5 years (interquartile range [IQR] 15.2–17.6) and 30.8 years (IQR 22.7–43.8), respectively. Median semen volume was 1.0mL (interquartile range [IQR] 0.5–2.0) versus 2.5mL (IQR 1.5–3.5), p<0.001. Median sperm concentration was 30 million/mL (IQR 10–57) versus 39 million/mL (IQR 14–57), p=0.2. Median sperm motility was 39% (IQR 20–55) versus 45% (IQR 35–55), p=0.01. Median total motile sperm count was 11 million (IQR 1.4–33) for adolescents versus 29 million (IQR 13–69) for adults, p<0.001.
Conclusion:
Young adolescent males had higher prevalence of azoospermia and lower semen parameters compared to adults. In conjunction with physical examination, Tanner stage, and specific clinical context, these data can help to inform patients and their families about potential for fertility preservation, even in very young adolescent patients.
Introduction
While semen parameters are not diagnostic of male infertility, they are crucial drivers of treatment decisions in the management of adolescent male patients with fertility concerns.(1) Varicocele is associated with impaired semen parameters, and varicocele repair is often pursued with the goal of improved semen parameters and fertility.(2) Likewise, semen parameters may impact fertility preservation among adolescents with malignancy, such as the decision to pursue testis biopsy, the extent of sperm cryopreservation.(3,4) However, the lack of established reference values for adolescent semen parameters substantially impairs their utility in these clinical settings.
The commonly referenced World Health Organization (WHO) criteria are based on fertile adult men only, and semen parameters consistent with subfertility in adults may not harbor the same prognostic significance in adolescents on the spectrum of puberty and Tanner stage development.(5) Attempts to collect semen analyses from healthy adolescents to establish reference ranges will be hampered by barriers pertaining to practical and ethical concerns of internal review boards, patient and parental consent.(6) While prior studies have characterized adolescent semen parameters in the setting of fertility preservation, the lack of a comparison cohort renders it difficult to discern the extent to which impaired adolescent semen parameters are due to puberty or the presence of malignancy.(3,7)
Therefore, we sought to compare the distribution of semen parameters in a multicenter, international cohort of adolescent and adult males presenting for fertility preservation in order to inform patients and providers confronted with fertility preservation prior to cancer therapy, as well as to potentially offer insight into the management of other conditions, such as varicocele, pertaining to fertility in the adolescent male population.
Materials and Methods
We performed a retrospective review of all adolescent males age 11 through 19 years who had semen analysis (SA) at one of three tertiary care centers in two countries (United States and United Kingdom).(8) Patients presented for sperm banking prior to treatment of malignancy from 2010 to 2017. Those with a history of testis cancer or systemic chemotherapy prior to SA were excluded as these clinical characteristics may impact semen parameters.(9–11) Patients who had SA for evaluation of varicocele were also excluded. A comparison adult cohort consisted of men who presented for sperm banking prior to treatment of malignancy at a single institution (Houston, TX) during the study period with the same exclusion criteria.
Semen parameters (semen volume, sperm concentration, motility) were measured at the local laboratory for each participating site. Each patient provided at least one semen sample. In patients who subsequently provided additional samples, the mean semen parameters of all samples for that individual was utilized for the analysis. Initial evaluation was performed on the uncentrifuged specimen. In the event that cryptozospermia or azoospermia was noted, the specimen was centrifuged for further analysis.
The prevalence of azoospermia in the adult and adolescent cohorts was determined based upon SA at presentation. Patients with azoospermia on one sample but presence of sperm on prior or subsequent samples were considered oligospermic. Prevalence of azoospermia in the adolescent cohort was compared to that of the adult cohort using chi-squared test. This comparison was performed to aid in determining the extent to which azoospermia in the adolescent population was due to the patients being pre-pubescent or due to an effect of the patients’ cancer diagnoses. If azoospermia in adolescents was predominantly driven by malignancy, we would expect similar rates of azoospermia cross all adolescent and adult age groups. In contrast, if azoospermia in adolescents was predominantly driven by progression of puberty or lack thereof, we would expect variation in the prevalence of azoospermia across age groups. While use of age as a surrogate for puberty is confounded by the lack of Tanner stage and data regarding the presence of varicocele, it can provide insight with regard to potential etiology of azoospermia.
The prevalence of azoospermia in the adolescent cohort was further stratified by age at one-year intervals in order to provide a more detailed assessment of differences between younger and older adolescents, though no statistical testing was performed as a result of power concerns secondary to the low number of young adolescents in the cohort. For descriptive analyses of cohort semen parameters, patients with azoospermia were excluded since they will likely represent distinct pathologies such as puberty or genetic abnormalities. Cohort demographic and clinical characteristics including age and duration of abstinence prior to SA were described for all non-azoospermic adolescent patients.
Semen parameters for the overall cohort were described according to quartile with additional reporting of the 5th and 95th percentile as a reference given the methodology of the aforementioned WHO guidelines, which utilize the 5th percentile as the lower bound of “normal.”(12) Distribution of semen parameters between adolescents and adults was compared using the Wilcoxon rank-sum test.
Statistical significance for all testing was determined at a p-value of 0.05. All statistical analysis was performed using Stata version 13.0 (StataCorp, College Station, TX). Institutional review board (IRB) approval was obtained for each participating institution.
Results
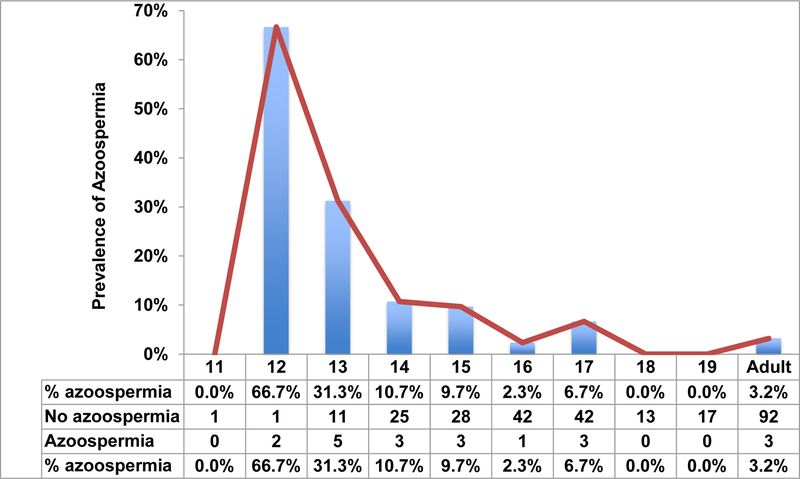
A total of 197 adolescent males underwent SA for fertility preservation during the study period of whom 17 (8.6%) had azoospermia. As expected, there was a decline in the prevalence of azoospermia with increasing age (Figure 1). In comparison, a total of 95 adult men with cancer had SA in whom 3 (3.2%) had azoospermia (p=0.08).
Figure 1:
Prevalence of azoospermia among adolescent males according to age
After exclusion of adolescents with azoospermia, the adolescent cohort was comprised of 180 patients from the United States (N=84, 46.7%) and the United Kingdom (N=96, 53.3%). Median age at presentation was 16.5 years (interquartile range [IQR] 15.2–17.6)
After exclusion of adults with azoospermia, the adult cohort was comprised of 92 patients. Median age at presentation was 30.8 years (IQR 22.7–43.8).
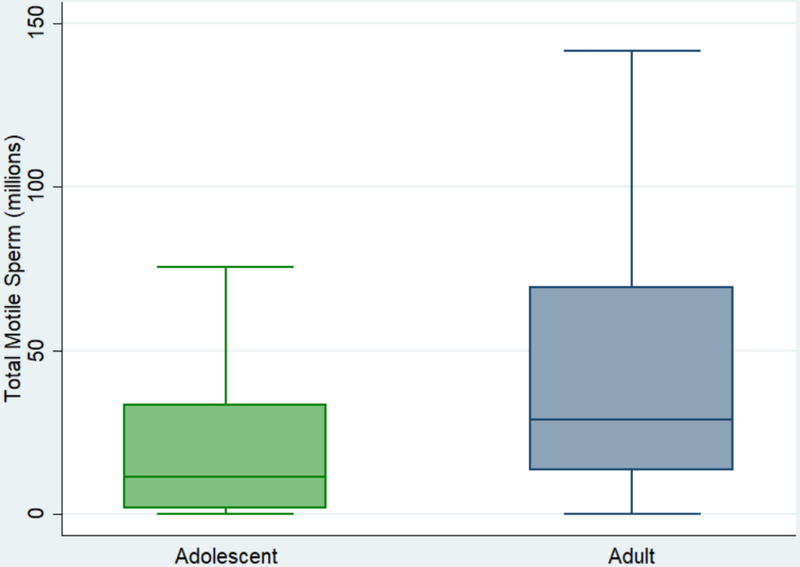
Semen parameters for the adolescent and adult cohorts are presented in Table 1. Median abstinence prior to SA was 4 days (IQR 3–5) for adolescents and 5 days (IQR 3–7) for adults. Median semen volume was 1.0mL (interquartile range [IQR] 0.5–2.0) for adolescents versus volume 2.5mL (IQR 1.5–3.5) for adults, p<0.001. Median sperm concentration was 30 million/mL (IQR 10–57) for adolescents versus 39 million/mL (IQR 14–57) for adults, p=0.2. Median sperm motility was 39% (IQR 20–55) for adolescents versus 45% (IQR 35–55) for adults, p=0.01. Median total motile sperm count was 11 million (IQR 1.4–33) for adolescents versus 29 million (IQR 13–69) for adults, p<0.001. A boxplot presenting the distributions of total motile sperm count is presented in Figure 2.
Table 1:
Adolescent and adult semen parameters
| 25th Percentile | 50th Percentile | 75th Percentile | p-value^ | ||||
|---|---|---|---|---|---|---|---|
| Adolescent | Adult | Adolescent | Adult | Adolescent | Adult | ||
| Concentration (mil/mL) | 10 | 14 | 30 | 39 | 57 | 57 | 0.2 |
| Volume (mL) | 0.5 | 1.5 | 1.0 | 2.5 | 2.0 | 3.5 | <0.001 |
| Motility (%) | 20 | 35 | 39 | 45 | 55 | 55 | 0.01 |
| Total motile count (mil) | 1.5 | 13 | 11 | 29 | 33 | 69 | <0.001 |
Wilcoxon-Mann-Whitney test
Figure 2:
Boxplot distribution of total motile sperm count, adolescent versus adult (box represents median and interquartile range)
The 5th percentile for all semen parameters among adolescents and adults is presented in Table 2. Across all semen parameters, both adolescent and adult parameters were lower than the WHO reference ranges.
Table 2:
5th percentile lower bound of “normal” SA for adolescent, adult, and World Health Organization (WHO) cohorts10
| Adolescent | Adult | WHO* | WHO^ | |
|---|---|---|---|---|
| Concentration (million/mL) | 1 | 3 | 9 | 15 |
| Volume (mL) | 0.2 | 0.6 | 1.2 | 1.5 |
| Motility (%) | 0 | 13 | 26 | 40 |
| Total motile count (mil) | 0 | 1.3 | - | - |
General population of unscreened men;
Fertile men whose partners had time-to-pregnancy of less than 12 months
Discussion
There is a paucity of data examining semen parameters in adolescent males, which is further complicated by the inherent variation in timing and progression of puberty among adolescents. The complexity of this issue and absence of adolescent-specific parameters is particularly challenging for management of adolescent conditions such as varicocele and malignancy, wherein future fertility potential is a crucial component of clinical decision-making. Studies of adolescent semen parameters have been hindered by practical and ethical concerns from internal review boards, providers, and parents. The ideal study would evaluate middle- and high-school volunteers with history, physical exam, and two semen samples after a pre-defined period of abstinence. This would also enable the exclusion of patients with cancer, varicocele, or other potential confounding medical history. As such a study is not technically feasible due to the aforementioned barriers, we sought to retrospectively compare semen parameters in adolescents and adults presenting for fertility preservation in the context of malignancy.
Two recent retrospective studies have examined semen parameters in an adolescent fertility preservation cohort. Dinofia et al. identified adolescents who were at least Tanner stage III and newly diagnosed with malignancy, some of whom had already received chemotherapy.(3) The study was limited by exclusion of adolescents with early Tanner stage who may have had spermatogenesis, as spermarche is an early pubertal event.(13) Daudin et al. reported data from a large, multi-center, national cohort over three decades.(7) While the largest series in the literature, the study did not report the prevalence of azoospermia in the adolescent fertility preservation cohort. Furthermore, the data was accrued across many different laboratories and over the course of a long time period, which may render the results subject to substantial variation in laboratory techniques and data collection. Nonetheless, both studies described baseline semen parameters in adolescents with malignancy, thereby helping to establish a reference range for these patients. The current study supports and expands upon the findings of these prior studies.
We found that the prevalence of azoospermia in adolescents decreased substantially with age and ultimately approached those of adults. We hypothesized that the prevalence of azoospermia in the adolescent population was likely driven by puberty. However, to exclude the possibility that azoospermia in the adolescent cohort was driven by a diagnosis of malignancy in a substantial portion of patients, we assembled a comparison cohort of adult men with malignancy. Using age as a surrogate for pubertal state, the higher prevalence of azoospermia in the adolescent cohort suggested that this was likely due to variations in onset of puberty. If azoospermia in adolescents was driven by malignancy, we would have expected similar rates of azoospermia across all adolescent and adult age groups, though this assumption is confounded by the inherent differences in the effects of adolescent and adult malignancies on spermatogenesis and the lack of physical exam (Tanner stage and varicocele).(14–16) These findings are consistent with DiNofia et al. showing decreasing prevalence of azoospermia across increasing age groups, though the absolute rates of azoospermia are lower in the current study.(3)
Figure 1 is very useful for counseling younger patients age 11–13 who present with malignancy or other fertility-related concerns. In the former circumstance, these data demonstrate that even very young adolescent boys may have spermatogenesis. Whereas some providers or family members might overlook the potential for fertility preservation in these young adolescent boys, the current data reinforce the importance of discussing and offering fertility preservation in even the youngest patients. In the latter circumstance, most commonly a clinical varicocele, these data can provide some reassurance that azoospermia is likely related to puberty rather than varicocele pathology, particularly when this is corroborated by Tanner stage. Though lack of Tanner stage and varicocele data in the current study unfortunately precludes the specific association of azoospermia with puberty, the high prevalence of azoospermia in younger patients relative to older patients suggests that azoospermia in this age range is likely to be puberty-related.
We found that the distribution of semen volume, sperm motility, and total motile sperm count in adolescents was significantly different from those of adults. These data are consistent with those of DiNofia et al. and Daudin et al., who previously demonstrated a correlation between these semen parameters and age, which likely reflects progression of puberty.(3,7) In contrast, whereas both Dinofia et al. and Daudin et al. did observe a slight association between age and sperm concentration, we did not observe a difference in sperm concentration between the two groups.(3,7) In the current study, there does appear to be a much lower sperm concentration in both adult and adolescent fertility preservation groups compared to the WHO reference ranges, which may be due to the inherent effects of malignancy on testicular function.(16) Similar to these findings in the context of malignancy, sperm concentration is lower but not correlated with age amongst adolescent varicocele patients, whereas semen volume and motility are positively correlated with age.(17) In aggregate, these findings suggest that sperm concentration may be most dependent upon non age-related gonadotoxic risk factors such as malignancy and varicocele, whereas semen volume and motility are age-dependent phenomenon, increasing with age and progression of puberty irrespective of potential gonadotoxic insult.
Our results must be evaluated in the context of the strengths and limitations of our study design. The multi-institutional and international nature of our cohort mitigates confounders and renders the results broadly applicable and generalizable. We attempted to minimize heterogeneity by excluding adolescent and adult men with known varicoceles or pertinent history such as testis cancer, cryptorchidism or orchiectomy that may affect spermatogenesis. Additionally, the inclusion of a comparison adult cohort sheds light upon the etiology of azoospermia in the adolescent population. In this regard, the findings further support and expand upon those of prior studies.(3,7)
The results are limited by the lack of specific clinical data such as Tanner stage, type of malignancy, and physical examination for the presence of varicocele. Ultimately, the interpretation of these results is contingent upon use of age as a surrogate for pubertal status in the absence of these data and without controlling for these potential confounders. Variability in laboratory sample processing and reporting (ex: counting chambers) across sites introduced variability, thereby limiting regional comparisons and reducing the accuracy of results from the overall cohort.(18) We evaluated semen parameters from a single SA in the majority of patients, which likely decreased the precision of semen parameter measurement given the substantial variation in semen parameters between consecutive samples from the same patient.(19) Additionally, the distinct malignancy profiles of adolescents versus adults likely contributed to variation between the two groups.
Conclusions
We present the first international, multi-institutional comparison of adolescent and adult semen parameters in the setting of fertility preservation. Young adolescent males had a higher prevalence of azoospermia compared to adults, and the distribution of semen parameters was significantly lower in adolescents compared to adults. The current data build upon prior studies to establish a frame of reference for providers, patients, and families who are confronted with fertility concerns in the context of adolescent malignancy and may further provide insight for patients presenting with azoospermia in the context of adolescent varicocele. In conjunction with physical examination, Tanner stage, and specific clinical context, these data can help to inform patients and their families about potential for fertility preservation, even in very young adolescent patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001. November 8;345(19):1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Chu DI, Zderic SA, Shukla AR, Srinivasan AK, Tasian GE, Weiss DA, et al. Does varicocelectomy improve semen analysis outcomes in adolescents without testicular asymmetry? J Pediatr Urol. 2017. February;13(1):76.e1–76.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiNofia AM, Wang X, Yannekis G, Ogle S, Hobbie WL, Carlson CA, et al. Analysis of semen parameters in a young cohort of cancer patients. Pediatr Blood Cancer. 2017. February;64(2):381–386. [DOI] [PubMed] [Google Scholar]
- 4.Ming JM, Chua ME, Lopes RI, Maloney AM, Gupta AA, Lorenzo AJ. Cryopreservation of testicular tissue in pre-pubertal and adolescent boys at risk for infertility: A low risk procedure. J Pediatr Urol. 2018. June;14(3):274.e1–274.e5. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz MP, Zurakowski D, Rosoklija I, Bauer SB, Borer JG, Johnson KL, et al. Semen parameters in adolescents with varicocele: association with testis volume differential and total testis volume. J Urol. 2015. May;193(5 Suppl):1843–1847. [DOI] [PubMed] [Google Scholar]
- 6.Fine RG, Gitlin J, Reda EF, Palmer LS. Barriers to use of semen analysis in the adolescent with a varicocele: Survey of patient, parental, and practitioner attitudes. J Pediatr Urol. 2016. February;12(1):41.e1–6. [DOI] [PubMed] [Google Scholar]
- 7.Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, et al. Sperm cryopreservation in adolescents and young adults with cancer: results of the French national sperm banking network (CECOS). Fertil Steril. 2015. February;103(2):478–86.e1. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Adolescent Health [Internet]. [cited 2018 Jul 15]. Available from: http://www.who.int/topics/adolescent_health/en/
- 9.Djaladat H, Burner E, Parikh PM, Beroukhim Kay D, Hays K. The association between testis cancer and semen abnormalities before orchiectomy: systematic review. J Adolesc Young Adult Oncol. 2014. December 1;3(4):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semet M, Paci M, Saïas-Magnan J, Metzler-Guillemain C, Boissier R, Lejeune H, et al. The impact of drugs on male fertility: a review. Andrology. 2017. June 16;5(4):640–663. [DOI] [PubMed] [Google Scholar]
- 11.Williams DH, Karpman E, Sander JC, Spiess PE, Pisters LL, Lipshultz LI. Pretreatment semen parameters in men with cancer. J Urol. 2009. February;181(2):736–740. [DOI] [PubMed] [Google Scholar]
- 12.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010. June;16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen CT, Skakkebaek NE, Richardson DW, Darling JA, Hunter WM, Jørgensen M, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986. March;62(3):532–535. [DOI] [PubMed] [Google Scholar]
- 14.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014. April;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 15.Paoli D, Rizzo F, Fiore G, Pallotti F, Pulsoni A, Annechini G, et al. Spermatogenesis in Hodgkin’s lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod. 2016. February;31(2):263–272. [DOI] [PubMed] [Google Scholar]
- 16.Meirow D, Schenker JG. Cancer and male infertility. Hum Reprod. 1995. August;10(8):2017–2022. [DOI] [PubMed] [Google Scholar]
- 17.Keene DJ, Cervellione RM. Total motile count increases with age in adolescentvaricocele patients; sperm concentration is an age independent parameter. European Society of Paediatric Urology; 2018. [Google Scholar]
- 18.Filimberti E, Degl’Innocenti S, Borsotti M, Quercioli M, Piomboni P, Natali I, et al. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): the experience of an external quality control (EQC) programme. Andrology. 2013. May;1(3):401–407. [DOI] [PubMed] [Google Scholar]
- 19.Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril. 2006. January;85(1):128–134. [DOI] [PubMed] [Google Scholar]